Abstract
Aims
There are few proven strategies to enhance physical activity and cardiometabolic profiles in patients with type 2 diabetes and hypertension. We examined the effects of physician‐delivered step count prescriptions and monitoring.
Methods
Participants randomized to the active arm were provided with pedometers and they recorded step counts. Over a 1‐year period, their physicians reviewed their records and provided a written step count prescription at each clinic visit. The overall goal was a 3000 steps/day increase over 1 year (individualized rate of increase). Control arm participants were advised to engage in physical activity 30 to 60 min/day. We evaluated effects on step counts, carotid femoral pulse wave velocity (cfPWV, primary) and other cardiometabolic indicators including haemoglobin A1c in diabetes (henceforth abbreviated as A1c) and Homeostasis Model Assessment‐Insulin Resistance (HOMA‐IR) in participants not receiving insulin therapy.
Results
A total of 79% completed final evaluations (275/347; mean age, 60 years; SD, 11). Over 66% of participants had type 2 diabetes and over 90% had hypertension. There was a net 20% increase in steps/day in active vs control arm participants (1190; 95% CI, 550‐1840). Changes in cfPWV were inconclusive; active vs control arm participants with type 2 diabetes experienced a decrease in A1c (−0.38%; 95% CI, −0.69 to −0.06). HOMA‐IR also declined in the active arm vs the control arm (ie, assessed in all participants not treated with insulin; −0.96; 95% CI, −1.72 to −0.21).
Conclusions
A simple physician‐delivered step count prescription strategy incorporated into routine clinical practice led to a net 20% increase in step counts; however, this was below the 3000 steps/day targeted increment. While conclusive effects on cfPWV were not observed, there were improvements in both A1c and insulin sensitivity. Future studies will evaluate an amplified intervention to increase impact.
Keywords: arterial stiffness, carotid femoral pulse wave velocity, diabetes, hypertension, pedometer, physical activity, step counter
1. INTRODUCTION
In adults with type 2 diabetes, self‐reported regular walks lead to a greater‐than‐40% reduction in both mortality and vascular event rates over the decade that follows the walking assessment.1, 2, 3 Pedometers and accelerometers capture “steps” in real time; in recent years, there has been a surge of popular interest in these devices as a means of tracking physical activity, setting targets and, thereby, improving health. In a longitudinal evaluation among adults with prediabetes, a 2000 steps/day increase over 1 year was associated with an 8% reduction in vascular complications over an average of 6 years.4 These findings support the case for integrating step count monitoring into the clinical management of individuals at high cardiometabolic risk.
Pedometer‐based intervention trials have generally focused on group‐based sessions led by a facilitator who works with group members to set goals and develop action plans. Such trials demonstrate an increase of roughly 2000 steps/day over a 3 to 6‐month intervention period.5, 6 Unfortunately, many pedometer‐based interventions are time‐limited, and there is evidence that step counts decline over time once the intervention ends.7 In contrast, patients have long‐term relationships with their treating physicians and there are no direct costs to patients associated with clinical follow‐up in a publicly‐funded health care system. Thus, physician‐delivered interventions are potentially sustainable. However, physicians cite many barriers to providing counseling concerning physical activity, including lack of resources, effective tools, time and training.8, 9, 10
With an eye to sustainability and feasibility, we developed a pedometer‐based intervention that was designed to be easily integrated into clinic visits for patients with type 2 diabetes and/or hypertension. The SMARTER trial (Step Monitoring to improve ARTERial health; ClinicalTrials.gov NCT0147520) evaluated a pedometer‐based intervention that was integrated into real‐world clinical practice settings where adults with type 2 diabetes and/or hypertension are followed. The intervention, delivered by the patient's own physician, was designed to be short, simple and easily integrated into clinic visits, to allow for sustainability. It involved goal‐setting, summarized in a “step count prescription.” “Steps” may be easier for physicians to prescribe than more complex forms of exercise. The change in step counts over the 1‐year intervention was compared between active and control arms.
Our aim was not only to capture an impact of the intervention on physical activity, but also to gauge its biological effects. Thus, we evaluated changes in several cardiometabolic measures. Primary outcome was change in carotid femoral pulse wave velocity (cfPWV). This is a non‐invasive measure that reflects the composite effects of all vascular risk factors on arterial health, and is considered to be a summative indicator of arterial health. It is an independent predictor of cardiovascular events.11, 12, 13
2. MATERIALS AND METHODS
The study design and methods have been described previously (SMARTER; Step Monitoring to improve ARTERial health; Clinicaltrials.gov NCT01475201; registered November 16, 2011; first patient recruited February 14, 2012).14 Briefly, SMARTER was a prospective, randomized, open‐label, blinded‐endpoint (PROBE) trial. Written informed consent was obtained and trial procedures were approved by McGill University's Faculty of Medicine Institutional Review Board (A08‐M76‐11B) and participating institutions (McGill University Health Centre, St. Mary's Hospital, Jewish General Hospital, Institut de Recherches Cliniques de Montréal).
A total of 74 physicians identified potentially eligible participants during routine clinic visits (2012‐2015). Eligibility criteria included a diagnosis of type 2 diabetes, hypertension, or both; age of ≥18 years; body mass index (BMI) of 25 to 40 kg/m2; and absence of gait impairment. Individuals with co‐morbid conditions with potential to impact procedures/outcomes (eg, active malignancy, pregnancy) were not enrolled, and those who reported 150 minutes or more of leisure‐time physical activity/week were also excluded. If cfPWV could not be assessed at the baseline evaluation (eg, because of atrial fibrillation or other arrhythmias), candidates were not randomized. Similarly, candidates were excluded if pedometer‐recorded step counts were 10 000/day or more during the 1‐week evaluation phase that was a component of the baseline assessment. Although increases in physical activity are arguably beneficial, irrespective of baseline activity levels, the greatest benefits are likely to be realized by the least fit and least active.15, 16
2.1. Ethics approval and consent to participate
Written informed consent was obtained and trial procedures were approved by McGill University's Faculty of Medicine Institutional Review Board (A08‐M76‐11B) and participating institutions (McGill University Health Centre, St. Mary's Hospital, Jewish General Hospital, Institut de Recherches Cliniques de Montréal).
2.2. Measurements
Participants completed questionnaires addressing demographic factors and medical history. Health behaviours and medications were queried and measurements were completed at both baseline and final assessments. Type 2 diabetes, hypertension and dyslipidemia diagnoses were based on physician‐reported diagnosis, use of corresponding medications and/or participant‐reported diagnosis. Duration of disease was acquired by participant report. Participants wore a Yamax SW‐701 pedometer at the waist for one week. The viewing window was concealed with a snap‐on cover and tamper‐proof seal.14 The pedometer was sent to the study centre with an unused pedometer (pre‐stamped envelope) that captured the “extra” step counts registered during mailing. These extra steps were subtracted from the value on the pedometer that was worn and steps/day were computed.
All measurements were performed in the morning under standardized conditions at the Vascular Health Unit, Montreal General Hospital, Montreal (Director, S.S. Daskalopoulou). Participants were fasting and were specifically instructed to abstain from caffeinated beverages, ethanol intake and smoking for at least 12 hours prior to assessment. All usual medications, other than antihyperglycaemic agents, were taken the morning of assessment. cfPWV was assessed in duplicate in a supine position after a 10‐minute rest using applanation tonometry (SphygmoCor system, AtCor Medical, Sydney, Australia) and values were averaged.17, 18, 19 Specifically, a micromanometer‐tipped tonometer (SPC‐301; Millar Instruments, Houston, Texas) was placed on the skin overlaying the carotid artery and the femoral artery. With this instrument and a 3‐lead electrocardiogram (ECG), the PWV was automatically calculated from measurements of the pulse transit time and the distance between the 2 recording sites [PWV = distance (m)/transit time (s)].17, 18, 19 Transit time was measured from the foot of the carotid waveform to that of the femoral waveform (foot‐to‐foot method) using sequential recordings referenced to the ECG. The distance was defined as (distance from the suprasternal notch to femoral artery) – (distance from carotid artery to the suprasternal notch), and was measured directly with a measuring tape.17, 18, 19 cfPWV was selected as the primary outcome because it is a composite indicator of arterial health and has the potential to capture the effects of physical activity on arterial health, including both those mediated by traditional cardiometabolic risk factors and those operating through other pathways.20, 21 Height, weight and waist and hip circumference were measured using standard procedures. BMI and waist‐to‐hip ratio were calculated. Peripheral blood pressure and heart rate were assessed with an automated oscillometric BpTRU Blood Pressure Monitor (BpTRU Medical Devices Ltd, BC, Canada; 6 automated measures, the first discarded and the average of the final 5 measures separately generated for systolic and diastolic blood pressure values).22 Fasting venous blood samples were drawn in the morning to measure total cholesterol, high density lipoprotein cholesterol (HDL) and triglycerides using spectrophotometry. Haemoglobin A1c (in diabetes patients; henceforth abbreviated as A1c) was measured with a high‐performance liquid chromatography analyser. In participants with type 2 diabetes who were not undergoing insulin therapy, and in all participants without type 2 diabetes, fasting glucose and insulin values were also assessed and were used to compute the Homeostatic Model Assessment‐Insulin Resistance value (HOMA‐IR).23 Low‐density lipoprotein cholesterol levels (LDL) were calculated using the Friedewald equation.
2.3. Randomization
Eligible individuals were randomized to either the control arm or the active trial arm using Dacima Clinical software (ie, individual‐level randomization with no stratification; random permuted blocks with randomly‐varied block sizes of 2, 4 and 6).
2.4. Interventions
Participants were typically seen by their physician in a clinical setting 3 to 4 times over a 12 to 15‐month period. The control arm received advice to engage in 30 to 60 minutes of activity daily, consistent with usual care. In the active arm, the physician wrote a step count prescription at each visit, as previously described in our protocol.14 At the first visit, the physician received a package with a pedometer and step count log for the participant, a package of “step count prescription” scripts and the baseline steps/day as assessed during baseline evaluation. The aim was to achieve a net increase over baseline of 3000 steps/day over 1 year. A step count increment of 2500 to 3000 steps is roughly equivalent to 30 minutes of walking at a moderate pace, as established through direct counts of individuals walking on a treadmill at a workload of 3 metabolic equivalents (METS)/minute.24
The speed at which the overall step target was achieved was determined during discussions between the patient and physician at the initial and follow‐up clinic visits, with some general guidelines provided. We recommended a slower rate of increase for those with lower step counts.14 Specifically, the time‐frame for an increase of 3000 steps/day was recommended for 10 months for sedentary participants (<5000 steps/day), for 7 months for low‐active participants (5000‐7499 steps/day) and for 5 months for somewhat active participants (7500‐9999 steps/day). A SMARTER trial research assistant reminded physicians when an active trial participant should be seen, either personally or by telephone or email. The SMARTER intervention did not include specific counseling related to dietary intake and eating behaviour. However, the treating physician could provide dietary counselling similarly in both trial arms according to usual care practice.
2.5. Statistical analysis
Means, SD, number and proportions were calculated, as appropriate, for all variables measured, separately for those in the active and control arms. We calculated average between‐arm differences in “after minus before values,” with 95% CIs for cfPWV, steps/day, other cardiometabolic risk factors (systolic and diastolic blood pressure, A1c, LDL, HDL, total cholesterol, triglycerides, HOMA‐IR, BMI, waist circumference, waist‐to‐hip ratio) and changes in numbers of antihypertensive, antihyperglycemic and lipid lowering medications. Differences in mean changes between the active and control arms were not adjusted; the mean changes for each trial arm were mean within‐individual changes and both arms were similar in terms of baseline cfPWV, step counts and other cardiometabolic risk factors. Intent‐to‐treat analyses with complete cases (ie, outcome assessment) were conducted for all endpoints. Additionally, for changes in cfPWV and pedometer‐assessed step counts, we used multiple imputations with ignorable and non‐ignorable mechanisms. We compared point estimates across these models (intent‐to‐treat analyses). Imputations were performed using WinBUGS version 1.4.3 and other analyses were conducted with SAS software (Version 9.3).
In terms of process evaluation and fidelity to trial intervention strategies, we examined the time interval between baseline and final assessments, and the number of clinical visits during the intervention period. For participants from whom we were able to obtain the final written prescription and who completed the final evaluation, we calculated the difference between the final step count prescription and target step counts (ie, baseline steps + 3000 steps/day) and the difference between the final evaluation of steps/day and the final prescription (mean and SD).
3. RESULTS
Among the 692 individuals who underwent preliminary assessment of eligibility by telephone or in clinical waiting areas, 392 were invited for a baseline assessment and 369 completed this evaluation (Figure 1). Our original recruitment target was 364 individuals.14 Among those who completed the baseline evaluation, 22 were determined to have an average step count that was ≥10 000 steps/day and were excluded. The remaining 347 were randomized. Final assessments were completed by 275 participants (79%).
Figure 1.
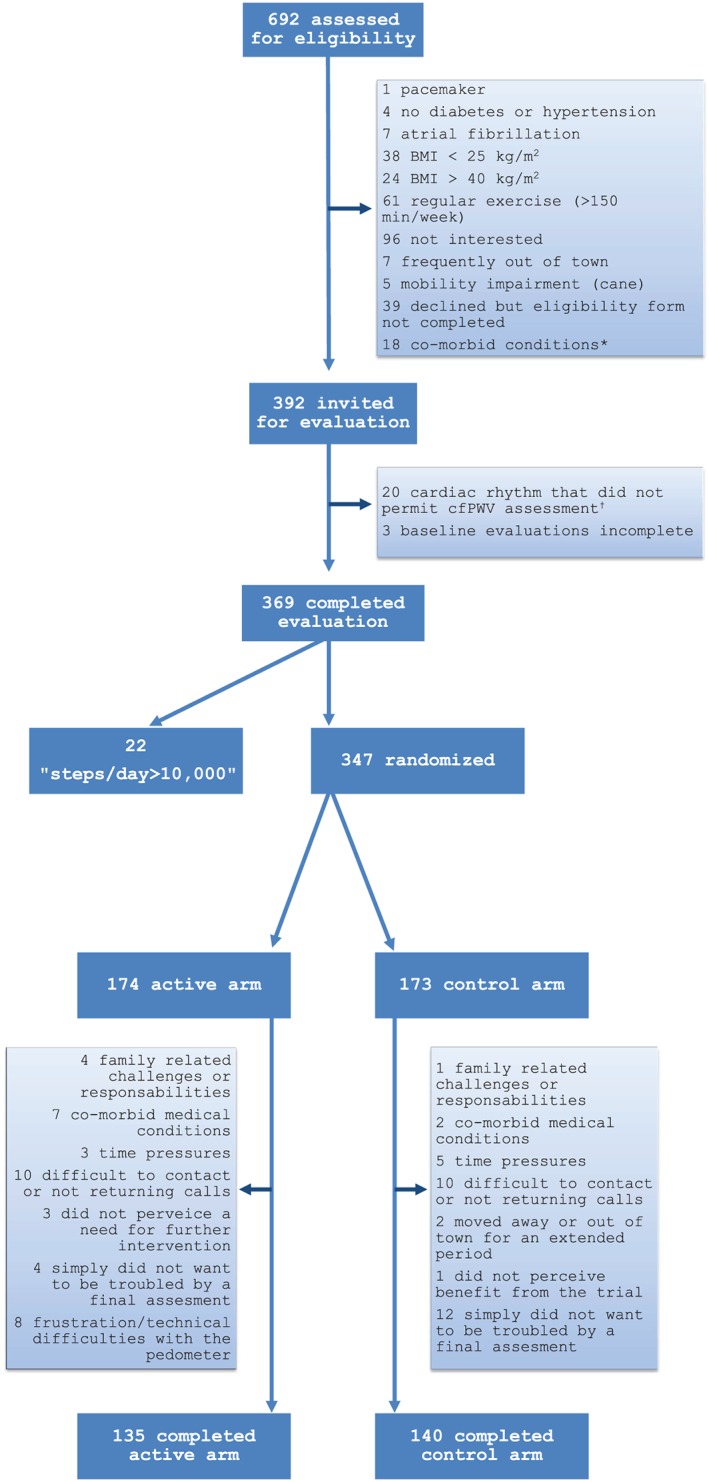
Participant flow. * angina (1), chronic obstructive pulmonary disease (6), inflammatory arthritis (2), cancer (4), renal disease (1), liver disease (1), depression (1), urinary impairment (2). † PVCs, irregular sinus rhythm.
Participants averaged 60 years of age (SD 11) and over half were women (Table 1). Based on physician‐reported diagnosis, use of relevant medications and/or self‐report, more than 65% of participants had type 2 diabetes; a similar proportion had dyslipidaemia and close to 90% had hypertension. Step counts, cfPWV and cardiometabolic risk factors were very similar between the 2 trial arms (Table 2). Steps/day were in the sedentary to low range, at under 5000 steps/day, and cfPWV was high, at close to 10 m/s. BMI averaged in the stage 1 obesity range. Systolic blood pressure was well‐controlled at a mean value of under 130 mm Hg; LDL‐C was below 2.5 mmol/L and A1c was under 8% in participants with diabetes. HOMA‐IR was elevated in those with type 2 diabetes who were not undergoing insulin therapy at above 4, and in those without type 2 diabetes at roughly 3. Medication profiles were similar between trial arms (Table S1).
Table 1.
Demographic characteristics, smoking status, cardiovascular risk factor and disease prevalence, and pregnancy cardiometabolic complication history
| Active arm N = 174 | Control arm N = 173 | |
|---|---|---|
| Demographic factors | ||
| Age, years, mean (SD) | 60.0 (11.2) | 59.4 (11.4) |
| Women, no. (%) | 99 (56.9) | 91 (52.6) |
| Post secondary education, no. (%) | 117 (70.1) | 123 (75.0) |
| White, no. (%) | 110 (63.6) | 98 (57.0) |
| Immigrant, no. (%) | 90 (51.7) | 96 (55.5) |
| Married/common‐law, no. (%) | 115 (74.2) | 115 (75.2) |
| Employed or student, no. (%) | 101 (58.7) | 98 (58.0) |
| Menopause (women) | 69 (69.7) | 70 (76.9) |
| Smoking history, no. (%) | ||
| Current smoker | 11 (6.3) | 8 (4.7) |
| Past smoker | 62 (35.6) | 58 (33.7) |
| Type 2 diabetes | 116 (66.7) | 123 (71.1) |
| Duration, years, mean (SD) | 10.5 (7.5) | 10.6 (8.3) |
| Hypertension, no. (%) | 161 (92.5) | 151 (87.3) |
| Duration, years, mean (SD) | 12.4 (11.4) | 12.7 (10.3) |
| Dyslipidemia, no (%) | 116 (66.7) | 123 (71.1) |
| Duration, years, mean (SD) | 9.6 (8.8) | 9.5 (7.7) |
| Cardiovascular disease | 35 (20.1) | 28 (16.3) |
| Pregnancy‐related complication in women with previous pregnancy, n = 155 | ||
| Gestational diabetes | 18 (22.8) | 19 (28.0) |
| Hypertensive disorder of pregnancy | 20 (25.3) | 13 (19.1) |
Type 2 diabetes, hypertension and dyslipidemia diagnoses were based on participant‐reported diagnosis, physician‐reported diagnosis and/or use of corresponding medications. Duration of disease was determined by participant report.
Among the 74 physicians involved in the SMARTER trial, 32 (43%) followed at least 4 participants and 9 physicians (12%) followed 9 or more participants. The average time interval (SD) between baseline and final assessment was 1 year and 2 months for both trial arms (423 [59] days in the active arm; 426 [70] days in the control arm). During the trial intervention period, the average number of clinical visits (SD) was 3.5 (0.7) in the active arm and 3.1 (1.0) in the control arm. Among the 134 active arm participants from whom the final step count prescription was retrieved and who completed the final assessment, prescribed step counts at the final clinical visit were 71 steps/day lower (SD 1963) than the 1‐year target of baseline steps/day + 3000 steps/day. The steps/day achieved as per final assessment was 1712 (SD 2971) lower than prescribed. Among these participants, 43% had a final prescription that was at or above target and 26% had a final step count value that was at or above the value prescribed at the final clinical visit. In complete case analysis, point estimates suggested a net reduction in cfPWV in the active arm vs control arm participants (−0.28 m/s; 95% CI, −0.68 to 0.13; Table 3); this was not conclusive and CIs were wider with imputation (−0.03 m/s; 95% CI, −0.22 to 0.17 with ignorable mechanisms; −0.01; 95% CI, −0.18 to 0.16 with non‐ignorable mechanisms).
In complete case analysis, the difference in changes in step counts between active and control arm participants was 1190 steps/day (95% CI, 550‐1840; Table 3). This represented a 20% increase in step counts over baseline values. A net increase was confirmed using imputation with ignorable mechanisms (619; 95% CrI, 327‐911) and non‐ignorable mechanisms (443; 95% CrI, 181‐706).
In an analysis evaluating the overall relationship between changes in step counts and changes in cfPWV for all complete cases, a 1000 steps/day increase was associated with a −0.068 m/s change in cfPWV (95% CI, −0.15 to 0.01) in an age‐ and sex‐adjusted model.
Complete case analyses were used for the remaining secondary outcomes (Table 3). There were net reductions in both systolic and diastolic blood pressure in active vs control arms but these differences were not conclusive (systolic, −2.59 mm Hg; 95% CI, −5.66 to 0.47; diastolic, −1.21 mm Hg; 95% CI, −3.04 to 0.62). There were no differences between active and control arms in terms of change in number of antihypertensive agents (−0.08 agents; 95% CI, −0.31 to 0.15; Table S2). There was no important difference in BMI change between active and control arms (−0.15 kg/m2; 95% CI, −0.43 to 0.15) and, similarly, there were no important between‐arm differences in terms of changes in waist circumference and waist‐to‐hip ratio. Lipid profiles changes did not differ between the active and control arms among those who completed trial procedures (eg, HDL, 0.01; 95% CI, −0.03 to 0.05; LDL, −0.02; −0.19, 0.15). There were no differences in change in number of lipid‐lowering medications in the active and control arms (−0.06; 95% CI, −0.22 to 0.09).
There was evidence of an impact on glucose handling. Among those with type 2 diabetes, there was a reduction in A1c among those in the active arm compared to the control arm (−0.38%; 95% CI, −0.69 to −0.06). There were no differences between the active and control arms in terms of change in number of glucose‐lowering agents (0.15 agents; 95% CI, −0.15 to 0.44; Table S2). Among active arm participants, none developed new type 2 diabetes and 4 developed impaired fasting glucose (6.1‐6.9 mmol/L). Among control arm participants, 4 developed type 2 diabetes and 3 developed impaired fasting glucose. Among those in whom insulin resistance was assessed (ie, patients with no diabetes or with diabetes but not undergoing insulin therapy), there was a reduction in insulin resistance in the active arm compared to the control arm (HOMA‐IR, −0.96; 95% CI, −1.72 to −0.21). A1c25 and HOMA‐IR26 are independent predictors of cardiovascular disease.
4. DISCUSSION
Our physician‐delivered step count prescription and monitoring strategy increased daily step counts by approximately 1200 over 1 year. This represents a 20% increase from the baseline 5000 steps/day. Physicians prescribed steps slightly below the 1‐year target of 3000 steps/day above baseline values. The net change in steps/day was below the number of steps prescribed, but a net increase was observed. In terms of biological effects, cfPWV was lowered but this was not conclusive. There were, however, improvements in both haemoglobin A1c (0.38% reduction) and insulin resistance (0.96 reduction in HOMA‐IR). Thus, a simple physician‐delivered step count prescription strategy incorporated into routine clinical practice can augment physical activity and confer some favourable cardiometabolic changes in sedentary overweight adults with type 2 diabetes and/or hypertension.
Step count monitoring offers a concrete and measurable metric to facilitate goal‐setting for physical activity. Previous pedometer‐based interventions that were evaluated in patients with type 2 diabetes have been more complex than the SMARTER intervention, involving a facilitator and, frequently, group sessions. Two meta‐analyses6, 27 indicate that such interventions achieve approximately 2000 steps/day more than the control arms over a 3 to 6‐month period, but there are declines in effect thereafter.7 In a single study that compared group‐based vs physician‐delivered pedometer‐based counseling (no written prescription) over 3 months, the group‐based strategy had greater impact.28 The effects of such short‐term interventions, however, are not sustained over time. In contrast, our intervention, being integrated into clinical practice, can be sustained beyond a few months and, in fact, led to a between‐arm difference at 1 year. Thus, there may be a trade‐off between a short, more powerful intervention that cannot be continued over time compared to a strategy with more modest effects but adapted to current models of care. Highlighting the potential impact of smaller activity increments, in a large observational cohort study conducted in Taiwan, a 15‐minute increment in physical activity was associated with long‐term reductions in mortality.29
The SMARTER intervention achieved a step count increase but this was less than the target of 3000 steps/day. The steps prescribed were close to this target but there remained an important gap between steps prescribed and steps achieved. To close the gap, there may be a need for more motivational support, a key need voiced by patients.30 In future studies, we aim to enhance our intervention by combining it with such components as telephone‐based support from other health professionals,31 peer support32 and automated messaging.33 Interventions using such strategies have demonstrated promise in prior studies but have not been combined with a physician‐delivered step‐count prescription.
Although our results suggested a reduction in cfPWV, we were underpowered to discern a conclusive impact. When designing SMARTER, we based our sample size calculations on the single existing physical activity trial at that time, with cfPWV as the outcome. This previous trial compared usual care to a 3‐month supervised exercise intervention34 among adults with diabetes, hypertension and dyslipidemia. The reported effect was a roughly 20% between‐arm difference in change in cfPWV (ie, equivalent to 2 m/s from a baseline of approximately 12 m/s); we aimed to detect a 10% difference to be conservative. Since then, other relevant studies have been conducted and these suggest a more modest impact of physical activity interventions on cfPWV. For example, a 2014 meta‐analysis35 of supervised exercise interventions demonstrated a 0.4 m/s reduction in cfPWV in the active arm compared to the control arm. Consistent with this meta‐analysis, we detected a 0.28 m/s reduction in cfPWV in the active arm vs the control arm in complete case analysis, although our findings were not conclusive (95% CI, −0.68 to 0.13). Interestingly, a previous cohort study that examined the relationship between step counts and cfPWV did not demonstrate a conclusive inverse linear relationship at baseline, but such a conclusive relationship did emerge at the 4‐year follow‐up visit.36 There was slower progression of cfPWV for those with higher baseline step counts (ie, increase in cfPWV was 0.1 m/s less per additional 1000 steps/day at baseline). It is possible, thus, that there is a cumulative impact of increases in step count, and that it may take more time for the step count increase we observed to translate into a conclusive cfPWV reduction .
Table 2.
Baseline step counts, carotid femoral pulse wave velocity and cardiometabolic and anthropometric measures
| Mean (SD) | Active arm N = 174 | Control arm N = 173 |
|---|---|---|
| Steps/day | 4550 (2230) | 5000 (2360) |
| Carotid femoral pulse wave velocity, m/s | 9.8 (2.4) | 9.8 (2.1) |
| Systolic/diastolic blood pressure, mm Hg | 124 (15.8)/76 (10.7) | 124 (13.2)/76 (9.5) |
| Heart rate, bpm | 72 (12.4) | 73 (11.9) |
| Glucose control and insulin resistance | ||
| Hemoglobin A1c (all type 2 diabetes), % | 7.6 (1.3) | 7.7 (1.3) |
| Fasting glucose, mmol/L | ||
| Type 2 diabetes; not on insulin1 | 8.2 (2.5) | 7.4 (1.9) |
| No type 2 diabetes2 | 5.6 (0.84) | 5.5 (0.95) |
| Fasting insulin, pmol/L | ||
| Type 2 diabetes; not on insulin1 | 79.0 (44.4) | 73.2 (52.0) |
| No type 2 diabetes2 | 72.1 (42.3) | 66.0 (39.0) |
| HOMA‐IR, mean3 (SD) | ||
| Type 2 diabetes; not on insulin1 | 4.88 (3.43) | 4.15 (3.52) |
| No type 2 diabetes2 | 3.05 (1.99) | 2.78 (1.94) |
| Lipid profile, mean (SD), mmol/L | ||
| Total cholesterol | 4.60 (1.37) | 4.49 (1.22) |
| HDL | 1.27 (0.34) | 1.26 (0.37) |
| LDL | 2.45 (1.0) | 2.34 (1.0) |
| Triglycerides, mmol/L | 1.73 (3.01) | 1.64 (1.10) |
| Anthropometric measures | ||
| Body mass index, kg/m2 | 31.7 (4.5) | 31.8 (4.5) |
| Weight, kg | 86.7 (15.7) | 87.5 (15.5) |
| Waist circumference, cm | 104.2 (10.8) | 104.1 (12.2) |
| Women | 102.0 (10.5) | 100.7 (10.9) |
| Men | 107.1 (10.6) | 107.8 (12.6) |
| Waist to hip ratio | 0.94 (0.07) | 0.94 (0.08) |
| Women | 0.91 (0.06) | 0.89 (0.07) |
| Men | 0.99 (0.06) | 0.99 (0.06) |
There were 61 diabetes patients in the control arm and 72 in the active arm, in whom fasting insulin, glucose and HOMA‐IR were measured/computed. These were the individuals with type 2 diabetes who were not undergoing insulin therapy.
There were 74 individuals without diabetes in the control arm and 74 in the active arm; fasting insulin, glucose and HOMA‐IR were measured/computed in these individuals.
Mean HOMA‐IR was computed among patients not taking exogenous insulin.
Conversion factors: 1 mmol/L glucose = 18.02 mg/dL; 1 pmol/L insulin = 0.14 µIU/mL; 1 mmol/L HDL, LDL, or total cholesterol = 38.6 mg/dL; 1 mmol/L triglycerides = 88.5 mg/dL.
In a meta‐analysis of pedometer‐based interventions across clinical populations (including 1 type 2 diabetes trial)5 there was a −3.8 mm Hg (95% CI, −1.7 to −5.9) difference in systolic blood pressure change in active vs control arms. In our trial, similar to cfPWV, there was a greater reduction in both systolic (−2.59 mm Hg; 95% CI, −5.66, 0.47) and diastolic (−1.21 mm Hg; 95% CI, −3.04, 0.62) blood pressure in active vs control arms, although this was not conclusive. The lack of conclusive effect is probably attributable to well‐controlled blood pressure at baseline (124/76 mm Hg average). Point estimates for impact on HDL and LDL were in a favourable direction, but were arguably trivial.
Table 3.
Changes in outcomes among participants who completed final evaluations
| Mean (95% CI) | Active arm | Control arm | Difference |
|---|---|---|---|
| Steps/day | 1220 (760, 1690) | 30 (−420, 480) | 1190 (550, 1840) |
| Carotid femoral pulse wave velocity, m/s | −0.17 (−0.50, 0.17) | 0.13 (−0.16, 0.42) | −0.30 (−0.74, 0.14) |
| Blood pressure, mm Hg | |||
| Systolic | −2.23 (−4.64, 0.18) | 0.36 (−1.56, 2.29) | −2.59 (−5.66, 0.47) |
| Diastolic | −1.45 (−2.86, −0.04) | −0.24 (−1.42, 0.93) | −1.21 (−3.04, 0.62) |
| Heart rate, bpm | −0.16 (−1.65, 1.33) | 0.81 (−0.76, 2.4) | −0.97 (−3.13, 1.18) |
| Glycemic control and insulin resistance | |||
| A1c, % (in type 2 diabetes) | −0.20 (−0.46, 0.06) | 0.18 (−0.01, 0.86) | −0.38 (−0.69, −0.06) |
| In type 2 diabetes patients not on insulin and in participants without type 2 diabetes | |||
| Fasting insulin, pmol/L | −2.15 (−8.61, 4.31) | 10.4 (0.81, 20.07) | −12.6 (−24.1, −1.1) |
| Fasting glucose, mmol/L | −0.08 (−0.33, 0.18) | 0.43 (0.17,0.68) | −0.50 (−0.86, −0.15) |
| HOMA‐IR | −0.16 (−0.51, 0.18) | 0.8 (0.12, 1.48) | −0.96 (−1.72, −0.21) |
| Lipid profile, mmol/L | |||
| Total cholesterol, mmol/L | −0.13 (−0.28, 0.01) | −0.10 (−0.25,0.05) | −0.03 (−0.24, 0.18) |
| HDL, mmol/L | 0.01 (−0.02, 0.04) | 0 (−0.03, 0.03) | 0.01 (−0.03, 0.05) |
| LDL, mmol/L | −0.10 (−0.22, 0.03) | −0.08 (−0.19, 0.04) | −0.02 (−0.19, 0.15) |
| Triglycerides, mmol/L | −0.15 (−0.44, 0.3) | 0.02 (−0.09, 0.13) | −0.17 (−0.47, 0.12) |
| Anthropometric measures | |||
| Body mass index, kg/m2 | −0.18 (−0.43, 0.07) | −0.03 (−0.23, 0.16) | −0.15 (−0.47, 0.17) |
| Weight, kg | −0.66 (−1.35, 0.03) | −0.11 (0.65, 0.42) | −0.55 (−1.41, 0.32) |
| Waist circumference, cm | 0.07 (−0.71, 1.14) | 0.22 (−0.85, 0.99) | −0.15 (−1.45, 1.15) |
| Waist to hip ratio | 0 (−0.01, 0.01) | 0.01 (−0.01, 0.02) | −0.01 (−0.02, 0.01) |
Conversion factors: 1 mmol/L glucose = 18.02 mg/dL; 1 pmol/L insulin = 0.14 µIU/mL; 1 mmol/L HDL, LDL, or total cholesterol = 38.6 mg/dL; 1 mmol/L triglycerides = 88.5 mg/dL.
There were conclusive effects on glucose handling. Among SMARTER trial participants with type 2 diabetes, there was a greater reduction in A1c in active arm compared to control arm participants (−0.38%; 95% CI, −0.69 to −0.06). This is close to the A1c reduction consistently demonstrated in gym‐based supervised exercise intervention studies.37, 38, 39, 40 In a previously cited meta‐analysis in pedometer‐based interventions in type 2 diabetes,6 no difference in A1c emerged between intervention arms, but the authors noted that baseline A1c was well controlled (6.64%‐8.0% on average); this may have limited the ability to detect differences. The baseline A1c in our SMARTER trial was at the upper limit of this range. There was other supporting evidence of favourable glucose handling effects in our trial, including a conclusive reduction in insulin resistance (between‐group difference in HOMA‐IR change, −0.96; 95% CI, −1.72 to −0.21) as well as the development of type 2 diabetes during the trial in 4 control arm participants, as compared to none in active arm participants. There were no between‐arm differences in changes in number of anti‐hyperglycaemic medications.
Adults with type 2 diabetes are among the most sedentary1, 2 and thus require tailored strategies to facilitate adherence to physical activity recommendations. When compared to aerobic exercise interventions, pedometer‐based approaches have been shown to lead to greater adherence over a 6‐month period (92% vs 77%).41 However, most pedometer studies have been short‐term in nature, and have not assessed longer term changes in physical activity and cardiometabolic profiles. SMARTER examined a 1‐year step count prescription strategy delivered by physicians during routine clinical visits. Our rationale was that, although physicians are not typically expert in physical activity counseling, patients do take doctors’ advice seriously.42, 43 Unfortunately, only 10% of physicians prescribe physical activity.44 In the SMARTER approach, the prescription delivered by physicians is simple and feasible; it meets the patient's need for accountability and support, without requiring additional resources and staffing. Most studies in physical activity indicate that the greatest benefits are realized by the least active participants.45 Consistent with this, SMARTER participants were sedentary to low active at baseline (mean, approximately 5000 steps/day). We demonstrated both an increase in step counts and improvements in glucose handling.
Our trial had some limitations. The challenges in demonstrating a conclusive impact on cfPWV were probably related to failing to achieve the target of a 3000 steps/day increase, well‐controlled baseline blood pressure values and to an underestimation of required sample size based on data available at the time of the trial design, as discussed. We would note, however, that there was a conclusive increase in step counts despite failure to reach targets. This was also confirmed through imputation with ignorable and nonignorable mechanisms, an approach more thorough than those in previous pedometer‐based studies. Furthermore, there was a decrease in both cfPWV and blood pressure values, although these were not conclusive. There was an impact on glucose handling, proven through subgroup analyses; this was consistent in both those with type 2 diabetes (ie, reduction in A1c) and those not undergoing insulin therapy (ie, reduction in HOMA‐IR). There were some baseline differences between trial arms. However, baseline steps/day, cfPWV and cardiometabolic profiles were similar between arms. As in any trial, there was some attrition, but nearly 80% of participants completed the final assessment and drop‐out rates were similar between arms.
In conclusion, a simple physician‐delivered step count prescription strategy incorporated into routine clinical practice led to a net increase of 20% in step counts; however, this was below the targeted increment of 3000 steps/day. While conclusive effects on cfPWV were not observed, there were conclusive improvements in both A1c and insulin sensitivity. Future studies will evaluate an amplified intervention to further increase step counts, to achieve greater impact on cardiovascular health.
Supporting information
Table S1. Medication profiles at baseline.
Table S2. Medications at baseline and final evaluations by trial arm.
ACKNOWLEDGMENTS
The SMARTER trial group includes Susan Kahn (Professor of Medicine, McGill University) and Louise Pilote (Physician Scientist; Professor of Medicine, McGill University) who provided important comments and feedback during protocol development. Recruitment and assessment assistance was provided by Cindy Ibberson, Corinne Suppère, Samantha Hajna, Anne‐Sophie Brazeau, Marie‐Eve Robillard, Yessica Haydee Gomez Sandoval, Alexandra Cooke and Rani Cruz. Patrick Belisle was our statistical programmer.
Critical to the success of this trial are our study participants and our network of collaborating physicians, including Catherine Kudo1, Natasha Garfield1, Les Meissner9, Roy Eappen9, Andrea Lalonde6, Donald Sproule2 , Ghislaine Roederer7, Pierre Larochelle7, Candace Lee, David Shannon3, Susan Still2, Vivian Petropoulos8, Maureen Doyle2 ,Leonora Lalla2, Lynn McLaughlin2, Walter Gregory1 , Sara Meltzer1, David Morris1, Jean‐Marie Boutin7, Juan Rivera1, Margaret Hughes2, Raymond Sorge2, Alexis Baass1,7, Stravroula Christopoulos4, Barry Posner1, Khue Ly3, John Hughes2, Reuben Ostrofsky2, Karen Dahan10, Robert Diez d'Aux2, Maxine Dumas‐Pilon2, Pnina Wasser2, Timothy Meagher3, Sofia Hussaini2, Kimberly Munro2, Robert Wistaff7, Agnieska Majdan4, Wen Hu1, April Kinghorn2, Mark Yaffe2, Joanna Caron2, Jean‐Francois Yale1, Carolina Capelle2, Sabiha Awan2, Paul Cruvellier2 , Tina Kader4, Laurence Green3, Alicia Schiffrin4, Nilay Ozen8, Samantha Sacks2, Zachary Weinstein2, Hortensia Mircescu7, Michel Bertrand7, Goldie Marmor, Roxanne Arel2 , Eva Wesolowska7, Ashley Martin3, Leah Feldman2, Marie‐Luce Chen2 , Hans Zingg1, Marie Weber2, Brent Richards4, Isabelle Leblanc2, Suzanne Morin3, Murray Vasilevsky5, David Blank1, Sandra Morris2, Aileen Roman2, Dominique Garrell7, Evelyn Kyle2, François Larivère7, Richard Mackler, Ania Tissakht2, Jeffrey Wiseman3, Renata Sava2, Kurt Jansen10.
1Division of Endocrinology, Department of Medicine, McGill University Health Centre and McGill University; 2Department of Family Medicine, St. Mary's Hospital, Montreal, Quebec and McGill University; 3Division of Internal Medicine, Department of Medicine, McGill University Health Centre and McGill University; 4Division of Endocrinology, Department of Medicine, Sir Mortimer Davis Jewish General Hospital and McGill University; 5Division of Nephrology, Department of Medicine, McGill University Health Centre and McGill University; 6Division of Internal Medicine, Department of Medicine, Sir Mortimer Davis Jewish General Hospital and McGill University; 7Institut de Recherches Cliniques de Montréal (IRCM); 8Division of Cardiology, Department of Medicine, St. Mary's Hospital, Montreal, Quebec and McGill University; 9Division of Endocrinology, St. Mary's Hospital, Montreal, Quebec and McGill University; 10Division of Primary Care, Queen Elizabeth Health Complex (QEHC), Department of Family Medicine, McGill University Health Centre.
Conflict of interest
All authors declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Author contributions
K. D. and S. S. D. conceived the study, supervized recruitment and data collection, oversaw analyses and interpreted findings. K. D. wrote the first draft of the manuscript, which was revised with S. S. D., with important input from S. L. B., L. J., A. B. C., E. R., L. T., D. C., M. S. and R. R.‐L. who also assisted with interpretation of findings. D. C. performed recruitment and data collection. L. J. supervised statistical analyses.
Dasgupta K, Rosenberg E, Joseph L, Cooke AB, Trudeau L, Bacon SL, Chan D, Sherman M, Rabasa‐Lhoret R, Daskalopoulou SS and SMARTER Trial Group . Physician step prescription and monitoring to improve ARTERial health (SMARTER): A randomized controlled trial in patients with type 2 diabetes and hypertension. Diabetes Obes Metab. 2017;19:695–704. https://doi.org/10.1111/dom.12874
Trial registration: Clinicaltrials.gov NCT01475201; registered November 16, 2011.
Funding information Funding for this trial is from the Canadian Institutes of Health Research (MOPP 114996; Nominated Principal Investigator, K. D.; Co‐Principal Investigators, S. S. D. and E. R.) and a grant from the Heart and Stroke Foundation (HSF G‐12‐000251; Principal Investigator, K. D.). K. D. and S. S. D. both hold clinical investigator salary awards from the Fonds de recherche du Québec‐Santé. A. C. holds a doctoral award from the Canadian Institutes of Health Research. The study funders had no role in collection, analysis, interpretation of data, writing of the report or decision to submit the article for publication
REFERENCES
- 1. Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan KM. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med. 2003;163:1440‐1447. [DOI] [PubMed] [Google Scholar]
- 2. Hu FB, Stampfer MJ, Solomon C, et al. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med. 2001;134:96‐105. [DOI] [PubMed] [Google Scholar]
- 3. Smith TC, Wingard DL, Smith B, Kritz‐Silverstein D, Barrett‐Connor E. Walking decreased risk of cardiovascular disease mortality in older adults with diabetes. J Clin Epidemiol. 2007;60:309‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yates T, Haffner SM, Schulte PJ, et al. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet. 2014;383:1059‐1066. [DOI] [PubMed] [Google Scholar]
- 5. Bravata DM, Smith‐Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296‐2304. [DOI] [PubMed] [Google Scholar]
- 6. Qiu S, Cai X, Chen X, Yang B, Sun Z. Step counter use in type 2 diabetes: a meta‐analysis of randomized controlled trials. BMC Med. 2014;12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tudor‐Locke C, Bell RC, Myers AM, et al. Controlled outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type II diabetes. Int J Obes Relat Metab Disord. 2004;28:113‐119. [DOI] [PubMed] [Google Scholar]
- 8. Douglas F, Torrance N, van Teijlingen E, Meloni S, Kerr A. Primary care staff's views and experiences related to routinely advising patients about physical activity. A questionnaire survey. BMC Public Health. 2006;6:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campkin L, Doyle‐Baker PK. Exercise counselling and use of exercise professionals by physicians: Findings from a scoping review. Alberta Centre for Active Living 2015; July 8, 2015. https://www.centre4activeliving.ca/news/2015/07/physician‐exercise‐counselling/. Accessed December 12, 2016.
- 10. Patel A, Schofield GM, Kolt GS, Keogh JW. General practitioners’ views and experiences of counselling for physical activity through the New Zealand Green Prescription program. BMC Fam Pract. 2011;12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mattace‐Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657‐663. [DOI] [PubMed] [Google Scholar]
- 13. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318‐1327. [DOI] [PubMed] [Google Scholar]
- 14. Dasgupta K, Rosenberg E, Daskalopoulou SS. Step Monitoring to improve ARTERial health (SMARTER) through step count prescription in type 2 diabetes and hypertension: trial design and methods. Cardiovasc Diabetol. 2014;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793‐801. [DOI] [PubMed] [Google Scholar]
- 16. Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laurent S, Cockcroft J, Van BL, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588‐2605. [DOI] [PubMed] [Google Scholar]
- 19. Doonan RJ, Scheffler P, Yu A, et al. Altered arterial stiffness and subendocardial viability ratio in young healthy light smokers after acute exercise. PLoS One. 2011;6:e26151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110‐2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bowles DK, Laughlin MH. Mechanism of beneficial effects of physical activity on atherosclerosis and coronary heart disease. J Appl Physiol. 2011;111(1):308‐310. doi:10.1152/japplphysiol.00634.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Daskalopoulou SS, Khan NA, Quinn RR, et al. The 2012 Canadian hypertension education program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol. 2012;28:270‐287. [DOI] [PubMed] [Google Scholar]
- 23. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412‐419. [DOI] [PubMed] [Google Scholar]
- 24. Marshall SJ, Levy SS, Tudor‐Locke CE, et al. Translating physical activity recommendations into a pedometer‐based step goal: 3000 steps in 30 minutes. Am J Prev Med. 2009;36:410‐415. [DOI] [PubMed] [Google Scholar]
- 25. Stevens RJ, Coleman RL, Adler AI, Stratton IM, Matthews DR, Holman RR. Risk factors for myocardial infarction case fatality and stroke case fatality in type 2 diabetes: UKPDS 66. Diabetes Care. 2004;27:201‐207. [DOI] [PubMed] [Google Scholar]
- 26. Bonora E, Formentini G, Calcaterra F, et al. HOMA‐estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135‐1141. [DOI] [PubMed] [Google Scholar]
- 27. Vaes AW, Cheung A, Atakhorrami M, et al. Effect of ‘activity monitor‐based’ counseling on physical activity and health‐related outcomes in patients with chronic diseases: a systematic review and meta‐analysis. Ann Med. 2013;45:397‐412. [DOI] [PubMed] [Google Scholar]
- 28. De Greef K, Deforche B, Tudor‐Locke C, De Bourdeaudhuij I. Increasing physical activity in Belgian type 2 diabetes patients: a three‐arm randomized controlled trial. Int J Behav Med. 2011;18:188‐198. [DOI] [PubMed] [Google Scholar]
- 29. Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244‐1253. [DOI] [PubMed] [Google Scholar]
- 30. Casey D, De Civita M, Dasgupta K. Understanding physical activity facilitators and barriers during and following a supervised exercise programme in Type 2 diabetes: a qualitative study. Diabet Med. 2010;27:79‐84. [DOI] [PubMed] [Google Scholar]
- 31. Kirkman MS, Weinberger M, Landsman PB, et al. A telephone‐delivered intervention for patients with NIDDM. Effect on coronary risk factors. Diabetes Care. 1994;17:840‐846. [DOI] [PubMed] [Google Scholar]
- 32. Foster G, Taylor SJ, Eldridge SE, Ramsay J, Griffiths CJ. Self‐management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev. 2007;4:CD005108. [DOI] [PubMed] [Google Scholar]
- 33. Arambepola C, Ricci‐Cabello I, Manikavasagam P, Roberts N, French DP, Farmer A. The impact of automated brief messages promoting lifestyle changes delivered via mobile devices to people with Type 2 diabetes: a systematic literature review and meta‐analysis of controlled trials. J Med Internet Res. 2016;18:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short‐term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care. 2009;32:1531‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ashor AW, Lara J, Siervo M, Celis‐Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta‐analysis of randomized controlled trials. PLoS One. 2014;9:e110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jennersjo P, Ludvigsson J, Lanne T, Nystrom FH, Ostgren CJ. Pedometer‐determined physical activity level and change in arterial stiffness in Type 2 diabetes over 4 years. Diabet Med. 2012;29:1119–1125. [DOI] [PubMed] [Google Scholar]
- 37. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta‐analysis. JAMA. 2011;305:1790‐1799. [DOI] [PubMed] [Google Scholar]
- 38. Boule N, Kenny G, Haddad E, Wells G, Sigal R. Meta‐analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia. 2003;46:1071‐1081. [DOI] [PubMed] [Google Scholar]
- 39. Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta‐analysis. Diabetes Care. 2006;29:2518‐2527. [DOI] [PubMed] [Google Scholar]
- 40. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;3:CD002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bell GJ, Harber V, Murray T, Courneya KS, Rodgers W. A comparison of fitness training to a pedometer‐based walking program matched for total energy cost. J Phys Act Health. 2010;7:203‐213. [DOI] [PubMed] [Google Scholar]
- 42. Hesse BW, Nelson DE, Kreps GL, et al. Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Arch Intern Med. 2005;165:2618‐2624. [DOI] [PubMed] [Google Scholar]
- 43. Kao AC, Green DC, Davis NA, Koplan JP, Cleary PD. Patients’ trust in their physicians: effects of choice, continuity, and payment method. J Gen Intern Med. 1998;13:681‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teoh H, Despres JP, Dufour R, et al. A comparison of the assessment and management of cardiometabolic risk in patients with and without type 2 diabetes mellitus in Canadian primary care. Diabetes Obes Metab. 2013;15:1093‐1100. [DOI] [PubMed] [Google Scholar]
- 45. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446‐1461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Medication profiles at baseline.
Table S2. Medications at baseline and final evaluations by trial arm.


