ABSTRACT
The nematode Caenorhabditis elegans utilizes gap junctions in different fashions in virtually all of its cells. This model animal has a surprisingly large number of innexin genes within its genome, and many nematode cell types can express multiple innexins at once, leading to the formation of diverse junction types and enough redundancy to limit the effect of single gene knockdowns on animal development or behavioral phenotypes. Here, we review the general properties of these junctions, their expression patterns, and their known roles in tissue development and in the animal's connectome. © 2016 Wiley Periodicals, Inc. Develop Neurobiol 77: 587–596, 2017
Keywords: innexin, nematode, heteromeric, heterotypic
INTRODUCTION
The gap junctions (electrical synapses) of the nematode, an invertebrate, are not formed by connexin proteins, but by innexins. The innexin proteins bear no specific sequence homologies to vertebrate connexins, but form intercellular membrane channels with similarities in structure and function to those in vertebrate tissues. There is distant homology between the innexin genes of Caenorhabditis elegans and the pannexin protein channels of vertebrates (Phelan and Starich, 2001; Baranova et al., 2004; Penuela et al., 2013). The general makeup of innexin proteins and channels in C. elegans has been reviewed elsewhere (Liu et al., 2006; Bao et al., 2007; Norman and Villu Maricq, 2007; Altun et al., 2009; Simonsen et al., 2014). Here, we will restrict ourselves to discussing how gap junction channels are deployed and utilized in various nematode tissues as intercellular channels. Similar to the case of pannexins in vertebrates, some innexins may also subserve other forms of communication, perhaps as hemichannels in a plasma membrane to release ATP, or as intramembrane hemichannels in cytoplasmic organelles (cf., Luo and Turnbull, 2011). However there is relatively little experimental evidence in C. elegans to demonstrate those potential roles.
GAP JUNCTIONS IN C. ELEGANS ARE SMALL, UBIQUITOUS, AND DIVERSE
Innexin genes in C. elegans show a similar diversity in number and organization to the connexin family in vertebrates and are surprisingly numerous compared to some other invertebrates such as the fruit fly Drosophila or the planarian Dugesia. The C. elegans genome encodes 25 innexin genes, and virtually every cell type in the animal appears to express at least one innexin protein, often expressing multiple different innexin genes per cell (Altun et al., 2009). The multiplicity of innexin expression underlies the formation of heterotypic and heteromeric gap junctions, perhaps several types per cell (Liu et al., 2013; Starich et al., 2014) [Fig. 1(A)]. This may also be true in many C. elegans tissues, but relatively little is known about which combinations of innexin subunits are compatible within a single channel—much less than what is known for vertebrate gap junctions (Koval et al., 2014). We anticipate that diverse heteromeric gap junction channels will become apparent in other invertebrates as well (cf., Nogi and Levin, 2005; Lehmann et al., 2006). Heterotypic and heteromeric channels offer unique opportunities for developmental modulation of channel properties in a manner parallel to what is becoming well known for other forms of intercellular membrane channels, such as glutamate or NMDA receptors (Liu and Zukin, 2007; Rodenas‐Ruano et al., 2012). The small size and inaccessible nature of cells inside living nematodes, and the difficulties in expressing multiple innexin subunits in cultured cells or frog oocytes has frustrated physiological studies of C. elegans gap junctions. Further studies of this type are surely overdue.
Figure 1.
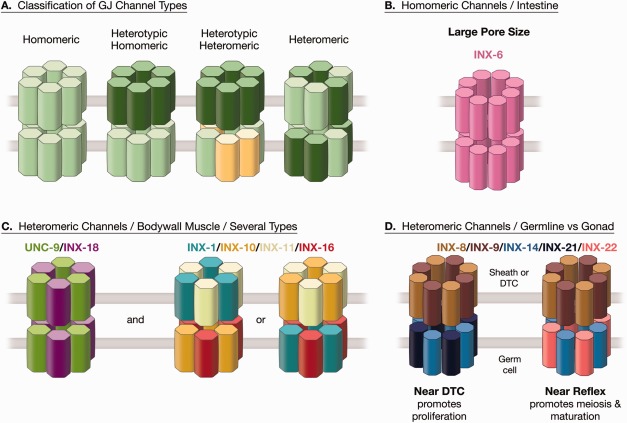
Models of gap junction composition for C. elegans. (A) Classification of gap junction channels according to their subunit composition as homomeric, heterotypic, or heteromeric (after Koval et al., 2014). Rather little is yet known about which combinations of innexin subunits are capable of associating within a hemichannel (in one membrane) or of docking across the membrane with a partner in the opposing membrane. (B) Model of the homomeric innexin channel in C. elegans intestine (after Oshima et al., 2016). (C) Models of possible innexin heteromeric channels in bodywall muscle, assuming six subunits per hemichannel, utilizing two innexins in one subtype and four other innexins in a second subtype (after Liu et al, 2013). (D) Models of possible innexin heteromeric channels in distal gonad, assuming eight subunits per hemichannel (after Starich et al, 2014). At present, we do not know the number of subunits per channel, and the possible arrangements shown here are among many possibilities. [Color figure can be viewed at wileyonlinelibrary.com.]
Some fundamental distinctions between vertebrate and invertebrate gap junctions became apparent before any molecular details were available. Although gap junctions can appear essentially equivalent even at the ultrastructural level using standard electron microscopy (TEM) in thin sections, the junctions of invertebrate tissues stand apart from those in vertebrates when investigated by the freeze fracture (FF) technique (Staehelin, 1974; Lane, Skaer and Swales, 1977). Vertebrate gap junction channels are seen by FF to be grouped into well‐ordered clusters of intramembrane particles (IMPs), with sixfold symmetry reflecting their internal composition of six subunits per hemichannel. Invertebrate gap junctions often show larger IMPS and some may utilize more subunits per hemichannel. INX‐6 channels in the C. elegans intestine involve eight subunits per hemichannel rather than six, forming larger IMPs and probably a wider channel pore size (Oshima et al., 2013, 2016) [Fig. 1(B)]. Oshima et al. (2016) argue that as many invertebrate gap junctions feature relatively large IMPs when viewed by FF, this eightfold arrangement may be commonplace for innexin‐based channels. Unfortunately, truly high resolution studies of native innexin gap junctions have rarely been performed, so the nature of most invertebrate gap junctions remains to be explored at this level of detail. Vertebrate gap junctions always consist of IMPs cleaving to the “P‐face” of the plasma membrane replica, with corresponding “E‐face” pits seen in a matching pattern to the IMPs. However, invertebrate gap junctions often consist of mixtures of particles and pits in both replica faces, sometimes with most IMPs cleaving to the E‐face (Lane et al., 1977). The planarian Dugesia was the first invertebrate where it became clear that individual tissues could show unique patterns in this E‐face/P‐face distribution when compared by FF (Quick and Johnson, 1977). Early FF results in C. elegans revealed a similar diversity (Hall, 1987). Although the IMPs in many nematode tissues appear to show similar diameters and similar packing densities, the ratio of E‐face to P‐face particles is tissue specific and the number of IMPs per array varies widely (Table 1).
Table 1.
Gap Junction Features Viewed by Freeze Fracture
| Nematode Tissue | Intramembrane Particles | ||
|---|---|---|---|
| P‐Face (%) | Packing Density | Plaque Size | |
| Hypodermis | 90 | Low | Medium |
| Muscle | 90 | High | Large |
| Intestine | 90 | High | Large |
| Neuron | ∼50 | Low | Small |
| Distal germline | 90 | Low | Small |
| Proximal germline | 80 | High | Large |
Given the small size of nematode cells, most IMP arrays are necessarily relatively small. Some classes of gap junctions in C. elegans are so small in size that they can only be revealed by the FF technique, but are never large enough to be seen in TEM by thin section (Starich et al., 2014). The small size of neuronal gap junctions in C. elegans has been a major concern in trying to describe the full connectome of the nematode nervous system (Hall, 1977; White et al., 1986; Hall and Russell, 1991; Jarrell et al., 2012). Because neuronal gap junctions are difficult to fully enumerate in even the best thin section series, all investigators have cautioned that some smaller gap junctions were missed when trying to list them all among identified cells.
The FF technique has also proven valuable when trying to distinguish gap junction from several other types of cell‐cell junctions in the nematode. Adherens junctions are a common feature in all epithelial tissues in C. elegans, taking the place of tight junctions, which are unknown here (Hall et al., 1999; Koeppen et al., 2001; Hall and Altun, 2008). Tight junctions in vertebrate and insect tissues are characterized by very close approach (i.e., no “gap”) of the apposed plasma membranes of two cells, as seen in thin section, and by parallel strands of IMPs when viewed by FF (Staehelin, 1974; Lane et al., 1977). Tight junctions create both an adhesive attachment between epithelial cells, and an extracellular seal to prevent leakage of fluids between basal and apical environments for the region bounded by an epithelium. In adherens junctions in the nematode, the two plasma membranes again are rather closely apposed, but generally a small gap is still apparent. The width of that gap is tissue‐specific, being the widest in adherens junctions between intestinal epithelia, and narrowest in hypodermis. Adherens junctions typically show an electron dense layer on their cytoplasmic surfaces, where they attach to the apical actin cytoskeleton, and whose thickness is more substantial in the larger junctions. Thus, they too provide an adhesive structure similar to the tight junction. Unlike tight junctions, adherens junctions do not show any IMPS in FF. Adherens junctions generally lie at the extreme apical borders of epithelia, whereas gap junctions typically lie along the lateral borders, at a variable distance beneath the apical adherens junctions.
A few nematode epithelia also display other types of cell‐cell junctions with even wider gaps. Pleated septate junctions and smooth septate (continuous) junctions are prominent features of the spermathecal epithelia (Hall and Altun, 2008). Pleated septate junctions lie apically, more apical than nearby adherens junctions, with visible septa spanning the gap. Cryptic septa are found at smooth septate junctions, which lie deeper on lateral borders. These latter junctions are best revealed by lanthanum infiltration, which helps to outline the septations (cf., Lane et al., 1977; Hall, unpublished observations). Continuous septate junctions also occur between intestinal cells in C. elegans, generally lying not far beneath their robust adherens junctions. FF of the Ascaris intestine first gave evidence for strands of IMPs corresponding to their continuous septate junctions (Davidson, 1983).
Careful anatomical studies of the entire adult of both sexes have revealed that gap junctions can be seen in virtually all tissue types, and in almost every cell in C. elegans (Hall and Altun, 2008). In some larval tissues, gap junctions are seen early in development, only to disappear when groups of epithelial cells fuse to form larger syncytia in the adult (Nguyen et al., 1999). When viewed globally across C. elegans tissues, the pattern of innexin expression across neighboring cells suggests that heteromeric and heterotypic gap junction channels will be common in this animal (Altun et al, 2009). As an example, the pharynx of the animal is responsible for the ingestion and preliminary processing of its main food, small bacteria, by very rapid contractions of coordinated muscle groups, well connected by gap junctions. Virtually all pharyngeal muscle groups are organized in segmental fashion (pm1 to pm8), and connected to their neighboring segments, including support cells, by a multiplicity of innexin channels (Fig. 2). Pharyngeal contractions are too rapid to be explained by chemical synaptic inputs from motor neurons, although pharyngeal neurons may influence the pharynx to change from one mode of action to the next (Raizen and Avery, 1994; Trojanowski and Fang‐Yen, 2015). Instead, spontaneous contractility of the individual muscle types must drive the rate of action. Indeed it has been shown that virtually all pharyngeal neurons can be laser‐ablated, individually or en masse, without abolishing the basal rhythm of muscle contraction (Avery and Horvitz, 1989). Pharyngeal muscles are divided into eight small groups of cells along the length of the organ (Fig. 2). Within one cell group (segment), all muscles appear to express the same set of innexins, and in virtually all cases they express several innexins either at high levels or at lower levels (Altun et al., 2009). Along the length of the pharynx, neighboring segmental groups express different assortments of innexins, so that heteromeric gap junctions between segments seem likely to be the rule here rather than the exception (Fig. 2). At present, there is no experimental evidence demonstrating the makeup of the channels between pharyngeal muscle segments, but recent expression studies and genetic ablation results in bodywall muscles are instructive (Liu et al., 2013).
Figure 2.
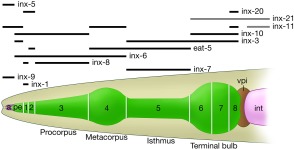
Innexin expression pattern in the pharynx (Reproduced with permission from Altun et al, Developmental Dynamics, 2009, 238, 1936–1950). Expression patterns for innexin genes are mapped versus the pharyngeal muscle segments, pm1 to pm8, illustrating which are highly expressed (dark bars) or weekly expressed (lighter bars) in the adult hermaphrodite. While many muscles express similar sets of innexins, each segment of pm muscles tends to express a different combination than its nearest neighbors. Within a segment, pharyngeal muscles lie in cell pairs which are often syncytial to their nearest neighbor, and form gap junctions to nearby marginal cells (not shown). Between segments, each muscle cell forms gap junctions to the muscles in the neighboring segment. Arcade cells, purple; pharyngeal epithelium (pe) and pm muscles (1–8), green; valve cells, brown; and intestine, pink. [Color figure can be viewed at wileyonlinelibrary.com.]
In the bodywall muscles along the length of the animal, 95 muscles are grouped into four quadrants, with a double row of muscles lying within each quadrant, effectively creating 12 slightly staggered segments along the main body axis (cf., figure 5.13 in Hall and Altun, 2008). These body muscles all express at least six innexins per cell, generally including the same set in all muscles for any stage in development (Liu et al., 2013). The cells are electrically coupled by gap junctions that are generally restricted to “muscle arms” that extend from each cell toward the motor nerve cords. Here, each muscle arm is contacted by neuromuscular junctions (NMJs) from several categories of principal motor neurons (White et al., 1976; Liu et al., 2007; Hall and Altun, 2008). Few gap junctions are present at other locations closer to the muscle sarcomeres, although some have been reported between muscle “bellies” on their lateral borders within a muscle quadrant (White et al., 1986). The layout of NMJ inputs to all bodywall muscle cells within a “segment” should insure that all nearby cells on the ventral side (i.e., both ventral quadrants) of the body will act in synchrony, and antiphasic to all muscle cells within the corresponding segment on the dorsal side. Indeed the NMJs from a fascicle of motor axons are grouped near muscle arm branches in a manner at each motor nerve's “muscle plate” where all muscles within the ventral segment may receive some fractional share of each quantal release of neurotransmitter at the muscle plate (Liu et al., 2007), and similar sharing of transmitter release occurs at the dorsal muscle plate for all dorsal bodywall muscles. While these multiplex NMJs should help to keep all muscles in synchrony locally, there is perhaps a stronger input via electrical signaling among the converging muscle arms themselves. Moreover, as each muscle cell tends to have arms extending from the extreme ends of the full cell length, and because there is some overlap at these endpoints to muscles of the next “segment,” it is clear that electrical signals should rapidly conduct within a quadrant from muscle to muscle along the length of the body to modulate contractility of the whole animal and its body shape. Genetic knockdown of any of six different innexin genes can partially inhibit this coupling, but there is no single innexin knockout that can fully extinguish coupling, as measured by intracellular recordings in a partially dissected preparation (Liu et al., 2013). Among these six innexins, the patterns of physiological deficits judged from such recordings suggest that there may be two different classes of heteromeric gap junctions here, one class involving two different innexins, and the second class involving four other innexins [Fig. 1(C)].
If we carry this lesson back to the pharynx, we anticipate an even more complex scene, potentially involving heterotypic/heteromeric gap junctions between pharyngeal segments, but perhaps some homotypic innexin combinations for those gap junctions linking muscles and support cells within a pharyngeal segment. Relative expression levels within a given muscle cell can change over developmental time, so that the exact combinations of innexins within gap junctions might be regulated during each larval stage, or possibly according to different conditions (on food, off food, or in dauer larvae, for example) to modify contractility as the animal encounters different environments, during molting, or reaches satiety. This latter aspect is purely speculation for the moment, but the complexity of gene expression within the pharynx and from one developmental stage to next offers a compelling scenario for further investigation.
Similar complex expression patterns for multiple innexins have been seen in small gap junctions between germline and somatic gonad, with several important developmental consequences. The somatic sheath cells and distal tip cell create a niche environment required for the development of the germline (Hall et al., 1999; Pepper et al., 2003; Byrd et al., 2014; Starich et al., 2014). Although larger GJs have been found between germline and soma in the proximal arm of the gonad (Hall et al, 1999), a new class of very small gap junctions has been discovered in the distal arm using FF and antibody staining. In the distal gonad arm, all individual junctions are too tiny to be discerned by standard TEM in thin sections (Starich et al., 2014). Some of these junctions connect the distal tip cell to the dividing germ cells at the distal end of the gonad arm, while similar small junctions connect the somatic sheath cells to the developing germline closer to the bend in the gonad arm (aka the “reflex”). These gap junctions individually are composed of very small numbers of channels (IMPs per array seen by FF), but are collectively numerous where they connect germ cells to the overlying somatic gonad. Genetic knockdown of any one of five innexin genes leads to systematic defects in germ cell maturation, and the evidence suggests that a typical gap junction channel consists of two different innexin proteins in one hemichannel (on the germline side) and a different pair of innexin channels in the opposite hemichannel (on the gonad sheath side) [Fig. 1(D)]. The mixture of innexin usage differs gradually along the length of the gonad arm, so that a fifth innexin gradually substitutes at hemichannels at the opposite end of the extended chain of sheath cells. Communication via innexin channels here is necessary for the germline cells to switch from mitosis to meiosis as they move along within the gonad arm. Interestingly, as these individual germ cells each slowly move relative to the overlying gonad sheath, they must break and reform gap junctions continuously as they traverse the length of the gonad arm and around the bend toward the uterus. The same germ cells are also connected to nearby neighbors within the germline via a central syncytium, the acellular “rachis” (Hall et al., 1999). This open door between all germ cells negates the chance that their gap junctions are allowing electrical signals to propagate, but to allow small molecules to be relayed between soma and germline. The dynamics of this situation are quite exciting, and much remains to be explored about how these gap junctions operate.
GAP JUNCTIONS IN THE NERVOUS SYSTEM AND MUSCLE
Although there are only 302 neurons and 56 glia in the adult C. elegans hermaphrodite (White et al., 1986), the diversity of innexin expression within them is currently unmatched in any other model organism. Fully 20 of the 25 innexin genes have been shown to be expressed in one or more cells in the nervous system (Altun et al., 2009). Some innexins appear to be expressed in a very restricted set of cells. INX‐14 is expressed only in the GABAergic inhibitory motor neuron classes, DD and VD. INX‐5 is expressed mostly in glial cells, but in very few neurons. INX‐2 is expressed only in AVK, and INX‐1 only in AIB and briefly in AIY neurons. But eight innexin genes are expressed in 15–30 neuron classes each. Furthermore, some neurons express groups of different innexins at once, and a few neurons may express as many as a dozen innexin genes. Among the six innexin genes not expressed in the nervous system, several are expressed in muscle. Considering how few tissues are involved in building the anatomy of the nematode, this abundance of different gap junction components suggests that the functions and regulation of these channels could be elaborate and flexible, perhaps serving different requirements during development, or to meet various environmental challenges.
As virtually all of the 302 neurons are expected to form gap junctions with other neurons, the issue of heteromeric and heterotypic channels arises immediately. Early hints for innexin mixtures were suggested from genetic studies of “uncoordinated” animals, where single mutations of different innexin genes gave rise to no obvious phenotype, or to only mild or moderate dysfunction in neurons and muscles (Starich et al., 1996). This suggests that redundancies must blunt single gene mutant phenotypes. Despite trouble in finding the smallest junctions by TEM, we have counted about 6,000 neuronal gap junctions in the hermaphrodite, and about 10,000 in the adult male (Hall and Russell; 1991; White et al., 1996; Jarrell et al., 2012; Emmons, Cook, and Hall, work in progress). Is this a simple system? For the moment, we do not know the true mixtures of innexins at most neuronal gap junctions, but the potential mixtures are impressive, and present a broad horizon for future physiological studies. It would be wonderful to utilize a technical method similar to GRASP or iBLINC (Feinberg et al., 2008; Desbois et al., 2015) to tease out pairwise relationships between adjoining cells, but clearly one would also hope to uncover the combinations of innexins at one side of each channel as well.
In many instances within the nematode connectome, one finds that the pattern of gap junction connectivity is quite similar or parallel to the pattern of chemical synaptic contacts (White et al., 1986). However, there are certain levels in sensory processing where gap junctions tend to dominate. For instance, a “hub‐and‐spoke” pattern has been suggested for the convergence of multiple head sensors to communicate via gap junctions onto a single interneuron, RMG (Macosko et al., 2009). This arrangement may facilitate coordination of several classes of sensory neuron activities, allowing the level of RMG activity to synchronize or facilitate the animal's responses to different modes of input toward a common output. In this case, RMG activity is apparently governing the animal's choice between social behavior and solitary behavior, that is, encouraging the animal to aggregate with other nematodes. Elsewhere gap junctions ought to allow for better synchronization and faster responses in decision making as synaptic delay is minimized. Gap junctions frequently link bilateral pairs of neurons, perhaps to coordinate activity levels between left/right sensory inputs.
In some circumstances, gap junctions between specialized muscle cells permit the coordinated contractions of many cells where only a minority of the muscles receive direct chemical synaptic inputs from neurons. The sex‐specific muscles controlling egg‐laying in the hermaphrodite are a good example. None of the uterine muscles receive any NMJs, and only a subset of the vulval muscles is directly innervated. However, gap junctions connect all muscle cells of both groups (White et al., 1986). Chemical synapses (NMJs) from a few neurons to some vulval muscles result in coordinated stimulation of all vulval and uterine muscles, squeezing the uterus and opening the vulval lips to push a fertilized egg out from the uterus. The prominent gap junctions within the pharynx also help to coordinate contractions among all cells within a region of the muscles there, allowing rapid waves of contraction to sweep along the length of the pharynx (Fang‐Yen et al., 2009).
GAP JUNCTIONS IN DEVELOPMENT
The roles that gap junctions play in tissue development may be diverse, but rather few have been studied closely yet in C. elegans. Interestingly, few innexin mutants have proven to be lethal, although some alleles do produce low levels of dead embryos. For instance, inx‐3 mutant alleles yield occasional dead embryos in which the pharynx becomes detached from the intestine, apparently due to the weakening of tissue linkages at the pharyngeal valve (Starich et al., 2003). Indeed, INX‐3 protein is expressed everywhere in the early embryo, and can be detected in small plaques ubiquitously even before the embryo begins gastrulation. It would appear that at this early stage, all cells may be communicating with neighbors via gap junctions, at or near the time when these cells are undergoing “global cell sorting” to migrate from the place of their birth to form functional groupings before tissues begin to form (Bischoff and Schnabel, 2006). It is worth remembering that in C. elegans, every cell division is pre‐programmed and predictable, and most daughter cells have fixed cell fates (Sulston and Horvitz, 1977). Sister cells often have different fates, and some individual cells always undergo apoptosis. In order for development to progress, many cells must separate from their sisters, and migrate to locate their proper partners before tissue morphogenesis can begin. Although unproven, it seems reasonable that gap junctions may play an accessory role in intercellular communication among undifferentiated cells to foster cell sorting, or to enhance cell clustering at the outset of morphogenesis. Stronger coupling might then help to synchronize or coordinate the morphogenesis within cell groups. Alternately, gap junctions may play an adhesive role during cell motility at this early stage in embryogenesis.
Coincident with the early wave of INX‐3 expression, INX‐8 and INX‐9 expression in the early embryo is associated with proper maturation of the eggshell (Starich et al., 2014; Stein and Golden, 2015). Mutations in either gene lead to leaky eggshells that permit diffusion of DAPI into the early embryo, with defects noted as early as the four cell stage. Other early defects in these mutants include failures in cytokinesis during early cell divisions, and the extrusion of polar bodies just beneath the eggshell. It remains unclear whether INX‐8 and INX‐9 are functioning here in gap junctions between embryonic cells, or possibly acting as hemichannels facing the eggshell?
As tissue development proceeds, virtually all cell types express one or more innexins, and gap junctions have been detected anatomically at the borders of most epithelial cells where they contact their neighbors within an epithelium. As the early embryonic pharynx defect in inx‐3 mutants suggests, gap junctions may also play a structural role in tissue integrity by linking one tissue to its neighbor, although adherens junctions are also widely utilized in the same role (Koeppen et al., 2001). The nematode body plan involves many syncytial epithelia, and gap junctions have been seen by TEM along cell borders in advance of targeted cell fusions (including self‐fusions) both in the embryonic excretory system (Stone et al., 2009; Abdus‐Saboor et al., 2011; Mancuso et al., 2012), and in hypodermal cells in the late larval male tail (Nguyen et al., 1999). Thus communication across gap junctions may help to guide certain steps in tissue morphogenesis.
Intercellular communication between neuron cell bodies via the innexin NSY‐5 (aka INX‐19) has been shown to regulate final cell fates in one sensory neuron class during later stages of morphogenesis in the late embryo (Chuang et al., 2007). In this case, the transitory gap junctions form between two homologous sense cells, born from two parallel lineages. The embryonic cells must migrate from their places of birth to meet and form soma‐to‐soma gap junctions near the developing nerve ring, allowing the cell pair to communicate and select between two alternate cell fates. Either AWCL or AWCR can randomly be induced to express the odorant receptors for butanone while the other AWC cell will instead express receptors for benzaldehyde and isoamyl alcohol. The gap junctions formed by NSY‐5 take part in this intercellular competition along with a claudin protein NSY‐4, so that only one AWC cell adopts each potential cell fate. Many more embryonic neuron cell bodies lying close to AWCs also form gap junctions based on NSY‐5 expression, but none are known to be involved in similar cell fate choices (Chuang et al., 2007). All these neurons also extend axons into the neighboring nerve ring, the central neuropil of the nematode brain, where they form additional synapses and gap junctions with many non‐homologous neurons which are retained into adulthood (White et al., 1986).
GAP JUNCTIONS ALLOW TRANSFER OF SMALL MOLECULES
Besides their roles in electrical signaling, gap junctions can permit the transfer of small molecules or fluid between tissues. This is well established for connexin‐based junctions in vertebrates (Goldberg et al., 2004), but is not well established for many invertebrate innexin channels. The relatively large physical pore size of some innexin channels should favor passage of larger molecules and solutes (cf., Oshima et al., 2013, 2016). Although the permeability and gating of most innexin channels remains to be carefully explored, some prominent C. elegans gap junctions are already implicated in metabolic processes. For instance, the gap junctions between the excretory canal cell and the hypodermis are especially large and collectively occupy a substantial fraction of the membrane surface area where these two tissues meet (Buechner et al., 1999; Hall and Altun, 2008). The canal cell extends lateral arms from its cell body to the far reaches of the head and tail, and operates as the kidney for C. elegans, removing excess fluid from the body and excreting this fluid through the excretory duct. Deeply embedded into the surrounding hypodermis, the excretory canals collect fluids, potentially via their prominent gap junctions with the hypodermis. Those fluids are then filtered via the elaborate canaliculi from the canal cytoplasm into the luminal space within the extended canals, before export via the excretory duct. Mutations that disrupt the continuity of the excretory duct cause lethal consequences in the embryo and early L1 larval stage, due to a fluid buildup that swells the animal into a “lethal rod” phenotype (Stone et al., 2009). Some mutant alleles in inx‐12 and inx‐13, two main innexins expected to form heterotypic/heteromeric junctions between hypodermis and the canal cell (Altun et al., 2009), also result in dead L1 larvae exhibiting lethal rod morphology (Todd Starich, pers. comm.). Although there are other possible explanations, these results suggest that INX‐12/INX‐13 junctions may facilitate water transport from hypodermis to canal cell.
Many classes of epithelial cells with C. elegans are also linked to their nearest neighbors via gap junctions (Hall and Altun, 2008). Depending on the cell type, these junctions may be large or small, but many can be seen easily by TEM. This is true for hypodermis, intestine, and the anterior epithelial cells of the buccal cavity and pharynx, none of which is expected to electrically excitable. In each case, it seems more likely that cells within an epithelial compartment can exchange small molecules to like cells. The small gap junctions discussed above between soma and germline in the nematode gonad also seem to involve a metabolic relationship rather than electrical signaling. In the case of the intestine, a calcium wave is seen to pass along the chain of intestinal cells via homomeric INX‐16 gap junctions that help to coordinate the defecation cycle (Peters et al., 2007). Additional innexins are also expressed by the intestinal cells that still permit dye coupling even in inx‐16 mutants, but INX‐16 alone seems to be required for normal propagation of calcium waves (Peters et al., 2007).
CONCLUSIONS
The gap junction gene family is surprisingly large and highly expressed across virtually every cell type in the nematode, C. elegans. Patterns of innexin expression are complex and overlapping in many cell types. These overlapping expression patterns allow cells to build heteromeric and heterotypic channels involving 2–4 different innexin proteins in a single channel. The physiological characteristics of such diverse subunit combinations have never been explored in invertebrates. They may offer opportunities for subtle regulation of channel gating and permeability during the brief life history of this animal. The profusion of so many innexins in some cell types suggests that not all innexins are utilized solely as intercellular gap junctions. Instead, some innexins may perform as hemichannels, either at the plasma membrane, or perhaps within subcellular organelles.
ACKNOWLEDGMENT
I thank Todd Starich for sharing unpublished data and Chris Crocker for his help with figures.
REFERENCES
- Abdus‐Saboor I, Mancuso V, Palozola K, Murray J, Howell K, Huang K, Norris CR, et al. 2011. Notch and Ras promote sequential steps of excretory tube development in C. elegans . Development 138:3545–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun ZF, Chen B, Thomas JH, Wang Z‐W, Hall DH. 2009. A high‐resolution map of C. elegans gap junction proteins. Dev Dyn 238:1936–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. 1989. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans . Neuron 3:473–485. [DOI] [PubMed] [Google Scholar]
- Bao L, Samuels S, Locovei S, Macagno ER, Muller KJ, Dahl G. 2007. Innexins form two types of channels. FEBS Lett 581:5703–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, et al. 2004. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83:706–716. [DOI] [PubMed] [Google Scholar]
- Bischoff M, Schnabel R. 2006. Global cell sorting is mediated by local cell‐cell interactions in the C. elegans embryo. Dev Biol 294:432–444. [DOI] [PubMed] [Google Scholar]
- Buechner M, Hall DH, Bhatt H, Hedgecock EM. 1999. Cystic canal mutants in C. elegans are defective in the apical membrane domain of the renal (excretory) cell. Dev Biol 214:227–241. [DOI] [PubMed] [Google Scholar]
- Byrd DT, Knobel K, Affeldt K, Crittenden SL, Kimble J. 2014. A DTC niche plexus surrounds the germline stem cell pool in C. elegans . PLoS One 9:e88372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C‐F, VanHoven MK, Fetter RD, Verselis VK, Bargmann CI. 2007. An innexin‐dependent cell network establishes left/right neuronal asymmetry in C. elegans . Cell 129:787–799. [DOI] [PubMed] [Google Scholar]
- Davidson LA. 1983. A freeze fracture and thin section study of intestinal cell membranes and intercellular junctions of a nematode, Ascaris . Tissue Cell 15:27–37. [DOI] [PubMed] [Google Scholar]
- Desbois M, Cook SJ, Emmons SW, Buelow HE. 2015. Directional trans‐synaptic labeling of specific neuronal connections in live animals. Genetics 200:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang‐Yen C, Avery L, Samuel ADT. 2009. Two size‐selective mechanisms specifically trap bacteria‐sized food particles in C. elegans . Proc Natl Acad Sci USA 106:20093–20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg EH, Vanhoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmann CI. 2008. GFP reconstitution across synaptic partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 57:353–363. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Valiunas V, Brink PR. 2004. Selective permeability of gap junction channels. Biochim Biophys Acta 1662:96–101. [DOI] [PubMed] [Google Scholar]
- Hall DH. 1977. The posterior nervous system of the nematode, Caenorhabditis elegans. Ph.D. Thesis, Caltech, Pasadena, CA.
- Hall DH. 1987. Freeze fracture and freeze etch studies of the nematode, Caenorhabditis elegans . N Y Acad Sci 494:215–217. [Google Scholar]
- Hall DH, Altun Z. 2008. C. elegans Atlas. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 340 p. [Google Scholar]
- Hall DH, Winfrey VP, Blauer G, Hoffman L, Furuta T, Rose KL, Hobert O, et al. 1999. Ultrastructural features of the adult hermaphrodite gonad of C. elegans: Relations between the germ line and soma. Dev Biol 212:101–123. [DOI] [PubMed] [Google Scholar]
- Hall DH, Russell RL. 1991. The posterior nervous system of the nematode Caenorhabditis elegans: Serial reconstruction of identified neurons and complete pattern of synaptic interactions. J Neurosci 11:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell TA, Wang Y, Bloniarz AE, Brittin CA, Xu M, Thomson JN, Albertson DG, et al. 2012. The connectome of a decision making neuronal network. Science 337:437–444. [DOI] [PubMed] [Google Scholar]
- Koeppen M, Simske J, Sims P, Firestein BL, Hall DH, Radice A, Rongo C, et al. 2001. Cooperative regulation of AJM‐1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat Cell Biol 3:983–991. [DOI] [PubMed] [Google Scholar]
- Koval M, Molina SA, Burt JM. 2014. Mix and match: Investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS Lett 588:1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane NJ, Skaer HL, Swales LS. 1977. Intercellular junctions in the central nervous system of insects. J Cell Sci 26:175–199. [DOI] [PubMed] [Google Scholar]
- Lehmann C, Lechner H, Loer B, Knieps M, Herrmann S, Famulok M, Bauer R, et al. 2006. Heteromerization of innexin gap junction proteins regulates epithelial tissue organization in Drosophila . Mol Biol Cell 17:1676–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Chen B, Gaier E, Joshi J, Wang Z‐W. 2006. Low conductance gap junctions mediate specific electrical coupling in the body wall muscle cells of C. elegans . J Biol Chem 281:7881–7889. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. 2007. Ca2+ permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30:126–134. [DOI] [PubMed] [Google Scholar]
- Liu Q, Chen B, Hall DH, Wang Z. 2007. A quantum of neurotransmitter causes minis in multiple postsynaptic cells at the C. elegans neuromuscular junction. Dev Neurobiology 67:123–128. [DOI] [PubMed] [Google Scholar]
- Liu P, Chen B, Altun ZF, Gross MJ, Shan A, Schuman B, Hall DH, et al. 2013. Six innexins contribute to electrical coupling of C. elegans bodywall muscle. PLos One 8:e76877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Turnbull MW. 2011. Characterization of nonjunctional hemichannels in caterpillar cells. J Insect Sci 11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. 2009. A hub‐and‐spoke circuit drives pheromone attraction and social behavior in C. elegans . Nature 458:1171–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso VP, Parry JM, Storer L, Poggioli C, Nguyen KCQ, Hall DH, Sundaram MV. 2012. Components of the apical extracellular matrix are required to maintain epithelial junction integrity in C. elegans . Development 139:979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CQ, Hall DH, Yang Y, Fitch DHA. 1999. Morphogenesis of the C. elegans male tail tip. Dev Biol 207:86–106. [DOI] [PubMed] [Google Scholar]
- Nogi T, Levin M. 2005. Characterization of innexin gene expression and functional roles of gap‐junctional communication in planarian regeneration. Dev Biol 287:314–335. [DOI] [PubMed] [Google Scholar]
- Norman KR, Villu Maricq A. 2007. Innexin function: Minding the gap junction. Curr Biol 17:R812–R814. [DOI] [PubMed] [Google Scholar]
- Oshima A, Matsuzawa T, Nishikawa K, Fujiyoshi Y. 2013. Oligomeric structure and functional characterization of the C. elegans innexin‐6 gap junction protein. J Biol Chem 288:10513–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima A, Matsuzawa T, Murata K, Tani K, Fujiyoshi Y. (2016) Hexadecameric structure of an invertebrate gap junction channel. J Mol Biol 428:1227–1236. [DOI] [PubMed] [Google Scholar]
- Penuela S, Gehl R, Laird DW. 2013. The biochemistry and function of pannexin channels. Biochem Biophys Acta 1828:15–22. [DOI] [PubMed] [Google Scholar]
- Pepper ASR, Lo T‐W, Killian DJ, Hall DH, Hubbard EJA. 2003. The establishment of C. elegans germline pattern is controlled by overlapping proximal and distal somatic gonad signals. Dev Biol 259:336–350. [DOI] [PubMed] [Google Scholar]
- Peters MA, Teramoto T, White JQ, Iwasaki K, Jorgensen EM. 2007. A calcium wave mediated by gap junctions coordinates a rhythmic behavior in C. elegans . Curr Biol 17:1601–1608. [DOI] [PubMed] [Google Scholar]
- Phelan P, Starich TA. 2001. Innexins get into the gap. Bioessays 23:388–396. [DOI] [PubMed] [Google Scholar]
- Quick DC, Johnson RG. 1977. Gap junctions and rhombic particle arrays in planaria. J Ultrastruct Res 60:348–361. [DOI] [PubMed] [Google Scholar]
- Raizen DM, Avery L. 1994. Electrical activity and behavior in the pharynx of C. elegans . Neuron 12:483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenas‐Ruano A, Chavez AE, Cossio MJ, Castillo PE, Zukin RS. 2012. REST‐dependent epigenetic remodeling promotes the developmental switch in synaptic NMDA receptors. Nat Neurosci 15:1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen KT, Moerman DG, Naus CC. 2014. Gap junctions in C. elegans . Front Physiol 5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA. 1974. Structure and function of intercellular junctions. Int Rev Cytol 39:191–283. [DOI] [PubMed] [Google Scholar]
- Starich T, Miller A, Nguyen RL, Hall DH, Shaw JE. 2003. The C. elegans innexin gene product INX‐3 is localized to gap junctions and is essential for embryonic development. Dev Biol 256:403–417. [DOI] [PubMed] [Google Scholar]
- Starich TA, Lee RY, Panzarella C, Avery L, Shaw JE. 1996. eat‐5 and unc‐7 represent a multigene family in C. elegans involved in cell‐cell coupling. J Cell Biol 134:537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starich TA, Hall DH, Greenstein D. 2014. Two classes of gap junction channels mediate soma‐ germline interactions essential for germline proliferation and gametogenesis in C. elegans . Genetics 198:1127–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KK, Golden A. (2015) The C. elegans eggshell. WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.179.1. Available at: http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Stone CE, Hall DH, Sundaram MV. 2009. Lipocalin signaling controls unicellular tube development in Caenorhabditis elegans . Dev Biol 329:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. 1977. Post‐embryonic cell lineages of the nematode C. elegans . Dev Biol 56:110–156. [DOI] [PubMed] [Google Scholar]
- Trojanowski NF, Fang‐Yen C. 2015. Simultaneous optogenetic stimulation of individual pharyngeal neurons and monitoring of feeding behavior in intact C. elegans . Methods Mol Biol 1327:105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. 1976. The structure of the ventral cord of C. elegans . Philos Trans R Soc Lond 275:327–348. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. 1986. The structure of the nervous system of the nematode C. elegans . Philos Trans R Soc Lond 314B 1–340. [DOI] [PubMed] [Google Scholar]


