Summary
A primary analysis of the ASPIRE study found that the addition of carfilzomib to lenalidomide and dexamethasone (carfilzomib group) significantly improved progression‐free survival (PFS) compared with lenalidomide and dexamethasone alone (control group) in patients with relapsed multiple myeloma (RMM). This post hoc analysis examined outcomes from ASPIRE in patients categorised by age. In the carfilzomib group, 103/396 patients were ≥70 years old, and in the control group, 115/396 patients were ≥70 years old. Median PFS for patients <70 years old was 28·6 months for the carfilzomib group versus 17·6 months for the control group [hazard ratio (HR), 0·701]. Median PFS for patients ≥70 years old was 23·8 months for the carfilzomib group versus 16·0 months for the control group (HR, 0·753). For patients <70 years the overall response rate (ORR) was 86·0% (carfilzomib group) and 66·9% (control group); for patients ≥70 years old the ORR was 90·3% (carfilzomib group) and 66·1% (control group). Within the carfilzomib group, grade ≥3 cardiovascular adverse events occurred more frequently among patients ≥70 years old compared with patients <70 years old. Carfilzomib‐lenalidomide‐dexamethasone has a favourable benefit‐risk profile for patients with RMM, including elderly patients ≥70 years old. Trial Registration: clinicaltrials.gov identifier: NCT01080391.
Keywords: carfilzomib, lenalidomide, dexamethasone, relapsed multiple myeloma, clinical trial
Carfilzomib is a second‐generation proteasome inhibitor that binds irreversibly to the β5 subunit of the 20s proteasome and exhibits increased selectivity compared with bortezomib (McBride et al, 2015). Based on results from the phase 2 PX‐171‐003‐A1 trial, carfilzomib was initially approved in the US in 2012 as a single agent for patients with relapsed or refractory myeloma who received at least two prior lines of therapy (including bortezomib and an immunomodulatory agent) and had progressive disease ≤60 days from the last therapy (McCormack, 2012; Siegel et al, 2012). In 2015, the indication for carfilzomib use was expanded to include its combination with lenalidomide and dexamethasone for patients with relapsed myeloma who received 1–3 prior lines of therapy. This expanded approval was based on results from the phase III ASPIRE trial, which compared a regimen of carfilzomib, lenalidomide and dexamethasone (carfilzomib group) to lenalidomide and dexamethasone (control group) (Stewart et al, 2015). In a pre‐planned interim analysis of the ASPIRE trial, progression‐free survival [PFS; hazard ratio (HR) = 0·69; P = 0·0001) and overall response rate (ORR; 87·1% vs. 66·7%; P < 0·001) were significantly higher in the carfilzomib group vs. the control group (Stewart et al, 2015).
The median age of diagnosis for multiple myeloma (MM) is 69 years, and treatment of MM within the elderly patient population remains a challenge (Johnson, 2014). Many patients over 65 years of age (and some younger patients in poor health) are not eligible for autologous stem cell transplantation (Palumbo & Magarotto, 2011). Age‐related physiological changes in the elderly can influence the pharmacokinetics of drugs and can potentially lead to increased toxicity, and high comorbidity burden among the elderly may affect their ability to tolerate cancer treatment (Vestal, 1997; Yancik et al, 2001; Du et al, 2002; John et al, 2003; Repetto, 2003). Regimens containing newer agents, such as the triplets bortezomib plus melphalan‐prednisone (VMP) and melphalan plus prednisone‐thalidomide (MPT) have shown clinical efficacy in the treatment of elderly patients and have improved disease management in this population (Mateos et al, 2008; Hulin et al, 2009; De La Rubia & Sanz, 2011). Survival rates for elderly patients with MM have not improved as much as those for younger patients, based on data in the Surveillance, Epidemiology, and End Results database from 1998 to 2007 (Pulte et al, 2011). A recent meta‐analysis of elderly MM patients treated with VMP or MPT found that patients aged 75 years or older had worse survival than those under 75 years old; renal failure and cardiac or gastrointestinal grade 3–4 adverse events were found to negatively correlate with survival among elderly patients with MM (Bringhen et al, 2013). As the elderly population is heterogeneous, a frailty score (based on age, comorbidities, and cognitive and physical conditions) that can be used to predict survival and risk of toxicity among elderly MM patients has recently been developed (Palumbo et al, 2015).
Clinical studies have demonstrated efficacy of regimens containing the first‐generation proteasome inhibitor bortezomib in elderly populations of patients with both newly diagnosed and relapsed MM (Mateos et al, 2006; Richardson et al, 2007). Although the occurrence of peripheral neuropathy appeared high among elderly patients receiving bortezomib, a less frequent dosing regimen was found to decrease rates of toxic effects (Mateos et al, 2006, 2010). Subcutaneous administration of bortezomib rather than intravenous administration has also been shown to lower rates of peripheral neuropathy (Moreau et al, 2011). Some single‐arm trials have demonstrated clinical efficacy of the second‐generation proteasome inhibitor carfilzomib in elderly patients with newly diagnosed MM, when used in combination with melphalan and prednisone or with lenalidomide and low‐dose dexamethasone (Dytfeld et al, 2014; Moreau et al, 2015). In these early phase studies, carfilzomib was generally well tolerated in elderly patients; grade 3/4 adverse events were primarily haematological, and incidence of peripheral neuropathy was low. At present, limited information exists regarding carfilzomib use among elderly patients in the relapsed MM setting.
In this study we performed a post hoc subgroup analysis of patients <70 and ≥70 years of age who were enrolled in the ASPIRE study to examine the efficacy and safety of carfilzomib in an elderly population with relapsed MM.
Methods
The study design, patient eligibility criteria, and assessment measures for the randomised, open‐label, phase 3 ASPIRE trial (NCT01080391) have previously been described (Stewart et al, 2015). Adult patients with relapsed MM who had received 1–3 prior treatments were eligible. Patients could have prior exposure to bortezomib if they had not progressed during treatment, and they could have prior exposure to lenalidomide and dexamethasone if they had not progressed during the first 3 months of therapy or discontinued due to intolerance. Patients were required to have adequate renal, haematological and hepatic function. Patients were excluded if they had significant peripheral neuropathy (grade 3/4, or grade 2 with pain), myocardial infarction within 4 months prior to randomization, New York Heart Association (NYHA) class III or IV heart failure, history of severe coronary artery disease, uncontrolled angina, severe uncontrolled ventricular arrhythmias, sick sinus syndrome, or electrocardiographic evidence of acute ischaemia or grade 3 conduction‐system abnormalities (unless subject had a pacemaker). Institutional review boards of all participating institutions approved the study protocol, and informed consent was provided by all patients.
Patients were randomised 1:1 to the carfilzomib group (carfilzomib, lenalidomide, and dexamethasone) or the control group (lenalidomide and dexamethasone). Stratification factors used for randomization were prior bortezomib therapy, prior lenalidomide therapy and β2‐microglobulin level. Patients received treatment in 28‐day cycles until withdrawal of consent, disease progression or unacceptable toxicity; carfilzomib was discontinued after cycle 18, after which patients in the carfilzomib group received just lenalidomide and dexamethasone until disease progression. Carfilzomib was given on days 1, 2, 8, 9, 15 and 16 of cycles 1–12, and on days 1, 2, 15 and 16 of cycles 13–18. Carfilzomib was administered as a 10‐min infusion at a starting dose of 20 mg/m2 on days 1 and 2 of cycle 1, and at a target dose of 27 mg/m2 for all subsequent infusions. In both the carfilzomib group and the control group, lenalidomide (25 mg) was given on days 1–21 and dexamethasone (40 mg) was given on days 1, 8, 15 and 22.
Patients in the intent‐to‐treat population were assigned to one of two cohorts/subgroups based on age: <70 years of age or ≥70 years of age. Outcome measures assessed in this post hoc subgroup analysis were PFS, ORR, duration of response (DOR) and safety. Efficacy analyses were performed using the intent‐to‐treat population, and safety analyses were performed using all patients who received at least one dose of study treatment. Responses and disease progression were assessed in a blinded manner by an independent review committee using the International Myeloma Working Group Uniform Response Criteria (Durie et al, 2006). PFS and DOR were summarised using the Kaplan‐Meier method. HRs were estimated with a stratified Cox proportional‐hazards model for PFS and the Mantel–Haenszel method for ORR in the overall population analysis, and with an unstratified Cox proportional‐hazards model for PFS and the unstratified Chi‐square test for ORR in the subgroup analyses. The European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Module (QLQ‐C30) questionnaire was used to assess health‐related quality of life (HRQOL) (Aaronson et al, 1993). Assessments were made on day 1 of cycles 3, 6, 12 and 18. As there was no pre‐specified hypothesis to test in this subgroup analysis, all comparisons between treatment arms were performed descriptively. All analyses for this study were performed by the sponsor [Onyx Pharmaceuticals, Inc., (South San Francisco, CA, USA) an Amgen subsidiary].
Results
ASPIRE was a multicentre trial with patients enrolled at sites in North America, Europe and the Middle East. The data reported here are from a planned interim analysis (date of first recruitment, 14 July 2010; data cut‐off date, 16 June 2014). A total of 792 patients were enrolled and randomised 1:1 to either the carfilzomib group or the control group. In the carfilzomib group, 103 out of 396 patients (26·0%) were ≥70 years of age, and in the control group, 115 out of 396 patients (29·0%) were ≥70 years of age (Table 1). In both the <70 and ≥70 years of age subgroups, baseline patient characteristics were generally well balanced between treatment arms (Table 1). The proportion of patients with high‐risk cytogenetic risk status at study entry was lower among patients in the ≥70 years of age subgroup than those in the <70 years of age subgroup for both treatment arms. Median duration of treatment was longer for patients receiving carfilzomib than for those receiving control treatment in both the <70 years of age subgroup (97·0 vs. 57·0 weeks) and the ≥70 years of age subgroup (74·0 vs. 57·6 weeks).
Table 1.
Patient demographics and baseline disease characteristics
| Age <70 years | Age ≥70 years | |||
|---|---|---|---|---|
| Carfilzomib group (n = 293) | Control group (n = 281) | Carfilzomib group (n = 103) | Control group (n = 115) | |
| Age, median years (range) | 60·0 (38·0–69·0) | 62·0 (31·0–69·0) | 74·0 (70·0–87·0) | 74·0 (70·0–91·0) |
| ECOG PS, n (%) | ||||
| 0 or 1 | 267 (91·1) | 262 (93·2) | 89 (86·4) | 99 (86·1) |
| 2 | 26 (8·9) | 19 (6·8) | 14 (13·6) | 16 (13·9) |
| Cytogenetic risk by FISH at study entry, n (%) | ||||
| High risk | 42 (14·3) | 43 (15·3) | 6 (5·8) | 9 (7·8) |
| Standard risk | 102 (34·8) | 111 (39·5) | 45 (43·7) | 59 (51·3) |
| Unknown | 149 (50·9) | 127 (45·2) | 52 (50·5) | 47 (40·9) |
| Creatinine clearance, n (%) | ||||
| 30 to <50 ml/min | 10 (3·4) | 17 (6·0) | 15 (14·6) | 14 (12·2) |
| ≥50 ml/min | 283 (96·6) | 260 (92·5) | 87 (84·5) | 98 (85·2) |
| Unknown/other value | 0 | 4 (1·4) | 1 (1·0) | 3 (2·6) |
| Serum β2‐microglobulin level, n (%) | ||||
| <2·5 mg/l | 61 (20·8) | 61 (21·7) | 16 (15·5) | 16 (13·9) |
| ≥2·5 mg/l | 232 (79·2) | 220 (78·3) | 87 (84·5) | 99 (86·1) |
| Prior therapy, n (%) | ||||
| Bortezomib | 197 (67·2) | 186 (66·2) | 64 (62·1) | 74 (64·3) |
| Lenalidomide | 56 (19·1) | 52 (18·5) | 23 (22·3) | 26 (22·6) |
ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridization.
The addition of carfilzomib to lenalidomide and dexamethasone improved PFS in both the <70 years of age subgroup and the ≥70 years of age subgroup. In the <70 years of age subgroup, patients receiving carfilzomib had a median PFS of 28·6 months compared with 17·6 months in the control group [HR, 0·70; 95% confidence interval (CI), 0·56–0·88] (Fig 1). In the ≥70 years of age subgroup, patients receiving carfilzomib had a median PFS of 23·8 months compared with 16·0 months in the control group (HR, 0·75; 95% CI, 0·53–1·08) (Fig 1). Among the patients receiving carfilzomib, median PFS was higher in the <70 years of age subgroup than the ≥70 years of age subgroup. A similar trend was observed for patients receiving lenalidomide and dexamethasone alone.
Figure 1.
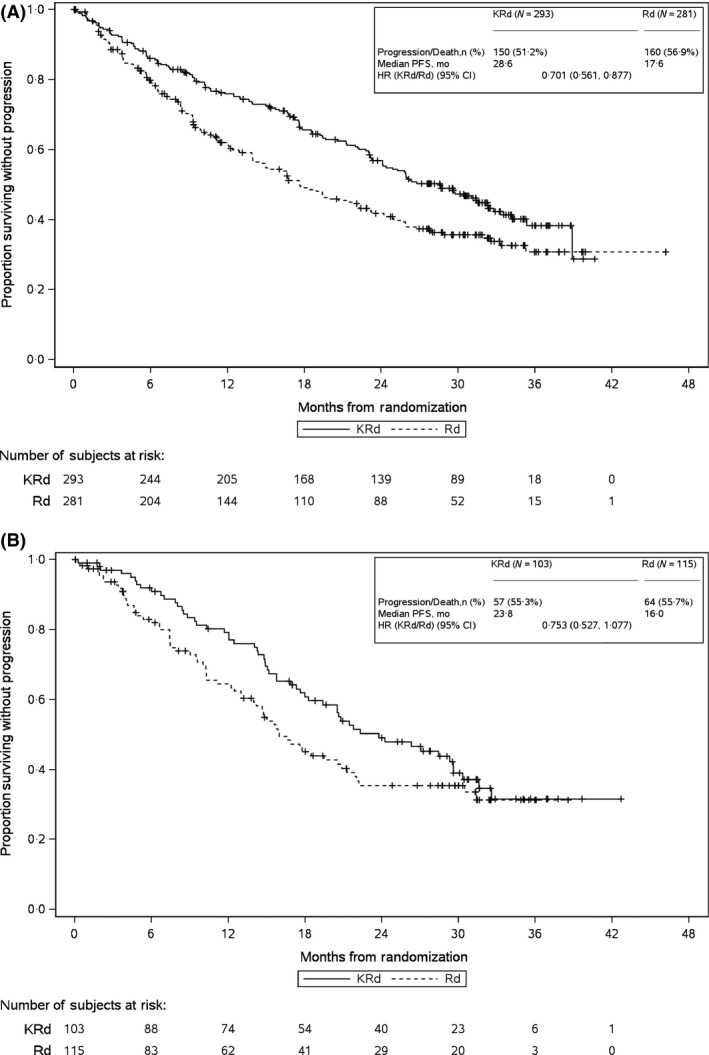
Kaplan–Meier PFS Curves for (A) <70 years of Age Subgroup and (B) ≥70 years of Age Subgroup. CI, confidence interval; HR, hazard ratio; KRd, carfilzomib‐lenalidomide‐dexamethasone; mo, months; PFS, progression‐free survival; Rd, lenalidomide‐dexamethasone
Response rates were higher in the carfilzomib group than the control group, regardless of age. Among patients <70 years of age, those in the carfilzomib treatment arm had a higher ORR (86·0% vs. 66·9%) and a higher rate of complete response or better (29·4% vs. 11·4%) than those in the control arm (Table 2). Among patients ≥70 years of age, those in the carfilzomib treatment arm had a higher ORR (90·3% vs. 66·1%) and a higher rate of complete response or better (38·8% vs. 4·3%) than those in the control arm (Table 2). Addition of carfilzomib also led to a longer median DOR in both subgroups (<70 years of age: 30·4 vs. 23·1 months; ≥70 years of age: 23·3 vs. 16·7 months) (Table 2).
Table 2.
Responses by age subgroup
| Age <70 years | Age ≥70 years | |||
|---|---|---|---|---|
| Carfilzomib group (n = 293) | Control Group (n = 281) | Carfilzomib group (n = 103) | Control group (n = 115) | |
| Best overall response, n (%)a | ||||
| Stringent complete response | 40 (13·7) | 16 (5·7) | 16 (15·5) | 1 (0·9) |
| Complete response | 46 (15·7) | 16 (5·7) | 24 (23·3) | 4 (3·5) |
| Very good partial response | 116 (39·6) | 83 (29·5) | 35 (34·0) | 40 (34·8) |
| Partial response | 50 (17·1) | 73 (26·0) | 18 (17·5) | 31 (27·0) |
| Overall response rate, % (95% CI)a | 86·0 (81·5–89·8) | 66·9 (61·1–72·4) | 90·3 (82·9–95·2) | 66·1 (56·7–74·7) |
| Median duration of response, months (95% CI) | 30·4 (25·1–36·1) | 23·1 (17·3–32·3) | 23·3 (17·2–29·4) | 16·7 (12·9–30·5) |
CI, confidence interval.
Patients evaluated for overall response rate had a best overall response of partial response or better.
Forty‐three patients in the carfilzomib group and 53 patients in the control group were ≥75 years of age. As age ≥75 years was identified as a poor prognostic factor in a recent meta‐analysis (Bringhen et al, 2013) we also assessed efficacy outcomes in this smaller subset of patients. Among patients aged ≥75 years, the median PFS (carfilzomib arm vs. control arm) was 30·3 months vs. 16·6 months (HR, 0·62; 95% CI, 0·36–1·08) and the ORR was 86·0% vs. 62·3%.
In the <70 years of age subgroup, the frequency of grade ≥3 hypophosphataemia was higher in the carfilzomib arm than the control arm (Table 3). The frequencies of grade ≥3 cardiac failure, grade ≥3 thrombocytopenia, grade ≥3 neutropenia, and grade ≥3 hypokalaemia were higher in the carfilzomib arm than the control arm (by at least five percentage points) in patients ≥70 years of age, but frequencies of these adverse events were similar between treatment arms in the younger patients (Table 3). Among patients receiving carfilzomib, cardiovascular adverse events (including hypertension, cardiac failure, pulmonary embolism and ischaemic heart disease) occurred more frequently in the ≥70 years of age subgroup than the <70 years of age subgroup (Table 3). Additionally, discontinuation of carfilzomib due to a cardiovascular adverse event of any grade occurred more frequently in the ≥70 years of age subgroup than the <70 years of age subgroup (6·8% vs. 1·4%). However, a trend for increased frequency of cardiovascular adverse events among elderly patients compared with younger patients was not observed in the control group. Grade ≥3 peripheral neuropathy occurred infrequently in this study (<3·0% of patients in each subgroup, regardless of treatment arm or age). In the <70 years of age subgroup, 23·2% of carfilzomib group patients and 20·9% of control group patients discontinued any study drug due to adverse events. In the ≥70 years of age subgroup, 34·0% of carfilzomib group patients and 34·8% of control group patients had a treatment discontinuation due to adverse events. Death while receiving study treatment or within 30 days of last dose occurred at similar rates between study arms in both subgroups [<70 years age subgroup, carfilzomib = 5·2% (adverse events, 4·5%; progressive disease, 0·3%; other, 0·3%), control = 7·2% (adverse events, 6·1%; progressive disease, 0·7%; other, 0·4%); ≥70 years of age subgroup, carfilzomib = 14·6% (adverse events, 13·6%; progressive disease, 1·0%), control = 11·6% (adverse events, 8·9%; progressive disease, 2·7%)]. These data also reveal that the rate of death was higher among patients ≥70 years old than patients <70 years old in both treatment groups. Among the patients who received carfilzomib, 31 (10·7%) patients <70 years of age and 22 (21·4%) patients ≥70 years of age required a carfilzomib dose reduction (the majority of dose reductions in both subgroups were due to adverse events). In the control group, 37·9% of patients <70 years of age and 53·6% of patients ≥70 years of age required a lenalidomide dose reduction; in the carfilzomib group, 40·5% of patients <70 years of age and 60·2% of patients ≥70 years of age required a lenalidomide dose reduction.
Table 3.
Adverse events by age subgroup
| Age <70 years | Age ≥70 years | |||
|---|---|---|---|---|
| Carfilzomib group (n = 289) | Control group (n = 277) | Carfilzomib group (n = 103) | Control group (n = 112) | |
| Any‐grade AE, n (%) | 278 (96·2) | 267 (96·4) | 102 (99·0) | 111 (99·1) |
| Grade ≥3 AE, n (%) | 236 (81·7) | 215 (77·6) | 92 (89·3) | 99 (88·4) |
| Grade ≥3 AEs reported in ≥5% of patients in any subgroup, n (%) | ||||
| Neutropenia | 78 (27·0) | 77 (27·8) | 38 (36·9) | 26 (23·2) |
| Anaemia | 45 (15·6) | 44 (15·9) | 25 (24·3) | 23 (20·5) |
| Thrombocytopenia | 44 (15·2) | 31 (11·2) | 21 (20·4) | 17 (15·2) |
| Pneumonia | 33 (11·4) | 25 (9·0) | 16 (15·5) | 16 (14·3) |
| Hypophosphatemia | 26 (9·0) | 7 (2·5) | 7 (6·8) | 11 (9·8) |
| Hypokalaemia | 21 (7·3) | 12 (4·3) | 16 (15·5) | 7 (6·3) |
| Fatigue | 16 (5·5) | 13 (4·7) | 14 (13·6) | 12 (10·7) |
| Hyperglycaemia | 12 (4·2) | 11 (4·0) | 8 (7·8) | 7 (6·3) |
| Asthenia | 7 (2·4) | 5 (1·8) | 7 (6·8) | 3 (2·7) |
| Hypertension | 11 (3·8) | 5 (1·8) | 6 (5·8) | 2 (1·8) |
| Leucopenia | 10 (3·5) | 9 (3·2) | 2 (1·9) | 7 (6·3) |
| Rash | 3 (1·0) | 0 | 2 (1·9) | 6 (5·4) |
| Grade ≥3 AEs of interest | ||||
| Cardiac failurea | 6 (2·1) | 5 (1·8) | 9 (8·7) | 2 (1·8) |
| Ischaemic heart diseaseb | 8 (2·8) | 7 (2·5) | 5 (4·9) | 1 (0·9) |
| Pulmonary embolism | 7 (2·4) | 8 (2·9) | 5 (4·9) | 1 (0·9) |
| Acute renal failurec | 9 (3·1) | 6 (2·2) | 4 (3·9) | 6 (5·4) |
AE, adverse event.
The category of cardiac failure included (in descending order of frequency) cardiac failure, congestive cardiac failure, pulmonary oedema, hepatic congestion, cardiopulmonary failure, acute pulmonary oedema, acute cardiac failure and right ventricular failure.
The category of ischaemic heart disease included (in descending order of frequency) angina pectoris, myocardial infarction, acute myocardial infarction, increased blood creatinine phosphokinase, coronary artery disease, myocardial ischaemia, coronary artery occlusion, increased troponin, increased troponin T, acute coronary syndrome, abnormal cardiac stress test, cardiomyopathy stress, unstable angina, coronary artery stenosis, abnormal electrocardiogram ST‐T segment and abnormal electrocardiogram T wave.
The category of acute renal failure included (in descending order of frequency) acute renal failure, renal failure, renal impairment, azotemia, oliguria, anuria, toxic nephropathy and pre‐renal failure.
The EORTC QLQ‐C30 Global Health Status/Quality of Life scale was used to assess HRQOL. As measured by change from the mean baseline score, patients in the <70 years of age subgroup who received carfilzomib reported greater improvements in HRQOL than control patients at all cycles examined (cycles 3, 6, 12 and 18) (Fig 2A). Among patients ≥70 years of age, a decrease in HRQOL scores from baseline was observed in both the carfilzomib group and the control group at cycles 3, 12 and 18 (mean scores were similar to baseline for both treatment groups at cycle 6). A greater decrease in HRQOL was reported for control patients compared with carfilzomib patients at cycle 12, but control patients and carfilzomib patients had similar changes in HRQOL at cycles 12 and 18 (Fig 2B). However, comparisons between treatment arms within the ≥70 years of age subgroup should be interpreted with caution due to small sample sizes (for example, at cycle 18, data were available for just 50 carfilzomib patients and 38 control patients) (Fig 2B).
Figure 2.
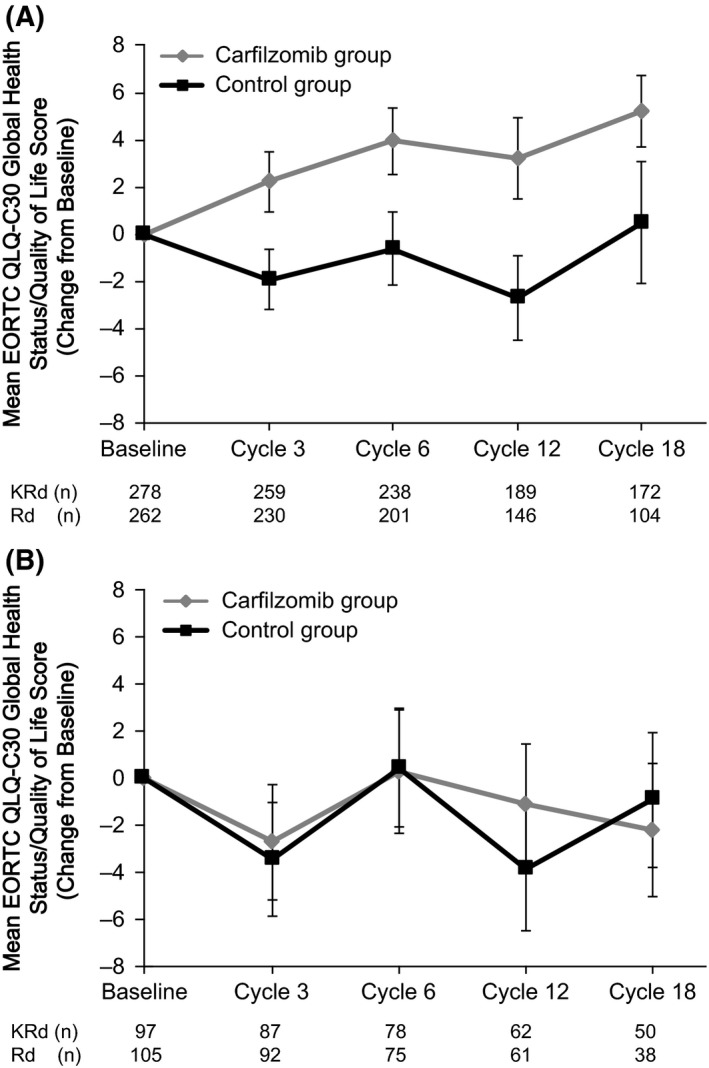
HRQOL for (A) <70 years of Age Subgroup and (B) ≥70 years of Age Subgroup. Mean scores (reported as change from baseline) from the EORTC QLQ‐C30 scale and standard errors are shown. Higher scores denote better quality of life. HRQOL, health‐related quality of life; KRd, carfilzomib‐lenalidomide‐dexamethasone; Rd, lenalidomide‐dexamethasone.
Discussion
This post hoc subgroup analysis found that addition of carfilzomib to lenalidomide and dexamethasone led to a clinically meaningful improvement in PFS in both age subgroups, including elderly patients (≥70 years of age) with relapsed MM. The HR for progression or death (carfilzomib versus control) among elderly patients was 0·75, similar to that among the younger individuals (0·70). Consistent results were obtained for carfilzomib versus control when efficacy outcomes were assessed in a smaller subset of elderly patients ≥75 years of age (HR for progression or death, 0·62). ORRs were also higher among patients receiving carfilzomib regardless of age. The efficacy findings from the subgroup analyses described here are consistent with results from the primary analysis of the ASPIRE trial (Stewart et al, 2015). Elderly patients with MM generally have poorer outcomes than younger patients, and there are challenges inherent in treating the elderly population. Selection of the carfilzomib‐lenalidomide‐dexamethasone triplet for high‐risk or less fit populations, such as the elderly, is supported by the results of this subgroup analysis.
Within the carfilzomib group, response rates (ORR, ≥ complete response) were similar for patients <70 years old and ≥70 years old, but median PFS was higher for patients <70 years old than for patients ≥70 years old; therefore, although the carfilzomib treatment effect was consistent across the two subgroups, there was a trend for the magnitude of PFS improvement to differ from ORR benefit with age. This may be explained in part by the observations that patients <70 years old had a longer DOR than patients ≥70 years old, and patients ≥70 years old had higher rates of treatment discontinuation, carfilzomib dose reduction, lenalidomide dose reduction and death than patients <70 years old. This is consistent with the observation that within the carfilzomib group, patients ≥70 years old had a shorter median treatment duration than patients <70 years old.
In the safety analysis, several adverse events of grade ≥3 (including thrombocytopenia, neutropenia, and hypokalaemia) occurred ≥5·0% more frequently among elderly patients (≥70 years of age) in the carfilzomib arm than among elderly patients in the control arm. An increased incidence of haematological adverse events has been reported in other clinical studies of carfilzomib, and additional research is needed to understand the mechanistic basis of this toxicity (Harvey, 2014). Additionally, findings from this study point toward increased cardiovascular toxicity associated with carfilzomib in elderly patients compared with younger patients. The frequencies of grade ≥3 cardiovascular adverse events (hypertension, cardiac failure, pulmonary embolism, ischaemic heart disease) were similar between treatment arms among younger patients, but tended to be higher in the carfilzomib arm than the control arm among patients ≥70 years. Furthermore, within the carfilzomib group, grade ≥3 cardiovascular events (hypertension, cardiac failure, pulmonary embolism, ischaemic heart disease) were observed more frequently among patients ≥70 years of age than among patients <70 years of age, and discontinuation of carfilzomib due to any‐grade cardiovascular adverse event occurred more frequently among patients ≥70 years of age than among patients <70 years of age.
In both subgroups (<70 years of age and ≥70 years of age), rates of treatment discontinuation due to an adverse event were similar between treatment arms. Additionally, deaths while receiving treatment or within 30 days after treatment discontinuation occurred at similar frequencies between the carfilzomib arm and the control arm in both subgroups, although the overall incidence of death was higher in patients ≥70 years of age than in those <70 years of age. Overall, the carfilzomib‐lenalidomide‐dexamethasone triplet had acceptable toxicity, even for elderly patients with relapsed MM. Patients in the ASPIRE trial were generally in good health, as patients with renal or hepatic dysfunction and/or with NYHA class III or IV heart failure were excluded. Additional research is needed to understand the safety profile of carfilzomib among frail elderly patients. In the <70 years of age subgroup, patients receiving carfilzomib reported improved HRQOL compared with control patients, but improved HRQOL among carfilzomib‐treated patients was not observed in the ≥70 years of age subgroup.
Some prior single‐arm studies have demonstrated high clinical activity of triplets containing carfilzomib for the front‐line treatment of MM in elderly patients (Dytfeld et al, 2014; Moreau et al, 2015). Moreau et al (2015) reported an ORR (defined as partial response or better) of 90% for patients >65 years of age with newly diagnosed MM treated with carfilzomib plus melphalan and prednisone. Jakubowiak et al (2012) studied a combination of carfilzomib, lenalidomide and dexamethasone in patients with newly diagnosed MM and reported that this regimen was well tolerated and produced a high rate of deep responses; in an updated subgroup analysis of this study Dytfeld et al (2014) reported that 100% of patients ≥65 years of age with newly diagnosed MM achieved a partial response or better to the carfilzomib‐lenalidomide‐dexamethasone regimen. To the best of our knowledge, the present study is the first to report on a carfilzomib‐containing regimen in elderly patients with relapsed MM. It is difficult to directly compare efficacy data from the relapsed MM setting to that in the newly diagnosed MM setting as the depth of response for MM patients tends to diminish with successive lines of therapy (Kurtin, 2013). Although the ORR we observed here (90·3% in the ≥70 years of age group) was slightly lower than that observed by Dytfeld et al (2014) for the carfilzomib‐lenalidomide‐dexamethasone regimen in the front‐line treatment of elderly patients with MM, our results suggest that carfilzomib remains highly active for elderly MM patients in the relapsed MM setting. Future research could investigate what therapeutic regimens elderly patients with MM would use following discontinuation of carfilzomib due to progression or toxicity.
Some prior studies have reported the clinical activity of bortezomib‐containing regimens in elderly patients with relapsed MM, including bortezomib, melphalan, thalidomide and dexamethasone [84% partial response or better, 19% complete response (Azarm et al, 2012)]; bortezomib and dexamethasone [65·4% partial response or better, 11% complete response (Castelli et al, 2015)]; bortezomib, dexamethasone and cyclophosphamide [83% partial response or better, 11% complete response (Mele et al, 2010)]; bortezomib, melphalan and prednisone [57% partial response or better, complete response not reported (Petrucci et al, 2013)]; and bendamustine, bortezomib and dexamethasone [57·6% partial response or better, 10·9% complete response (Rodon et al, 2015)]. The complete response rate we observed here for a carfilzomib‐containing regimen in a population of elderly patients with relapsed MM (38·8% complete response + stringent complete response) is higher than that observed in several other studies of bortezomib‐containing regimens in elderly patients with relapsed and/or refractory MM. However, it is difficult to make direct comparisons between these previous studies and the present study because of differences in study population, age cut‐offs, and treatment regimens.
In conclusion, we observed clinically meaningful improvements in PFS and ORR among elderly patients with relapsed MM treated with carfilzomib. As this was a post hoc subgroup analysis and statistical significance testing was not performed, the findings reported here need to be interpreted with caution. Although the sample size overall and within each subgroup allowed for meaningful comparisons, these results need to be replicated in a prospective study to confirm the benefits of using carfilzomib to treat relapsed MM in an elderly population.
Author contributions
Antonio Palumbo, A. Keith Stewart, Roman Hájek, Ruben Niesvizky, Jesus San‐Miguel, Heinz Ludwig, Sanjay Aggarwal, Philippe Moreau and Meletios A. Dimopoulos helped design the study. Antonio Palumbo, A. Keith Stewart, Tamás Masszi, Ivan Špička, Albert Oriol, Roman Hájek, Laura Rosiñol, David Siegel, Georgi G. Mihaylov, Vesselina Goranova‐Marinova, Péter Rajnics, Aleksandr Suvorov, Ruben Niesvizky, Andrzej Jakubowiak, Jesus San‐Miguel, Heinz Ludwig, Philippe Moreau and Meletios A. Dimopoulos were involved in the acquisition of data. Mihaela Obreja performed data analysis. Antonio Palumbo, A. Keith Stewart, Tamás Masszi, Ivan Špička, Albert Oriol, Roman Hájek, Laura Rosiñol, David Siegel, Georgi G. Mihaylov, Vesselina Goranova‐Marinova, Péter Rajnics, Aleksandr Suvorov, Ruben Niesvizky, Andrzej Jakubowiak, Jesus San‐Miguel, Heinz Ludwig, Mihaela Obreja, Sanjay Aggarwal, Philippe Moreau and Meletios A. Dimopoulos interpreted the data and participated in review and revision of the manuscript.
Disclosures
Meletios A. Dimopoulos: Honoraria – Celgene, Onyx; Consulting or Advisory Role – Celgene, Onyx. A. Keith Stewart: Honoraria – Amgen, Celgene, Onyx; Consulting or Advisory Role – Celgene, Janssen, Takeda. Tamás Masszi: Consulting or Advisory Role – Amgen, Bristol‐Myers Squibb, Janssen‐Cilag, Takeda. Ivan Špička: Honoraria – Celgene, Janssen‐Cilag, Janssen R & D; Research Funding – Celgene. Albert Oriol: Consulting or Advisory Role – Amgen, Celgene, Janssen; Speakers' Bureau – Amgen, Celgene, Janssen. Roman Hájek: Honoraria – Celgene, Janssen, Merck, Onyx. Laura Rosiñol: Honoraria – Celgene, Janssen. David Siegel: Honoraria – Celgene, Millennium, Onyx; Speaker's Bureau – Celgene, Millennium, Onyx. Georgi G. Mihaylov: No relationships to disclose. Vesselina Goranova‐Marinova: No relationships to disclose. Péter Rajnics: No relationships to disclose. Aleksandr Suvorov: No relationships to disclose. Ruben Niesvizky: Consulting or Advisory Role – Celgene, Millennium, Onyx; Speakers' Bureau – Celgene, Millennium, Onyx. Andrzej Jakubowiak: Honoraria – Onyx; Consulting or Advisory Role – Onyx; Speakers' Bureau – Onyx; Research Funding – Onyx. Jesus San‐Miguel: Consulting or Advisory Role – Bristol‐Myers Squibb, Celgene, Janssen, Millennium, Merck Sharp & Dohme, Novartis, Onyx. Heinz Ludwig: Speakers' Bureau ‐ Amgen, Bristol‐Myers Squibb, Celgene, Novartis, Takeda; Study Support – Takeda. Antonio Palumbo: Honoraria – Amgen, Celgene, Onyx; Consulting or Advisory Role – Amgen, Celgene, Onyx; Employment – Takeda (Antonio Palumbo is currently a Takeda employee; all data reported in this manuscript were generated during his previous employment at the University of Torino). Mihaela Obreja: Employment – Amgen. Sanjay Aggarwal: Employment – Amgen. Philippe Moreau: Honoraria – Celgene, Janssen, Millennium, Novartis, Onyx; Consulting or Advisory Role – Celgene, Janssen, Millennium, Novartis, Onyx.
Acknowledgments
The authors would like to thank Jesse Potash of Amgen Inc. for medical writing assistance. The ASPIRE study was supported by Onyx Pharmaceuticals, Inc. an Amgen subsidiary.
References
- Aaronson, N.K. , Ahmedzai, S. , Bergman, B. , Bullinger, M. , Cull, A. , Duez, N.J. , Filiberti, A. , Flechtner, H. , Fleishman, S.B. , de Haes, J.C. , Kaasa, S. , Klee, M. , Osoba, D. , Razavi, D. , Rofe, P.B. , Schraub, S. , Sneeuw, K. , Sullivan, M. & Takeda, F. for the European Organization for Research and Treatment of Cancer Study Group on Quality of Life . (1993) The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute, 85, 365–376. [DOI] [PubMed] [Google Scholar]
- Azarm, T. , Akbari, M. , Azarm, A. & Mohager, H. (2012) Bortezomib in combination with low‐dose oral melphalan, dexamethasone and thalidomide for relapsed elderly patients with multiple myeloma. Journal of Research in Medicine Sciences, 17, 8–14. [PMC free article] [PubMed] [Google Scholar]
- Bringhen, S. , Mateos, M.V. , Zweegman, S. , Larocca, A. , Falcone, A.P. , Oriol, A. , Rossi, D. , Cavalli, M. , Wijermans, P. , Ria, R. , Offidani, M. , Lahuerta, J.J. , Liberati, A.M. , Mina, R. , Callea, V. , Schaafsma, M. , Cerrato, C. , Marasca, R. , Franceschini, L. , Evangelista, A. , Teruel, A.I. , van der Holt, B. , Montefusco, V. , Ciccone, G. , Boccadoro, M. , San Miguel, J. , Sonneveld, P. & Palumbo, A. (2013) Age and organ damage correlate with poor survival in myeloma patients: meta‐analysis of 1435 individual patient data from 4 randomized trials. Haematologica, 98, 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli, R. , Pantaleo, G. , Gallipoli, P. , Gidaro, A. , Arquati, M. , Wu, M.A. & Lambertenghi Deliliers, G. (2015) Salvage therapy with bortezomib and dexamethasone in elderly patients with relapsed/refractory multiple myeloma. Anti‐Cancer Drugs, 26, 1078–1082. [DOI] [PubMed] [Google Scholar]
- De La Rubia, J. & Sanz, M.A. (2011) Treatment of multiple myeloma in the elderly: realities and hopes. Leukemia & Lymphoma, 52, 9–14. [DOI] [PubMed] [Google Scholar]
- Du, X.L. , Osborne, C. & Goodwin, J.S. (2002) Population‐based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. Journal of Clinical Oncology, 20, 4636–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie, B.G. , Harousseau, J.L. , Miguel, J.S. , Blade, J. , Barlogie, B. , Anderson, K. , Gertz, M. , Dimopoulos, M. , Westin, J. , Sonneveld, P. , Ludwig, H. , Gahrton, G. , Beksac, M. , Crowley, J. , Belch, A. , Boccadaro, M. , Cavo, M. , Turesson, I. , Joshua, D. , Vesole, D. , Kyle, R. , Alexanian, R. , Tricot, G. , Attal, M. , Merlini, G. , Powles, R. , Richardson, P. , Shimizu, K. , Tosi, P. , Morgan, G. & Rajkumar, S.V. & International Myeloma Working Group . (2006) International uniform response criteria for multiple myeloma. Leukemia, 20, 1467–1473. [DOI] [PubMed] [Google Scholar]
- Dytfeld, D. , Jasielec, J. , Griffith, K.A. , Lebovic, D. , Vesole, D.H. , Jagannath, S. , Al‐Zoubi, A. , Anderson, T. , Detweiler‐Short, K. , Stockerl‐Goldstein, K. , Ahmed, A. , Jobkar, T. , Durecki, D.E. , McDonnell, K. , Mietzel, M. , Couriel, D. , Kaminski, M. , Vij, R. & Jakubowiak, A.J. (2014) Carfilzomib, lenalidomide, and low‐dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica, 99, e162–e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey, R.D. (2014) Incidence and management of adverse events in patients with relapsed and/or refractory multiple myeloma receiving single‐agent carfilzomib. Journal of Clinical Pharmacology, 6, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulin, C. , Facon, T. , Rodon, P. , Pegourie, B. , Benboubker, L. , Doyen, C. , Dib, M. , Guillerm, G. , Salles, B. , Eschard, J.P. , Lenain, P. , Casassus, P. , Azais, I. , Decaux, O. , Garderet, L. , Mathiot, C. , Fontan, J. , Lafon, I. , Virion, J.M. & Moreau, P. (2009) Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. Journal of Clinical Oncology, 27, 3664–3670. [DOI] [PubMed] [Google Scholar]
- Jakubowiak, A.J. , Dytfeld, D. , Griffith, K.A. , Lebovic, D. , Vesole, D.H. , Jagannath, S. , Al‐Zoubi, A. , Anderson, T. , Nordgren, B. , Detweiler‐Short, K. , Stockerl‐Goldstein, K. , Ahmed, A. , Jobkar, T. , Durecki, D.E. , McDonnell, K. , Mietzel, M. , Couriel, D. , Kaminski, M. & Vij, R. (2012) A phase 1/2 study of carfilzomib in combination with lenalidomide and low‐dose dexamethasone as a frontline treatment for multiple myeloma. Blood, 120, 1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John, V. , Mashru, S. & Lichtman, S. (2003) Pharmacological factors influencing anticancer drug selection in the elderly. Drugs and Aging, 20, 737–759. [DOI] [PubMed] [Google Scholar]
- Johnson, T.M. (2014) Multiple myeloma treatment and management in the elderly. The Consultant Pharmacist, 29, 434–438, 440–444, 446–451. [DOI] [PubMed] [Google Scholar]
- Kurtin, S.E. (2013) Relapsed or relapsed/refractory multiple myeloma. Journal of the Advanced Practitioner in Oncology, 4, 5–14. [PMC free article] [PubMed] [Google Scholar]
- Mateos, M.V. , Hernandez, J.M. , Hernandez, M.T. , Gutierrez, N.C. , Palomera, L. , Fuertes, M. , Diaz‐Mediavilla, J. , Lahuerta, J.J. , de la Rubia, J. , Terol, M.J. , Sureda, A. , Bargay, J. , Ribas, P. , de Arriba, F. , Alegre, A. , Oriol, A. , Carrera, D. , Garcia‐Larana, J. , Garcia‐Sanz, R. , Blade, J. , Prosper, F. , Mateo, G. , Esseltine, D.L. , van de Velde, H. & San Miguel, J.F. (2006) Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood, 108, 2165–2172. [DOI] [PubMed] [Google Scholar]
- Mateos, M.V. , Hernandez, J.M. , Hernandez, M.T. , Gutierrez, N.C. , Palomera, L. , Fuertes, M. , Garcia‐Sanchez, P. , Lahuerta, J.J. , de la Rubia, J. , Terol, M.J. , Sureda, A. , Bargay, J. , Ribas, P. , Alegre, A. , de Arriba, F. , Oriol, A. , Carrera, D. , Garcia‐Larana, J. , Garcia‐Sanz, R. , Blade, J. , Prosper, F. , Mateo, G. , Esseltine, D.L. , van de Velde, H. & San Miguel, J.F. (2008) Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: updated time‐to‐events results and prognostic factors for time to progression. Haematologica, 93, 560–565. [DOI] [PubMed] [Google Scholar]
- Mateos, M.V. , Oriol, A. , Martinez‐Lopez, J. , Gutierrez, N. , Teruel, A.I. , de Paz, R. , Garcia‐Larana, J. , Bengoechea, E. , Martin, A. , Mediavilla, J.D. , Palomera, L. , de Arriba, F. , Gonzalez, Y. , Hernandez, J.M. , Sureda, A. , Bello, J.L. , Bargay, J. , Penalver, F.J. , Ribera, J.M. , Martin‐Mateos, M.L. , Garcia‐Sanz, R. , Cibeira, M.T. , Ramos, M.L. , Vidriales, M.B. , Paiva, B. , Montalban, M.A. , Lahuerta, J.J. , Blade, J. & Miguel, J.F. (2010) Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. The Lancet Oncology, 11, 934–941. [DOI] [PubMed] [Google Scholar]
- McBride, A. , Klaus, J.O. & Stockerl‐Goldstein, K. (2015) Carfilzomib: a second‐generation proteasome inhibitor for the treatment of multiple myeloma. American Journal of Health‐System Pharmacy, 72, 353–360. [DOI] [PubMed] [Google Scholar]
- McCormack, P.L. (2012) Carfilzomib: in relapsed, or relapsed and refractory, multiple myeloma. Drugs, 72, 2023–2032. [DOI] [PubMed] [Google Scholar]
- Mele, G. , Giannotta, A. , Pinna, S. , Loseto, G. , Coppi, M.R. , Brocca, C.M. , Melpignano, A. & Quarta, G. (2010) Frail elderly patients with relapsed‐refractory multiple myeloma: efficacy and toxicity profile of the combination of bortezomib, high‐dose dexamethasone, and low‐dose oral cyclophosphamide. Leukemia & Lymphoma, 51, 937–940. [DOI] [PubMed] [Google Scholar]
- Moreau, P. , Pylypenko, H. , Grosicki, S. , Karamanesht, I. , Leleu, X. , Grishunina, M. , Rekhtman, G. , Masliak, Z. , Robak, T. , Shubina, A. , Arnulf, B. , Kropff, M. , Cavet, J. , Esseltine, D.L. , Feng, H. , Girgis, S. , van de Velde, H. , Deraedt, W. & Harousseau, J.L. (2011) Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non‐inferiority study. The Lancet Oncology, 12, 431–440. [DOI] [PubMed] [Google Scholar]
- Moreau, P. , Kolb, B. , Attal, M. , Caillot, D. , Benboubker, L. , Tiab, M. , Touzeau, C. , Leleu, X. , Roussel, M. , Chaleteix, C. , Planche, L. , Chiffoleau, A. , Fortin, J. , Avet‐Loiseau, H. , Mary, J.Y. , Hulin, C. & Facon, T. (2015) Phase 1/2 study of carfilzomib plus melphalan and prednisone in patients aged over 65 years with newly diagnosed multiple myeloma. Blood, 125, 3100–3104. [DOI] [PubMed] [Google Scholar]
- Palumbo, A. & Magarotto, V. (2011) Novel treatment paradigm for elderly patients with multiple myeloma. American Journal of Blood Research, 1, 190–204. [PMC free article] [PubMed] [Google Scholar]
- Palumbo, A. , Bringhen, S. , Mateos, M.V. , Larocca, A. , Facon, T. , Kumar, S.K. , Offidani, M. , McCarthy, P. , Evangelista, A. , Lonial, S. , Zweegman, S. , Musto, P. , Terpos, E. , Belch, A. , Hajek, R. , Ludwig, H. , Stewart, A.K. , Moreau, P. , Anderson, K. , Einsele, H. , Durie, B.G. , Dimopoulos, M.A. , Landgren, O. , San Miguel, J.F. , Richardson, P. , Sonneveld, P. & Rajkumar, S.V. (2015) Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood, 125, 2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucci, M.T. , Levi, A. , Bringhen, S. , Scotti, S. , Gentilini, F. , Russo, S. , Siniscalchi, A. , Larocca, A. , Grammatico, S. , Boccadoro, M. , Foa, R. & Palumbo, A. (2013) Bortezomib, melphalan, and prednisone in elderly patients with relapsed/refractory multiple myeloma: a multicenter, open label phase 1/2 study. Cancer, 119, 971–977. [DOI] [PubMed] [Google Scholar]
- Pulte, D. , Gondos, A. & Brenner, H. (2011) Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. Oncologist, 16, 1600–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetto, L. (2003) Greater risks of chemotherapy toxicity in elderly patients with cancer. Journal of Supportive Oncology, 1, 18–24. [PubMed] [Google Scholar]
- Richardson, P.G. , Sonneveld, P. , Schuster, M.W. , Irwin, D. , Stadtmauer, E.A. , Facon, T. , Harousseau, J.L. , Ben‐Yehuda, D. , Lonial, S. , San Miguel, J.F. , Cavenagh, J.D. & Anderson, K.C. (2007) Safety and efficacy of bortezomib in high‐risk and elderly patients with relapsed multiple myeloma. British Journal of Haematology, 137, 429–435. [DOI] [PubMed] [Google Scholar]
- Rodon, P. , Hulin, C. , Pegourie, B. , Tiab, M. , Anglaret, B. , Benboubker, L. , Jardel, H. , Decaux, O. , Kolb, B. , Roussel, M. , Garderet, L. , Leleu, X. , Fitoussi, O. , Chaleteix, C. , Casassus, P. , Lenain, P. , Royer, B. , Banos, A. , Benramdane, R. , Cony‐Makhoul, P. , Dib, M. , Fontan, J. , Stoppa, A.M. , Traulle, C. , Vilque, J.P. , Petillon, M.O. , Mathiot, C. , Dejoie, T. , Avet‐Loiseau, H. & Moreau, P. (2015) Phase II study of bendamustine, bortezomib and dexamethasone as second‐line treatment for elderly patients with multiple myeloma: the Intergroupe Francophone du Myelome 2009‐01 trial. Haematologica, 100, e56–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, D.S. , Martin, T. , Wang, M. , Vij, R. , Jakubowiak, A.J. , Lonial, S. , Trudel, S. , Kukreti, V. , Bahlis, N. , Alsina, M. , Chanan‐Khan, A. , Buadi, F. , Reu, F.J. , Somlo, G. , Zonder, J. , Song, K. , Stewart, A.K. , Stadtmauer, E. , Kunkel, L. , Wear, S. , Wong, A.F. , Orlowski, R.Z. & Jagannath, S. (2012) A phase 2 study of single‐agent carfilzomib (PX‐171‐003‐A1) in patients with relapsed and refractory multiple myeloma. Blood, 120, 2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, A.K. , Rajkumar, S.V. , Dimopoulos, M.A. , Masszi, T. , Spicka, I. , Oriol, A. , Hajek, R. , Rosinol, L. , Siegel, D.S. , Mihaylov, G.G. , Goranova‐Marinova, V. , Rajnics, P. , Suvorov, A. , Niesvizky, R. , Jakubowiak, A.J. , San‐Miguel, J.F. , Ludwig, H. , Wang, M. , Maisnar, V. , Minarik, J. , Bensinger, W.I. , Mateos, M.V. , Ben‐Yehuda, D. , Kukreti, V. , Zojwalla, N. , Tonda, M.E. , Yang, X. , Xing, B. , Moreau, P. & Palumbo, A. (2015) Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. New England Journal of Medicine, 372, 142–152.25482145 [Google Scholar]
- Vestal, R.E. (1997) Aging and pharmacology. Cancer, 80, 1302–1310. [DOI] [PubMed] [Google Scholar]
- Yancik, R. , Ganz, P.A. , Varricchio, C.G. & Conley, B. (2001) Perspectives on comorbidity and cancer in older patients: approaches to expand the knowledge base. Journal of Clinical Oncology, 19, 1147–1151. [DOI] [PubMed] [Google Scholar]


