Abstract
The study of adult neural cell production has concentrated on neurogenesis. The mechanisms controlling adult gliogenesis are still poorly understood. Here, we provide evidence for a homeostatic process that maintains the population of glial cells in the Drosophila adult brain. Flies lacking microRNA miR‐31a start adult life with a normal complement of glia, but transiently lose glia due to apoptosis. miR‐31a expression identifies a subset of predominantly gliogenic adult neural progenitor cells. Failure to limit expression of the predicted E3 ubiquitin ligase, Rchy1, in these cells results in glial loss. After an initial decline in young adults, glial numbers recovered due to compensatory overproduction of new glia by adult progenitor cells, indicating an unexpected plasticity of the Drosophila nervous system. Experimentally induced ablation of glia was also followed by recovery of glia over time. These studies provide evidence for a homeostatic mechanism that maintains the number of glia in the adult fly brain.
Keywords: astrocyte, glia, microRNA, neurogenesis, stem cell
Subject Categories: Development & Differentiation, Neuroscience
Introduction
Glia outnumber neurons in the human brain and are critical for providing passive trophic support for neurons. Glia also serve important active roles in sculpting the nervous system and modulating synaptic connectivity (Allen et al, 2012; Schafer et al, 2012; Chung et al, 2013). Glia play important roles during development and to support normal functioning of the adult brain. Despite recent advances in understanding the functions of glia, little is known about the mechanisms underlying their development or their maintenance in the adult.
Recent work has shown that Drosophila melanogaster glia perform functions very similar to those in mammals. Like mammalian astrocytes, Drosophila astrocytes encourage synapse formation (Ullian et al, 2001; Muthukumar et al, 2014; Tasdemir‐Yilmaz & Freeman, 2014). The molecular mechanisms underlying glial function in the brains of mammals are also conserved. The Draper signalling pathway was shown to regulate glial phagocytosis in Drosophila (MacDonald et al, 2006; Ziegenfuss et al, 2008), and the mammalian orthologue of Draper, megf10, was identified as the critical receptor mediating phagocytosis of synapses by astrocytes in the central nervous system of rodents (Chung et al, 2013).
In mammals, glia and neurons are produced during development from the same progenitor cells, known as radial glia (Rowitch & Kriegstein, 2010). Neural stem cells also exist in the adult brain (Doetsch et al, 1999), and considerable progress has been made in studying neurogenesis by adult neural stem cells. Apart from one report on the generation of adult astrocytes (Awasaki et al, 2008), little is known about the extent of gliogenesis in the adult brain or the mechanisms by which these progenitor cells are controlled. In this report, we provide evidence for a distinct subset of adult neural progenitor cells that are bipotent but predominantly gliogenic. These cells are identified by expression of the microRNA miR‐31a. Mutants lacking miR‐31a do not show a developmental defect in production of glia, but some of these cells are transiently lost in the central brain of adult miR‐31a mutants and recover thereafter. The defect in the miR‐31a mutant provided evidence for ongoing gliogenesis in the adult brain. Glia also recover following induced ablation in the young adult, providing evidence for a homeostatic mechanism to maintain an appropriate number of glia in the adult brain.
Results
Loss of astrocytes in miR‐31a mutants
We made use of mutants from a collection of targeted miRNA knockout alleles (Chen et al, 2014) to examine the organization of the adult brain. Mutants lacking miR‐31a had fewer cells expressing the glial gene reversed polarity (repo), visualized with anti‐repo antibody labelling (Figs 1A and B, and EV1A and B, and Appendix Fig S1). In Canton S (CS) control animals, the number of adult glia was relatively constant with age, ranging from an average of 610 ± 130 at day 2 to 688 ± 127 at day 7 and 668 ± 185 at day 21 (Fig 1B and Appendix Fig S1A and B). The number of repo‐expressing cells was comparable to the controls in 2‐day‐old miR‐31a mutants, but dropped to ~60% of the Canton S control number by day 7 (Fig 1B and Appendix Fig S1A). For ease of comparison, the data are represented as a percentage of the average of the Canton S controls. The observation that glia were present in normal numbers at day 2 suggests that the defect does not reflect a failure to produce adult glia in normal numbers during pupal development, when the majority of adult glia are born (Awasaki et al, 2008; Omoto et al, 2015). Instead, it appears that glia were lost in the mutant.
Figure 1. Loss of astrocytes in miR‐31a mutant brains.
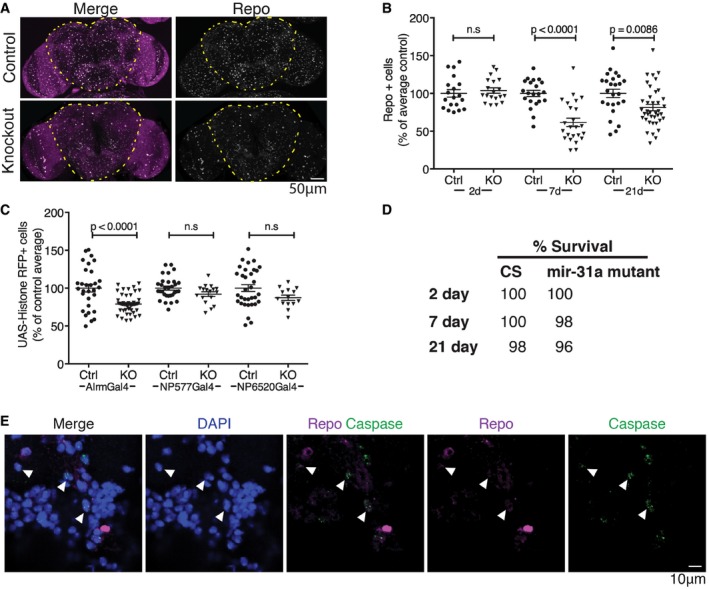
- Representative images of 7‐days adult brains labelled with anti‐repo to visualize glia and with DAPI to label nuclei (magenta). The images show maximum projections of stacks of optical sections. The central brain region in which glia were counted is outlined.
- Number of anti‐repo‐positive glia in the central brain region at 2, 7 and 21 days. The number of glia is represented as a percentage of the average number of glia in central brains of controls for each age. P‐values were determined using t‐test (unpaired, two‐tailed) comparing control and mutant at each age. Error bars represent SEM.
- Astrocytes were labelled using Alrm‐Gal4, and cortex glia using NP577‐Gal4 to drive UAS‐Histone‐RFP. Ensheathing glia were labelled using NP6520‐Gal4 driving UAS‐Histone‐RFP. Ctrl indicates Canton S control flies. KO indicates the miR‐31a mutant background. P‐values were determined using t‐test (unpaired, two‐tailed) comparing control and mutant for each driver. ns: no significant difference. Animals were analysed at 7 days post‐eclosion. Error bars represent SEM.
- Comparison of survival rate of Canton S controls and miR‐31a mutants at 2, 7 and 21 days post‐eclosion.
- Antibody to activated caspase‐3 (green) was used to visualize apoptotic cells in 4‐days post‐eclosion miR‐31a mutant brains. Glia were labelled with anti‐repo (purple). White arrowheads point to caspase‐3‐positive, repo‐positive cells. Nuclei were labelled with DAPI. Images are single confocal slices.
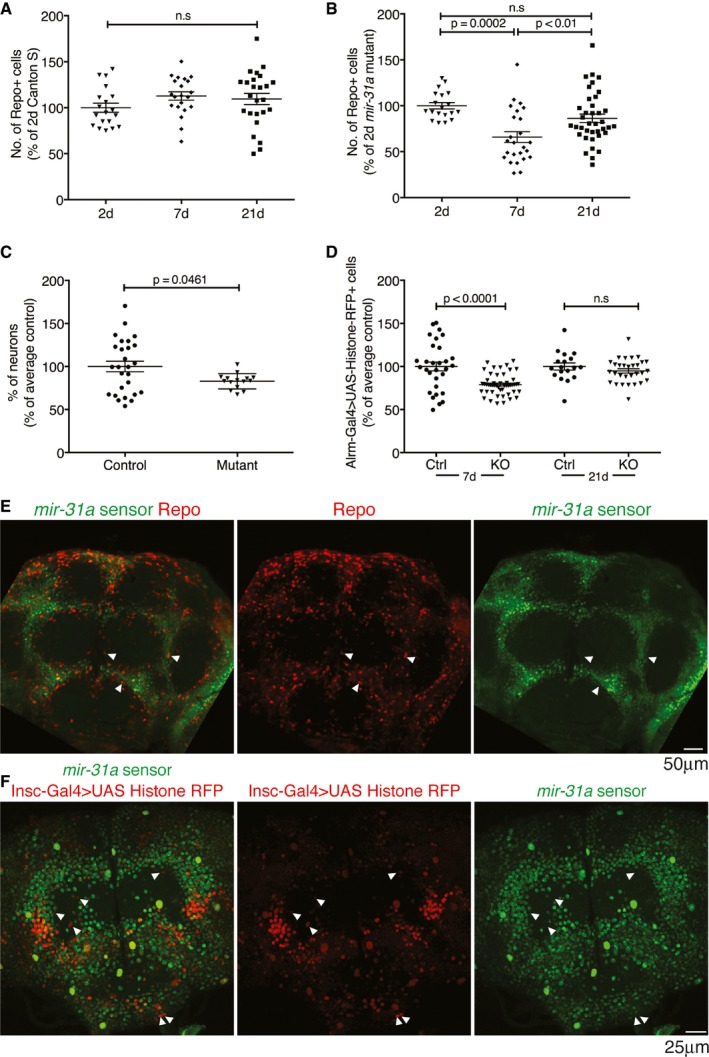
Figure EV1. miR‐31a is expressed in adult progenitor cells that give rise to glia (related to Fig 1).
-
A, BNumber of glia at 2, 7 and 21 days post‐eclosion represented as a percentage of the number in 2‐day‐old flies. Error bars represent SEM. Data were analysed using one‐way ANOVA. (A) Canton S controls. (B) miR‐31a mutants.
-
CSmall significant difference in number of neurons in the central brain in 7‐day‐old adults was observed. Data are represented as a percentage of the average number of neurons in Canton S control animals. Data were quantified with Imaris (Bitplane). Unpaired Student's t‐test was used for analysis. Error bars represent SEM.
-
DAstrocyte numbers (Alrm‐Gal4 > UAS‐Histone‐RFP) in 7‐days and 21‐days controls (Ctrl) and miR‐31a mutants (KO) represented as a percentage of the number in the CS controls. Unpaired Student's t‐test was used for analysis. Error bars represent SEM.
-
EmiR‐31a sensor in a 2‐days post‐eclosion adult brain. miR‐31a activity is indicated by the absence of GFP expression. White arrowheads point to example cells where GFP co‐localizes with anti‐repo (red), indicating low miRNA activity in the mature glia.
-
FmiR‐31a sensor (GFP) expression is excluded from some Insc‐Gal4 > UAS‐Histone‐RFP‐expressing cells (white arrowheads) in the brains of 2‐days post‐eclosion adults.
By 21 days, the number of glia in the mutant brains was on average ~80% of that in the controls, compared to ~60% at 7 days of age (Fig 1B). These observations suggested the possibility of a homeostatic mechanism by which lost glia were gradually replaced. We observed a small but significant reduction in the number of neurons between 7‐day‐old Canton S controls and miR‐31a mutants (P = 0.046, Fig EV1C).
To determine which type of glia were lost in the miR‐31a mutant, we made use of Gal4 drivers to label different glial subtypes by expression of UAS‐Histone‐RFP and compared number of Gal4‐positive cells in control and mutant backgrounds. Alrm‐Gal4 labels astrocytes (Doherty et al, 2009). NP577‐Gal4 labels cortex glia, and NP6250‐Gal4 labels ensheathing glia (Awasaki et al, 2008). There was no significant decrease in the number of cortex glia or ensheathing glia, but the number of Alrm‐Gal4‐expressing astrocytes was significantly reduced in 7‐day‐old miR‐31a mutants (Figs 1C and EV1D, and Appendix Fig S2). Loss of Alrm‐Gal4‐expressing astrocytes accounts for approximately half of the missing repo‐positive glia. The identity of the other missing glia has not been determined.
By 21 days, the number of Alrm‐Gal4 > UAS‐Histone‐RFP‐expressing cells was not significantly different in mutants and control brains (Fig EV1D and Appendix Fig S2B). There was no difference in the survival of flies between Canton S controls and miR‐31a mutant flies at 2, 7 and 21 days (Fig 1D). Thus, differences in viability cannot account for the loss and recovery of glia observed in the miR‐31a mutants during the first 3 weeks of adult life. We detected activated caspase‐3 in repo‐expressing glia (Fig 1E), suggesting that glia were lost by apoptosis in the mutant and subsequently replaced.
Additional controls were performed to test whether glia might be losing repo expression in older animals, hence leading us to conclude incorrectly that there were fewer glia in the brain. We used flies carrying Gal80ts and repo‐Gal4 to drive G‐Trace [UAS‐RFP, UAS‐Flp, Ubi‐p63E(FRT.Stop)GFP] in the adult. Flies were reared at 18°C until eclosion and shifted to 29°C to activate Gal4 in the newly eclosed adults. In this experiment, GFP serves as a permanent lineage tag for cells that expressed repo‐Gal4 in the adult. 94 ± 3% of GFP‐expressing cells also expressed repo‐Gal4, indicating that very few glial cells lose repo expression. There was also a high concordance between RFP and GFP expression indicating that few cells lose repo‐Gal4 activity. Thus, glia in the adult central brain do not lose repo expression at a rate that could affect the interpretation of our results (Fig EV2A).
Figure EV2. Rchy1, the target of miR‐31a, causes death of glia by apoptosis (related to Figs 2 and 3).
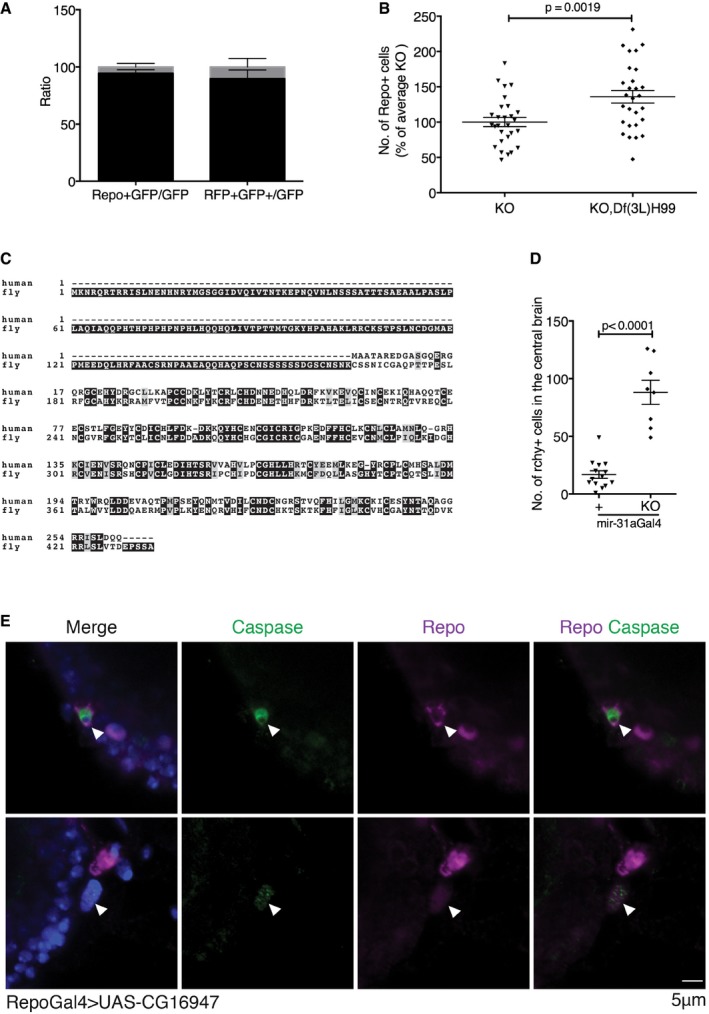
- Left panel: number of repo‐expressing and lineage‐tagged GFP+ cells. Right panel: lineage‐tagged GFP+ cells with active repo‐Gal4. Data are presented as a ratio of the total number of GFP cells in the central brain. Error bars represent SEM.
- Number of glia in miR‐31a mutants at 7 days with and without Df(3L)H99. Introducing Df(3L)H99 prevented glial loss. Data were analysed with an unpaired Student's t‐test. Error bars represent SEM.
- Alignment of fly and human Rchy1 proteins showing regions of sequence similarity. The antibody to human Rchy1 was raised against a peptide containing residues 87–167.
- The number of Rchy1‐expressing cells in the central brain was greater in the miR‐31a mutants than in Canton S controls (88 ± 0.4 vs. 17 ± 3). Data were analysed with an unpaired Student's t‐test. Error bars represent SEM.
- Activated caspase‐3‐positive (green) glial cells stained with anti‐repo (purple) are observed in the brains of 2‐days post‐eclosion adult repo‐Gal4 > UAS‐CG16947 brains. White arrowheads point to anti‐repo‐positive cells that are activated caspase‐3‐positive.
miR‐31a is required in adult neural progenitors to maintain glial number
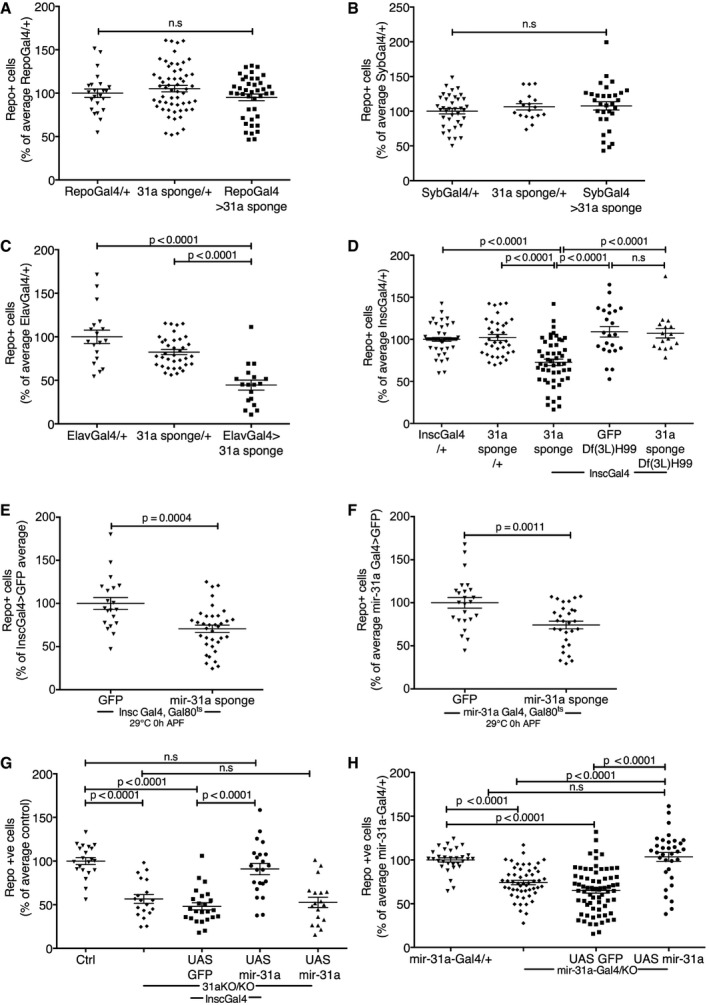
To determine where miR‐31a activity was required, we made a microRNA sponge transgene to selectively deplete miR‐31a in specific cell types. Depleting miR‐31a in mature glia with repo‐Gal4 did not affect the number of surviving glia (Fig 2A and Appendix Figs S3A and S4). We confirmed that miR‐31a activity was not detected in mature glia using a miR‐31a sensor transgene in 2‐days adult brains (Fig EV1E). Depleting miR‐31a in post‐mitotic neurons with synaptobrevin‐Gal4 also did not affect the number of surviving glia (Syb‐Gal4, Fig 2B and Appendix Figs S3B and S4). However, depleting miR‐31a with elav‐Gal4 (embryonic lethal abnormal visual system) led to a significant reduction in the number of glia (Fig 2C and Appendix Figs S3C and S4). elav‐Gal4 is expressed in post‐mitotic neurons in the larval and adult stages (Osterwalder et al, 2001; Izergina et al, 2009; Li et al, 2013). In the embryonic CNS, elav‐Gal4 has also been shown to be expressed in neural progenitor cells as well as in post‐mitotic neurons (Berger et al, 2007).
Figure 2. miR‐31a is required in adult glial progenitor cells to prevent glial apoptosis.
-
A–DNumber of anti‐repo‐positive glia at 7 days of age. Glia counts in the experimental samples are represented as a percentage of the average number of glia in central brains of the Gal4/+ controls for each panel. Data were analysed using one‐way ANOVA with post hoc Tukey analysis. Error bars represent SEM. For each Gal4 driver tested, Gal4/+ was compared with the UAS‐miR‐31a sponge transgene/+ and with Gal4 directing expression of the sponge (Gal4 > 31a sponge). (A) repo‐Gal4, (B) Syb‐Gal4, (C) elav‐Gal4, (D) Insc‐Gal4, Df(3L)H99 indicate flies carrying one copy of this deletion, to limit apoptosis in the presence of the UAS‐miR‐31a sponge or UAS‐GFP.
-
E, FNumber of anti‐repo‐positive glia following adult‐specific depletion of miR‐31a. Flies carrying Gal80 ts with Insc‐Gal4 (E) or miR‐31a‐Gal4 (F) and the UAS‐miR‐31a sponge or UAS‐GFP were raised at 18°C until adults had eclosed, and were then shifted to 29°C to allow Gal4 activity. UAS‐GFP was used as a control. Flies were examined 7 days after Gal4 activation. Data were analysed using an unpaired two‐tailed Student's t‐test. Error bars represent SEM.
-
G, HNumber of anti‐repo‐positive glia in brains at 7 days. UAS‐GFP was used as a control for expression of UAS‐miR‐31a transgene. Ctrl: Canton S control. 31a KO/KO indicates the homozygous mutant. Data were analysed using one‐way ANOVA with post hoc Tukey analysis. Error bars represent SEM. (G) Insc‐Gal4 was used to direct transgene expression. (H) The miR‐31a‐Gal4 allele was used to direct transgene expression. Gal4/+ indicates miR‐31a‐Gal4 allele in trans to wild‐type. miR‐31a‐Gal4/KO indicates the Gal4 allele in trans to the deletion allele.
The effect of depleting the miRNA with elav‐Gal4 suggested that miR‐31a expression might be important in progenitor cells. To further explore this, we used Inscuteable‐Gal4 to label adult neuroglial progenitors (Omoto et al, 2015). Depletion of miR‐31a in Insc‐Gal4 cells led to a significant reduction in the number of glia (P < 0.0001, Fig 2D and Appendix Figs S3D, S4 and S5). Using the miR‐31a sensor, we observed overlap between Insc‐Gal4 > UAS‐Histone‐RFP expression and miR‐31a‐positive (GFP‐negative) in cells clustering around the antennal lobe of 2‐days adult brains (Fig EV1F). We hypothesize that these overlap cells might represent an adult glial progenitor population.
Although the miR‐31a sensor showed no overlap with mature glia expressing repo, we observed overlap with Alrm‐Gal4 > UAS‐Histone‐RFP expression in the antennal lobe (Appendix Fig S6B and C). We hypothesize that this subset of Alrm‐Gal4 cells might be immature astrocytes that have not yet begun to express repo. Alternatively, Alrm‐Gal4 might be expressed in adult glial progenitors that express miR‐31a.
The number of glia was comparable in newly eclosed Canton S control and miR‐31a mutant flies, and caspase labelling suggested that reduction in glial number in 7‐days miR‐31a mutant adults might be due to cell death (Fig 1). To investigate this further, we reduced the expression of miR‐31a in progenitor cells of flies that carried one copy of the Df(3L)H99 deficiency. This chromosomal deletion removes three apoptosis regulators, head involution defect (hid), reaper and grim, and can be used to limit apoptosis in vivo (White et al, 1994). There was no loss of glia upon expression of the miRNA sponge in this background, indicating that loss of these cells in the mutant was due to apoptosis. Df(3L)H99 did not alter glial numbers in the control genotype where GFP was expressed instead of the miR‐31a sponge (Fig 2D and Appendix Figs S3D, S4 and S5), indicating that the Df(3L)H99 deficiency did not affect glial number on its own. Df(3L)H99 was also introduced into the miR‐31a mutant background, where it also suppressed glial loss (Fig EV2B and Appendix Fig S7A).
The observation that glial number was normal in the mutant at 2 days but lower at 7 days suggested that the miRNA is required in the adult. To test this, we made use of temperature‐sensitive Gal80 ts to allow temporal control of Gal4 activity. Flies carrying Gal80 ts together with a Gal4 driver and the UAS‐miR‐31a sponge transgene were raised at 18°C until adults eclosed and were then shifted to 29°C to allow Gal4 activity. Adult‐specific depletion of miR‐31a in Insc‐Gal4‐expressing cells led to a significant reduction in glia number compared to control animals (Fig 2E and Appendix Figs S3E and S5). Similarly, adult‐specific depletion of miR‐31a in miR‐31a‐Gal4 cells led to a significant reduction in glia number compared to control animals (Fig 2F and Appendix Figs S3F and S5; the miR‐31a‐Gal4 allele was generated with a vector that allows recombinase‐mediated cassette exchange to replace the endogenous miR‐31a hairpin with Gal4 sequences).
As a further test of whether the glial phenotype was due to loss of miR‐31a expression in progenitor cells, we drove expression of UAS‐miR‐31a with Insc‐Gal4 in the miR‐31a mutant background. Restoring miR‐31a expression in Insc‐Gal4 cells suppressed the glial loss phenotype (Fig 2G and Appendix Figs S3G and S5). This was not observed when UAS‐GFP was expressed instead. Expressing the UAS‐miR‐31a transgene in miR‐31a‐Gal4‐expressing cells also proved to be sufficient to rescue the loss of glia in the mutant (Fig 2H and Appendix Figs S3H and S6A). Furthermore, using the progenitor‐specific driver Worniu‐Gal4 to direct expression of the miR‐31a sponge recapitulated the glial loss phenotype (Appendix Figs S6D and E, and S9).
These findings suggest that the miR‐31a‐Gal4 cells comprise a subset of the neuroglial progenitor population defined by Insc‐Gal4 expression. Although the miRNA does not appear to be required in mature glia, its depletion from this progenitor cell population resulted in loss of ~40% of glia in early adulthood. These observations suggest that miR‐31a is required in the progenitor cells to support subsequent survival of newly generated glia.
miR‐31a targets a predicted E3 ubiquitin ligase
MicroRNAs typically downregulate their target transcripts, so it is expected that functional targets will be overexpressed in miRNA mutant tissue. Using targetscan.org, we obtained a list of predicted miR‐31a targets and tested transcript levels with quantitative RT–PCR on RNA isolated from control and miR‐31a mutant heads. 19 out of the 53 predicted targets tested showed an increase of more than 1.5‐fold in the mutant (Table EV1). These were selected for in vivo functional tests using RNAi transgenes to offset the increase in target RNA levels. If overexpression of the target contributes to the mutant phenotype, then reducing its level by RNAi should decrease the severity of the phenotype.
The RNAi transgenes were expressed in the miR‐31a mutant using Insc‐Gal4. Only CG16947 RNAi showed a significant increase in the number of glia in the mutant brain under these conditions (Fig 3A and Appendix Figs S7B and S8). We did not observe any reduction in the severity of the mutant phenotype with RNAi lines for any of the other miR‐31a‐predicted targets. Depletion of CG16947 in an otherwise normal background did not affect the number of glia (Fig 3B and Appendix Figs S7C and S8). These observations suggested that the increased expression of CG16947 in the glial progenitor population was responsible for the mutant phenotype. Consistent with this, overexpression of CG16947 with Insc‐Gal4 in an otherwise normal background was sufficient to reduce the number of adult glia (Fig 3C and Appendix Figs S7D and S8). Comparable results were obtained when we used miR‐31a‐Gal4 to direct transgene expression selectively in the smaller population of miR‐31a‐expressing cells (Fig 3D–F and Appendix Figs S7E–G, S8 and S9). Taken together, these findings indicate that overexpression of CG16947 contributes to the miR‐31a mutant phenotype.
Figure 3. An E3 ubiquitin ligase gene, CG16947, is the target of miR‐31a .
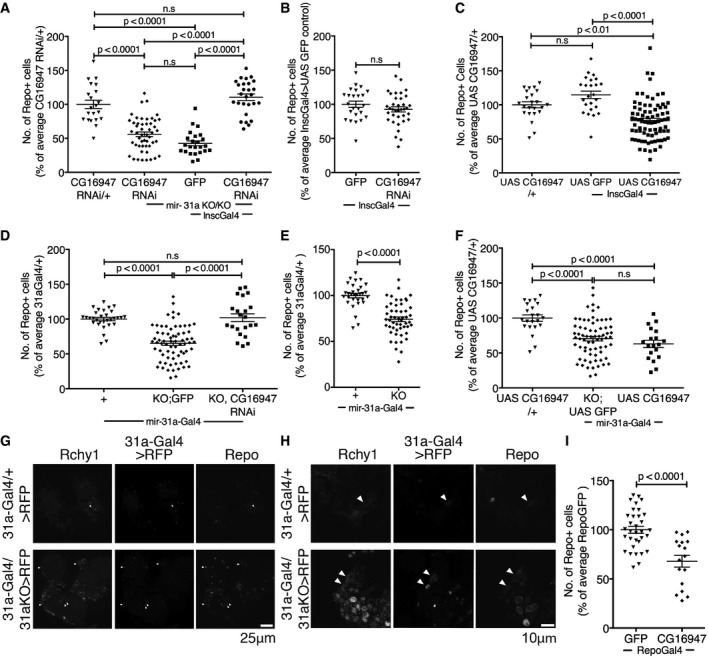
-
A–FNumber of anti‐repo‐positive glia in adult brains at 7 days. Glia counts in the experimental samples are represented as a percentage of the average number of glia in central brains of the indicated controls. Data were analysed using one‐way ANOVA with post hoc Tukey analysis (A, C, D, F) or with an unpaired Student's t‐test (B, E). Error bars represent SEM. (A) The UAS‐CG16947 RNAi transgene without a Gal4 driver was used as a control. miR‐31a KO/KO indicates the homozygous deletion mutant. A UAS‐GFP transgene was used as a control for expression of the RNAi transgene with Insc‐Gal4 in the mutant background. ns: not significant. See also Appendix Fig S1 and Table EV1. (B) Expression of UAS‐GFP or UAS‐CG16947 RNAi using Insc‐Gal4 cells in an otherwise normal background. (C) The UAS‐CG16947 transgene without a Gal4 driver was used as a control. UAS‐GFP was used as a control for expression of the UAS‐CG16947 transgene with Insc‐Gal4 in an otherwise normal background. (D) All samples carried one copy of the miR‐31a‐Gal4 allele. KO;GFP indicates the deletion allele and a UAS‐GFP transgene. KO,CG16947 RNAi indicates the deletion allele and the UAS‐RNAi transgene to deplete CG16947 mRNA. (E) All flies carried the miR‐31a‐Gal4 allele. KO indicates the deletion allele. (F) The UAS‐CG16947 transgene without a Gal4 driver was used as a control. UAS‐GFP in the mutant background was used for comparison to expression of UAS‐CG16947 transgene with miR‐31a‐Gal4 in an otherwise normal background.
-
G, HOptical sections of 7‐days adult brains labelled with antibody to Rchy1 protein. miR‐31a‐Gal4 was used to drive the expression of UAS‐Histone‐RFP. Glia were visualized with anti‐repo. More cells expressed Rchy1, Histone‐RFP and repo in the mutant brains (miR‐31a‐Gal4/KO > RFP) than in the heterozygous controls (miR‐31a‐Gal4/+ > RFP, white arrowheads). (H) Higher magnification images from the samples in (G).
-
INumber of anti‐repo‐positive glia in 7‐days post‐eclosion brains from controls expressing UAS‐GFP or UAS‐CG16947 in glia under repo‐Gal4 control. Data were analysed using an unpaired Student's t‐test. Error bars represent SEM.
The human orthologue of CG16947 is a predicted RING finger and CHY zinc finger domain containing 1, E3 ubiquitin protein ligase (Rchy1). The Drosophila and human Rchy1 proteins share considerable similarity (Fig EV2C), so we used an antibody raised against a peptide including residues 87–167 of human Rchy1. Labelling with this antibody was more intense in the miR‐31a mutant brain compared to the control, and more cells were labelled (Fig 3G and H; 17 ± 3 cells were detectably labelled in the miR‐31a‐Gal4/+ heterozygous control animals compared to 88 ± 10 in the mutants, Fig EV2D). As the number of cells showing elevated Rchy1 expression exceeded the number of miR‐31a‐Gal4‐expressing progenitor cells (Fig 3G), we infer that loss of the miRNA in the progenitors allows stable inheritance of Rchy1 in their progeny. Accordingly, the number of cells co‐expressing Rchy1 and repo increased in the mutant (Fig 3G and H).
To ask whether expression of Rchy1 causes loss of glial cells, we drove CG16947 expression in glia with repo‐Gal4 in an otherwise normal background. This caused a significant reduction in the number of glia in the brain compared to the UAS‐GFP control (Fig 3I and Appendix Figs S7H and S9A). This is consistent with the hypothesis that inheritance of Rchy1 protein from the progenitor cells leads to apoptosis of their glial progeny. Additionally, we observed the presence of activated caspase in glia labelled with anti‐repo in 2‐days repo‐Gal4 UAS‐CG16947 adult brains, indicating that overexpression causes death of glia by apoptosis (Fig EV2E).
miR‐31a‐expressing cells are bipotent neural progenitors that are predominantly gliogenic
To explore the potential of the miR‐31a‐Gal4‐expressing cells as progenitors in the adult, we performed clonal analysis using MARCM, which permanently labels their progeny with GFP. We induced clones in adult brains on the day of eclosion and labelled after 1 day with anti‐elav to mark neurons (Fig 4A) or anti‐repo to label glia (Figs 4B and EV3A). We observed both anti‐repo‐positive and anti‐elav‐positive GFP‐expressing clones, indicating that the miR‐31a‐Gal4 cells can divide in the adult to produce glial and neuronal progeny.
Figure 4. Adult glial progenitor cells give rise to both neurons and glia.
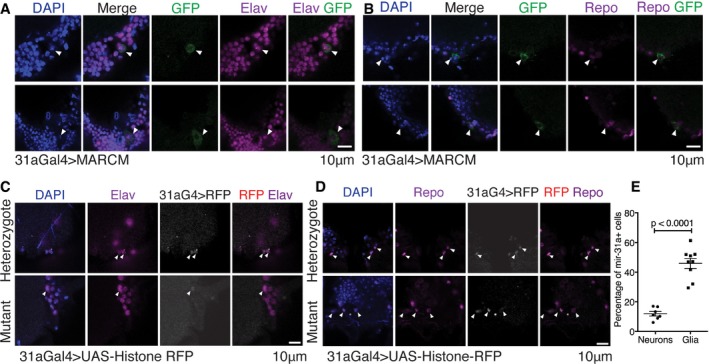
-
AMARCM clonal analysis using miR‐31a‐Gal4. Recombination was induced by heat shock at 1 day post‐eclosion to induce GFP expression in the progeny of miR‐31a‐Gal4‐expressing cells. Flies were dissected the day after MARCM induction. Images show neurons expressing elav and GFP. Arrowheads indicate anti‐elav‐positive and GFP‐expressing cells.
-
BMARCM clonal analysis as in (A) using miR‐31a‐Gal4. Images show glial cells expressing repo and GFP. Arrowheads indicate anti‐repo‐positive and GFP‐expressing cells.
-
C, DmiR‐31a‐Gal4 (31aG4)‐expressing cells were visualized with UAS‐Histone‐RFP at 1 day post‐eclosion. Anti‐elav was used to label neurons (C). Anti‐repo was used to label glia (D). Nuclei were labelled with DAPI. Heterozygote: miR‐31a‐Gal4/+. Mutant: miR‐31a‐Gal4/miR‐31a KO allele. Figure EV3B and C shows lineage tagging using miR‐31a‐Gal4, Gal80 ts with G‐Trace after 1 day or 3 days at 29°C. Arrowheads point to anti‐elav‐positive and UAS‐Histone‐RFP‐positive cells (C) or anti‐repo‐positive and UAS‐Histone‐RFP‐positive cells (D).
-
EPercent of miR‐31a‐Gal4‐expressing cells that were labelled with anti‐elav (neurons) or anti‐repo (glia) in 1‐day post‐eclosion miR‐31a‐Gal4/+ > UAS‐Histone‐RFP brains. Error bars represent SEM. Unpaired Student's t‐test was used.
Figure EV3. Adult progenitor cells give rise to both neurons and glia (related to Figs 4 and 5).
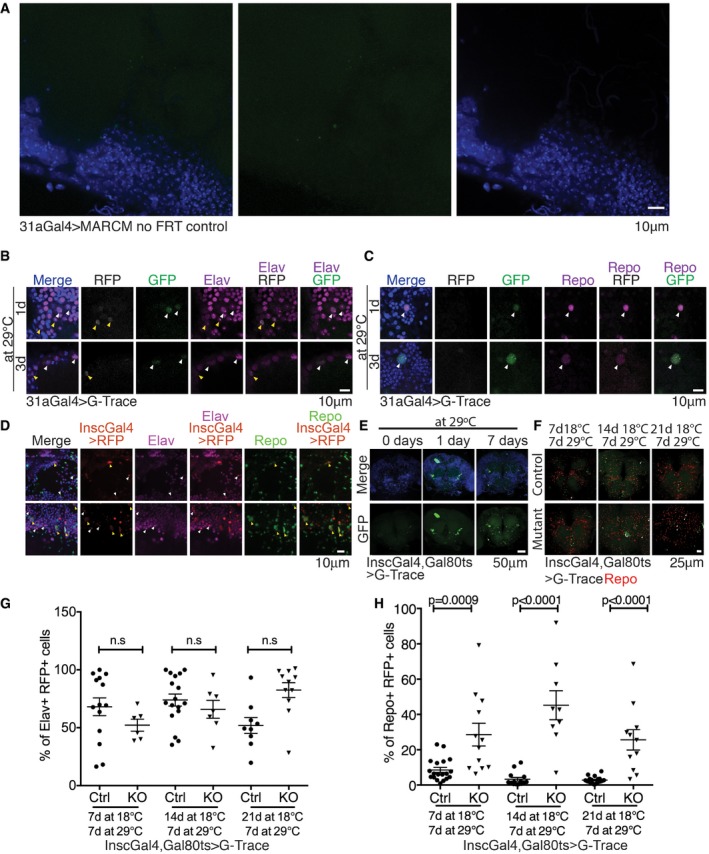
-
AmiR‐31a‐Gal4 stocks lacking FRT42D were crossed to UAS‐CD8‐GFP,hsFlp;FRT42D, tubGal80 flies. Heat shock was induced 1 day post‐eclosion and the flies dissected 1 day later. The bright green spots seen in the images are not correlated with DAPI, indicating that these are non‐specific bright GFP spots. The control shows that the clones that we observe in the MARCM stocks are not phantom clones as no clones are observed in the no‐FRT42D control.
-
B, CG‐Trace [UAS‐RFP, UAS‐Flp, Ubi‐p63E(FRT.Stop)GFP] lineage analysis of miR‐31a‐Gal4‐expressing cells in the adult. Gal4 activity was limited to adult stages with Gal80ts. RFP represents ongoing Gal4 activity (or recent activity allowing for perdurance). GFP is a permanent lineage tag for cells that have expressed Gal4. (B) Anti‐elav was used to label neurons. Yellow arrowheads point to RFP+ elav+ cells. White arrowheads point to GFP+ elav+ cells. (C) Anti‐repo was used to label glia. White arrowheads point to GFP+ repo+ cells.
-
DInsc‐Gal4‐expressing cells visualized with UAS‐Histone‐RFP gave rise to elav‐expressing neurons (purple; white arrowheads) and repo‐expressing glia (green; yellow arrowheads). Elav+ RFP+ and repo+ RFP+ cells represent non‐overlapping populations.
-
EImages showing the central brain regions of flies carrying G‐Trace [UAS‐RFP, UAS‐Flp, Ubi‐p63E(FRT.Stop)GFP] with Insc‐Gal4 and Gal80 ts. Flies were reared at 18°C until eclosion. Flies that were not shifted to the permissive temperature of 29°C (left) showed very few GFP‐expressing clones in the central brain. Flies that were reared right after eclosion for 7 days at 29°C had more clones than those that had been reared for only 1 day at 29°C.
-
F–HFlies carrying G‐Trace with the Insc‐Gal4 driver and Gal80 ts were raised at 18°C until the indicated age, after which Gal4 was activated by shifting to 29°C for a further 7 days. Brains were labelled with anti‐repo or anti‐elav. RFP expression represents ongoing or recent Gal4 activity. GFP is a permanent lineage tag for cells that have expressed Gal4. Ctrl indicates the Canton S control background. KO indicates the miR‐31a mutant background. (F) Images of brains shifted to activate Gal4 at 7, 14 and 21 days. Clones expressing repo and GFP are labelled with white arrowheads. (G) Cells expressing elav and RFP represented as a percentage of RFP‐expressing cells. Although it appears that there may be a trend towards fewer elav‐expressing cells in the earlier time points in the mutant brains, the scatter in the data is large, and the differences were not statistically significant. (H) Cells expressing repo and RFP represented as a percentage of RFP‐expressing cells. The number of cells expressing repo was significantly increased in the mutant brains. Unpaired Student's t‐test was used. Error bars represent SEM.
As another means to explore the potential of these cells, we used miR‐31a‐Gal4 to drive expression of UAS‐Histone‐RFP and stained the brains with antibody to repo or elav. The perdurance of the Gal4 and RFP proteins allowed their detection together with elav protein (Fig 4C) and with repo (Fig 4D). We counted the number of miR‐31a‐Gal4‐expressing cells that co‐labelled with repo or elav at 2 days of age. Many more of the miR‐31a‐Gal4‐expressing cells were labelled with anti‐repo than with anti‐elav (Fig 4E), suggesting that these cells are biased towards production of glial progeny.
Next, we carried out lineage tagging using G‐Trace [UAS‐RFP, UAS‐Flp, Ubi‐p63E(FRT.Stop)GFP], which allows Gal4‐regulated heritable expression of GFP (Evans et al, 2009). Flies carrying G‐Trace with miR‐31a‐Gal4 and Gal80 ts were raised at 18°C to keep Gal4 inactive during development. Newly eclosed adults were shifted to 29°C to allow Gal4 activity and to start lineage tagging. Brains were labelled with anti‐elav and anti‐repo after 1 or 3 days at 29°C. GFP‐expressing progeny were found that co‐expressed elav, indicating formation of neuronal progeny (Fig EV3B). GFP‐expressing progeny were found that expressed repo, indicating formation of glial progeny (Fig EV3C).
Having established that the miR‐31a‐expressing cells can serve as adult progenitors for glia and neurons, we were interested in comparing their potential with those of the adult progenitors expressing Insc‐Gal4. Using perdurance of Insc‐Gal4 and UAS‐Histone‐RFP to label recently born progeny of the Insc‐Gal4 cells, we observed neuronal progeny expressing RFP and elav, and glial progeny expressing RFP and repo. As shown in Fig EV3D, these cells represent non‐overlapping populations.
Next, we combined G‐Trace with Insc‐Gal4 and Gal80 ts and shifted flies to 29°C to activate Gal4 after eclosion. Very little background is observed in animals that were reared at 18°C until eclosion (Fig EV3E). GFP‐labelled clones were detected after 1 day at 29°C. Their number had increased after 7 days, indicating ongoing proliferation of the Insc‐Gal4 progenitors in the adult (Fig EV3E). The G‐Trace analysis was extended to later activation of Gal4 after 7, 14 or 21 days (Fig EV3F), after which flies were raised for an additional 7 days at 29°C before labelling brains with anti‐elav or anti‐repo. Approximately 50% of clones in the control flies produced elav‐positive neurons at all three ages (Figs 5A and B, and EV3G). At day 7, ~20% of clones produced repo‐positive glial progeny, but this dropped to ~7–8% of clones at days 14 and 21 (Figs 5C and D, and EV3H).
Figure 5. Lineage tracing reveals adult glia homeostasis.
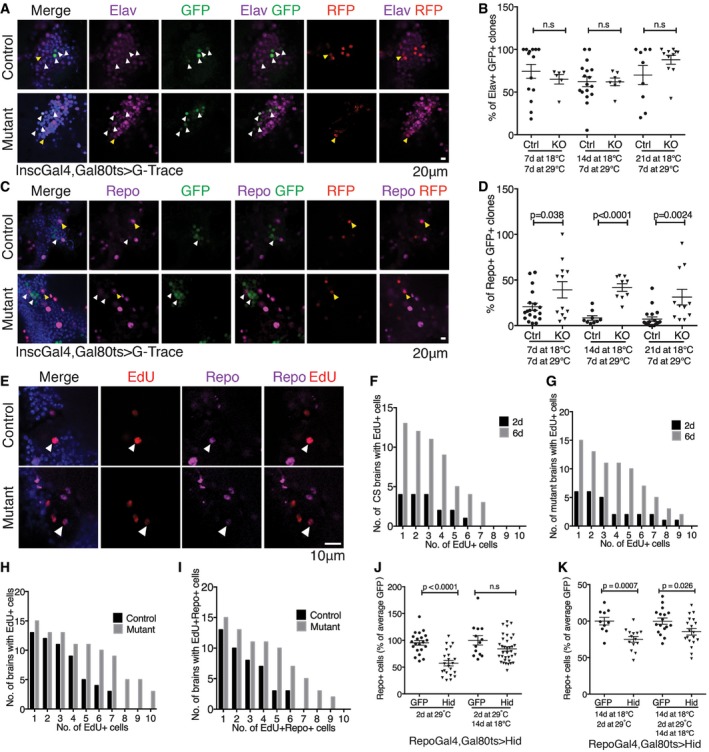
-
A–DPermanent lineage tags were induced in Insc‐Gal4‐expressing cells in the adult by controlling the expression of G‐Trace [UAS‐RFP, UAS‐Flp, Ubi‐p63E(FRT.Stop)GFP] with Gal80 ts. RFP expression reflects ongoing Gal4 activity, and GFP serves as a permanent tag for cells that have expressed Gal4. Flies were shifted to 29°C at 7, 14 or 21 days and aged for 7 days at 29°C before processing. Otherwise wild‐type controls were compared with miR‐31a mutants (KO). There was very little background leakiness of Gal4 activity in flies kept at 18°C (Fig EV3E). Data were analysed using an unpaired Student's t‐test (two‐tailed). ns: not significant. Error bars represent SEM. (A, C) Optical sections through the central brain of adult flies shifted at 14 days. (A) Neurons were labelled with anti‐elav (purple). (B) Percent of clones expressing the GFP lineage tag and elav. (C) Glia were labelled with anti‐repo (purple). (D) Percent of clones expressing GFP and repo.
-
E–IEdU labelling was used to visualize cells undergoing DNA synthesis. Cells were counted in the central brain. (E) Images are single optical images of EdU‐treated Canton S (Control) and miR‐31a mutant brains from 6‐day‐old flies, 3 days after the end of EdU exposure. Note the presence of cells expressing repo and labelled with EdU (white arrowheads). (F) Number of EdU‐positive cells at 2 days or 6 days after eclosion in control animals. (G) Number of EdU‐positive cells at 2 days or 6 days after eclosion in mutant animals. (H) Counts of EdU‐positive cells in Canton S controls and miR‐31a mutants 6 days post‐eclosion. (I) Counts of anti‐repo‐positive and EdU‐positive in the central brains of Canton S controls and miR‐31a mutants 6 days post‐eclosion. Paired t‐test was used.
-
J, KFlies carrying repo‐Gal4 and Gal80 ts were reared at 18°C until eclosion (J) or 14 days of age (K) to keep Gal4 inactive. This was followed by 2 days at 29°C to express UAS‐hid or UAS‐GFP as a control. Left: flies were examined immediately after 2 days of transgene expression. Right: flies were allowed to recover for 14 days at 18°C before processing. Anti‐repo was used to visualize the glia. Data were analysed using an unpaired t‐test (two‐tailed). ns: not significant. Error bars represent SEM.
These data provide evidence for distinct types of multipotent progenitor cells in the adult brain. The miR‐31a‐Gal4 progenitor population is predominantly gliogenic, but can also produce neurons. The Insc‐Gal4‐expressing cells are more abundant and produce more neuronal progeny than glial progeny. Insc‐Gal4‐expressing progenitor cells produced more glial progeny in the miR‐31a mutant brains compared to the control animal brains (Fig 5D), but there was no significant change in the production of neurons (Fig 5B).
Loss of glia in the adult central brain leads to reactivation of adult progenitor cells
To further examine the proliferation of these progenitor cells, we performed a 5‐ethynyl‐2′‐deoxyuridine (EdU) incorporation experiment to label cells undergoing DNA synthesis. Flies were fed with EdU‐containing food from 24 to 54 h after eclosion. The numbers of EdU‐positive cells were then counted in the central brain at the end of the feeding period or after a 3‐day chase on normal food. The number of EdU‐positive cells increased between day 2 and day 6 in the Canton S control and in the mutants (Fig 5F; P = 0.006 for the CS controls. G: P = 0.001 for the miR‐31a mutants). There were significantly more EdU‐positive cells in the mutant that in the controls (P = 0.0002, Figs 5H and EV4A), indicating that there was more progenitor cell proliferation in mutant brains. These preparations were also labelled with anti‐repo to mark glia. Figure 5I shows that there were more EdU‐positive cells overall in the mutant brains and that a greater proportion of these cells had adopted glial identity, based on repo expression (82 ± 3% in the mutant vs 76 ± 6% in the control, P = 0.0004). This experiment provides additional evidence for production of new glia in the adult brain. Furthermore, it shows that the proportion of proliferating cells that make glia was higher in the miR‐31a mutants than in the controls.
Figure EV4. Active division of progenitor cells is observed in the adult brain of Drosophila melanogaster (related to Fig 5).

- EdU‐labelling experiment. Overview confocal stack images of EdU and repo immunoreactivity in the central brains of control and miR‐31a mutant brains (mutant).
- Raw counts of anti‐repo‐positive glia in the central brain in glial ablation experiments where flies carrying repo‐Gal4 and Gal80 ts were reared at 18°C until eclosion to keep Gal4 inactive. This was followed by 2 days at 29°C to express UAS‐hid or UAS‐GFP as a control. Left panels: flies were examined immediately after 2 days of transgene expression. Right panels: flies were allowed to recover for 14 days at 18°C before processing. Data were analysed using an unpaired t‐test (two‐tailed). ns: not significant. Error bars represent SEM.
Increased proliferation and increased production of glia in the miR‐31a mutant suggest an underlying homeostatic system that maintains glial number. If so, elimination of glia by an independent genetic means should also increase production of new glia. To test this, we used repo‐Gal4 and Gal80ts to transiently express the pro‐apoptotic gene hid in adult glia. The flies were raised at 18°C until eclosion and shifted to 29°C for 2 days to express hid in repo‐Gal4‐expressing glia. Flies were sampled at this time or allowed to recover for 14 days at 18°C before labelling with anti‐repo. After 2 days at 29°C, the number of glia was reduced by half compared to the GFP‐expressing controls (Figs 5J and EV4B). There was no significant difference from the control after the 14‐day recovery period, indicating recovery of the missing glia (Figs 5K and EV4B). To investigate whether glial recovery was retained in older animals, we induced hid expression for 2 days at 14 days post‐eclosion. This reduced the number of glia, but the subsequent recovery was incomplete (Fig 5K and Appendix Fig S10B). These data provide evidence for a glial homeostatic mechanism that maintains the requisite number of glia in the brain. The effectiveness of glial recover after ablation was less in older flies, suggesting loss of plasticity with age.
Discussion
miR‐31a acts in adult glial progenitors to support glial cell survival
Despite the critical role that glia play in the proper functioning of neurons, study of adult neural cell production has focused mainly on neurons. Much less is known about gliogenesis in the adult brain. Omoto et al (2015) and Awasaki et al (2008) recently reported that production of adult glia occurs during pupal development. In this report, we provide several lines of evidence for ongoing gliogenesis in the adult. These data include labelling the newly synthesized DNA of newly born cells in the adult showed the production of glia as well as neurons. Genetic mosaic analysis to produce clones of marked cells in the adult brain showed the production of new neurons and new glia from progenitor cells expressing Insc‐Gal4 as well as a newly identified population of progenitors that express the miR‐31a microRNA. Interestingly, the miR‐31a‐expressing progenitors appear to be mainly gliogenic, while those expressing Insc‐Gal4 are mainly neurogenic. Given that both progenitors can make neurons and glia, we suggest that the miR‐31a‐expressing progenitor cells may be a specialized subset of Insc‐Gal4‐expressing neuroglial progenitors. This is supported by the observation of miR‐31a sensor activity in a subset of Insc‐Gal4 cells.
In the absence of miR‐31a expression, glial number is affected. We noted that the number of glia is normal in young adult miR‐31a mutants, drops sharply over several days and then recovers. This pattern implies an active process of glial turnover in the young adult brain, which is impacted by the absence of the miRNA in the progenitor population. We have provided evidence that this defect is due to the requirement for the miRNA in progenitor cells, but not in mature glia or neurons.
Misexpression of an E3 ubiquitin ligase compromises glial homeostasis
Our findings show that miR‐31a acts through regulation of Rchy1, a predicted E3 ubiquitin ligase. Although many transcripts are misregulated in the mutant, restoring expression of Rchy1 towards normal levels effectively suppressed the glial defect in the mutant. This implies that the overexpression of Rchy1 contributes to the failure of these cells to produce sufficient glia during the remodelling process that we observed in the young brain. In miR‐31a mutants, Rchy1 protein was detected at elevated levels in the progeny of the miR‐31a progenitor cells. Excess Rchy1 appears to be detrimental to the survival of the glial progeny, in that loss of glia can be suppressed by blocking apoptosis. There was only a small change in the number of neurons in adult brains of miR‐31a mutants compared to wild‐type animals. But, we note that the miR‐31a progenitor cells are few in number compared to the predominantly neurogenic Insc‐Gal4‐expressing progenitors. Therefore, an effect of excess Rchy1 in these cells on production of neurons cannot be ruled out.
Ubiquitin‐mediated protein turnover has been shown to play several important roles during CNS development in Drosophila. Ubiquitination and proteosomal degradation of glial cells missing allow embryonic glia to exit the cell cycle and begin differentiation (Ho et al, 2009). Additionally, neural cell fate can be controlled by ubiquitin‐dependent regulation of protein translation (Hindley et al, 2011; Werner et al, 2015). Our study shows that a microRNA is responsible for regulating the level of a predicted E3 ubiquitin ligase in a neural progenitor cell, which affects the viability of its progeny. Identification of the targets of Rchy1 in neural progenitor cells may provide new insights into the control of their differentiation and survival.
Adult glial homeostasis
Loss of glia in the miR‐31a mutant points to a previously unidentified plasticity of the young adult brain in Drosophila. Our findings imply ongoing replacement of glia in the young adult brain. Furthermore, they provide evidence that the brain can detect when there are too few glia and that this can trigger progenitor cells to increase production of new glia, by increasing the proportion of their progeny that differentiate into glia. How the number of glia is monitored in the brain will be a topic for future study.
Approximately half of the glia affected in the miR‐31a mutant were astrocytes. In this context, parallels to the mammalian brain may be interesting. In mammals, most astrocytes contact a blood vessel. Since astrocytes rely on the vasculature for survival, it is speculated that there is a matching of astrocytes to blood vessels (Foo et al, 2011). The fly does not have a closed circulatory system, so there are no blood vessels in the brain. There must be other mechanisms by which astrocyte number is monitored. An obvious possibility is that the need for astrocytes (or their survival) is linked to the number of neurons with which they make contact.
Two recent studies have shown that mammalian glia, specifically oligodendrocyte precursor cells (OPC) and microglia, can repopulate the brain after induced loss of the entire populations of these two cell types (Hughes et al, 2013; Elmore et al, 2014, 2015). Hughes et al (2013) demonstrate that OPC differentiation to oligodendrocytes occurs throughout life. As such, the homeostatic response to maintain OPCs in the brain likely reflects the need to replace the OPCs that have either differentiated or died. Their data also demonstrate that there is a continuous turnover of oligodendrocytes in the adult. Astrogenesis has been observed to occur in the prefrontral cortex of mice in response to voluntary exercise (Mandyam et al, 2007). Our findings provide evidence that this normal turnover of astrocytes also occurs in the Drosophila brain and that specific neural progenitor cells maintain an ongoing homeostatic control of astrocyte numbers in the adult Drosophila brain. It will be interesting to learn whether a comparable progenitor population exists in mammals to support astrocyte turnover.
Materials and Methods
Fly stocks
Flies were grown at 25°C or 18°C using standard fly media.
The miR‐31a‐targeted knockout mutant flies were made by homologous recombination, described in Chen et al (2014). A mini‐white reporter was inserted in the place of the miR‐31a hairpin. The RMCE mutant allele was made by targeted homologous recombination using the pRMCE vector described in Weng et al (2009), as described in (Chen et al, 2011). The 3,923‐bp 5′ homology arm was amplified with 5′gcggccgcGGCGCAGAATGAGTATTGGT3′ and 5′gcggccgcTGGTTCACAATTCACCGAAA3′. The 2,294‐bp 3′ homology arm was amplified with 5′tgtgtccgtcagtacctgcaggCAGCGAGATCGAACAGAA3′ and 5′ggcgcgcctgcaggCAATCGACAGTGGTGTTTCCT3′. Following recombination, the 180‐bp region encompassing the miR‐31a hairpin was replaced with a mini‐white cassette flanked by attP sites. The mini‐white cassette was subsequently replaced with an attB‐flanked Gal4 cassette to generate the miR‐31a‐Gal4 allele. The direction of Gal4 insertion was verified by PCR.
To make the miR‐31a sponge, the reverse complement of the mature dme‐miR‐31a 5p sequence was used. The sequence, TCAGCTATGACGACATCTTGCCA, was used with a point mutation made in the tenth position to change a C to an A. A fragment containing 10 copies of this sequence was synthesised with the NotI enzyme site added to the 5′ end and the XbaI enzyme site added to the 3′ end. With these two restriction enzyme sites, the miR‐31a sponge was cloned into the pCaSpeR3 vector, downstream of EGFP. The vector was then injected into w 1118 flies and transgenic lines recovered.
To make the miR‐31a sensor, two copies of the reverse complement of the mature sequence of miR‐31a were cloned with XbaI and XhoI sites flanking the sequence into a plasmid downstream of GFP under the control of the tubulin promoter.
The following fly stocks were used: for astrocytes, Alrm‐Gal4 (astrocyte leucine‐rich repeat molecule; Doherty et al, 2009; kind gift from M. Freeman, University of Massachusetts); for cortex glia, NP577‐Gal4; and for ensheathing glia, NP6520‐Gal4 (Awasaki et al, 2008) (kind gifts from T. Lee, Janelia Farm). Insc‐Gal4 (inscuteable) (kind gift from H. Reichert, Biozentrum, University of Basel) and UAS‐hid (kind gift from Herman Stellar, The Rockefeller University). UAS‐CD8‐GFP, repo‐Gal4 (glia), elav‐Gal4, CG16947 RNAi, EGFR RNAi, CG1103 RNAi, UAS‐Histone‐RFP, UAS‐tubGal80ts, FRT42Dtubulin Gal80, FRT42D and G‐Trace (Evans et al, 2009) fly stocks were obtained from the Bloomington Drosophila Stock Center. UAS CG5021 RNAi was obtained from the Vienna Drosophila RNAi Centre. UAS‐CG16947 was obtained from the Harvard Exelixis Collection stocks.
EdU labelling
Flies were reared under identical conditions at 25°C until eclosion. Male flies were picked immediately after eclosion. Flies were starved for 12 h on 1% agar, 24 h after eclosion. Thereafter, flies were fed a mixture of yeast containing 5% sucrose, 1% food colouring and 2 mg/ml of EdU (Click‐iT EdU Alexa Fluor 555 Imaging Kit, Thermo Fisher, MA, USA) for 30 h. The flies were then transferred to normal fly food and dissected 72 h after the end of EdU exposure.
MARCM
UAS‐CD8‐GFP,hsFlp; FRT42D tubGal80 flies were crossed to miR‐31a‐Gal4, FRT42D flies. The flies were reared at 25°C and upon eclosion were heat‐shocked at 37°C for 2 h. Thereafter, the flies were kept for an additional day at 25°C before dissection and immunostaining. A control stock of miR‐31a‐Gal4 lacking FRT42D (no FRT control) was used to show that no phantom clones were observed.
Immunostaining and analysis
Anti‐repo (1:20, 8D12) and Anti‐elav (1:20, 7E8A10) antibodies were obtained from the Developmental Studies Hybridoma Bank, IA, USA. Rabbit anti‐RCHY1 (1:100, Millipore, HPA030339, Sigma Darmstadt, Germany), chicken anti‐GFP (1:500, AB13970, Abcam, Cambridge, UK), rabbit anti‐activated caspase‐3 (1:500, 9578S, 9661S, Cell Signaling Technology, Danvers, MA). Alexa Fluor or DyLight highly cross‐adsorbed anti‐mouse and anti‐rabbit secondary antibodies were used (1:1,000) (Thermo Fisher, MA, USA). Alexa Fluor anti‐rat secondary antibodies were used (1:1,000). Brains were dissected in cold PBS and fixed for 20 min at room temperature with 4% paraformaldehyde and 0.2% Tween 20 in PBS. The brains were blocked in 3% bovine serum albumin in PBS for 30 min at room temperature. Brains were incubated in primary antibodies diluted in antibody buffer (150 mM NaCl, 50 mM Tris base, 1% BSA, 100 mM L‐lysine, 0.04% azide, pH 7.4) over 36–48 h at 4°C and in secondary antibody for 24 h at 4°C in antibody buffer. The brains were washed in between antibodies four to five times with PBST (PBS with 0.1% Triton X) and whole‐mounted in anti‐fade mounting media (0.2 M Tris–HCL, pH 8.5, 2.5% n‐propyl gallate and 90% glycerol; Stork et al, 2012). Images were taken using a Zeiss confocal Imager M2. ImageJ (NIH) was used with the ITCN plugin (Centre for Bio‐image Informatics as UC Santa Barbara) to count the number of glial cells in the central brain. Co‐localization analysis was performed with the Coloc function of Imaris (Bitplane, CT USA), and the Spots function was used to count the number of cells in the brains used for co‐localization analysis.
Quantitative PCR
Total RNA was extracted with TRIzol from the frozen heads of 20–30 heads miR‐31a KO/KO or Canton S control flies. The heads had been flash‐frozen in liquid nitrogen and were not thawed prior to extraction. For target RNA screening, the RNA was treated with DNase using the RNA cleanup protocol from the RNeasy mini kit (Qiagen). RNA was quantitated via spectrophotometry (NanoDrop, Thermo Scientific). The 260/280 ratio of the RNA samples was ~2.0. Samples ranged in concentration from 191 ng/μl to 424 ng/μl in 35 μl of water. 1 μg of total RNA for each sample was converted to cDNA. First strand DNA synthesis was done with Superscript RT‐III (Invitrogen) and 1 μl of a 50 mM stock of oligo dT primers. The oligo dT primers were first incubated with 1 mM of dNTP mix and 1 μg of RNA diluted in water at 65°C for 5 min in a reaction volume totalling 13 μl. The samples were then incubated on ice for 1 min after which a reaction mixture of Superscript RT‐III, DTT and RNAase OUT was added to each sample. The samples were then incubated at 50°C for 50 min and the reaction terminated at 85°C for 5 min. All steps were performed in a PCR machine.
Quantitative RT–PCR was done with Fast SYBR Green Master Mix (#4385612, Applied Biosystems) in the Applied Biosystems 7500 Fast Real‐Time PCR machine. The data were normalized to the reference genes, actin (F 5′ GCTTCGCTGTCTACTTTCCA, R 5′ CAGCCCGACTACTGCTTAGA), GAPDH (F 5′ CCACTGCCGAGGAGGTCAACTAC, R 5′ ATGCTCAGGGTGATTGCGTATGC) and RP49 (F 5′ GCTAAGCTGTCGCACAAA, R 5′ TCCGGTGGGCAGCATGTG). PCR primer efficiency was determined for each primer pair, and primers were only used if the efficiency exceeded 85%. Data represent the average of three independent biological replicates.
| Quantitative RT–PCR conditions | |
|---|---|
| Holding stage | Step 1: 95°C 20 s |
| Cycling stage | Step 2: 95°C 3 s |
| Step 3: 60°C 30 s | |
| Return to step 2: 40 times | |
| Melt curve stage | Step 4: 95°C 15 s |
| Step 5: 60°C 60 s | |
| Step 6: 95°C 15 s | |
| Step 7: 60°C 15 s | |
Box‐shade analysis
http://www.ch.embnet.org/software/BOX_form.html was used to compare the aligned protein sequences of Drosophila melanogaster CG16947 and human RCHY1.
Statistics
Prism 6 (GraphPad, CA, USA) was used to conduct statistical analysis. Unpaired student's t‐test and one‐way ANOVA with post hoc Tukey analysis were used. For t‐tests, Cohen's d (1.6) was used to calculate the effect size; sample size for experiments presented exceeded this value. Refer to scatter plot graphs for number of n per experiment. The F‐test was used to compare the variance between two samples. For one‐way ANOVA tests, Bartlett's test was used. For EdU studies, paired test was used. Cell counts for Figs 1, 2, 3 were done with the experimenter blinded. All brains dissected from live animals on the day of collection were included in the analysis; no brains were excluded from analysis. For the EdU experiments, only flies that had an ingested comparable amount of EdU as indicated by the redness of the belly from the red food dye added to the yeast paste were dissected and analysed. SEM values and sample size for all experiments are in Table EV2.
Author contributions
LCF performed the experiments. LCF and SMC designed the experiments and wrote the manuscript. SS contributed to producing the miR‐31a RMCE allele.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Table EV1
Table EV2
Review Process File
Acknowledgements
This work was supported by core funding from IMCB. SC is supported by a grant from the Novo Nordisk Foundation (NNF12OC0000552). We thank Nivasini Kumar and Tan Kah Junn for technical support.
The EMBO Journal (2017) 36: 1215–1226
Contributor Information
Lynette Caizhen Foo, Email: lynettefoo@gmail.com.
Stephen Michael Cohen, Email: scohen@sund.ku.dk.
References
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA (2012) Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486: 410–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Lai SL, Ito K, Lee T (2008) Organization and postembryonic development of glial cells in the adult central brain of Drosophila . J Neurosci 28: 13742–13753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Renner S, Lüer K, Technau GM (2007) The commonly used marker ELAV is transiently expressed in neuroblasts and glial cells in the Drosophila embryonic CNS. Dev Dyn 236: 3562–3568 [DOI] [PubMed] [Google Scholar]
- Chen Y‐W, Weng R, Cohen SM (2011) Protocols for use of homologous recombination gene targeting to produce microRNA mutants in Drosophila . Methods Mol Biol 732: 99–120 [DOI] [PubMed] [Google Scholar]
- Chen Y‐W, Song S, Weng R, Verma P, Kugler J‐M, Buescher M, Rouam S, Cohen SM (2014) Systematic study of Drosophila microRNA functions using a collection of targeted knockout mutations. Dev Cell 31: 784–800 [DOI] [PubMed] [Google Scholar]
- Chung W‐S, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504: 394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim D, Garcia‐Verdugo J, Alvarez‐Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703–716 [DOI] [PubMed] [Google Scholar]
- Doherty J, Logan MA, Tasdemir OE, Freeman MR (2009) Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci 29: 4768–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MRP, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN (2014) Colony‐stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82: 380–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MRP, Lee RJ, West BL, Green KN (2015) Characterizing newly repopulated microglia in the adult mouse: impacts on animal behavior, cell morphology, and neuroinflammation. PLoS One 10: e0122912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Olson JM, Ngo KT, Kim E, Lee NE, Kuoy E, Patananan AN, Sitz D, Tran P, Do M‐T, Yackle K, Cespedes A, Hartenstein V, Call GB, Banerjee U (2009) G‐TRACE: rapid Gal4‐based cell lineage analysis in Drosophila . Nat Methods 6: 603–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo LC, Allen NJ, Bushong EA, Ventura PB, Chung W‐S, Zhou L, Cahoy JD, Daneman R, Zong H, Ellisman MH, Barres BA (2011) Development of a method for the purification and culture of rodent astrocytes. Neuron 71: 799–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley CJ, McDowell GS, Wise H, Philpott A (2011) Regulation of cell fate determination by Skp1‐Cullin1‐F‐box (SCF) E3 ubiquitin ligases. Int J Dev Biol 55: 249–260 [DOI] [PubMed] [Google Scholar]
- Ho MS‐C, Chen H, Chen M, Jacques C, Giangrande A, Chien C‐T (2009) Gcm protein degradation suppresses proliferation of glial progenitors. PNAS 106: 6778–6783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE (2013) Oligodendrocyte progenitors balance growth with self‐repulsion to achieve homeostasis in the adult brain. Nat Neurosci 16: 668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izergina N, Balmer J, Bello B, Reichert H (2009) Postembryonic development of transit amplifying neuroblast lineages in the Drosophila brain. Neural Dev 4: 44–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Erclik T, Bertet C, Chen Z, Voutev R, Venkatesh S, Morante J, Celik A, Desplan C (2013) Temporal patterning of Drosophila medulla neuroblasts controls neural fates. Nature 498: 456–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J, Beach M, Porpiglia E, Sheehan A, Watts R, Freeman M (2006) The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron 50: 869–881 [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF (2007) Methamphetamine self‐administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci 27: 11442–11450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumar AK, Stork T, Freeman MR (2014) Activity‐dependent regulation of astrocyte GAT levels during synaptogenesis. Nat Neurosci 17: 1340–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto JJ, Yogi P, Hartenstein V (2015) Origin and development of neuropil glia of the Drosophila larval and adult brain: two distinct glial populations derived from separate progenitors. Dev Biol 404: 2–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H (2001) A conditional tissue‐specific transgene expression system using inducible GAL4. Proc Natl Acad Sci USA 98: 12596–12601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH, Kriegstein AR (2010) Developmental genetics of vertebrate glial–cell specification. Nature 468: 214–222 [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron 74: 691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork T, Bernardos R, Freeman M (2012) Analysis of glial cell development and function in Drosophila . Cold Spring Harb Protoc doi:10.1101/pdb.top067587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdemir‐Yilmaz OE, Freeman MR (2014) Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev 28: 20–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian E, Sapperstein S, Christopherson K, Barres B (2001) Control of synapse number by glia. Science 291: 657–661 [DOI] [PubMed] [Google Scholar]
- Weng R, Chen YW, Bushati N, Cliffe A, Cohen SM (2009) Recombinase‐mediated cassette exchange provides a versatile platform for gene targeting: knockout of miR‐31b. Genetics 183: 399–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A, Iwasaki S, McGourty CA, Medina‐Ruiz S, Teerikorpi N, Fedrigo I, Ingolia NT, Rape M (2015) Cell‐fate determination by ubiquitin‐dependent regulation of translation. Nature 525: 523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Grether M, Abrams J, Young L, Farrell K, Steller H (1994) Genetic control of programmed cell death in Drosophila . Science 264: 677–683 [DOI] [PubMed] [Google Scholar]
- Ziegenfuss JS, Biswas R, Avery MA, Hong K, Sheehan AE, Yeung Y‐G, Stanley ER, Freeman MR (2008) Draper‐dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature 453: 935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Table EV1
Table EV2
Review Process File


