Abstract
Psoriasis, a chronic, immune‐mediated skin disease characterized by red, scaly plaques, affects approximately 0.3% of the population in Japan. The aim of this open‐label study was to evaluate the long‐term efficacy and safety of ixekizumab, a humanized, anti‐interleukin‐17A monoclonal antibody, in Japanese patients with plaque psoriasis (n = 78, including 11 psoriatic arthritis), erythrodermic psoriasis (n = 8) and generalized pustular psoriasis (n = 5). Ixekizumab was administrated s.c. at baseline (week 0, 160 mg), from weeks 2 to 12 (80 mg every 2 weeks), and from weeks 16 to 52 (80 mg every 4 weeks). At week 52, 92.3% of patients with plaque psoriasis achieved Psoriasis Area and Severity Index (PASI) 75, 80.8% achieved PASI 90, 48.7% achieved PASI 100, and 52.6% had remission of plaques (by static Physician Global Assessment, sPGA [0]). Difficult to treat areas of psoriasis (nail or scalp) also responded to ixekizumab. All patients with psoriatic arthritis who were assessed (5/5) achieved an American College of Rheumatology 20 response. Most patients with erythrodermic psoriasis or generalized pustular psoriasis responded to ixekizumab and the clinical outcome was maintained over 52 weeks (75% and 60% of patients achieved sPGA [0, 1] at week 52, respectively). Mostly mild or moderate treatment‐emergent adverse events were reported by 79 of 91 patients; the most common were nasopharyngitis, eczema, seborrheic dermatitis, urticaria and injection site reactions. In conclusion, 52‐week ixekizumab treatment was efficacious and well tolerated in Japanese patients with plaque psoriasis. Efficacy was also observed in patients with erythrodermic psoriasis, generalized pustular psoriasis and psoriatic arthritis.
Keywords: erythrodermic psoriasis, generalized pustular psoriasis, ixekizumab, Japan, plaque psoriasis
Introduction
In Japan, it has been estimated that psoriasis, a chronic, immune‐mediated skin disease characterized by red, scaly plaques, affects approximately 0.3% of the population.1 In 2010–2011, more than 400 000 patients were diagnosed with plaque psoriasis, and approximately 2000 and 5000 were diagnosed with the more severe forms of psoriasis, erythrodermic psoriasis and generalized pustular psoriasis, respectively.1 Psoriasis is associated with several comorbidities, including psoriatic arthritis and psychological disorders, and can have a significant impact on quality of life.2
Ixekizumab, a humanized, anti‐interleukin (IL)‐17A monoclonal antibody, is a promising treatment for psoriasis. In a phase 2, double‐blind, placebo‐controlled study of American and Danish patients with moderate to severe plaque psoriasis, a 12‐week treatment with ixekizumab (75 mg every 4 weeks, which is similar to dosing in many phase 3 trials) improved clinical symptoms of psoriasis (by Psoriasis Area and Severity Index [PASI] 75) in up to 82.8% of patients.3 This effect was maintained for 52 weeks, with no unexpected safety signals.4 In addition, in multinational, phase 3, double‐blind, controlled studies of patients with plaque psoriasis, a 12‐week treatment with ixekizumab (80 mg every 2 weeks) had greater efficacy (PASI 75 response rate 89.7%) than placebo (2.4%) or the tumor necrosis factor‐α antagonist etanercept (41.6%).5
In Japanese patients, Saeki et al.6 reported that ixekizumab for 24 weeks improved clinical symptoms of psoriasis in nearly all patients with moderate to severe plaque psoriasis (n = 78).6 In addition, ixekizumab was efficacious in a small group of Japanese patients with erythrodermic psoriasis (n = 8) and generalized pustular psoriasis (n = 5). Here, we report the results of the extension study of Saeki et al., which aimed to evaluate the long‐term (52‐week) efficacy and safety of ixekizumab in Japanese patients with plaque psoriasis (including psoriatic arthritis), erythrodermic psoriasis and generalized pustular psoriasis.
Methods
Study design
This was a phase 3, multicenter (28 centers), single‐arm, open‐label, long‐term study (Clinicaltrials.gov number NCT01624233; first patient enrolled 21 June 2012; data‐lock 17 June 2014). The protocol for this study was approved by local institution ethics committees and conforms to the provisions of the Declaration of Helsinki. All patients who received treatment provided written informed consent. This study is an extension of the study reported by Saeki et al.6
Study population
The study enrolled male and female Japanese patients with psoriasis who were aged 20 years or older, were candidates for phototherapy or systemic therapy, and had a confirmed diagnosis at least 6 months before baseline of either: (i) plaque psoriasis (PASI score, ≥ 12; static Physician Global Assessment [sPGA] score, ≥3, and ≥10% body surface area [BSA] involvement at screening and baseline); (ii) erythrodermic psoriasis (≥80% BSA involvement with inflammatory erythema at screening and baseline); or (iii) generalized pustular psoriasis (met criteria set by Japanese Ministry of Health, Labor and Welfare).7 For the plaque psoriasis group, patients with and without psoriatic arthritis were included (diagnosis of psoriatic arthritis was confirmed using the Classification Criteria for Psoriatic Arthritis [CASPAR]).8
The exclusion criteria included a clinically significant flare within 12 weeks of baseline (for patients with plaque psoriasis) and limitations on prior and concomitant medications (see Saeki et al.6 for details).
Treatment protocol
Ixekizumab (Eli Lilly and Company, Indianapolis, IN, USA) was administrated s.c. as follows: at baseline (week 0), 160 mg of ixekizumab; from weeks 2 to 12, 80 mg every 2 weeks (induction dosing period); and from weeks 16 to 52, 80 mg every 4 weeks (maintenance dosing period).
Outcome measures
The following efficacy outcome measures were assessed: changes from baseline in PASI; proportion of patients with at least 75% reduction of PASI scores (PASI 75), at least 90% reduction (PASI 90) and a 100% reduction (PASI 100) (where PASI 100 refers to complete resolution of plaques); proportion of patients achieving sPGA (0, 1); proportion of patients achieving remission of psoriasis plaques by sPGA (0); percentage of BSA involved; Nail Psoriasis Severity Index (NAPSI; for patients with nail psoriasis at baseline);9 Psoriasis Scalp Severity Index (PSSI; for patients with scalp psoriasis at baseline); Itch Numeric Rating Scale (Itch NRS; scale from “no itch” to “worst itch imaginable”);10 Dermatology Life Quality Index (DLQI);11 Global Improvement Scores (for patients with erythrodermic psoriasis or generalized pustular psoriasis; psoriatic lesions rated from resolved to worsened); assessment of skin symptoms (for patients with generalized pustular psoriasis; based on practice guidelines7 where skin symptoms are evaluated by areas of erythema, confluent pustules and skin edema); and joint pain (pain visual analog scale [VAS] score), American College of Rheumatology 20 (ACR20), and ACR core set of symptoms (for patients with psoriatic arthritis and ≥3 tender joint count and ≥3 swollen joint count at screening and baseline).12
The safety outcome measures included treatment‐emergent adverse events (TEAE; coded and summarized using the Medical Dictionary for Regulatory Activities, Version 17.0), TEAE of special interest (cytopenias, liver function [hepatic] tests, infection, injection site reactions, allergic reactions/hypersensitivities, cerebrocardiovascular events, malignancies, depression, Pneumocystis jirovecii pneumonia, interstitial lung disease), serious adverse events (SAE), immunogenicity (antibody production against ixekizumab defined as treatment‐emergent antidrug antibody [TE‐ADA]) and laboratory parameters (changes in laboratory measures were also assessed by Common Terminology Criteria for Adverse Events [CTCAE] Grade). TEAE were included regardless of their relationship with the study drug.
Statistical analysis
All analyses used the full analysis set, defined as all patients who received at least one dose of ixekizumab and who had at least one post‐baseline PASI measurement. Sample size determination is described in Saeki et al.6 No statistical comparative tests were conducted. Continuous data were summarized by descriptive statistics, and categorical data were summarized by frequency counts and percentages. For clinical response, a non‐responder imputation was calculated at each visit. For continuous variables, a last observation carried forward approach was used for imputation of missing data. Analyses were conducted using SAS version 9.2 (Cary, NC, USA).
Results
Patient disposition and characteristics
The majority of patients completed the 52 weeks of treatment (Fig. 1). Of the 78 patients with plaque psoriasis who entered the study, 70 (89.7%) completed the 52‐week treatment period. Of the patients with erythrodermic psoriasis or generalized pustular psoriasis who entered the study, all (8/8 and 5/5, respectively) completed the 52‐week treatment period. The baseline demographic and clinical characteristics of the study population are presented in Table 1 (modified from Saeki et al.).6 In summary, most patients were male (76.9%), less than 65 years old (90.1%) and had previously received psoriasis therapy (96.7%). Mean bodyweight was lower for patients with generalized pustular psoriasis than for patients with plaque psoriasis or erythrodermic psoriasis.
Figure 1.
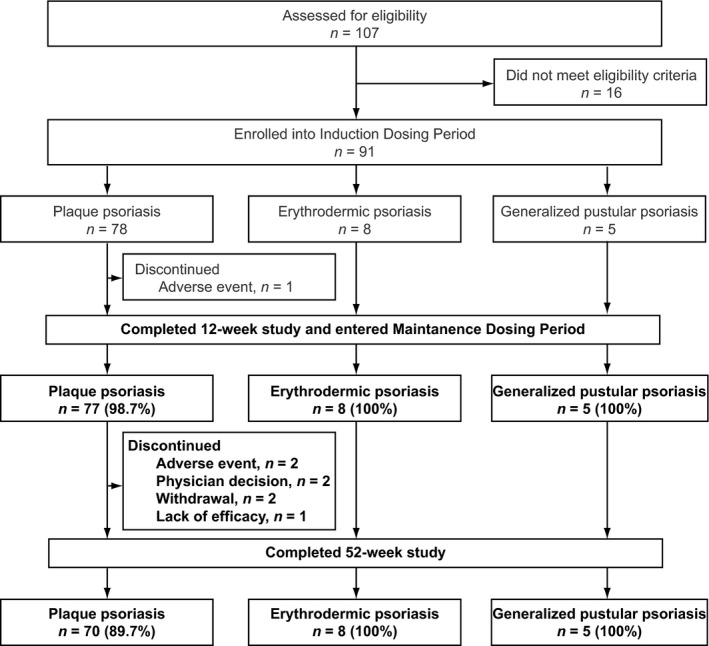
Patient flow diagram for the 52‐week study of Japanese patients with plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis treated with ixekizumab.
Table 1.
Patient demographics and baseline characteristics
| Characteristics | Plaque psoriasis (n = 78) | Erythrodermic psoriasis (n = 8) | Generalized pustular psoriasis (n = 5) |
|---|---|---|---|
| Age, years | 45.7 ± 11.6 | 50.2 ± 12.9 | 48.2 ± 15.6 |
| Sex, male, n (%) | 61 (78.2) | 7 (87.5) | 2 (40.0) |
| Weight, kg | 73.0 ± 16.1 | 78.6 ± 19.5 | 55.8 ± 10.2 |
| Duration of psoriasis, years | 15.1 ± 9.5 | 18.4 ± 14.0 | 21.3 ± 15.7 |
| Prior biologic treatment, n (%) | 14 (17.9) | 3 (37.5) | 2 (40.0) |
| Prior non‐biologic systemic treatment, n (%) | 57 (73.1) | 8 (100) | 5 (100) |
| PASI | 26.6 ± 8.8 | 42.8 ± 11.6 | 12.8 ± 5.5 |
| sPGA | 3.8 ± 0.6 | 4.5 ± 0.5 | 3.4 ± 0.9 |
| Nail Ps, n (%) | 44 (56.4) | 8 (100) | 4 (80.0) |
| NAPSI in patients with nail Ps | 31.7 ± 23.7 | 30.9 ± 15.1 | 24.8 ± 18.9 |
| Scalp Ps, n (%) | 77 (98.7) | 8 (100) | 5 (100) |
| PSSI in patients with scalp Ps | 26.4 ± 15.5 | 39.8 ± 17.5 | 15.0 ± 8.6 |
| Assessment of skin symptoms (0–9) | NA | NA | 2.8 ± 1.9 |
| DLQI | 10.9 ± 6.5 | 11.4 ± 7.6 | 9.6 ± 6.5 |
| Itch NRS | 6.1 ± 2.5 | 4.5 ± 2.5 | 7.2 ± 2.4 |
| PsA, n (%) | 11 (14.1) | NA | NA |
| Pain VAS in patients with PsA | 62.4 ± 23.8 | NA | NA |
Data are presented as mean ± SD unless otherwise stated. DLQI, Dermatology Life Quality Index; NA, not applicable; NAPSI, Nail Psoriasis Severity Index; NRS, Numeric Rating Scale; PASI, Psoriasis Area and Severity Index; Ps, psoriasis; PsA, psoriatic arthritis; PSSI, Psoriasis Scalp Severity Index; SD, standard deviation; sPGA, static Physician Global Assessment; VAS, visual analog scale.
Efficacy
Patients with plaque psoriasis
Patients with plaque psoriasis responded to ixekizumab treatment and the clinical outcome was maintained over 52 weeks (Fig. 2, Table 2). At week 52, 92.3% of patients achieved PASI 75, 80.8% of patients achieved PASI 90 and 48.7% achieved PASI 100. In subgroup analyses, the PASI 75 response rate was not affected by baseline characteristics, such as age, baseline BSA or previous treatment (data not shown).
Figure 2.
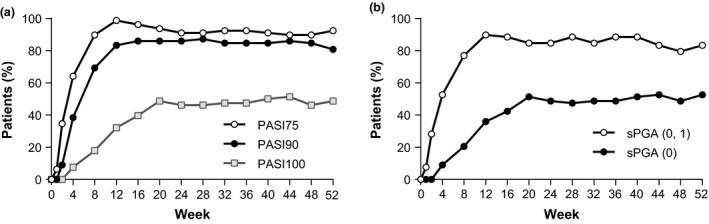
(a) Proportion of patients with plaque psoriasis with PASI 75, PASI 90, PASI 100 and (b) sPGA (0, 1), sPGA (0) response rates during the 52‐week study of Japanese patients. For these outcomes, missing data were imputed with non‐responder imputation. PASI, Psoriasis Area and Severity Index; sPGA, static Physician Global Assessment.
Table 2.
Efficacy outcomes at week 52 of ixekizumab treatment in patients with plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis
| Outcome | Plaque psoriasis (n = 78) | Erythrodermic psoriasis (n = 8) | Generalized pustular psoriasis (n = 5) |
|---|---|---|---|
| PASI (0–72) | |||
| Change from baseline | −25.40 ± 8.792 | −39.81 ± 9.894 | −11.0 ± 3.072 |
| Minimum, maximum | −53.1, −10.8 | −56.4, −28.2 | −16.2, −8.0 |
| PASI 75, n (%) | 72 (92.3) | 8 (100) | 4 (80.0) |
| PASI 90, n (%) | 63 (80.8) | 6 (75.0) | 3 (60.0) |
| PASI 100, n (%) | 38 (48.7) | 1 (12.5) | 2 (40.0) |
| sPGA (0), n (%) | 41 (52.6) | 1 (12.5) | 2 (40.0) |
| sPGA (0, 1), n (%) | 65 (83.3) | 6 (75.0) | 3 (60.0) |
| BSA % | 2.2 ± 5.1 | 6.1 ± 8.0 | 1.4 ± 1.7 |
| NAPSI (0–80)† | |||
| Change from baseline | −23.4 ± 23.67 | −20.9 ± 14.83 | −16.5 ± 15.20 |
| Minimum, maximum | −80, 35 | −42, 0 | −31, −1 |
| PSSI (0–72)‡ | |||
| Change from baseline | −23.3 ± 16.48 | −35.5 ± 16.95 | −12.8 ± 7.73 |
| Minimum, maximum | −72, 15 | −60, −16 | −22, −5 |
| Assessment of skin symptoms (0–9) | NA | NA | 0.8 ± 0.84 |
| Global improvement scores | |||
| Resolved, n (%) | NA | 1 (12.5) | 2 (40.0) |
| Improved, n (%) | NA | 7 (87.5) | 3 (60.0) |
| DLQI (0–30) | |||
| Change from baseline | −9.6 ± 6.24 | −9.5 ± 6.61 | −5.8 ± 3.90 |
| Minimum, maximum | −27, 1 | −24, −2 | −10, 0 |
| Itch NRS (0–10) | |||
| Change from baseline | −4.8 ± 2.83 | −3.4 ± 2.07 | −5.4 ± 3.13 |
| Minimum, maximum | −10, 2 | −6, −1 | −10, −2 |
† n = 44 for plaque psoriasis, n = 8 for erythrodermic psoriasis, n = 4 for generalized pustular psoriasis; ‡ n = 76 for plaque psoriasis, n = 8 for erythrodermic psoriasis, n = 5 for generalized pustular psoriasis. Continuous data are presented as mean ± SD unless otherwise stated. For continuous measures, missing data are imputed with LOCF; for categorical measures, missing data are imputed with NRI. BSA, body surface area; DLQI, Dermatology Life Quality Index; LOCF, last observation carried forward; NA, not applicable; NAPSI, Nail Psoriasis Severity Index; NRI, non‐responder imputation; NRS, Numeric Rating Scale; PASI, Psoriasis Area and Severity Index; PSSI, Psoriasis Scalp Severity Index; SD, standard deviation; sPGA, static Physician Global Assessment.
Improvements in PASI and sPGA scores were observed as early as 1 week after treatment initiation,6 peaked at approximately 12 weeks, and were maintained for the 52‐week treatment period (Fig. 2).
At 52 weeks, approximately half of the patients had a complete resolution or remission of psoriasis plaques with ixekizumab treatment (by PASI 100 or sPGA [0]; Fig. 2, Table 2). This is an improvement over the rate of complete resolution or remission at 12 weeks when approximately one‐third of patients had a PASI 100 or sPGA (0).6
Improvements in BSA percentage, DLQI and Itch NRS observed at 12 weeks6 were maintained over 52 weeks (Table 2). Difficult to treat areas of psoriasis also responded to ixekizumab treatment. For patients with nail psoriasis, NAPSI improved at 12 weeks6 and showed further improvements by 52 weeks (Table 2). For patients with scalp psoriasis, PSSI improved at 12 weeks6 and was maintained over 52 weeks (Table 2).
Patients with psoriatic arthritis
Patients with psoriatic arthritis responded to ixekizumab treatment and the clinical outcome was maintained over 52 weeks (Table 3). At week 12, four of five patients with ACR data achieved an ACR20 response and at week 52, five of five patients achieved an ACR20 response. Improvements in the ACR core set of symptoms were observed at 12 weeks and maintained over 52 weeks. By the patient's assessment of joint pain, VAS scores improved by week 12 and were maintained to week 52.
Table 3.
Response to ixekizumab treatment at weeks 12 and 52 in patients with psoriatic arthritis
| Measure | Week 12 | Week 52 |
|---|---|---|
| Patient's assessment of joint pain (VAS), change from baseline† | ||
| Mean ± SD | −47 ± 22.05 | −51 ± 19.05 |
| Minimum, maximum | −84, −10 | −90, −18 |
| ACR20 response rate, n (%)‡ | 4 (80.0) | 5 (100) |
| ACR core set of symptoms, median change from baseline (minimum, maximum)‡ | ||
| Tender joint count (68 joints) | −6 (−39, −5) | −9 (−43, −5) |
| Swollen joint count (66 joints) | −3 (−29, −1) | −6 (−35, −1) |
| Patient's global assessment of PsA disease activity (VAS) | −67 (−87, −31) | −68 (−87, −53) |
| Physician's global assessment of PsA disease activity (VAS) | −71 (−79, −4) | −73 (−94, −27) |
| HAQ‐DI | 0 (−1.63, 0) | −0.25 (−1.63, 0) |
| hsCRP (mg/dL) | −1.345 (−5.827, 0.056) | −1.903 (−5.432, 0.152) |
† n = 11, ‡ n = 5. Data are presented as median change from baseline (minimum, maximum) unless otherwise stated. For continuous measures, missing data are imputed with LOCF; for categorical measures, missing data are imputed with NRI. ACR, American College of Rheumatology; HAQ‐DI, Health Assessment Questionnaire – Disability Index; hsCRP, high‐sensitivity C‐reactive protein; LOCF, last observation carried forward; NRI, non‐responder imputation; PsA, psoriatic arthritis; SD, standard deviation; VAS, visual analog scale.
Patients with erythrodermic psoriasis or generalized pustular psoriasis
For patients with erythrodermic psoriasis or generalized pustular psoriasis, global improvement scores indicated that all patients either resolved or improved by week 52 of ixekizumab treatment (Table 2). An example of this improvement is provided in Figure 3. In addition, improvements in PASI, sPGA, PSSI, DLQI and Itch NRS were observed at 12 weeks6 and maintained for the 52‐week treatment period. For patients with nail psoriasis, NAPSI improved at 12 weeks6 and showed further improvements by 52 weeks (Table 2). For patients with generalized pustular psoriasis, skin symptoms improved at 12 weeks6 and showed further improvements by 52 weeks; over the 52 weeks, skin symptoms improved from severe6 to mild (Table 2).
Figure 3.
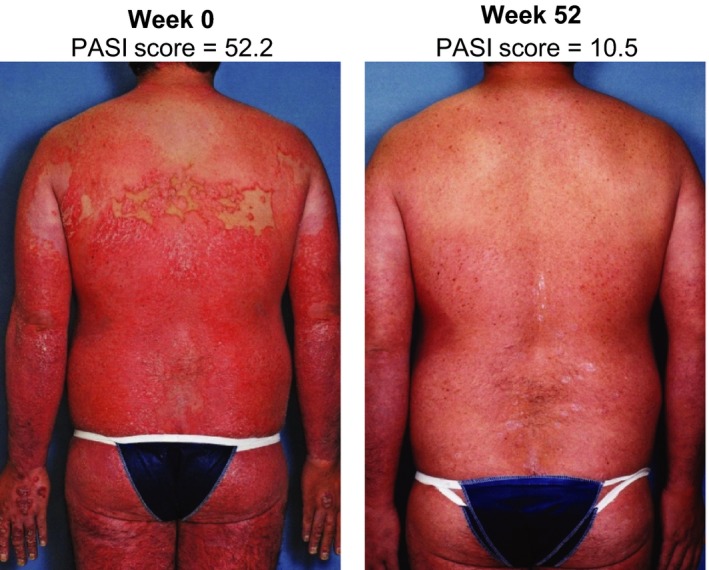
Representative photographs of a patient with erythrodermic psoriasis taken at baseline (week 0)6 and after 52 weeks of treatment with ixekizumab. PASI, Psoriasis Area and Severity Index.
Safety
Adverse events
During the 52‐week study, TEAE (regardless of their relationship with the study drug) were reported by 79 of the 91 patients (86.8%; Table 4). Among the patients with TEAE, most patients (75/79) reported TEAE that were mild or moderate in severity and there were no notable differences between plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis, although patient numbers for erythrodermic psoriasis and generalized pustular psoriasis were small. No deaths were reported. There were four SAE reported by three patients (n = 1 each of colon cancer, deep vein thrombosis, pulmonary embolism and sleep apnea syndrome); all were reported by patients with plaque psoriasis. There were three adverse events reported that led to discontinuation from the study (n = 1 each of neutropenia, colon cancer [SAE] and pulmonary embolism [SAE]); all were reported by patients with plaque psoriasis.
Table 4.
Safety overview during the 52‐week ixekizumab treatment period for patients with plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis
| Adverse event,a n (%) | Plaque psoriasis (n = 78) | Erythrodermic psoriasis (n = 8) | Generalized pustular psoriasis (n = 5) |
|---|---|---|---|
| Patients with ≥1 TEAE | 67 (85.9) | 7 (87.5) | 5 (100) |
| AE leading to discontinuation | 3 (3.8) | 0 | 0 |
| Deaths | 0 | 0 | 0 |
| SAE | 3 (3.8) | 0 | 0 |
| TEAE of special interest | |||
| Infections | 48 (61.5) | 6 (75.0) | 4 (80.0) |
| Allergic reactions/hypersensitivity | 22 (28.2) | 2 (25.0) | 2 (40.0) |
| Injection site reaction | 12 (15.4) | 0 | 2 (40.0) |
| Hepatic | 7 (9.0) | 1 (12.5) | 0 |
| Cytopenias | 3 (3.8) | 0 | 0 |
| Depression | 1 (1.3) | 0 | 0 |
| Malignancies | 1 (1.3) | 0 | 0 |
| Cerebrocardiovascular | 0 | 0 | 0 |
| Pneumocystis jirovecii pneumonia | 0 | 0 | 0 |
| Interstitial lung disease | 0 | 0 | 0 |
Adverse events were included regardless of their relationship with the study drug. AE, adverse event; SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
The most common TEAE overall were nasopharyngitis (36/91, 39.6%), eczema (11/91, 12.1%), seborrheic dermatitis (8/91; 8.8%), urticaria (8/91, 8.8%) and injection site reaction (8/91, 8.8%). The most common TEAE of special interest were infections, allergic reactions/hypersensitivity, injection site reaction and hepatic events (Table 4). The most common infection was nasopharyngitis. There was one report of oral candidiasis and no reported cases of tuberculosis or invasive fungal infections. All of the allergic reactions/hypersensitivity were non‐anaphylactic; the most common reactions were eczema, urticaria and contact dermatitis. All injection site reactions were mild or moderate. Most hepatic events were liver‐related laboratory test results; the most common was increased alanine aminotransferase (5/91, 5.5%). Of note, there were no reports of pneumocystis pneumonia, interstitial lung disease, cerebrocardiovascular events requiring adjudication, Crohn's disease or ulcerative colitis.
There were no clinically significant changes in laboratory parameters noted over the 52 weeks (data not shown). Although decreases in neutrophil, leukocyte and platelet counts, and increases in lymphocyte counts were observed, none of these changes were clinically meaningful. There were four patients with plaque psoriasis (5.1%) with a decrease in neutrophil grade to Common Terminology Criteria for Adverse Events (CTCAE) grade 2; of these, two patients decreased by two CTCAE grades from baseline and two patients decreased by one CTCAE grade from baseline.
Immunogenicity
During the 52‐week study, there were 10 of 91 (11%) TE‐ADA‐positive patients, all with plaque psoriasis, noted in the maintenance dosing period. Of the 10 TE‐ADA positive patients, one patient had transient TE‐ADA status and nine patients had persistent TE‐ADA status. All TE‐ADA were inconclusive for neutralizing antibody (i.e. neutralizing antibody could not be analyzed due to assay limitations in the presence of serum ixekizumab concentrations). There were no notable differences in PASI or sPGA response rates between TE‐ADA‐positive and ‐negative patients (data not shown).
Discussion
Long‐term (52‐week) ixekizumab treatment resulted in maintenance of clinically meaningful improvements in the signs and symptoms of plaque psoriasis in Japanese patients. In addition, the clinical symptoms of psoriasis improved in a small number of patients with erythrodermic psoriasis and generalized pustular psoriasis, and plaque psoriasis patients with psoriatic arthritis responded well to ixekizumab treatment. There were no unexpected safety signals through 52 weeks of ixekizumab treatment, and the safety profile of ixekizumab was similar to that previously reported after short‐term evaluation in Japanese patients,6 and as reported for another long‐term treatment study;4 no safety concerns specific to Japanese patients were raised. These results highlight the potential of ixekizumab as a long‐term treatment for Japanese patients with psoriasis.
The efficacy findings for patients with plaque psoriasis, including PASI, sPGA, percentage BSA, NAPSI, PSSI, DLQI and Itch NRS, were consistent with 12‐week phase 3 studies,5 the short‐term results in Japanese patients,6 the long‐term, 52‐week global study,4 and a post‐hoc analysis of scalp and nail psoriasis from a phase 2 psoriasis study.13 In mostly Caucasian patients, ixekizumab treatment for 52 weeks resulted in 77% of patients with a PASI 75 at the 52‐week end‐point.4 In our study in Japanese patients, ixekizumab treatment for 52 weeks resulted in 92.3% of patients with a PASI 75 at the 52‐week end‐point. Although a direct comparison of the Caucasian and Japanese studies is not possible, given differences in study population, study design, sample size, demographics and baseline disease characteristics (bodyweight, disease severity, previous psoriasis therapy), and dosing regimens, our results suggest that long‐term ixekizumab is effective in both Caucasian and Japanese patients.4 Our findings for patients with plaque psoriasis were also consistent with studies of other anti‐IL‐17 drugs. The PASI 75 response rates after treatment were 93.3% (at 48 weeks) for brodalumab14 and approximately 60–80% (at 52 weeks) for secukinumab,15 and the PASI 100 response rates after treatment were approximately 62% (at 48 weeks) for brodalumab14 and 20–40% (at 52 weeks) for secukinumab,15 which are similar to our findings.
In addition, in our study, ixekizumab was efficacious in a small number of patients with psoriatic arthritis and resulted in all patients with ACR data achieving an ACR20 response. As psoriatic arthritis can affect up to 20% of Japanese patients with psoriasis,16 this is a promising result that will be evaluated in larger clinical trials, including assessment of joint symptoms, structural damage and enthesitis. Other treatments for psoriatic arthritis,17 including other anti‐IL‐17 drugs,18, 19 are generally effective in approximately 50% of patients, although these have been evaluated in larger studies.
To our knowledge, this is the first study to document 52‐week efficacy and safety of an anti‐IL‐17 treatment in a small number of patients with erythrodermic psoriasis and generalized pustular psoriasis. In our study, all patients with these severe forms of psoriasis responded (improved or resolved) to ixekizumab treatment, no SAE were reported and no safety concerns were identified. Although our study only included a small number of patients, this is a promising result given that treatment options for these patients are limited.
The study is mainly limited by the small sample sizes for patients with erythrodermic psoriasis, generalized pustular psoriasis and psoriatic arthritis. Other limitations include the open‐label study design and the lack of a control group. However, the study was conducted in multiple centers and the discontinuation rate was very low.
In conclusion, long‐term, 52‐week ixekizumab treatment was efficacious and well tolerated in Japanese patients with plaque psoriasis, including those with psoriatic arthritis. Efficacy was also observed in a small number of patients with erythrodermic psoriasis and generalized pustular psoriasis. Controlled and comparative studies are ongoing to confirm the utility of ixekizumab as a treatment for patients with psoriasis.
Conflict of Interest
H. S. and H. N. have no conflicts of interest to declare; all other authors are employees of Eli Lilly, and K. N., G. S. C. and T. I. own stock in Eli Lilly.
Acknowledgments
This study was sponsored by Eli Lilly Japan K.K., manufacturer of ixekizumab. Medical writing assistance was provided by Janelle Keys, PhD, CMPP and Rebecca Lew, PhD, CMPP of ProScribe – Envision Pharma Group, and was funded by Eli Lilly Japan K.K. ProScribe's services that complied with international guidelines for Good Publication Practice (GPP3). Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis and preparation of the manuscript. Assistance with manuscript preparation, editing and review was provided by Keisuke Shinkai of Eli Lilly Japan K.K. All authors contributed to interpretation of the study findings. T. I. and T. A. participated in study design and Y. M. provided support for statistical analyses. The authors would like to thank all study participants and the Japanese Ixekizumab Study Group: Toshihide Akasaka of Iwate Medical University Hospital, Yoshihide Asano of The University of Tokyo Hospital, Takafumi Etoh of Tokyo Teishin Hospital, Yasuyuki Fujita of Hokkaido University Hospital, Takashi Hashimoto of Kurume University Hospital, Mari Higashiyama of Nissay Hospital, Atsuyuki Igarashi of NTT Medical Center Tokyo, Hironobu Ihn of Kumamoto University, Keiji Iwatsuki of Okayama University Hospital, Kenji Kabashima of Kyoto University Hospital, Akira Kawada of Kinki University Faculty of Medicine, Makoto Kawashima of Tokyo Women's Medical University, Koichiro Nakamura of Saitama Medical University Hospital, Yukari Okubo of Tokyo Medical University Hospital, Ryuhei Okuyama of Shinshu University School of Medicine, Akira Ozawa of Tokai University School of Medicine, Koji Sayama of Ehime University Graduate School of Medicine, Mariko Seishima of Gifu University Graduate School of Medicine, Tetsuo Shiohara of Kyorin University Hospital, Masakazu Takahara of Kyusyu University Hospital, Hidetoshi Takahashi of Asahikawa Medical University Hospital, Kazuhiko Takehara of Kanazawa University Hospital, Keiji Tanese of Keio University Hospital, Mamori Tani of Osaka University Hospital, Yoshinori Umezawa of The Jikei University Hospital, Hideaki Watanabe of Showa University School of Medicine and Keiichi Yamanaka of Mie University Graduate School of Medicine.
References
- 1. Kubota K, Kamijima Y, Sato T et al Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open 2015; 5: e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lonnberg AS, Zachariae C, Skov L. Targeting of interleukin‐17 in the treatment of psoriasis. Clin Cosmet Investig Dermatol 2014; 7: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leonardi C, Matheson R, Zachariae C et al Anti‐interleukin‐17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 2012; 366: 1190–1199. [DOI] [PubMed] [Google Scholar]
- 4. Gordon KB, Leonardi CL, Lebwohl M et al A 52‐week, open‐label study of the efficacy and safety of ixekizumab, an anti‐interleukin‐17A monoclonal antibody, in patients with chronic plaque psoriasis. J Am Acad Dermatol 2014; 71: 1176–1182. [DOI] [PubMed] [Google Scholar]
- 5. Griffiths CE, Reich K, Lebwohl M et al Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015; 386: 541–551. [DOI] [PubMed] [Google Scholar]
- 6. Saeki H, Nakagawa H, Ishii T et al Efficacy and safety of open‐label ixekizumab treatment in Japanese patients with moderate‐to‐severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol 2015; 29: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 7. Iwatsuki K, Terui T, Ozawa A. Practice guidelines for generalized pustular psoriasis (GPP): treatment guidelines incorporating TNF‐a inhibitor. Jpn J Dermatol 2010; 120: 815–839. [Japanese]. [Google Scholar]
- 8. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006; 54: 2665–2673. [DOI] [PubMed] [Google Scholar]
- 9. Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol 2003; 49: 206–212. [DOI] [PubMed] [Google Scholar]
- 10. Phan NQ, Blome C, Fritz F et al Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012; 92: 502–507. [DOI] [PubMed] [Google Scholar]
- 11. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)‐a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 12. Villaverde V, Balsa A, Cantalejo M et al Activity indices in rheumatoid arthritis. J Rheumatol 2000; 27: 2576–2581. [PubMed] [Google Scholar]
- 13. Langley RG, Rich P, Menter A et al Improvement of scalp and nail lesions with ixekizumab in a phase 2 trial in patients with chronic plaque psoriasis. J Eur Acad Dermatol Venereol 2015; 29: 1763–1770. [DOI] [PubMed] [Google Scholar]
- 14. Papp K, Leonardi C, Menter A et al Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol 2014; 71: 1183–1190. [DOI] [PubMed] [Google Scholar]
- 15. Langley RG, Elewski BE, Lebwohl M et al Secukinumab in plaque psoriasis‐results of two phase 3 trials. N Engl J Med 2014; 371: 326–338. [DOI] [PubMed] [Google Scholar]
- 16. Ohara Y, Kishimoto M, Takizawa N et al Prevalence and clinical characteristics of psoriatic arthritis in Japan. J Rheumatol 2015; 42: 1439–1442. [DOI] [PubMed] [Google Scholar]
- 17. Kang EJ, Kavanaugh A. Psoriatic arthritis: latest treatments and their place in therapy. Ther Adv Chronic Dis 2015; 6: 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McInnes IB, Sieper J, Braun J et al Efficacy and safety of secukinumab, a fully human anti‐interleukin‐17A monoclonal antibody, in patients with moderate‐to‐severe psoriatic arthritis: a 24‐week, randomised, double‐blind, placebo‐controlled, phase II proof‐of‐concept trial. Ann Rheum Dis 2014; 73: 349–356. [DOI] [PubMed] [Google Scholar]
- 19. Mease PJ, Genovese MC, Greenwald MW et al Brodalumab, an anti‐IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014; 370: 2295–2306. [DOI] [PubMed] [Google Scholar]


