Summary
This phase 1/2 study evaluated the safety, pharmacokinetic behavior and anti‐tumour activity of ublituximab, a unique type I, chimeric, glycoengineered anti‐CD20 monoclonal antibody, in rituximab‐relapsed or ‐refractory patients with B‐cell non‐Hodgkin lymphoma (B‐NHL) or chronic lymphocytic leukaemia (CLL). Induction therapy (doses of 450–1200 mg) consisted of 4 weekly infusions in cycle 1 for NHL and 3 weekly infusions in cycles 1 and 2 for CLL. Patients received ublituximab maintenance monthly during cycles 3–5, then once every 3 months for up to 2 years. Enrolled patients with B‐NHL (n = 27) and CLL (n = 8) had a median of 3 prior therapies. No dose‐limiting toxicities or unexpected adverse events (AEs) occurred. The most common AEs were infusion‐related reactions (40%; grade 3/4, 0%); fatigue (37%; grade 3/4, 3%); pyrexia (29%; grade 3/4, 0%); and diarrhoea (26%; grade 3/4, 0%). Common haematological AEs were neutropenia (14%; grade 3/4, 14%) and anaemia (11%; grade 3/4, 6%). The overall response rate for evaluable patients (n = 31) was 45% (13% complete responses, 32% partial responses). Median duration of response and progression‐free survival were 9·2 months and 7·7 months, respectively. Ublituximab was well‐tolerated and efficacious in a heterogeneous and highly rituximab‐pre‐treated patient population.
Keywords: anti‐CD20 monoclonal antibody, B‐cell lymphoma, LFB‐R603, TG‐1101, ublituximab
Monoclonal antibodies (MAb) targeting CD20 have improved the outcome of patients with B‐cell malignancies and represent a major advance in treatment (Dotan et al, 2010; Weiner et al, 2010; Singh et al, 2014). While the activity of single‐agent rituximab has been modest, its addition to standard chemotherapy markedly improves overall response rates (ORR), progression‐free survival (PFS) and overall survival (OS) across all subtypes of B‐cell non‐Hodgkin lymphoma (B‐NHL) and chronic lymphocytic leukaemia (CLL) (Habermann et al, 2006; Van Oers et al, 2006; Hochster et al, 2009; Parikh & Wierda, 2010). Maintenance therapy with rituximab following immunochemotherapy induction significantly improved responses and PFS in previously untreated and relapsed follicular lymphoma (FL) (Van Oers et al, 2006; Nastoupil et al, 2014). However, complete responses (CRs) with rituximab are rare, and indolent lymphomas, such as CLL and FL, remain incurable diseases, highlighting the need for well‐tolerated agents effective in the relapsed state (Khouri, 2006; Farren et al, 2015). The development of novel anti‐CD20 MAbs with activity in rituximab‐resistant disease represents a major advance in the care of these patients.
Rituximab depletes B cells through three primary mechanisms: complement‐dependent cytotoxicity (CDC), programmed cell death and antibody‐dependent cellular cytotoxicity (ADCC) (Oflazoglu & Audoly, 2010; Winiarska et al, 2011). Approximately half of all patients who are initially responsive to rituximab are refractory to retreatment (Davis et al, 2000). The mechanisms of rituximab resistance are hypothesised to revolve around loss of cell‐surface CD20 by transcriptional down‐regulation, selection of a CD20‐negative clone, ‘shaving’ of the CD20 antigen/antibody complex, or internalisation of the antigen/antibody complex into the target cell (Beum et al, 2006; Rezvani & Maloney, 2011; Winiarska et al, 2011; Vaughan et al, 2014). Efforts to overcome rituximab resistance have focused on the development of new CD20‐targeted MAbs that: (i) are active in ‘low’ CD20‐expressing tumours; (ii) bind to unique CD20 epitopes; and (iii) have increased ability to engage the innate immune system, particularly ADCC (Oflazoglu & Audoly, 2010; Winiarska et al, 2011).
Next‐generation anti‐CD20 MAbs have been engineered to have a variety of features that distinguish them from rituximab, many of which revolve around improving ADCC and CDC (Table SI). Ofatumumab, a second‐generation, fully human anti‐CD20 MAb optimised for high CDC activity, has shown efficacy as a single agent in CLL patients refractory to fludarabine and alemtuzumab (Wierda et al, 2010). Other approaches to enhance the interaction between anti‐CD20s and their targets include changing the amino acid sequence (Bowles et al, 2006) or glycosylation pattern of the IgG in a way that enhances interaction with the Fc receptor (FcR) on effector cells (Dalle et al, 2011). Such modifications can enhance the in vitro efficacy and appear to be safe in clinical trials (Salles et al, 2012). Third‐generation anti‐CD20 MAbs are Fc domain‐engineered antibodies designed to improve therapeutic activity by enhancing Fc‐gamma receptor‐IIIa (FcγRIIIa) interactions, improving ADCC activity (Winiarska et al, 2011). Obinutuzumab, a type II glycomodified MAb, has improved ADCC over rituximab, with less CDC than ofatumumab. It is a humanised IgG2 class MAb approved for the treatment of CLL (Cartron et al, 2014). Obinutuzumab is also unique in how it cross‐links CD20, resulting in enhanced direct cell death.
Ublituximab is a type I, chimeric, glycoengineered anti‐CD20 recombinant IgG1 MAb produced in the rat cell line YB2/0. Interestingly, ADCC activity is known to be dependent on the fucose content of oligosaccharides bound to anti‐CD20‐MAbs (Konno et al, 2012). Anti‐CD20 MAbs with low fucose content exhibit higher ADCC activity, while antibodies with high fucose content have less ADCC (Konno et al, 2012). The low fucose content in ublituximab's Fc region results in increased affinity for the FcγRIIIa (or CD16) (Le Garff‐Tavernier et al, 2014, 2015). This increased affinity is associated with potent in vitro ADCC against B cells compared with both rituximab and ofatumumab, particularly against tumour cells that express low levels of CD20, such as CLL (De Romeuf et al, 2008; Bellon et al, 2011; Le Garff‐Tavernier et al, 2011). Ublituximab differs from obinutuzumab in at least 3 distinct ways: (i) ublituximab is an IgG1 class MAb, which exhibits improved ADCC, in contrast to obinutuzumab, which is an IgG2 class MAb; (ii) ublituximab and obinutuzumab bind to distinctly different epitopes on the CD20 domain (Figure S1); and (iii) obinutuzumab is humanised, while ublituximab is chimeric. Murine xenograft models of FL and mantle cell lymphoma have demonstrated superior anti‐tumour activity and marked tumour‐growth delay with ublituximab compared with rituximab (Tourais Estaves et al, 2011). In a first‐in‐human phase 1 study of single‐agent ublituximab in heavily treated CLL‐patients, all of whom had relapsed following fludarabine‐based therapy and 58% of whom were refractory to prior rituximab, ublituximab was well‐tolerated and active at doses up to 450 mg per infusion (Cazin et al, 2011, 2013). Infusion‐related reactions (IRRs), pyrexia, neutropenia and thrombocytopenia were the most prevalent adverse events (AEs), with an ORR of 45%.
This phase 1/2 trial (ClinicalTrials.gov NCT01647971) was performed to determine the maximum tolerated dose (MTD), safety and efficacy of single‐agent ublituximab in patients with relapsed/refractory B‐cell NHL or CLL previously treated with rituximab.
Methods
Study design
This was an open‐label, phase 1/2 trial performed at 6 sites (Fig 1). Phase 1 employed a Fibonacci 3 + 3 dose‐escalation design (flat doses of 450, 600, 900 and 1200 mg). Patients with CLL/small lymphocytic lymphoma (SLL) received treatment on days 1, 8 and 15 during cycles 1 and 2 (28‐day cycles). In the absence of disease progression or unacceptable toxicity, patients received maintenance ublituximab on day 1 of cycles 3–5, then every 3 months for a maximum of 2 years. Patients with NHL received ublituximab weekly during cycle 1 (days 1, 8, 15 and 22), no dose in cycle 2, and then received maintenance infusions on day 1 of cycles 3–5, followed by a dose every 3 months for a maximum of 2 years (Fig 1). The CLL/SLL treatment schedule was selected based on pharmacokinetic/pharmacodynamic (PK/PD) results from the first‐in‐human phase 1 study indicating profound B‐cell depletion on a weekly‐times‐8 schedule. Since this was the first human experience with ublituximab in NHL, the dosing schedule for patients with NHL was selected to mimic the schedule used by other anti‐CD20 regimens, with the inclusion of maintenance infusions. Pre‐infusion treatment with an oral antihistamine and steroids was required. Flat doses of ublituximab were escalated as follows: (i) 450 mg, (ii) 600 mg, (iii) 900 mg and (iv) 1200 mg. During the phase 2 study, the recommended phase 2 dose (RP2D) was administered on the same schedule as in the phase 1. All patients provided written informed consent. The study protocol was approved by an Institutional Review Board and was conducted in accordance with the principles of Good Clinical Practice and the ethical principles outlined in the Declaration of Helsinki.
Figure 1.
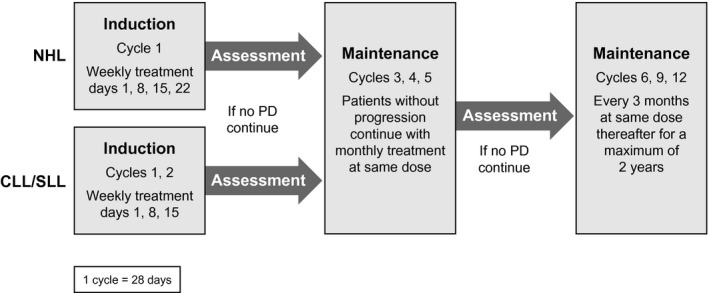
Study design for ublituximab. CLL, chronic lymphocytic leukaemia; NHL, aggressive non‐Hodgkin lymphoma; PD, progressive disease; SLL, small lymphocytic lymphoma.
Study objectives
The phase 1 objectives were to determine the dose‐limiting toxicity (DLT), MTD, RP2D and safety profile. Secondary objectives included determination of ORR, PK profiles, PFS, duration of response (DOR) and time to response. A DLT was defined as any of the following occurring within cycle 1 and considered related to therapy: grade 4 thrombocytopenia, grade 4 febrile neutropenia, grade 3 or greater non‐haematological AE, any grade 5 event or any persistent AE that delayed administration of the next dose by >14 days. The MTD was defined as the dose at which <2 patients experienced a DLT. CLL patients were enrolled in the 600 and 900 mg cohorts only and were evaluated for DLTs separately from NHL patients. The primary objective of phase 2 was determination of ORR. Secondary objectives included DOR, time to response, PFS and assessment of safety.
Inclusion criteria
Patients were required to have histologically confirmed relapsed or refractory B‐NHL or CLL/SLL, prior rituximab treatment, an Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and adequate organ/marrow function with baseline absolute neutrophil count >1 × 109/l and platelet count >50 × 109/l. ‘Rituximab‐refractory’ was defined as progression on or within 6 months of rituximab treatment. ‘Relapsed’ patients were defined as those who progressed more than 6 months after rituximab treatment. Patients with primary central nervous system lymphoma were eligible.
Exclusion criteria
These included pregnancy; active hepatitis B or C, human immunodeficiency virus or other acute infections; uncontrolled concurrent illness; severe allergy to human/mouse antibodies; history of malignancy (except basal cell carcinoma, in situ carcinoma of breast or cervix treated surgically with curative intent, or any malignancy that had been in CR for least 5 years); and chemotherapy or radiation ≤3 weeks or stem cell transplant ≤3 months prior to study entry.
Patient evaluations
Blood samples for complete blood count and chemistry were collected prior to each infusion up to cycle 12 and post‐infusion during cycles 1 and 5. Other evaluations included chest x‐ray, computed tomography (at screening, end of cycle 2 and approximately every 12 weeks thereafter), and positron emission tomography (PET, optional). Efficacy analyses were performed for any patient with one post‐baseline measurement. Safety analyses were based on all registered patients who received at least 1 dose of ublituximab.
Outcomes
Assessment of response was based on the International Working Group (IWG) criteria for NHL (Cheson et al, 2007) and CLL (Hallek et al, 2008). Response assessments occurred at week 8 and every 12 weeks thereafter. AEs were based on published criteria (Common Terminology Criteria for Adverse Events, Version 4.0, https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf). Duration of response, time to response and PFS were determined per standard IWG criteria (Cheson et al, 2007; Hallek et al, 2008).
Pharmacokinetic assessment
Serum concentrations of ublituximab were analysed at 3P Lab (University of Wisconsin, Carbone Comprehensive Cancer Center, Madison, WI, USA). Parameters evaluated included peak (C max) and trough concentrations, terminal half‐life, area under the concentration curve (AUC) and systemic clearance.
Blood samples were collected pre‐dose, 1 min prior to end of infusion (EOI) and 0·5, 6, 24, 72 and 168 h post‐EOI on days 1 and 22 of cycle 1 (C1D1 and C1D22) and C5D1. Samples were also collected pre‐dose, 1 min prior to EOI and 30 min and 6 h post‐dose on days 8 and 15 of cycle 1. Pre‐dose samples were also drawn prior to each maintenance infusion (cycles 3, 4, 6 and 9) for up to 1 year.
Ublituximab concentrations were evaluated by flow cytometry. The assay was validated over the range of 25–1500 ng/ml, with a correlation coefficient of >98% and per cent coefficient of variation (CV) ranging from 3·3% to 9·6%.
Standard PK parameters were determined using non‐compartmental methods. C max, and trough (C min) concentrations were determined by inspection of each individual's concentration‐time curve. If two peaks occurred in the same collection period, the first peak was recorded as the C max. Terminal disposition rate constants were estimated by linear regression analysis of the last two time points (72 and 168 h) of the log‐concentration versus time. Terminal half‐lives (t1/2) were calculated by dividing 0·693 by the elimination rate constant. The AUC was calculated using the linear trapezoidal rule up to the last collection time point (AUC0‐168 h), then extrapolated to infinity. Systemic clearance was determined by dividing dose by AUC. Differences among the kinetic parameter variables were evaluated using an unpaired two‐tailed t‐test.
Statistical analysis
Up to 80 patients could be enrolled. If 8 or more patients responded, the null hypothesis of ORR ≤5% would be rejected and replaced by the hypothesis that the response rate for the treatment was ≥15%. The planned sample size provided 90% power at alpha <5%. Early termination of the study would occur if no responders were observed among the first 20 enrolled patients.
PFS was determined using the methods of Kaplan–Meier (Kaplan & Meier, 1958). All statistical analyses were performed using a two‐sided hypothesis test at the 5% level of significance, using SAS (Cary, NC, USA) Version 9.2.
Results
Patients
Thirty‐five patients were enrolled between August 2012 and June 2015, and 29 discontinued before completing the planned 2 years of treatment. Seventy‐one per cent of patients had received ≥2 prior rituximab‐based regimens, and 43% were considered rituximab‐refractory (Table 1).
Table 1.
Baseline characteristics of the patients and prior therapy
| Characteristic | Phase I (N = 20) | Phase II (N = 15) | Patients (N = 35) |
|---|---|---|---|
| Median age – years (range) | 65·5 (50–88) | 69 (45–86) | 66 (45–88) |
| Gender – n (%) | |||
| Female | 8 (40) | 10 (67) | 18 (51) |
| Male | 12 (60) | 5 (33) | 17 (49) |
| ECOG – n (%) | |||
| 0 | 9 (45) | 4 (27) | 13 (37) |
| 1 | 10 (50) | 10 (67) | 20 (57) |
| 2 | 1 (5) | 1 (6) | 2 (6) |
| Subtype of lymphoma – n (%) | |||
| Indolent NHL | 10 (50) | 10 (67) | 20 (57) |
| Follicular | 7 (35) | 5 (33) | 12 (29) |
| Marginal zone | 3 (15) | 5 (33) | 8 (23) |
| CLL/SLL | 8 (40) | – | 8 (23) |
| Aggressive NHL | 2 (10) | 5 (33) | 7 (20) |
| Mantle Cell | 2 (10) | 3 (20) | 5 (14) |
| Diffuse Large B‐Cell | – | 2 (13) | 2 (6) |
| Prior therapy regimens – median (range) | 3·5 (1–6) | 2 (1–9) | 3 (1–9) |
| Prior therapy – n (%) | |||
| Rituximab | – | – | 35 (100) |
| Alkylating Agent (R‐CHOP, R‐CVP, R‐ICE, other) | – | – | 23 (66) |
| Bendamustine (± rituximab) | – | – | 12 (34) |
| Purine analogue | – | – | 10 (29) |
| Stem‐cell transplantation | – | – | 5 (14) |
| Bortezomib | – | – | 5 (14) |
| Experimental therapya | – | – | 6 (17) |
| Rituximab‐refractory – n (%) | 7 (35) | 8 (53) | 15 (43) |
| 2 or > prior rituximab regimens – n (%) | 14 (70) | 11 (73) | 25 (71) |
| Refractory to immediate prior therapy – n (%) | 7 (35) | 8 (53) | 15 (43) |
CLL, chronic lymphocytic leukaemia; ECOG, Eastern Cooperative Oncology Group; NHL, non‐Hodgkin lymphoma; R‐CHOP, rituximab, cyclophosphamide, hydroxydaunorubicin (doxorubicin), Oncovin (vincristine) and prednisone; R‐CVP, rituximab, cyclophosphamide, vincristine and prednisone; R‐ICE, rituximab, ifosfamide, carboplatin and etoposide; SLL, small lymphocytic lymphoma.
Includes bevacizumab, vorinostat, MLN4924, brentuximab, pralatrexate, lenalidomide.
Four patients discontinued prior to the first efficacy assessment and were not evaluable for response (2 for AEs not related to study drug; 1 for a serious AE [pneumonia]; and 1 patient withdrew consent). All 35 patients were evaluated for safety.
At the end of the study, 21/35 (60%) patients had discontinued treatment for progression, while 8/35 (23%) patients stopped treatment for other reasons [AE/serious AE (n = 3); withdrawal of consent (n = 1); or investigator/patient decision (n = 4)]. The remaining 6/35 (17%) completed all treatment.
Dose determination and treatment
No DLTs were observed, hence no MTD was identified (Table 2). There was no significant difference in the overall number of AEs among the four dose cohorts. There appeared to be no difference in ORR between 900 and 1200 mg, with a slightly higher incidence of haematological AEs observed (grade 3 neutropenia, anaemia, and thrombocytopenia) at the 1200‐mg dose level. Hence 900 mg was selected as the RP2D.
Table 2.
Ublituximab activity by dose cohort (Phase 1)
| Dose (mg) | Disease | DLT | Best response |
|---|---|---|---|
| 450 | MZL | No | CR |
| FL | No | PD | |
| MCL | No | PD | |
| 600 | FL | No | SD |
| FL | No | SD | |
| MZL | No | CR | |
| CLL | No | NE | |
| CLL | No | NE | |
| CLL | No | PR | |
| CLL | No | PR | |
| CLL | No | PR | |
| 900 | FL | No | CR |
| FL | No | CR | |
| MZL | No | PR | |
| CLL | No | SD | |
| CLL | No | SD | |
| CLL | No | SD | |
| 1200 | FL | No | SD |
| FL | No | PD | |
| MCL | No | SD |
CLL, chronic lymphocytic leukaemia; CR, complete response; FL, follicular lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; NE, not evaluable; PD, progressive disease; PR, partial response, SD, stable disease.
Note: Non‐evaluable patients discontinued study prior to first disease assessment (one patient with pneumonia deemed unrelated to study, and one patient withdrew consent).
Infusion times averaged 4 h for the first administration, 3 h for the second and third, and decreased to an average of 90 min for the fourth and all subsequent doses. Day 1 infusions were split for CLL patients with up to 150 mg administered on day 1 and the remaining dose administered on day 2 of cycle 1 only. Dose interruptions were permitted at the discretion of the investigator.
Safety
All 35 patients experienced ≥1 AE, with 17 (49%) reporting at least 1 grade 3/4 AE. Seventeen patients (49%) required ≥1 dose interruption, of which 11 were for IRRs. IRRs occurred in 14 (40%) patients (Table 3) and were more prevalent among patients with CLL. The majority of IRRs occurred on C1D1, with only 5 occurring on subsequent cycles. No episodes of grade ≥3 IRR were reported. All IRRs were manageable with infusion interruptions, and all patients recovered without repercussion.
Table 3.
Adverse events during treatmenta
| Adverse event (N = 35) | Any grade n (%) | Grade 3/4 n (%) |
|---|---|---|
| Infusion related reactionb | 14 (40) | 0 |
| Fatigue | 13 (37) | 1 (3) |
| Pyrexia | 10 (29) | 0 |
| Diarrhea | 9 (26) | 0 |
| Cough | 8 (23) | 0 |
| Insomnia | 6 (17) | 0 |
| Nausea | 6 (17) | 0 |
| Constipation | 5 (14) | 1 (3) |
| Neutropenia | 5 (14) | 5 (14) |
| Peripheral oedema | 5 (14) | 1 (3) |
| Urinary tract infection | 5 (14) | 1 (3) |
| Abdominal pain | 4 (11) | 0 |
| Decreased haemoglobin | 4 (11) | 2 (6) |
| Chills | 4 (11) | 0 |
| Rhinorrhoea | 4 (11) | 0 |
| Sinusitis | 4 (11) | 0 |
Adverse events that occurred in ≥10% of patients during treatment. Classification according to preferred term, Medical Dictionary for Regulatory Activities (MedDRA) version 18 (http://www.meddra.org/how-to-use/support-documentation). Patients who had multiple events within the same preferred‐term category were counted once in that category (at the highest grade reported).
Infusion‐related reaction includes chills, itching, dyspnea, and throat irritation.
Other AEs included fatigue, pyrexia and diarrhoea (Table 3). Laboratory abnormalities included neutropenia (14%; grade 3/4, 14%), thrombocytopenia (6%; grade 3/4, 6%) and anaemia (11%; grade 3/4, 6%). No infections were associated with grade 3/4 neutropenia, and no bleeding accompanied thrombocytopenia. All patients with grade 3/4 neutropenia received granulocyte colony‐stimulating factor (G‐CSF) and recovered without sequelae. No events of febrile neutropenia were reported. Urinary tract infection was reported in 5 patients, with only 1 grade 3/4 event. One event of hypogammaglobulinaemia was reported at the lowest dose studied (450 mg).
Serious AEs occurred in 13 patients (37%), the most frequent being pneumonia (n = 3). All other serious AEs occurred only once. No deaths were related to study treatment. One patient developed grade 3 serum sickness after 2 infusions, was hospitalised, permanently discontinued ublituximab, and recovered 15 days after the event was reported.
Efficacy
The best ORR to ublituximab among 31 patients evaluable for efficacy was 45% (Fig 2A). Forty‐five per cent of patients had stable disease (SD), and 10% experienced progressive disease (PD) at the first efficacy assessment. Five patients experienced improvement in their response during the maintenance phase as follows: three patients (one patient each with CLL, marginal zone lymphoma [MZL] and FL) improved from SD to partial response (PR); while two patients (one patient each with FL and MZL) improved their response from PR to complete response (CR). Only 15 (48%) patients progressed during the maintenance phase, supporting the potential merits of protracted dosing in this heavily treated population.
Figure 2.
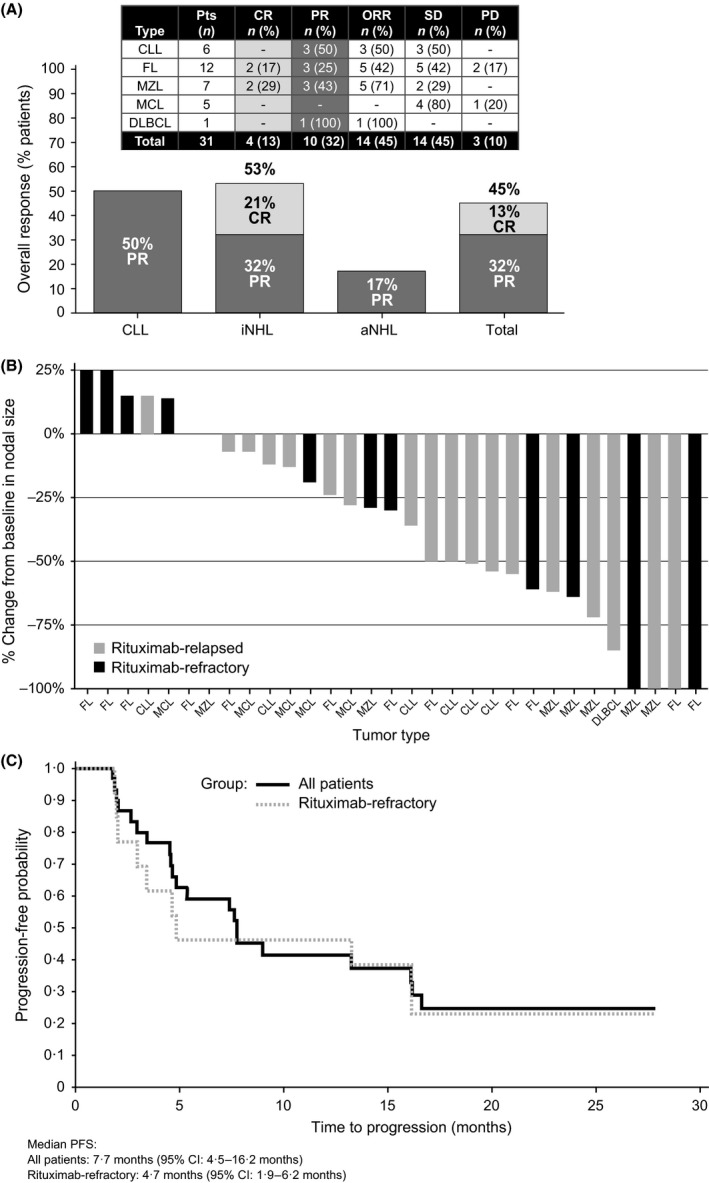
Treatment Response. (A) Overall response by lymphoma subtype. (B) Individual patient best percentage change from baseline in nodal size. (C) Progression‐free survival. aNHL, aggressive non‐Hodgkin lymphoma; CI, confidence interval; CLL, chronic lymphocytic leukaemia; CR, complete response; DLBCL, diffuse large B‐cell lymphoma; FL, follicular lymphoma; iNHL, indolent non‐Hodgkin lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; ORR, overall response rate; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
The ORR was 44% (11/25) and 50% (3/6) in patients with NHL and CLL, respectively (Fig 2A). Among the 6 evaluable CLL patients, 5 (83%) had an absolute lymphocyte count (ALC) >4·0 ×109/l at study entry (range 3·055–165·996 × 109/l). A rapid depletion in circulating lymphocytes was observed in all CLL patients, with most patients achieving a >50% reduction within 7 days of the first infusion, and all 6 patients achieved an ALC <4·0 ×109/l within the first cycle (Fig S2). Of the 13 evaluable patients who were rituximab‐refractory, 4 achieved an ORR of 31%, including 2 CRs, all in patients with indolent NHL. Change in tumour size from baseline is shown in Fig 2B.
The median time to response was 1·8 (range 1–11) months, with a median DOR of 9·2 (range 1–24) months. Median PFS for all evaluable patients was 7·7 (95% CI: 4·5–16·2) months with a median PFS for the rituximab‐refractory patients of 4·7 (95% CI: 1·9–16·2) months (Fig 2C).
Pharmacokinetic profile
Twenty patients had PK samples submitted and analysed (Table SII). Sixteen patients were evaluable for full PK analyses during C1D1, 14 during C1D22, and 10 during C5D1. Figs 3A and 3B present the free drug concentration as a function of time curves, showing a dose‐proportional relationship. There was a good relationship between dose and C max (R2 = 0·69, Fig S3A) and between dose and exposure (AUC0‐t, R2 = 0·38, Fig S3B) for C1D1 over the ublituximab dose range examined.
Figure 3.
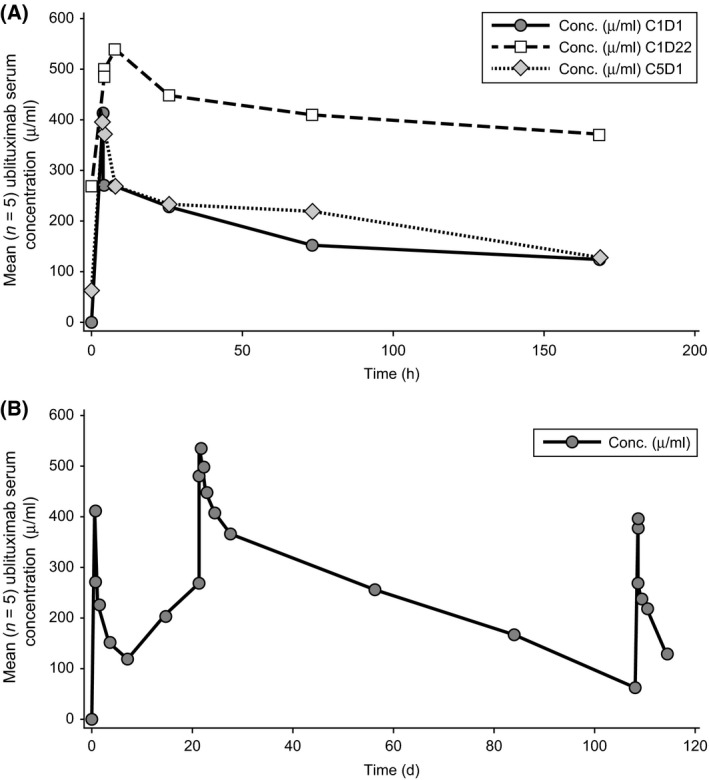
Ublituximab Serum Concentration‐Time Curves. (A) Mean serum concentration (Conc.) of ublituximab versus time immediately after dosing at different stages of treatment. (B) Mean serum concentration of ublituximab versus time over 4 months of treatment. C1D1: cycle 1, day 1; C1D22: cycle 1, day 22; C5D1: cycle 5 day 1; Conc.: concentration; h: hours
Discussion
The introduction of anti‐CD20 therapy into the treatment of B‐cell malignancies has improved clinical outcomes for patients with NHL and CLL. However, emergence of acquired resistance to rituximab is a significant clinical issue. Just as patients who become resistant to conventional chemotherapy require novel non–cross‐resistant treatment options, patients resistant to MAbs need effective biologicals with activity that can overcome previously acquired rituximab resistance.
The phase 1 trial established the safety of ublituximab on the prescribed schedules. The most common AE was grade 1/2 IRR, with no grade 3/4 IRRs. In contrast, obinutuzumab exhibited grade 3/4 IRR in 15% and 25% of CLL patients in the phase 1 and 2 trials, respectively (Cartron et al, 2014); in 5% of patients with indolent NHL; (Salles et al, 2013) and in 7·5% of patients with aggressive NHL (Morschhauser et al, 2013). Grade 3 IRRs were reported in 3% of patients receiving ofatumumab for FL (Czuczman et al, 2012). Infusion time for ublituximab, which decreased to an average of 90 min in later administrations, compares favourably to that seen with another anti‐CD20 MAb, ofatumumab (https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/arzerra.pdf). Collectively, these observations suggest a toxicity profile that is favourable or at least similar to that reported for other anti‐CD20 MAbs.
Ublituximab produced meaningful responses in heavily pre‐treated patients. The ORR was 45% among 31 evaluable patients and 31% among the 13 rituximab‐refractory patients. Because of the challenges in obtaining standard imaging prior to study enrolment, we defined the rituximab‐refractory population as being refractory to or relapsing within 6 months of rituximab, recognizing the differences between these two potential subpopulations. While it is difficult to make direct comparisons across studies, and taking into consideration the limited sample size for specific histologies in this study, the ublituximab response rates compare favourably to the ORRs of 11% and 44% seen with ofatumumab monotherapy in patients with rituximab‐refractory FL (Czuczman et al, 2012) and relapsed or refractory CLL (Coiffier et al, 2008), respectively. The responses are also comparable to ORRs of 55%, 30% and 42% seen in studies of obinutuzumab in relapsed or refractory patients with indolent NHL (Salles et al, 2013), aggressive NHL (Morschhauser et al, 2013), and CLL (Cartron et al, 2014), respectively. While the number of rituximab‐refractory patients was limited, the activity observed in these patients supports the development of novel biological agents. Enhanced anti‐CD20 MAbs that are well tolerated and active in rituximab‐resistant disease can provide meaningful clinical benefit to patients with limited treatment options.
Even considering the variability in sample size across subtypes, notable activity was observed in patients with indolent lymphoma, with 21% (4/19) of patients achieving a CR as their best response. In comparison, in the phase 2 obinutuzumab trials, CRs were achieved by 11% to 29% of patients with indolent lymphoma (Salles et al, 2013; Sehn et al, 2015). CR rates were lower with rituximab monotherapy (3–17%) (Feuring‐Buske et al, 2000; Foran et al, 2000; Cohen et al, 2003; Sehn et al, 2015). Similarly, rituximab monotherapy has shown modest activity in CLL (Huhn et al, 2001; Furman et al, 2014), which has been attributed to low CD20 antigen expression by CLL cells (Prevodnik et al, 2011). The collective experience with ublituximab supports improved ADCC and improved activity in low CD20‐expressing malignancies (De Romeuf et al, 2008; Bellon et al, 2011).
Anti‐CD20 therapy has demonstrated the greatest benefit in combination, traditionally with multi‐drug chemotherapy‐based regimens. While the introduction of novel targeted therapies has shifted the treatment paradigm of CLL and indolent lymphoma, the activity of these agents is likely to be potentiated by the addition of an anti‐CD20 MAb given their different mechanisms of action. Recently, a number of multi‐drug, non‐chemotherapy‐based regimens have emerged, which although they are in early stages, appear to offer the efficacy of standard chemotherapy without the toxicity (Furman et al, 2014; Burger et al, 2015; Jones et al, 2015).
In similar fashion, ublituximab is being evaluated for the treatment of NHL or CLL in combination with other agents, including lenalidomide (ClinicalTrials.gov NCT01744912), ibrutinib (ClinicalTrials.gov NCT02013128), and TGR‐1202 (PI3K‐inhibitor) with or without ibrutinib (ClinicalTrials.gov NCT02006485). A phase 3 study will compare treatment with ibrutinib versus ibrutinib plus ublituximab in patients with previously treated CLL who have high‐risk cytogenetic features (ClinicalTrials.gov NCT02301156).
Author contributions
DM, JGK, PS, and HPM were responsible for the conception and design of the study. Collection and assembly of data was done by AS, CMF, MTS, MYK, DM, CD, JEA, PGN, JMK, PS, HPM, and OAO. Data analysis and interpretation were performed by CMF, DM, JGK, PS, HPM, and OAO. Manuscript writing was accomplished by all authors, and all authors reviewed and approved the final manuscript.
Conflict of interest
MTS, CD, and OAO have received research funding from TG Therapeutics, Inc. (TGTX). OAO has also received funding for travel, accommodations, and expenses from TGTX, for which he has served as an unpaid consultant. PS and HPM are employees of TGTX who hold leadership roles and stock in the company. PGN has served as a consultant and received travel and accommodations expenses from Novartis and Boehringer Ingelheim (BI); he has received research funding from Novartis, BI, Genentech, TGTX, Lilly Newlink, Agendia, Abraxis, AstraZeneca, and Celgene. AS has received honoraria from Gilead Sciences. DM has been a speaker and received reimbursement for travel, accommodations, and expenses from Janssen and Alexion. JEA has received research funding from Acetylon. JMK has stock or other ownership to disclose with Helix Diagnostics. JGK has been a speaker for and received honoraria from Amgen, and has held a paid consulting or advisory role with TGTX. CMF and MYK have no conflicts of interest to declare.
Supporting information
Fig S1. Epitope binding sites on anti CD20 MAbs.
Fig S2. Change from baseline in absolute lymphocyte count (ALC) in patients with chronic lymphocytic leukemia (CLL).
Fig S3. Pharmacokinetic analysis. A. C max vs. dose. B. Exposure versus dose.
Table SI. Differences between selected anti‐CD20 monoclonal antibodies.
Table SII. Ublituximab pharmacokinetic summary.
Acknowledgements
This study was funded by TG Therapeutics, Inc. All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. The authors wish to thank Michael Chen, PhD, of TCM Group Inc. for statistical analysis. Editorial support (assembling tables and figures, collating author comments, copyediting, fact checking and referencing) and graphic services were provided by Susan Abulhawa, PhD, Nancy Price, PhD, and Elizabeth Rosenberg, PhD, of AOI Communications, L.P., and were funded by TG Therapeutics, Inc. We would also like to thank the Lymphoma Research Fund at Columbia University for partially supporting the Center for Lymphoid Malignancies at Columbia. The authors would like to thank the patients who participated in this investigation. Preliminary data from this study were presented as a poster at the 50th Annual American Society of Clinical Oncology Meeting in Chicago, IL, 30 May to 3 June 2014.
References
- Bellon, A. , Sadoun, A. , Grivel, K. , Moulard, M. , Brune, F. , Prost, J.‐F. & Salcedo, M. (2011) Comparison of cell lysis mediated by LFB‐R603 (ublituximab) with that mediated by ofatumumab against cells expressing low levels of CD20. [Abstract]. Blood, 118, 3913. [Google Scholar]
- Beum, P.V. , Kennedy, A.D. , Williams, M.E. , Lindorfer, M.A. & Taylor, R.P. (2006) The shaving reaction: rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP‐1 monocytes. The Journal of Immunology, 176, 2600–2609. [DOI] [PubMed] [Google Scholar]
- Bowles, J.A. , Wang, S.Y. , Link, B.K. , Allan, B. , Beuerlein, G. , Campbell, M.A. , Marquis, D. , Ondek, B. , Wooldridge, J.E. , Smith, B.J. , Breitmeyer, J.B. & Weiner, G.J. (2006) Anti‐CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood, 108, 2648–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger, J.A. , Tedeschi, A. , Barr, P.M. , Robak, T. , Owen, C. , Ghia, P. , Bairey, O. , Hillmen, P. , Bartlett, N.L. , Li, J. , Simpson, D. , Grosicki, S. , Devereux, S. , Mccarthy, H. , Coutre, S. , Quach, H. , Gaidano, G. , Maslyak, Z. , Stevens, D.A. , Janssens, A. , Offner, F. , Mayer, J. , O'dwyer, M. , Hellmann, A. , Schuh, A. , Siddiqi, T. , Polliack, A. , Tam, C.S. , Suri, D. , Cheng, M. , Clow, F. , Styles, L. , James, D.F. & Kipps, T.J. (2015) Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. New England Journal of Medicine, 373, 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron, G. , De Guibert, S. , Dilhuydy, M.S. , Morschhauser, F. , Leblond, V. , Dupuis, J. , Mahe, B. , Bouabdallah, R. , Lei, G. , Wenger, M. , Wassner‐Fritsch, E. & Hallek, M. (2014) Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood, 124, 2196–2202. [DOI] [PubMed] [Google Scholar]
- Cazin, B. , Lepretre, S. , Coiffier, B. , Aurran, T. , Cartron, G. , Feugier, P. , Brehar, O. , Segaud, F. , Sadoun, A. & Ribrag, V. (2011) Multicentre Phase I study with an 8‐dose regimen of single agent Anti‐CD20 monoclonal antibody LFB‐R603 in patients with relapsed chronic lymphocytic leukemia (CLL). [Abstract]. Blood, 118, 2862. [Google Scholar]
- Cazin, B. , Lepretre, S. , Coiffer, B. , Aurran, T. , Cartron, G. , Feugier, P. , Brehar, O. , Sadoun, A. , Sportelli, P. , Miskin, H. & Ribrag, V. (2013) Final results of a multicenter phase Ib single agent study with the Novel Anti‐CD20 monoclonal antibody Ublituximab (TG‐1101) in patients with relapsed chronic Lymphocytic Leukemia (CLL). presented at the Congress of the European Hematology Association, Stockholm, Sweden, June 13‐16, 2013. Haematologica, 98, 111. [Google Scholar]
- Cheson, B.D. , Pfistner, B. , Juweid, M.E. , Gascoyne, R.D. , Specht, L. , Horning, S.J. , Coiffier, B. , Fisher, R.I. , Hagenbeek, A. , Zucca, E. , Rosen, S.T. , Stroobants, S. , Lister, T.A. , Hoppe, R.T. , Dreyling, M. , Tobinai, K. , Vose, J.M. , Connors, J.M. , Federico, M. , Diehl, V. & Lymphoma, I.H.P.O. (2007) Revised response criteria for malignant lymphoma. Journal of Clinical Oncology, 25, 579–586. [DOI] [PubMed] [Google Scholar]
- Cohen, Y. , Solal‐Celigny, P. & Polliack, A. (2003) Rituximab therapy for follicular lymphoma: a comprehensive review of its efficacy as primary treatment, treatment for relapsed disease, re‐treatment and maintenance. Haematologica, 88, 811–823. [PubMed] [Google Scholar]
- Coiffier, B. , Lepretre, S. , Pedersen, L.M. , Gadeberg, O. , Fredriksen, H. , Van Oers, M.H. , Wooldridge, J. , Kloczko, J. , Holowiecki, J. , Hellmann, A. , Walewski, J. , Flensburg, M. , Petersen, J. & Robak, T. (2008) Safety and efficacy of ofatumumab, a fully human monoclonal anti‐CD20 antibody, in patients with relapsed or refractory B‐cell chronic lymphocytic leukemia: a phase 1‐2 study. Blood, 111, 1094–1100. [DOI] [PubMed] [Google Scholar]
- Czuczman, M.S. , Fayad, L. , Delwail, V. , Cartron, G. , Jacobsen, E. , Kuliczkowski, K. , Link, B.K. , Pinter‐Brown, L. , Radford, J. , Hellmann, A. , Gallop‐Evans, E. , Dirienzo, C.G. , Goldstein, N. , Gupta, I. , Jewell, R.C. , Lin, T.S. , Lisby, S. , Schultz, M. , Russell, C.A. & Hagenbeek, A. & 405 Study Investigators (2012) Ofatumumab monotherapy in rituximab‐refractory follicular lymphoma: results from a multicenter study. Blood, 119, 3698–3704. [DOI] [PubMed] [Google Scholar]
- Dalle, S. , Reslan, L. , Besseyre De Horts, T. , Herveau, S. , Herting, F. , Plesa, A. , Friess, T. , Umana, P. , Klein, C. & Dumontet, C. (2011) Preclinical studies on the mechanism of action and the anti‐lymphoma activity of the novel anti‐CD20 antibody GA101. Molecular Cancer Therapeutics, 10, 178–185. [DOI] [PubMed] [Google Scholar]
- Davis, T.A. , Grillo‐Lopez, A.J. , White, C.A. , Mclaughlin, P. , Czuczman, M.S. , Link, B.K. , Maloney, D.G. , Weaver, R.L. , Rosenberg, J. & Levy, R. (2000) Rituximab anti‐CD20 monoclonal antibody therapy in non‐Hodgkin's lymphoma: safety and efficacy of re‐treatment. Journal of Clinical Oncology, 18, 3135–3143. [DOI] [PubMed] [Google Scholar]
- De Romeuf, C. , Dutertre, C.A. , Le Garff‐Tavernier, M. , Fournier, N. , Gaucher, C. , Glacet, A. , Jorieux, S. , Bihoreau, N. , Behrens, C.K. , Beliard, R. , Vieillard, V. , Cazin, B. , Bourel, D. , Prost, J.F. , Teillaud, J.L. & Merle‐Beral, H. (2008) Chronic lymphocytic leukaemia cells are efficiently killed by an anti‐CD20 monoclonal antibody selected for improved engagement of FcgammaRIIIA/CD16. British Journal of Haematology, 140, 635–643. [DOI] [PubMed] [Google Scholar]
- Dotan, E. , Aggarwal, C. & Smith, M.R. (2010) Impact of Rituximab (Rituxan) on the treatment of B‐Cell Non‐Hodgkin's Lymphoma. P & T, 35, 148–157. [PMC free article] [PubMed] [Google Scholar]
- Farren, T.W. , Giustiniani, J. , Fanous, M. , Liu, F. , Macey, M.G. , Wright, F. , Prentice, A. , Nathwani, A. & Agrawal, S.G. (2015) Minimal residual disease detection with tumor‐specific CD160 correlates with event‐free survival in chronic lymphocytic leukemia. Blood Cancer Journal, 5, e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuring‐Buske, M. , Kneba, M. , Unterhalt, M. , Engert, A. , Gramatzki, M. , Hiller, E. , Trumper, L. , Brugger, W. , Ostermann, H. , Atzpodien, J. , Hallek, M. , Aulitzky, E. & Hiddemann, W. (2000) IDEC‐C2B8 (Rituximab) anti‐CD20 antibody treatment in relapsed advanced‐stage follicular lymphomas: results of a phase‐II study of the German Low‐Grade Lymphoma Study Group. Annals of Hematology, 79, 493–500. [DOI] [PubMed] [Google Scholar]
- Foran, J.M. , Gupta, R.K. , Cunningham, D. , Popescu, R.A. , Goldstone, A.H. , Sweetenham, J.W. , Pettengell, R. , Johnson, P.W. , Bessell, E. , Hancock, B. , Summers, K. , Hughes, J. , Rohatiner, A.Z. & Lister, T.A. (2000) A UK multicentre phase II study of rituximab (chimaeric anti‐CD20 monoclonal antibody) in patients with follicular lymphoma, with PCR monitoring of molecular response. British Journal of Haematology, 109, 81–88. [DOI] [PubMed] [Google Scholar]
- Furman, R.R. , Sharman, J.P. , Coutre, S.E. , Cheson, B.D. , Pagel, J.M. , Hillmen, P. , Barrientos, J.C. , Zelenetz, A.D. , Kipps, T.J. , Flinn, I. , Ghia, P. , Eradat, H. , Ervin, T. , Lamanna, N. , Coiffier, B. , Pettitt, A.R. , Ma, S. , Stilgenbauer, S. , Cramer, P. , Aiello, M. , Johnson, D.M. , Miller, L.L. , Li, D. , Jahn, T.M. , Dansey, R.D. , Hallek, M. & O'brien, S.M. (2014) Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. New England Journal of Medicine, 370, 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann, T.M. , Weller, E.A. , Morrison, V.A. , Gascoyne, R.D. , Cassileth, P.A. , Cohn, J.B. , Dakhil, S.R. , Woda, B. , Fisher, R.I. , Peterson, B.A. & Horning, S.J. (2006) Rituximab‐CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B‐cell lymphoma. Journal of Clinical Oncology, 24, 3121–3127. [DOI] [PubMed] [Google Scholar]
- Hallek, M. , Cheson, B.D. , Catovsky, D. , Caligaris‐Cappio, F. , Dighiero, G. , Dohner, H. , Hillmen, P. , Keating, M.J. , Montserrat, E. , Rai, K.R. & Kipps, T.J. (2008) Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute‐Working Group 1996 guidelines. Blood, 111, 5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochster, H. , Weller, E. , Gascoyne, R.D. , Habermann, T.M. , Gordon, L.I. , Ryan, T. , Zhang, L. , Colocci, N. , Frankel, S. & Horning, S.J. (2009) Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression‐free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. Journal of Clinical Oncology, 27, 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn, D. , Von Schilling, C. , Wilhelm, M. , Ho, A.D. , Hallek, M. , Kuse, R. , Knauf, W. , Riedel, U. , Hinke, A. , Srock, S. , Serke, S. , Peschel, C. & Emmerich, B. (2001) Rituximab therapy of patients with B‐cell chronic lymphocytic leukemia. Blood, 98, 1326–1331. [DOI] [PubMed] [Google Scholar]
- Jones, J.A. , Wach, M. , Robak, T. , Brown, J.R. , Menter, A.R. , Vandenberghe, E. , Ysebaert, L. , Wagner‐Johnston, N.D. , Polikoff, J. , Salman, H.S. , Taylor, K.M. , Coutre, S. , Spurgeon, S.E.F. , Kendall, S.D. , Flinn, I. , Dreiling, L. , Dubowy, R. , Cho, Y. , Peterman, S. & Owen, C. (2015) Results of a phase III randomized, controlled study evaluating the efficacy and safety of idelalisib (IDELA) in combination with ofatumumab (OFA) for previously treated chronic lymphocytic leukemia (CLL). Journal of Clinical Oncology, 33, Available at: http://meetinglibrary.asco.org/content/151048-156. (suppl abstr 7023). [Google Scholar]
- Kaplan, E.L. & Meier, P. (1958) Nonparametric estimation from incomplete observations. Journal of the American Statistical Association, 53, 457–481. [Google Scholar]
- Khouri, I.F. (2006) Reduced‐intensity regimens in allogeneic stem‐cell transplantation for non‐hodgkin lymphoma and chronic lymphocytic leukemia. American Society of Hematology Education Program, 2006, 390–397. [DOI] [PubMed] [Google Scholar]
- Konno, Y. , Kobayashi, Y. , Takahashi, K. , Takahashi, E. , Sakae, S. , Wakitani, M. , Yamano, K. , Suzawa, T. , Yano, K. , Ohta, T. , Koike, M. , Wakamatsu, K. & Hosoi, S. (2012) Fucose content of monoclonal antibodies can be controlled by culture medium osmolality for high antibody‐dependent cellular cytotoxicity. Cytotechnology, 64, 249–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Garff‐Tavernier, M. , Decocq, J. , De Romeuf, C. , Parizot, C. , Dutertre, C.A. , Chapiro, E. , Davi, F. , Debre, P. , Prost, J.F. , Teillaud, J.L. , Merle‐Beral, H. & Vieillard, V. (2011) Analysis of CD16 + CD56dim NK cells from CLL patients: evidence supporting a therapeutic strategy with optimized anti‐CD20 monoclonal antibodies. Leukemia, 25, 101–109. [DOI] [PubMed] [Google Scholar]
- Le Garff‐Tavernier, M. , Herbi, L. , De Romeuf, C. , Nguyen‐Khac, F. , Davi, F. , Grelier, A. , Boudjoghra, M. , Maloum, K. , Choquet, S. , Urbain, R. , Vieillard, V. & Merle‐Beral, H. (2014) Antibody‐dependent cellular cytotoxicity of the optimized anti‐CD20 monoclonal antibody ublituximab on chronic lymphocytic leukemia cells with the 17p deletion. Leukemia, 28, 230–233. [DOI] [PubMed] [Google Scholar]
- Le Garff‐Tavernier, M. , Herbi, L. , De Romeuf, C. , Azar, N. , Roos‐Weil, D. , Bonnemye, P. , Urbain, R. , Leblond, V. , Merle‐Beral, H. & Vieillard, V. (2015) The optimized anti‐CD20 monoclonal antibody ublituximab bypasses natural killer phenotypic features in Waldenstrom macroglobulinemia. Haematologica, 100, e147–e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschhauser, F.A. , Cartron, G. , Thieblemont, C. , Solal‐Celigny, P. , Haioun, C. , Bouabdallah, R. , Feugier, P. , Bouabdallah, K. , Asikanius, E. , Lei, G. , Wenger, M. , Wassner‐Fritsch, E. & Salles, G.A. (2013) Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large B‐cell lymphoma or mantle‐cell lymphoma: results from the phase II GAUGUIN study. Journal of Clinical Oncology, 31, 2912–2919. [DOI] [PubMed] [Google Scholar]
- Nastoupil, L.J. , Sinha, R. , Byrtek, M. , Zhou, X. , Taylor, M.D. , Friedberg, J.W. , Link, B.K. , Cerhan, J.R. , Dawson, K. & Flowers, C.R. (2014) The use and effectiveness of rituximab maintenance in patients with follicular lymphoma diagnosed between 2004 and 2007 in the United States. Cancer, 120, 1830–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oflazoglu, E. & Audoly, L.P. (2010) Evolution of anti‐CD20 monoclonal antibody therapeutics in oncology. Monoclonal Antibodies, 2, 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh, S.A. & Wierda, W.G. (2010) Role of CD20 monoclonal antibodies in previously untreated chronic lymphocytic leukemia. Clinical Lymphoma, Myeloma & Leukemia, 10, S27–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevodnik, V.K. , Lavrencak, J. , Horvat, M. & Novakovic, B.J. (2011) The predictive significance of CD20 expression in B‐cell lymphomas. Diagnostic Pathology, 6, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani, A.R. & Maloney, D.G. (2011) Rituximab resistance. Best Practice and Research. Clinical Haematology, 24, 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles, G. , Morschhauser, F. , Lamy, T. , Milpied, N. , Thieblemont, C. , Tilly, H. , Bieska, G. , Asikanius, E. , Carlile, D. , Birkett, J. , Pisa, P. & Cartron, G. (2012) Phase 1 study results of the type II glycoengineered humanized anti‐CD20 monoclonal antibody obinutuzumab (GA101) in B‐cell lymphoma patients. Blood, 119, 5126–5132. [DOI] [PubMed] [Google Scholar]
- Salles, G.A. , Morschhauser, F. , Solal‐Celigny, P. , Thieblemont, C. , Lamy, T. , Tilly, H. , Gyan, E. , Lei, G. , Wenger, M. , Wassner‐Fritsch, E. & Cartron, G. (2013) Obinutuzumab (GA101) in patients with relapsed/refractory indolent non‐Hodgkin lymphoma: results from the phase II GAUGUIN study. Journal of Clinical Oncology, 31, 2920–2926. [DOI] [PubMed] [Google Scholar]
- Sehn, L.H. , Goy, A. , Offner, F.C. , Martinelli, G. , Caballero, M.D. , Gadeberg, O. , Baetz, T. , Zelenetz, A.D. , Gaidano, G. , Fayad, L.E. , Buckstein, R. , Friedberg, J.W. , Crump, M. , Jaksic, B. , Zinzani, P.L. , Padmanabhan Iyer, S. , Sahin, D. , Chai, A. , Fingerle‐Rowson, G. & Press, O.W. (2015) Randomized Phase II trial comparing Obinutuzumab (GA101) with Rituximab in patients with relapsed CD20 + indolent B‐cell non‐Hodgkin lymphoma: final analysis of the GAUSS Study. Journal of Clinical Oncology, 33, 3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, V. , Gupta, D. , Arora, R. , Tripathi, R.P. , Almasan, A. & Macklis, R.M. (2014) Surface levels of CD20 determine anti‐CD20 antibodies mediated cell death in vitro. PLoS ONE, 9, e111113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourais Estaves, I. , Dumontet, C. , Herveau, S. , Reslan, L. , Brune, F. , Van Overtvelt, L. , Salcedo, M. & Fournes, B. (2011) LFB‐R603 (ublituximab), a third‐generation monoclonal anti‐CD20 antibody, displays additive antitumor activity with antileukemic chemotherapeutic agents in mouse xenograft models. [Abstract]. Blood, 118, 1660. [Google Scholar]
- Van Oers, M.H. , Klasa, R. , Marcus, R.E. , Wolf, M. , Kimby, E. , Gascoyne, R.D. , Jack, A. , Van't Veer, M. , Vranovsky, A. , Holte, H. , Van Glabbeke, M. , Teodorovic, I. , Rozewicz, C. & Hagenbeek, A. (2006) Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non‐Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood, 108, 3295–3301. [DOI] [PubMed] [Google Scholar]
- Vaughan, A.T. , Iriyama, C. , Beers, S.A. , Chan, C.H. , Lim, S.H. , Williams, E.L. , Shah, V. , Roghanian, A. , Frendeus, B. , Glennie, M.J. & Cragg, M.S. (2014) Inhibitory FcgammaRIIb (CD32b) becomes activated by therapeutic mAb in both cis and trans and drives internalization according to antibody specificity. Blood, 123, 669–677. [DOI] [PubMed] [Google Scholar]
- Weiner, L.M. , Surana, R. & Wang, S. (2010) Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Reviews Immunology, 10, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda, W.G. , Kipps, T.J. , Mayer, J. , Stilgenbauer, S. , Williams, C.D. , Hellmann, A. , Robak, T. , Furman, R.R. , Hillmen, P. , Trneny, M. , Dyer, M.J. , Padmanabhan, S. , Piotrowska, M. , Kozak, T. , Chan, G. , Davis, R. , Losic, N. , Wilms, J. , Russell, C.A. & Osterborg, A. (2010) Ofatumumab as single‐agent CD20 immunotherapy in fludarabine‐refractory chronic lymphocytic leukemia. Journal of Clinical Oncology, 28, 1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winiarska, M. , Glodkowska‐Mrowka, E. , Bil, J. & Golab, J. (2011) Molecular mechanisms of the antitumor effects of anti‐CD20 antibodies. Frontiers in Bioscience (Landmark Ed), 16, 277–306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Epitope binding sites on anti CD20 MAbs.
Fig S2. Change from baseline in absolute lymphocyte count (ALC) in patients with chronic lymphocytic leukemia (CLL).
Fig S3. Pharmacokinetic analysis. A. C max vs. dose. B. Exposure versus dose.
Table SI. Differences between selected anti‐CD20 monoclonal antibodies.
Table SII. Ublituximab pharmacokinetic summary.


