Abstract
Background
Spinal cord stimulation (SCS) has been shown to provide pain relief in painful diabetic polyneuropathy (PDPN). As the vasculature system plays a great role in the pathophysiology of PDPN, a potential beneficial side‐effect of SCS is peripheral vasodilation, with high frequency (HF) SCS in particular. We hypothesize that HF‐SCS (500 Hz), compared with conventional (CON) or low frequency (LF)‐SCS will result in increased alleviation of mechanical hypersensitivity in chronic experimental PDPN.
Methods
Diabetes was induced in 8‐week‐old female Sprague–Dawley rats with an intraperitoneal injection of 65 mg/kg of streptozotocin (n = 44). Rats with a significant decrease in mechanical withdrawal response to von Frey filaments over a period of 20 weeks were implanted with SCS electrodes (n = 18). Rats were assigned to a cross‐over design with a random order of LF‐, CON‐, HF‐ and sham SCS and mechanical withdrawal thresholds were assessed with von Frey testing.
Results
Compared with sham treatment, the average 50% WT score for 5 Hz was 4.88 g higher during stimulation (p = 0.156), and 1.77 g higher post‐stimulation (p = 0.008). CON‐SCS resulted in 50% WT scores 5.7 g, and 2.51 g higher during (p = 0.064) and after stimulation (p < 0.004), respectively. HF‐SCS started out with an average difference in 50% WT score compared with sham of 1.87 g during stimulation (p = 0.279), and subsequently the steepest rise to a difference of 5.47 g post‐stimulation (p < 0.001).
Conclusions
We demonstrated a delayed effect of HF‐SCS on mechanical hypersensitivity in chronic PDPN animals compared with LF‐, or CON‐SCS.
Significance
This study evaluates the effect of SCS frequency (5–500 Hz) on mechanical hypersensitivity in the chronic phase of experimental PDPN. High frequency (500 Hz) – SCS resulted in a delayed effect‐ on pain‐related behavioural outcome in chronic PDPN.
1. Introduction
Spinal cord stimulation (SCS) is a last resort treatment modality for patients suffering from persistent painful symptoms in neuropathic pain syndromes (Kemler et al., 2000; Kumar et al., 2007). Recently, the efficacy of conventional SCS (CON‐SCS) (30–80 Hz, 200–500 μs) has also been confirmed in patients with painful diabetic polyneuropathy (PDPN) in two ongoing randomized clinical trials (Slangen et al., 2014; de Vos et al., 2014; van Beek et al., 2015) and in the acute phase of experimental PDPN (Pluijms et al., 2013). Based on the complex pathophysiology of PDPN, including hyperglycaemia, vascular and neurotrophic‐support‐related mechanisms (Dobretsov et al., 2003; Eaton et al., 2003; Romanovsky et al., 2006), we hypothesize that further optimization of the SCS stimulation parameters might result in identification of additional beneficial effects in chronic diabetic animals.
Abundant literature suggests that optimization of the stimulation parameters of SCS in PDPN, and SCS frequency in particular, could focus on modulation of the vascular system and the release of neurotrophic factors. Cerebral spinal fluid (CSF) levels of various neurotrophic factors correlate with reported pain and SCS frequency that provides optimal pain relief in FBSS patients (McCarthy et al., 2013; McCarthy and McCrory, 2014). Furthermore, CON‐SCS has been shown to induce peripheral vasodilation in naive as well as diabetic rats (Wu et al., 2007). Increasing the pulse amplitude, thus increasing the total electrical charge, resulted in a further increased peripheral blood flow only in naive rats. High‐frequency SCS (HF‐SCS) (500 Hz) has been reported to increase vasodilation via antidromic stimulation of unmyelinated C‐fibre and release of calcitonin‐gene‐related peptide (CGRP) (Tanaka et al., 2003, 2004; Gao et al., 2010). Moreover, electrical stimulation of the sciatic nerve at 200 Hz has been shown to result in more vasodilation and nerve regeneration compared with lower frequencies (0, 2, 20 Hz) in diabetic rats with a sciatic nerve gap (Kao et al., 2013). These results indicate that higher frequencies could benefit SCS treatment in PDPN. Therefore, we recently investigated whether CON‐, HF‐ or low frequency (LF) (5 Hz) SCS alleviates mechanical hypersensitivity in an experimental model of acute PDPN (Pluijms et al., 2013). From this experimental study, it was concluded that CON‐, LF‐ and HF‐SCS all provided similar alleviation of mechanical hypersensitivity in the acute phase of experimental diabetes.
As PDPN follows a progressive course, vascular deterioration and nerve degeneration become increasingly important in a chronic stage of PDPN (Partanen et al., 1995; Al‐Delaimy et al., 2004; Litwak et al., 2013). In line with clinical observations, experimental work has demonstrated that pathophysiological changes in streptozotocin‐induced diabetic rats are progressive as well (Wei et al., 2003; Hassan et al., 2011). As SCS mediated vasodilation is CGRP receptor dependent, the potential benefit from improving blood flow might become apparent when receptor‐dependent vascular reactivity is affected over a longer period of time. Because HF‐SCS has been shown to increase vasodilation we hypothesize that HF‐SCS (500 Hz), compared with CON‐ or LF‐SCS will result in increased alleviation of mechanical hypersensitivity in a chronic stage (6 months) of experimental PDPN. As repetitive SCS has been shown to result in a cumulative pain relieving effect of SCS in a rat model of traumatic nerve injury, we further hypothesized that repetitive SCS, and in particular HF‐SCS, results in a cumulative alleviation of mechanical hypersensitivity in an experimental model of chronic PDPN (Maeda et al., 2008).
2. Methods
2.1. Animals
All experiments were performed using female Sprague–Dawley rats (n = 49), which were 8 weeks of age at the start of the experiment (180–220 g). Animals were housed on a reversed day night rhythm in transparent plastic cages with access to food and water ad libitum. The experiments were approved by the Animal Research Committee of the Maastricht University Medical Centre.
2.2. Induction of diabetes mellitus
Diabetes mellitus (DM) was induced with a single intraperitoneal (i.p.) injection of 65 mg/kg of streptozotocin (STZ) (Sigma‐Aldrich, Schnelldorf, Germany) (n = 44). A separate group of rats were injected with saline to serve as a control group over time for weight, glucose and mechanical hypersensitivity (n = 5). Before STZ injection, rats were weighed and fasted overnight. STZ was freshly dissolved in sterile 0.9% NaCl to a solution of 65 mg/mL. Four days after STZ injection, blood glucose level was determined in blood derived from the saphenous vein using a standard blood glucose meter (Accu‐Chek Aviva®, Roche Diagnostics GmbH, Mannheim, Germany). Rats with a glucose level of ≥15 mmol/L were considered diabetic (Calcutt, 2004) and were included in the study. Throughout the study additional blood glucose measurements were regularly performed to confirm the status of DM. When glucose levels exceeded 30 mmol/L, we placed a third of a slow releasing insulin pellet (LinShin Canada, Inc.) subcutaneously in the trunk.
2.3. Behavioural testing
Before the start of behavioural testing, rats were placed in a transparent box on an elevated mesh floor and were given 15 min to acclimate to the surroundings. Mechanical allodynia was assessed according to the ‘up‐down method’ (Chaplan et al., 1994). In short, von Frey filaments with incrementing stiffness (bending forces 1.2, 2.0, 3.6, 5.5, 8.5, 15.1 and 28.84 g) were applied to the hind paws of the rats for 5 s. If the hind paw was not withdrawn (=negative response), the next filament with higher bending force was applied, whereas the previous filament with lower bending force was applied if the hind paw was withdrawn (positive response). The 50% withdrawal threshold (50% WT) was calculated after completion of a sequence of six consecutive responses. A cut‐off value of 28.84 g was defined. Calculated 50% WTs were logarithmically transformed to a linear scale for statistical analysis. The experimenter was blinded to SCS settings during the behavioural analysis.
2.4. Development of mechanical hypersensitivity (‘allodynia’)
The mechanical withdrawal threshold was assessed before induction of DM to assess baseline mechanical sensitivity, throughout the study to determine the development of PDPN and before, during and after every SCS session. The maximum duration of the experiment was set to 24 weeks as STZ induced diabetic rats then started to develop cataracts. Only animals showing mechanical allodynia at 20 weeks were treated with SCS, whereas animals without mechanical allodynia were excluded from further study. The presence of mechanical allodynia was defined as a decrease ≥0.2 unit in log (50% WT) as compared with baseline.
2.5. Implantation of spinal cord stimulation electrode
The implantation of the SCS electrode was performed according to the standard protocol used in our institution, which was based on methods and techniques originally developed at the Karolinska Institutet (Stockholm, Sweden) and adapted to the use of a quadripolar lead (Medtronic, Minneapolis, MN, USA). In short, under general anaesthesia, a small laminectomy was made at level T13 and the electrode was inserted in the epidural space in rostral direction. The position of the electrode was adjusted so that the contacts were situated at the T10–12 spinal levels. The lead was fixed with sutures to the muscle and the wound was sutured in layers. The proximal end of the lead was tunnelled subcutaneously to exit through the neck skin.
2.6. Spinal cord stimulation
The leads exiting the neck skin were connected to an external neurostimulator (MultiStim model 3800, A‐M Systems, Sequim, WA, USA) fitted with a stimulus isolator (model 3820, A‐M Systems) The 1st and 3rd of 4 contacts from rostral were set as an anode, the 2nd and 4th were set as a cathode. The motor threshold (MT) was defined as the lowest current inducing symmetrical contractions of the lower trunk or hindlimbs and was determined at a frequency of 2 Hz and pulse width of 200 μs before each stimulation session. The current was then adjusted to 67% of the MT. Rats were randomly assigned to a cross‐over design of SCS sessions with 5‐, 50‐ or 500 Hz‐ or sham stimulation. A session consisted of four consecutive days with 40 min of SCS treatment per day The mechanical withdrawal threshold was assessed before the start of every SCS treatment and at 15‐, 30‐, 60‐ and 90 min after the start of SCS treatment. Hence, the measurements after 15‐ and 30 min of SCS were performed with the pulse generator switched on (apart from the sham SCS group) (Fig. 1). A responder to SCS treatment was defined as a rat showing an increase ≥0.2 units in log (50% WT). A minimum rest period of 3 days was maintained between sessions to serve as a wash out period.
Figure 1.
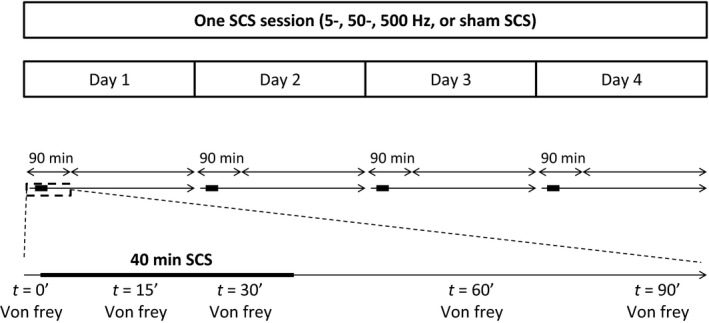
Schematic of one spinal cord stimulation (SCS) session. A session consisted of four consecutive days with 40 min of SCS treatment per day. The mechanical withdrawal threshold was assessed by means of Von Frey testing before the start, and 15‐, 30‐, 60‐ and 90 min after the start of SCS treatment on day 1, 2, 3 and 4. After a session with 5‐, 50‐, 500 Hz or sham SCS was successfully completed, a rest period of 3 days was maintained without any experiments before the next session was started.
2.7. Statistical analysis
Analyses of bodyweights, blood glucose levels and development of mechanical allodynia were performed by means of paired sampled T‐tests.
For the analysis of treatment outcomes, a mixed‐effects model was used to analyse mechanical hypersensitivity, adjusting for baseline mechanical hypersensitivity and treatment order as fixed effects and treating rats as random effects to account for the fact that each rat was measured multiple times and was exposed to multiple SCS frequencies. This approach guarantees that all rats are included in the multivariate analysis despite that some were not exposed to all frequencies. Data were logarithmically transformed to obtain a linear scale and to account for Weber's Law (Mills et al., 2012). First, we compared mechanical hypersensitivity across the different used frequencies during and after stimulation over all 4 stimulation days. Next, we assessed cumulative alleviation of continued treatment for every tested frequency by assessing treatment slopes over the 4‐day stimulation period.
All analyses were performed using SPSS (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY, USA). p‐Values ≤ 0.05 were considered to indicate statistical significance.
3. Results
3.1. Flow chart of animals
Injection of STZ (n = 44) resulted in DM in 39 rats. Normoglycaemic rats (n = 5) were added to the control group during the PDNP development phase of the study (first 20 weeks). Out of the group of diabetic rats, three died during the study as a result from STZ‐related health deterioration. Rats with DM had a significantly lower increase in bodyweight throughout the study and sustained higher blood glucose levels as compared with non‐diabetic rats (non‐diabetic rats showed a bodyweight increase from 215 to 303 g, while the bodyweight of diabetic rats increased only from 215 to 261 g (p < 0.0001) (Fig. 2). Average blood glucose levels increased significantly in diabetic rats as compared with non‐diabetic rats in the first week and sustained elevated throughout the study (non‐diabetic rats; 6–6.9 mmol/L, diabetic rats; 27–30.3 mmol/L, p < 0.001) (Fig. 3). Besides one rat with stable DM, all rats required insulin treatment during the study. Eighteen diabetic rats with a ≥ 0.2 unit decrease in log (50% WT) were selected for implantation of SCS electrodes. The mean MT increased until the third SCS session (session 1: 0.11 mA (SD 0.05); session 2: 0.16 mA (SD 0.05); session 3: 0.23 mA (SD 0.04); session 4: 0.22 mA (SD 0.09)).
Figure 2.
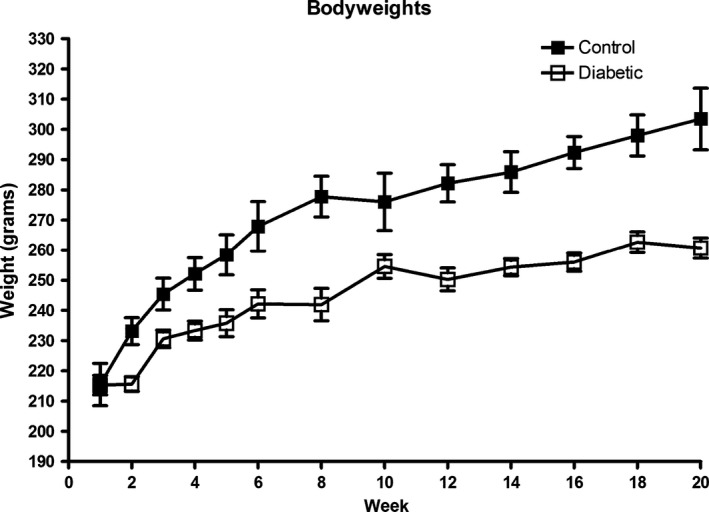
Bodyweight during the study (Mean ± SEM).
Figure 3.
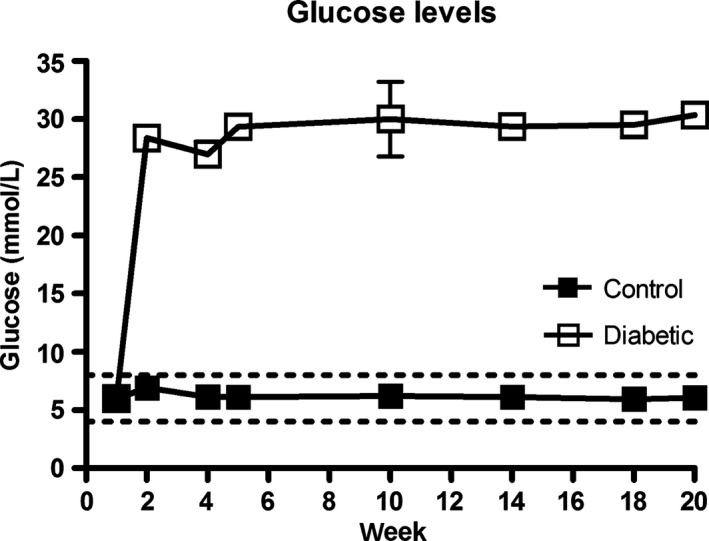
Blood glucose levels throughout the study (Mean ± SEM). The area between the dotted lines represents a normal physiological range.
3.2. Complications
Completion of the full stimulation paradigm (4 weeks) was limited due to damaging of the external part of the electrodes and, in some few cases, development of paraplegia due to compression of the spinal cord (as confirmed by post mortem examination). Data from paraplegic animals were excluded from analysis (n = 3). In total, 15 rats were included to undergo the 4‐week SCS paradigm. As not all rats underwent the full stimulation paradigm, the final analysis is based on 8, 7, 10 and 5 successfully completed SCS sessions with 5 Hz‐, 50 Hz‐, 500 Hz‐ and sham SCS, respectively.
3.3. Development of mechanical hypersensitivity (‘allodynia’)
Diabetic rats (n = 39) showed a significant decrease in average 50% WT from 18.2 g at baseline to 4.9 g at the time of implantation (p < 0.0001). There was no significant difference in 50% WT between baseline and time of implantation in non‐diabetic rats (p = 0.503) (Fig. 4.)
Figure 4.
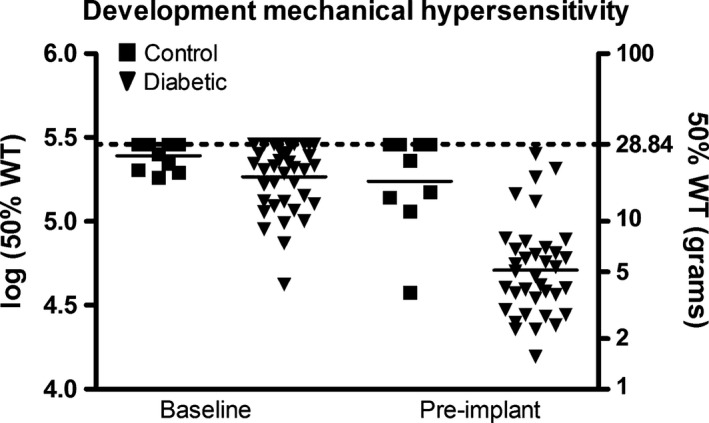
Development of mechanical hypersensitivity in diabetic and control animals. Baseline measurements and pre‐implantation measurements after 20 weeks are shown.
3.4. Effect of SCS on mechanical hypersensitivity (‘allodynia’)
The average baseline 50% WT score (i.e. before the first allocated treatment started) was 3.9 g (95% CI 1.95, 7.76). Moreover, there was no difference in baseline 50% WT scores between the first days of every SCS session (p = 0.238).
We observed only marginal differences in 50% WT scores between allocated treatments (overall p‐value = 0.063) during stimulation (average of 15′ and 30′ measurements) and post‐stimulation (average of 60′ and 90′ measurements). Compared with sham treatment, the average 50% WT score for 5 Hz was 4.88 g higher during stimulation (p = 0.156), and 1.77 g higher post‐stimulation (p = 0.008). For 50 Hz, the average 50% WT score was 5.7 g higher during stimulation (p = 0.064), and 2.51 g higher after stimulation (p < 0.004). Treatment with 500 Hz SCS started out with the lowest contrast to sham treatment. We observed an average difference in 50% WT score compared with sham of 1.87 g during stimulation (p = 0.279), and subsequently the steepest rise to a difference of 5.47 g post‐stimulation (p < 0.001) (Fig. 5 and Table 1).
Figure 5.
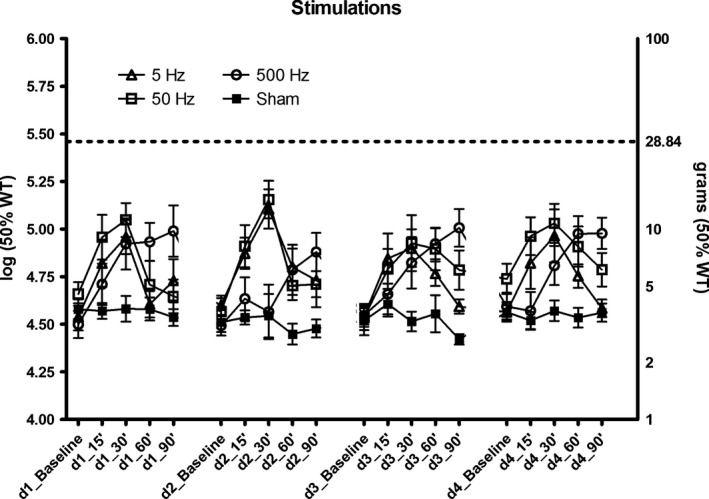
Four spinal cord stimulation (SCS) sessions plotted in one graph. One session consisted of four consecutive days with 40 min of SCS treatment per day with 5, 50, 500 Hz or sham stimulation. The mechanical withdrawal threshold was assessed before the start of SCS treatment and at 15‐, 30‐, 60‐ and 90 min after the start of SCS treatment.
Table 1.
Average 50% WT scores (grams) for all treatments, during (15′ and 30′) and post‐stimulation (60′ and 90′)
| During stimulation scorea (95% CI) | After stimulation scorea (95% CI) | |
|---|---|---|
| 500 Hz | 5.50 (3.09, 9.77) | 8.71 (6.03, 12.59) |
| 50 Hz | 9.33 (6.31, 13.80) | 5.75 (4.17, 7.94) |
| 5 Hz | 8.51 (6.17, 11.75) | 5.01 (4.07, 6.17) |
| Sham | 3.63 (3.09, 4.27) | 3.24 (2.75, 3.80) |
Mean values are corrected for differences in baseline 50% WT scores (grams).
3.4.1. Cumulative alleviation over the 4‐day SCS period
There was no evidence of a cumulative effect of treatment over the 4‐day period. The overall slope of time, as measured in days, was −0.004 (p = 0.688), indicating an extremely small, non‐significant decline of 50% WT score over the 4 days. This conclusion was not altered after including an interaction term of time in days and treatment (p = 0.608).
3.5. Responders and non‐responders to SCS
The average percentage of responders to treatment was 60% (five out of eight) during LF‐SCS, and decreased to 36% (three out of eight) post LF‐SCS. During CON‐SCS, the average percentage of responders to treatment was 65% (five out of seven) and decreased to 33% (two out of seven) post CON‐SCS. The average percentage of responders to treatment was 36% (four out of ten) during HF‐SCS, and increased to 70% (seven out of ten) post HF‐SCS. Average percentages of responders during and post sham SCS of 13% and 5%, respectively, were observed (Table 2). Out of five rats that completed at least all three sessions besides sham‐SCS, one rat showed no response to HF‐SCS at any given time point.
Table 2.
Numbers of rats responding to SCS treatment with a log (50% WT) increase ≥0.2 (%)
| 15′ | 30′ | 60′ | 90′ | ||
|---|---|---|---|---|---|
| 5 Hz | Day 1 | 5/8 (63) | 7/8 (88) | 2/8 (25) | 3/8 (38) |
| Day 2 | 4/8 (50) | 5/8 (63) | 4/8 (50) | 3/8 (38) | |
| Day 3 | 4/8 (50) | 6/8 (75) | 4/8 (50) | 2/8 (25) | |
| Day 4 | 3/8 (38) | 4/8 (50) | 4/8 (50) | 1/8 (13) | |
| 50 Hz | Day 1 | 4/7 (57) | 6/7 (86) | 3/7 (43) | 1/7 (14) |
| Day 2 | 4/6 (67) | 4/6 (67) | 1/6 (17) | 1/6 (17) | |
| Day 3 | 3/7 (43) | 4/7 (57) | 4/7 (57) | 2/7 (29) | |
| Day 4 | 5/7 (71) | 5/7 (71) | 3/7 (43) | 3/7 (43) | |
| 500 Hz | Day 1 | 4/10 (40) | 5/10 (50) | 6/10 (60) | 7/10 (70) |
| Day 2 | 3/10 (30) | 3/10 (30) | 6/10 (60) | 7/10 (70) | |
| Day 3 | 3/10 (30) | 5/10 (50) | 8/10 (80) | 7/10 (70) | |
| Day 4 | 2/10 (20) | 4/10 (40) | 7/10 (70) | 8/10 (80) | |
| Sham | Day 1 | 0/5 (0) | 1/5 (20) | 1/5 (20) | 0/5 (0) |
| Day 2 | 0/5 (0) | 1/5 (20) | 0/5 (0) | 0/5 (0) | |
| Day 3 | 1/5 (20) | 1/5 (20) | 1/5 (20) | 0/5 (0) | |
| Day 4 | 0/5 (0) | 1/5 (20) | 0/5 (0) | 0/5 (0) |
4. Discussion
The aim of this study was to evaluate the effect of SCS frequency (5–50–500 Hz) on mechanical hypersensitivity in the chronic phase of experimental PDPN. In addition, repetitive SCS was performed to evaluate a potential cumulative effect of SCS on mechanical allodynia. Data from this study demonstrate that SCS results in alleviation of mechanical hypersensitivity. However, the decrease in mechanical hypersensitivity induced by HF‐SCS (500 Hz) in the chronic phase of experimental PDPN follows a distinct and delayed time course when compared with LF‐ (5 Hz) or CON‐SCS (50 Hz). HF‐SCS resulted in increased WTs predominantly after cessation of SCS, whereas WTs returned towards pre‐SCS values after cessation of LF‐, and CON‐SCS. These findings are in contrast to our previous work where we demonstrated that alleviation of mechanical allodynia during HF‐SCS in the acute phase of experimental PDPN followed a similar time course as compared with LF‐, and CON‐SCS (Pluijms et al., 2013). It should be noted that the frequency range used in the acute PDPN study was smaller (4–375 Hz) compared with the frequency range used in this study (5–500 Hz) as in the former study rats showed signs of discomfort at higher frequencies. Since the same animal model is used in both experiments, signs of discomfort in the acute PDPN study are possibly explained by the fact that monophasic pulses were applied to the spinal cord through a monopolar electrode. In this study, biphasic pulses were applied through a quadripolar paddle lead. The total pulse width was 200 μs in both studies. Recently, Song and colleagues reported that 1 kHz SCS with mono‐ or biphasic pulse shape, with equal total pulse width (24 μs), results in alleviation of mechanical hypersensitivity only with monophasic stimulation in an animal model of neuropathic pain (Song et al., 2015). The authors argue that this could be the result of more cathodal current being delivered to the nervous tissue when applying monophasic pulses. Possibly, the use of monophasic HF‐SCS with a pulse width of 200 μs led to unpleasant paraesthesia in our former study. This might explain why no behavioural abnormalities were observed in this study when applying biphasic HF‐SCS with a pulse width of 200 μs. Nonetheless, such a delayed time course of increasing WTs after cessation of HF‐SCS has not been described before, regardless of the type of the electrode used, the generated pulse shape, pulse width and the frequency of stimulation. Therefore, a pathophysiological explanation for the delayed effectiveness of HF‐SCS in an experimental model of chronic PDNP seems more plausible. CON‐SCS has been shown to increase peripheral blood perfusion in the acute phase of streptozotocin induced diabetic rats (Wu et al., 2007; Gao et al., 2010). Peripheral blood perfusion, in addition to structural micro‐ and macrovascular abnormalities, is impaired especially in the chronic diabetic condition (Orchard et al., 1990; Tesfamariam et al., 1991; Creager et al., 2003; Wei et al., 2003; Cade, 2008; Hassan et al., 2011). A small prospective open‐label study in PDPN patients failed to demonstrate a significant effect of CON‐SCS on microcirculatory function, whereas these patients did experience pain relief (de Vos et al., 2009). On the other hand, several studies do suggest a correlation between increased blood perfusion and alleviation of clinical symptoms in conditions with peripheral artery disease (Petrakis and Sciacca, 1999, 2000b; Ubbink et al., 1999; Naoum and Arbid, 2013). As HF‐SCS has been demonstrated to result in increased vasodilatory responses compared with CON‐SCS, HF‐SCS in particular could improve SCS outcome in chronic PDPN (Gao et al., 2010). In this study we could demonstrate a delayed, albeit not a superior effect of HF‐SCS on mechanical hypersensitivity compared with LF‐, or CON‐SCS. Unfortunately, characterization of the wash out effect of HF‐SCS on mechanical hypersensitivity was not possible. Therefore, we could not calculate the area under the curve and compare the total effect size of the different frequencies. On the basis of the present results, we conclude that HF‐SCS results in delayed alleviation of mechanical hypersensitivity in an experimental model of chronic PDPN compared with LF‐, and CON‐SCS.
It is well possible that LF‐ and CON‐SCS provide optimal pain relief via a different mechanism compared with HF‐SCS. Maeda and colleagues have demonstrated that LF‐ and CON‐SCS were superior to SCS frequencies up to 250 Hz in a rat model of traumatic nerve injury (Maeda et al., 2008, 2009). The more vascular driven aetiology of PDPN, in contrast to traumatic nerve injury, could be responsible for the presence of an effect of HF‐SCS in a rat model of chronic PDPN. Streptozotocin‐induced PDPN is accompanied by severe DM pathology, including cataract which confirms the heavy impairment of the vascular system (Wei et al., 2003). Possibly, pain relief via spinal neuromodulation is not the primary mode of action of HF‐SCS. Instead, pain relief could predominantly result secondary to an effect of SCS on ischaemia which might explain why alleviation of mechanical hypersensitivity starts later as compared with LF‐ and CON‐SCS in an experimental model of chronic PDPN. Indeed, computer modelling suggests that SCS frequency partly determines the outcome of WDR neurons projecting to the brain. Zhang and colleagues showed that frequencies above 100 Hz were unable to inhibit WDR signalling, suggesting that these frequencies lead to pain relief via alternative mechanisms (Zhang et al., 2014). Further substantiating evidence that alternative working mechanisms are applicable to SCS in PDPN is the absence of a cumulative effect after 4 days of CON‐ and LF‐SCS, which was observed in an experimental model of traumatic nerve injury (Maeda et al., 2008). Further research on the accumulation of alleviation of painful symptoms with repetitive treatment with LF‐, and CON‐SCS could provide more insight into the differences in working mechanisms of SCS in pain syndromes from different origins.
Currently there is still no evidence for a causal relationship between vasodilation and the alleviation of painful symptoms in PDPN. It is well possible, and most probable, that SCS alleviates pain via spinal neuromodulation and induces vasodilation via an independent mechanism. Indeed, not all patients who experience pain relief with SCS show a vasodilatory response (de Vos et al., 2009). Furthermore, it was suggested long ago that diabetic patients with a significant increase in tcpO2 that is associated with a clinical improvement during the test period, and that not merely patients with pain relief alone, should be considered for permanent SCS device implantation (Petrakis and Sciacca, 2000a).
In this study we evaluated the effect of SCS frequency on behavioural outcome in an experimental model of chronic PDPN. In conclusion, we demonstrated a delayed, albeit not a superior effect of HF‐SCS on mechanical hypersensitivity as compared with LF‐, or CON‐SCS. Furthermore, cumulative effects after repetitive treatments with different frequencies (5–500 Hz) were absent in an experimental model of chronic PDPN. Future studies should combine in vivo macro‐ and microvasculature assessments and behavioural outcome parameters after long‐term HF‐SCS in chronic PDPN to validate behavioural findings and acquire more insight in the working mechanism behind HF‐SCS. Stimulation paradigms with a short duration and the need for anaesthetics make it difficult to link changes in blood perfusion of the hind paws to behavioural outcomes. Long‐term stimulation might provide opportunities to correlate behavioural outcome and blood perfusion at multiple time points, which could lead to more robust conclusions. Although requiring anaesthetics, Laser Doppler Imaging and Flowmetry are non‐invasive tools that could provide aid in determining total cutaneous blood perfusion and local responsiveness of the vascular bed to SCS in experimental PDPN, respectively (Wu et al., 2007; Rajan et al., 2009). Moreover, blocking vasodilatory mechanisms such as CGRP signalling, when applying HF‐SCS, could provide insight in the causal relationship between vasodilation and behavioural outcome.
The effect of HF‐SCS on neurotransmitter release in PDPN should not be overlooked (Wu et al., 2008). Indeed, several pharmacological treatments that intervene with neurotransmission provide pain relief in experimental PDPN, and a number of them are clinically established (Calcutt and Chaplan, 1997; Calcutt et al., 2004; Jensen et al., 2006; Jolivalt et al., 2008; Schiene et al., 2015). Post mortem analysis of peripheral and central neurotransmitter levels at different time points during or after SCS, and in particular HF‐SCS, could provide clues on the involvement of specific neurotransmitters in the delayed time course of pain relief after HF‐SCS.
Author contributions
M.v.B. oversaw the overall execution of the project, performed the experiments, contributed to the experimental design and interpretation of the results, analysed the results and wrote the manuscript. M.v.K. and B.L. discussed and contributed to the interpretation of the results and commented on the manuscript. S.M.J.v.K helped with analysing and interpreting the results. W.M.H. participated in the execution of the experiments. E.A.J. conceived and designed the experiments, contributed to the interpretation of the results and helped writing the manuscript.
Funding sources
This research was partially funded by a personal research grant from Medtronic to Prof. Dr. E.A. Joosten (NM2782). B. Linderoth serves as a consultant for Medtronic, St Jude Medical, Boston Sci and Elekta AB.
Conflicts of interest
None declared.
References
- Al‐Delaimy, W.K. , Merchant, A.T. , Rimm, E.B. , Willett, W.C. , Stampfer, M.J. , Hu, F.B. (2004). Effect of type 2 diabetes and its duration on the risk of peripheral arterial disease among men. Am J Med 116, 236–240. [DOI] [PubMed] [Google Scholar]
- van Beek, M. , Slangen, R. , Schaper, N.C. , Faber, C.G. , Joosten, E.A. , Dirksen, C.D. , van Dongen, R.T. , Kessels, A.G.H. , van Kleef, M. (2015). Sustained treatment effect of spinal cord stimulation in painful diabetic peripheral neuropathy: 24‐month follow‐up of a prospective two‐center randomized controlled trial. Diabetes Care 38, e132–e134. [DOI] [PubMed] [Google Scholar]
- Cade, W.T. (2008). Diabetes‐related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther 88, 1322–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcutt, N.A. (2004). Modeling diabetic sensory neuropathy in rats. Methods Mol Med 99, 55–65. [DOI] [PubMed] [Google Scholar]
- Calcutt, N.A. , Chaplan, S.R. (1997). Spinal pharmacology of tactile allodynia in diabetic rats. Br J Pharmacol 122, 1478–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcutt, N.A. , Freshwater, J.D. , Mizisin, A.P. (2004). Prevention of sensory disorders in diabetic Sprague‐Dawley rats by aldose reductase inhibition or treatment with ciliary neurotrophic factor. Diabetologia 47, 718–724. [DOI] [PubMed] [Google Scholar]
- Chaplan, S.R. , Bach, F.W. , Pogrel, J.W. , Chung, J.M. , Yaksh, T.L. (1994). Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53, 55–63. [DOI] [PubMed] [Google Scholar]
- Creager, M.A. , Lüscher, T.F. , Cosentino, F. , Beckman, J.A. (2003). Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 108, 1527–1532. [DOI] [PubMed] [Google Scholar]
- Dobretsov, M. , Hastings, S.L. , Romanovsky, D. , Stimers, J.R. , Zhang, J.M. (2003). Mechanical hyperalgesia in rat models of systemic and local hyperglycemia. Brain Res 960, 174–183. [DOI] [PubMed] [Google Scholar]
- Eaton, S.E. , Harris, N.D. , Ibrahim, S. , Patel, K.A. , Selmi, F. , Radatz, M. , Ward, J.D. , Tesfaye, S. (2003). Increased sural nerve epineurial blood flow in human subjects with painful diabetic neuropathy. Diabetologia 46, 934–939. [DOI] [PubMed] [Google Scholar]
- Gao, J. , Wu, M. , Li, L. , Qin, C. , Farber, J.P. , Linderoth, B. , Foreman, R.D. (2010). Effects of spinal cord stimulation with “standard clinical” and higher frequencies on peripheral blood flow in rats. Brain Res 1313, 53–61. [DOI] [PubMed] [Google Scholar]
- Hassan, Z. , Dewa, A. , Asmawi, M.Z. , Sattar, M.Z.A. (2011). Assessment of vascular reactivity at different time‐course on streptozotocin‐induced diabetic rats. J Exp Integr Med 1, 175–183. [Google Scholar]
- Jensen, T.S. , Backonja, M.M. , Hernandez Jimenez, S. , Tesfaye, S. , Valensi, P. , Ziegler, D. (2006). New perspectives on the management of diabetic peripheral neuropathic pain. Diab Vasc Dis Res 3, 108–119. [DOI] [PubMed] [Google Scholar]
- Jolivalt, C.G. , Lee, C.A. , Ramos, K.M. , Calcutt, N.A. (2008). Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium‐chloride co‐transporters. Pain 140, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao, C.‐H. , Chen, J.‐J.J. , Hsu, Y.‐M. , Bau, D.‐T. , Yao, C.‐H. , Chen, Y.‐S. (2013). High‐frequency electrical stimulation can be a complementary therapy to promote nerve regeneration in diabetic rats. PLoS ONE 8, e79078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler, M.A. , Barendse, G.A.M. , Kleef, M. , Vet, H.C.W. , Rijks, C.P.M. , Furnee, C.A. (2000). Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med 343, 618–624. [DOI] [PubMed] [Google Scholar]
- Kumar, K. , Taylor, R.S. , Jacques, L. , Eldabe, S. , Meglio, M. , Molet, J. , Thomson, S. , O'Callaghan, J. , Eisenberg, E. , Milbouw, G. , Buchser, E. , Fortini, G. , Richardson, J. , North, R.B. (2007). Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 132, 179–188. [DOI] [PubMed] [Google Scholar]
- Litwak, L. , Goh, S.‐Y. , Hussein, Z. , Malek, R. , Prusty, V. , Khamseh, M.E. (2013). Prevalence of diabetes complications in people with type 2 diabetes mellitus and its association with baseline characteristics in the multinational A1chieve study. Diabetol Metab Syndr 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, Y. , Wacnik, P.W. , Sluka, K.A. (2008). Low frequencies, but not high frequencies of bi‐polar spinal cord stimulation reduce cutaneous and muscle hyperalgesia induced by nerve injury. Pain 138, 143–152. [DOI] [PubMed] [Google Scholar]
- Maeda, Y. , Ikeuchi, M. , Wacnik, P. , Sluka, K.A. (2009). Increased c‐fos immunoreactivity in the spinal cord and brain following spinal cord stimulation is frequency‐dependent. Brain Res 1259, 40–50. [DOI] [PubMed] [Google Scholar]
- McCarthy, K.F. , McCrory, C. (2014). Cerebrospinal fluid levels of glial cell‐derived neurotrophic factor correlate with spinal cord stimulation frequency in patients with neuropathic pain: A preliminary report. Spinal Cord 52(Suppl 2), S8–S10. [DOI] [PubMed] [Google Scholar]
- McCarthy, K.F. , Connor, T.J. , McCrory, C. (2013). Cerebrospinal fluid levels of vascular endothelial growth factor correlate with reported pain and are reduced by spinal cord stimulation in patients with failed back surgery syndrome. Neuromodulation, 16, 519–522; discussion 522. [DOI] [PubMed] [Google Scholar]
- Mills, C. , Leblond, D. , Joshi, S. , Zhu, C. , Hsieh, G. , Jacobson, P. , Meyer, M. , Decker, M. (2012). Estimating efficacy and drug ED50's using von Frey thresholds: Impact of weber's law and log transformation. J Pain 13, 519–523. [DOI] [PubMed] [Google Scholar]
- Naoum, J.J. , Arbid, E.J. (2013). Spinal cord stimulation for chronic limb ischemia. Methodist DeBakey Cardiovasc J 9, 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard, T.J. , Dorman, J.S. , Maser, R.E. , Becker, D.J. , Drash, A.L. , Ellis, D. , LaPorte, R.E. , Kuller, L.H. (1990). Prevalence of complications in IDDM by sex and duration: Pittsburgh epidemiology of diabetes complications study II. Diabetes 39, 1116–1124. [DOI] [PubMed] [Google Scholar]
- Partanen, J. , Niskanen, L. , Lehtinen, J. , Mervaala, E. , Siitonen, O. , Uusitupa, M. (1995). Natural history of peripheral neuropathy in patients with non‐insulin‐dependent diabetes mellitus. N Engl J Med 333, 89–94. [DOI] [PubMed] [Google Scholar]
- Petrakis, I.E. , Sciacca, V. (1999). Epidural spinal cord electrical stimulation in diabetic critical lower limb ischemia. J Diabetes Complications 13, 293–299. [DOI] [PubMed] [Google Scholar]
- Petrakis, E. , Sciacca, V. (2000a). Prospective study of transcutaneous oxygen tension (TcPO2) measurement in the testing period of spinal cord stimulation in diabetic patients with critical lower limb ischaemia. Int Angiol 19, 18–25. [PubMed] [Google Scholar]
- Petrakis, I.E. , Sciacca, V. (2000b). Spinal cord stimulation in diabetic lower limb critical ischaemia: Transcutaneous oxygen measurement as predictor for treatment success. Eur J Vasc Endovasc Surg 19, 587–592. [DOI] [PubMed] [Google Scholar]
- Pluijms, W.A. , van Kleef, M. , Honig, W.M. , Janssen, S.P. , Joosten, E.A. (2013). The effect of spinal cord stimulation frequency in experimental painful diabetic polyneuropathy. Eur J Pain 17, 1338–1346. [DOI] [PubMed] [Google Scholar]
- Rajan, V. , Varghese, B. , van Leeuwen, T.G. , Steenbergen, W. (2009). Review of methodological developments in laser Doppler flowmetry. Lasers Med Sci 24, 269–283. [DOI] [PubMed] [Google Scholar]
- Romanovsky, D. , Cruz, N.F. , Dienel, G.A. , Dobretsov, M. (2006). Mechanical hyperalgesia correlates with insulin deficiency in normoglycemic streptozotocin‐treated rats. Neurobiol Dis 24, 384–394. [DOI] [PubMed] [Google Scholar]
- Schiene, K. , Tzschentke, T.M. , Schröder, W. , Christoph, T. (2015). Mechanical hyperalgesia in rats with diabetic polyneuropathy is selectively inhibited by local peripheral nociceptin/orphanin FQ receptor and μ‐opioid receptor agonism. Eur J Pharmacol 754, 61–65. [DOI] [PubMed] [Google Scholar]
- Slangen, R. , Schaper, N.C. , Faber, C.G. , Joosten, E.A. , Dirksen, C.D. , van Dongen, R.T. , Kessels, A.G. , van Kleef, M. (2014). Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: A prospective two‐center randomized controlled trial. Diabetes Care 37, 3016–3024. [DOI] [PubMed] [Google Scholar]
- Song, Z. , Meyerson, B.A. , Linderoth, B. (2015). High‐frequency (1 kHz) spinal cord stimulation—Is pulse shape crucial for the efficacy? A Pilot Study. Neuromodulation 18, 714–720. [DOI] [PubMed] [Google Scholar]
- Tanaka, S. , Barron, K.W. , Chandler, M.J. , Linderoth, B. , Foreman, R.D. (2003). Local cooling alters neural mechanisms producing changes in peripheral blood flow by spinal cord stimulation. Auton Neurosci 104, 117–127. [DOI] [PubMed] [Google Scholar]
- Tanaka, S. , Komori, N. , Barron, K.W. , Chandler, M.J. , Linderoth, B. , Foreman, R.D. (2004). Mechanisms of sustained cutaneous vasodilation induced by spinal cord stimulation. Auton Neurosci 114, 55–60. [DOI] [PubMed] [Google Scholar]
- Tesfamariam, B. , Brown, M.L. , Cohen, R.A. (1991). Elevated glucose impairs endothelium‐dependent relaxation by activating protein kinase C. J Clin Investig 87, 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubbink, D.T. , Spincemaille, G.H. , Prins, M.H. , Reneman, R.S. , Jacobs, M.J. (1999). Microcirculatory investigations to determine the effect of spinal cord stimulation for critical leg ischemia: The Dutch multicenter randomized controlled trial. J Vasc Surg 30, 236–244. [DOI] [PubMed] [Google Scholar]
- de Vos, C.C. , Rajan, V. , Steenbergen, W. , van der Aa, H.E. , Buschman, H.P. (2009). Effect and safety of spinal cord stimulation for treatment of chronic pain caused by diabetic neuropathy. J Diabetes Complications 23, 40–45. [DOI] [PubMed] [Google Scholar]
- de Vos, C.C. , Meier, K. , Zaalberg, P.B. , Nijhuis, H.J. , Duyvendak, W. , Vesper, J. , Enggaard, T.P. , Lenders, M.W. (2014). Spinal cord stimulation in patients with painful diabetic neuropathy: A multicentre randomized clinical trial. Pain 155, 2426–2431. [DOI] [PubMed] [Google Scholar]
- Wei, M. , Ong, L. , Smith, M.T. , Ross, F.B. , Schmid, K. , Hoey, A.J. , Burstow, D. , Brown, L. (2003). The streptozotocin‐diabetic rat as a model of the chronic complications of human diabetes. Heart Lung Circ 12, 44–50. [DOI] [PubMed] [Google Scholar]
- Wu, M. , Thorkilsen, M.M. , Qin, C. , Farber, J.P. , Linderoth, B. , Foreman, R.D. (2007). Effects of spinal cord stimulation on peripheral blood circulation in rats with streptozotocin‐induced diabetes. Neuromodulation 10, 216–223. [DOI] [PubMed] [Google Scholar]
- Wu, M. , Linderoth, B. , Foreman, R.D. (2008). Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: A review of experimental studies. Auton Neurosci 138, 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T.C. , Janik, J.J. , Grill, W.M. (2014). Modeling effects of spinal cord stimulation on wide‐dynamic range dorsal horn neurons: Influence of stimulation frequency and GABAergic inhibition. J Neurophysiol 112, 552–567. [DOI] [PubMed] [Google Scholar]


