Abstract
Membrane receptors and ion channels respond to various stimuli and relay that information across the plasma membrane by triggering specific and timed processes. These include activation of second messengers, allowing ion permeation, and changing cellular excitability, to name a few. Gaining control over equivalent processes is essential to understand neuronal physiology and pathophysiology. Recently, new optical techniques have emerged proffering new remote means to control various functions of defined neuronal populations by light, dubbed optogenetics. Still, optogenetic tools do not typically address the activity of receptors and channels native to neurons (or of neuronal origin), nor gain access to their signaling mechanisms. A related method—synthetic optogenetics—bridges this gap by endowing light sensitivity to endogenous neuronal receptors and channels by the appending of synthetic, light‐receptive molecules, or photoswitches. This provides the means to photoregulate neuronal receptors and channels and tap into their native signaling mechanisms in select regions of the neurons, such as the synapse. This review discusses the development of synthetic optogenetics as a means to study neuronal receptors and channels remotely, in their natural environment, with unprecedented spatial and temporal precision, and provides an overview of tool design, mode of action, potential clinical applications and insights and achievements gained.
Keywords: native receptors, neurons, optogenetics, photoswitches, synthetic optogenetics
Subject Categories: Methods & Resources, Neuroscience
Glossary
- 2PE
two‐photon excitation
- AAQ
acrylamide–azobenzene–QA
- ACh
acetylcholine
- AP
action potential
- BGAG
benzylguanine‐azoglutamate
- ChR2
channelrhodopsin‐2
- CNS
central nervous system
- DPCC
diphenylcarbamyl‐chloride
- ER
endoplasmic reticulum
- GABA
γ‐aminobutyric acid
- GIRK
G‐protein activated inwardly rectifying potassium channel
- GPCR
G‐protein‐coupled receptor
- LBD
ligand‐binding domain
- LiGluN
light‐gated NMDA glutamate receptors
- LiGluR
light‐gated kainate glutamate receptors
- LimGluRs
light‐gated metabotropic glutamate receptors
- L‐MAG
maleimide–azobenzene and 4'L stereochemistry glutamate
- LTP
long‐term potentiation
- mAChR
muscarinic acetylcholine receptor
- MAQ
maleimide–azobenzene–QA
- MscL
mechanosensitive channel of large conductance
- nAChR
nicotinic acetylcholine receptor
- nAChR
nicotinic acetylcholine receptors
- NMDAR
N‐methyl‐D‐aspartate receptors
- P2X
purinergic (ATP‐gated) receptors
- PALs
photoswitchable affinity labels
- PAPC
p‐azophenyldiphenylcarbamyl‐chloride
- PCL
photochromic ligand
- PM
plasma membrane
- PNS
peripheral nervous system
- PTL
photoswitchable tethered ligands
- PTX
pertussis toxin
- PX
photoswitchable tweezers
- QA
quaternary ammonium
- RGC
retinal ganglion cell
- SNAP
O6‐alkylguanine‐DNA alkyltransferase
- SPARK
synthetic photoisomerizable azobenzene‐regulated K+ channel
- TCP
targeted covalent photoswitch
- TM
transmembrane
- TREK
two‐pore‐domain potassium channel
- V‐LOGO
visible light operated GIRK opener
- wt
wild type
Introduction
The need to better understand the function and roles of neuronal membrane proteins, synaptic receptors and ion channels in particular, has become one of the more pressing goals in neuroscience for the reason that their function and dysfunction underlay some of the most prevalent and devastating neurodegenerative diseases, neuropsychiatric disorders, and synaptopathies 1, 2, 3, 4, 5, 6. The better we understand their mechanisms of actions, in health and disease, the better we may design novel drugs and treatments.
The study of neuronal receptors and channels has been extensively addressed over the years by conventional pharmacology, electrophysiology, structural studies, molecular and genetic manipulations, etc. 7, 8, 9, 10, 11, 12, 13. Despite the countless insights gained, these and other contemporary techniques display inherent drawbacks, such as specificity and off‐targets, slow kinetics, poor spatial confinement, lack of or slow reversibility, etc. These appreciably limit our understanding of the processes under study 14, 15, 16, 17. Consequently, new methods are still vigorously pursued.
One novel approach developed for the remote study of neurons is optogenetics 18, 19, 20. Optogenetics is a momentous and rapidly evolving field that leverages naturally occurring light‐sensitive proteins (hereinafter referred to as photoreceptors, see 21) and repurposes them into genetically encoded light‐gated cellular actuators. Thereby, optogenetic tools allow the user to control cellular activity by light. The type of modulation depends on the photoreceptor's biological function. For instance, the widespread optogenetic tool ChR2 is a light‐gated cation channel 22. When expressed at the membrane of neurons, light illumination causes rapid channel opening resulting in strong membrane depolarization and AP firing 23, 24, 25, 26, 27. This methodology has drastically transformed the way we approach and study biological systems, neuroscience in particular, owing to the method's non‐invasive and reversible nature and, owing to the inherent properties of light, fantastic spatiotemporal resolution. Notably, with cell type‐specific promoters 28, the expression of optogenetic tools can be confined to select cells and neuronal circuits to be exclusively interrogated by light. Optogenetic tools are extensively employed today to control neuronal circuits and behavior by regulating membrane excitability, though they can also control a wide array of additional cellular mechanisms ranging from protein trafficking, to inducing genomic engineering, to humbly name but a few of the achievements and applications described to date 19, 22, 23, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38.
However, optogenetic tools are not without fault. These tools may suffer from slow kinetics of activation or deactivation, small channel conductance, poor expression in mammalian cells, undesired secondary effects (e.g., acidifications), unwarranted basal activity prior to light illumination, unexpected inverse effects (i.e., depolarization instead of hyperpolarization), and more 19, 39, 40, 41, 42, 43, 44. With regard to neuronal synapses, optogenetic tools are limited in their ability to control endogenous signaling mechanisms. For instance, though several tools have been specifically expressed at the synapse (e.g., 39, 45, 46, 47, 48), very few were able to provide direct control over native components or endogenous signaling.
This gap can be bridged by an analogous method—synthetic optogenetics—that similarly employs light to regulate the function of proteins but, instead of the use of photoreceptors, relies on the appending of synthetic chromophores to “blind” proteins (i.e., proteins that do not naturally respond to light), denoted photoswitches.
There are many names by which this approach has been described (chemical‐optogenetics, photopharmacology, or optogenetic pharmacology 49, 50, 51). However, as new tools are appearing in the literature, most names no longer cover all cases. For example, although the method initially employed pharmacophores in the design of photoswitches, several recent advances show the possibility to design pharmacophore‐less photoswitches (e.g., photoswitchable tweezers, see below). Therefore, names embracing the term “pharmaco” are less relevant today, warranting the re‐naming of the approach. The name of synthetic optogenetics deems the most appropriate for several reasons: The method requires the usage of synthetic photoswitches, several key tools are denoted synthetic (e.g., SPARK, see below) and, importantly, as the field of optical control of cellular activity is widely recognized today by the name of optogenetics, the name “synthetic optogenetics” allows it to remain under this umbrella term, whilst emphasizing the distinction of the approach from others. In addition, we hypothesize that this name should also cover future developments in this area too (see discussion).
Here, we first review the historical development of the technique and proceed to provide an overview of modern developments of light‐gated channels and receptors, through examples of their usage in the field of neuroscience. Lastly, we discuss several limitations, how these could be overcome and end with future prospects.
Photosensitizing proteins by synthetic optogenetics
The approach of synthetic optogenetics combines chemical and genetic methods to render potentially any protein sensitive to light. In contrast to optogenetic tools incorporating naturally occurring chromophores, this method employs synthetic, light‐sensitive photoswitches 52. Akin to chromophores found in nature (e.g., retinal 53, 54), photoswitches too undergo chemical reactions following the absorption of specific wavelengths. One highly relevant reaction to the design of biological tools is the trans‐to‐cis isomerization of azobenzenes, resulting in a large geometrical change and significant reduction in the length of the molecule (Fig 1A) 55. Azobenzenes are mostly (> 95%) found in –trans in the dark or following green‐light illumination (e.g., ~500 nm), but isomerize to the shorter bent –cis form (~80%) following near‐UV light absorption (e.g., 350 nm; Fig 1B) 56. Therefore, isomerization can be utilized to alter/modulate a protein's function. For instance, if the azobenzene‐core is decorated with a specific receptor's ligand, isomerization from trans‐to‐cis could be used to expose the ligand making it accessible to the receptor, indirectly photoregulating it (Fig 1C). Other chemical headgroups can be added onto the azobenzene photoswitch, such as blockers, antagonists, allosteric modulators, conjugating moieties, lipophilic chains (see Fig 1, toolbox). These will typically determine the mechanism of action of the photoswitch. Today, the variety of the modes of action by which photoswitches can regulate protein function is extensive, involving varying the distance between a drug and its binding site, changing local polarity, inducing lateral pressure in the membrane, applying forceps‐like motion to gate channels, piecing a fragmented drug, etc. (Fig 1C–J). Notably, the number of mechanisms employed by photoswitches exceeds the list enumerated here and, in fact, is limited only by the creativity of the designers.
Figure 1. Isomerization of azobenzene to modulate protein function.
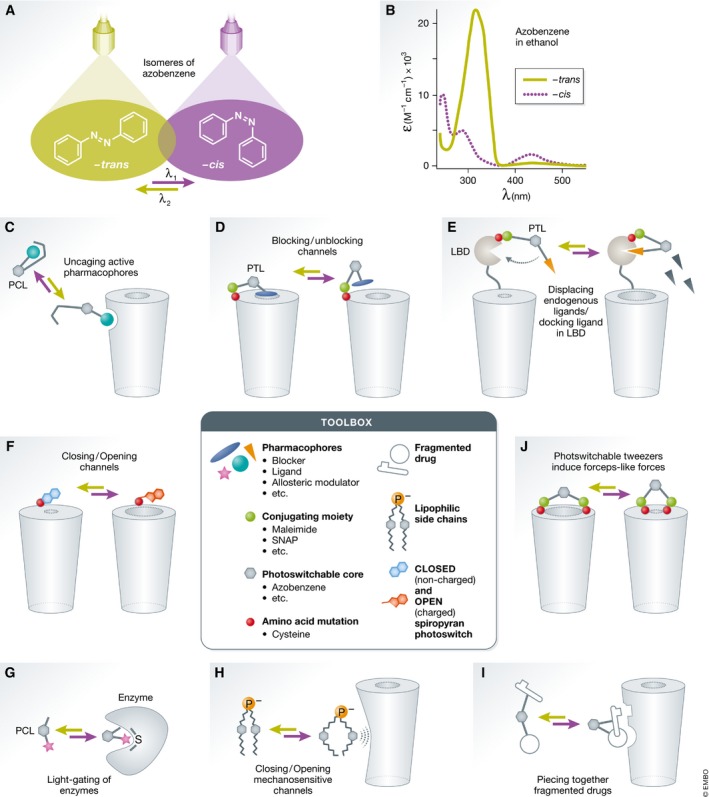
(A) Molecular structures of –trans and –cis isomers of azobenzene, depending on the wavelengths used. Near‐UV light (violet) induces isomerization to –cis, whereas green light reverts the molecule back to –trans. (B) Absorption spectra of –trans and –cis isomers of azobenzene in ethanol. (C–J) Mechanisms of actions of azobenzene‐based (gray hexagon) photoswitches. (middle) Toolbox: building blocks for the synthesis of photoswitches. Isomerization of PCLs can uncage an active pharmacophore (C). A PTL bearing a channel blocker (blue ellipse) is tethered to a modified channel (cysteine, red circle) via a conjugating moiety (maleimide, green circle). In –trans, the blocker reaches the pore of the channel (D). A receptor with a PTL bound to its LBD undergoing isomerization enables the ligand (orange triangle) to enter the LBD to activate or displace the endogenous ligand (black triangles) (E). Spiropyran undergoing isomerization from the closed (non‐charged, cyan) to the open (charged, red) state induces channel opening (F). Light‐gating of an enzyme by a PCL (G). Membrane incorporated photoswitches induce lateral mechanical pressure in the membrane during isomerization to open mechanosensitive channels (H). A fragmented drug is pieced together by the isomerization process (I). Photoswitchable tweezers bound to two cysteines (red circles) via maleimides (green circles) induce forceps‐like forces (J).
The historical development of synthetic optogenetics
Synthetic optogenetics was pioneered by the Erlanger group, which initially demonstrated the photocontrol over the enzyme chymotrypsin 57. Here, the authors synthesized a soluble photochromic ligand (PCL) composed of a light‐sensitive azobenzene‐core linked to diphenylcarbamyl‐chloride (DPCC—a specific inactivator of the enzyme) they termed PAPC (p‐azophenyldiphenylcarbamyl‐chloride; Fig 1G, purple). PCLs are advantageous as they do not require any genetic alterations of their target protein, enabling the regulation of wt proteins, wt‐chymotrypsin in this case. The authors envisioned that the light‐dependent isomerization of the azobenzene in PAPC could be used to gate (i.e., enable or disable) the ability of the pharmacophore (DPCC) to act on its site of action, a serine residue located deep within the enzyme. Indeed, they then showed that the –cis isomer of PAPC is five times more potent in inactivating chymotrypsin than the –trans isomer, suggesting that the DPCC has different pharmacological properties when the attached azobenzene is in the –trans or –cis conformation 57.
This report paved the way for other groups to render their favored enzyme photolabile. To this end, other groups have replaced DPCC with other specific compounds from the toolbox of available pharmacophores. This mix and match quickly yielded photoregulated acetylcholinesterases 58, 59, trypsin 60, aldolases 61, 62, urease, amylase, and more (see list in 63). Interestingly, the engineering of photoregulated enzymes subsided in the following years. However, the approach was not neglected, rather shifted from targeting soluble proteins to membrane proteins. The first demonstration of the photoswitching of membrane receptors was shown to involve nicotinic acetylcholine receptors (nAChR) 64. Here too, the authors exploited the changes in geometry of the azobenzene so that the PCL, here containing a carbachol headgroup, would differently interact and affect nAChR. When in –trans, the photoswitch inhibited the receptor more potently than when in –cis. This report established that the approach was not limited to soluble proteins, namely enzymes, and further showed its advantages over conventional pharmacological reagents. Indeed, though several specific drugs acting on nAChRs were available at the time, this approach enabled the study of nAChRs in their native environment with unprecedented temporal resolution, resulting in a flurry of publications 65, 66, 67, 68, 69.
During these years, another development laid the groundwork for what will become one of the most employed strategies in synthetic optogenetics: conjugating PCLs to their target. This involved the design of tethered photoswitches or photoswitchable tethered ligands (PTLs). PTLs are designed to irreversibly bind to their protein target, creating a high local concentration of the drug. To the best of our knowledge, the first tethered photoswitch was the cysteine‐reactive QBr photoswitch (3‐(alpha‐bromomethyl)‐3′‐[alpha‐(trimethylammonium)methyl] azobenzene bromide) 70. This azobenzene‐based photoswitch included an additional chemical moiety at the other end of the azobenzene that would covalently conjugate the photoswitch onto existing outer cysteine residues in nAChRs 70. Once bound, QBr selectively activated the channels when in its extended –trans form, and much less so when in –cis (e.g., Fig 1D). The use of PTLs is preferable over PCLs in certain instances as it keeps the photoswitch near the receptor at all times, granting a high local concentration of the chemical headgroup. This can enable efficient competition of the photoswitch with the soluble ligand (e.g., Fig 1E, black filled triangles). It also enables persistent perfusion of the preparation without necessitating constant supplementation of the photoswitch. Owing to these advantages, the photoregulation of enzymes was revisited in the following years with the use of PTLs (e.g., 61, 71, 72). Collectively, these pioneering experiments demonstrated the potential of the approach, laid the framework (i.e., toolbox) for the design of soluble (PCLs) or tethered (PTLs) photoswitches, and, importantly, hinted at the potential of the approach to render any blind protein light‐sensitive 73, 74, 75.
Synthetic optogenetics revisited
Despite the method's first appearance almost 50 years ago, the approach was gradually ignored and almost completely neglected for many years. However, in 2004, the spark was re‐ignited by a group of scientists from the University of California at Berkeley (amusingly befitting as UCB carries the motto “let there be light” on its seal). The Isacoff–Trauner–Kramer alliance (see perspective by 76) designed a Synthetic Photoisomerizable Azobenzene‐Regulated K+ channel based on the Shaker channel—dubbed SPARK—to control neuronal excitability 77. This was the first report using a PTL and a genetically modified channel to control mammalian neuron excitability, only two years after the first optogenetic demonstration using rhodopsin to “chARGe” (depolarize) neurons (33, but also see perspective by 78).
To confer light sensitivity to the Shaker channel, Banghart and colleagues synthesized a PTL consisting of a maleimide, azobenzene and a quaternary amine (QA—a K+ channel blocker) denoted MAQ 77. When in –trans, the end‐to‐end distance of MAQ spans ~17 Å. Knowledge of this distance was then used to locate an outer residue in the Shaker channel that was situated at a similar distance from the pore. This residue was mutated to a cysteine to conjugate the photoswitch (via its maleimide) so that when in –trans, the quaternary amine of MAQ would reach the pore and block the channel. In turn, this would inhibit membrane hyperpolarization resulting in AP firing by the neuron (Fig 2A and B). This process could be rapidly reversed by near‐UV light, as isomerization of MAQ to –cis shortened the molecule (to ~10 Å), physically removing the blocker from the pore, which opened the channel and electrically silenced the neuron. By toggling between green and near‐UV illumination, the authors were able to prevent or enable AP firing repeatedly and remotely with a high spatiotemporal resolution (Fig 2B). Importantly, the authors note that the molecular design of the channel was possible mainly because of the rise in the availability of detailed structural and molecular information of ion channels, and K+ channels in particular (e.g., 79, 80). In point of fact, SPARK emerged at the time when the Nobel prize in Chemistry was attributed to Roderick MacKinnon for solving the structures of K+ channels 81. Notably, the genetic manipulation of the channel enabled the exclusive expression of the channel in neurons, thereby limiting PTL conjugation to this defined population, unlike PCLs that cannot be typically confined to selected cells (but see below).
Figure 2. SPARK regulates neuronal excitability.
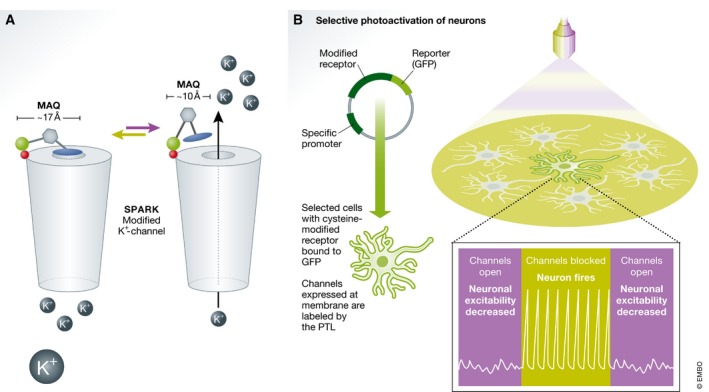
(A) SPARK, a modified K+ channel tethered to MAQ. In –trans (green arrow), MAQ extends to ~17 Å, enabling block of the pore and no ion conductance (open circles denoted with K+). During isomerization (violet arrow), the shorter –cis isomer (~10 Å) relieves the block and the channel opens, generating an efflux of K+ ions. (B) Selective photoactivation of neurons. The cysteine‐modified receptor (bound to GFP) is genetically targeted to a defined neuronal population (via specific promoters). Channels expressed at the membrane (green highlight) of selected cells are labeled by the PTL. During green‐light illumination (green bar, bottom), the channels are blocked, enabling firing by the neuron (bottom trace, APs). Neuronal excitability is decreased by isomerization back to –cis (by near‐UV light, violet bar) and opening of the channels. Cysteine, red circle; azobenzene, gray hexagon; maleimide, green circle; quaternary amine/QA, blue ellipse.
In parallel, two other groups were also toiling over the design of light‐gated channels. Interestingly, both groups made use of the bacterial mechanosensitive channel of large conductance (MscL). The group of Bert Poolman synthesized a new type of an azobenzene‐based photoswitch containing lipophilic phosphate tails, named 4‐Azo‐5P 82. This photoswitch would incorporate within the leaflets of the membrane so that during trans‐to‐cis isomerization, the photoswitch would exert lateral pressure in the membrane. This would mechanically gate the closely situated MscL channel (Fig 1H). In 2005, the Feringa group also designed a photoregulated MscL, using another clever approach 83. MscL, though mechanosensitive, also exhibits spontaneous channel opening when polar or charged amino acids are introduced next to the pore 84. Koçer exploited this phenomenon and synthesized a spiropyran‐based PTL. To note, in contrast to azobenzenes, the isomerization of spiropyrans involves the interconversion between closed‐ and open‐ring structures, which is accompanied by a large polarity change of the molecule 55. Thus, when this photoswitch was accurately tethered next to the pore (via an iodoacetate tether), its isomerization by light absorption resulted in a local polarity change that induced channel opening (Fig 1F) 83.
Contemporary light‐gated receptors and channels
The early developers of the method have provided the blueprint and building blocks for the development of the approach, which researchers are still using to date to devise more sophisticated photoswitches and light‐gating mechanisms. Of the different light‐sensitive cores (for detailed list see 55) and protein tethering methods available 85, azobenzenes and maleimides are used most in the synthesis of PCL and PTL photoswitches, for good reasons. Azobenzenes are easily synthesized, exhibit high photostationary states, undergo fast photoisomerization, and photobleach slowly. Analogously, maleimides are commercially available, thiol conjugation is covalent, and the reaction is moderately specific under physiological conditions (pH and temperature). From a protein‐engineering point of view, maleimide conjugation is highly favorable as it requires very minimal protein modification—a single amino acid substitution to cysteine. Even though maleimides can potentially interact with other existing cysteines, free cysteines (i.e., unconjugated and water accessible) are naturally of low abundance in proteins (< 2% 86). Nevertheless, it should be noted that maleimides hydrolyze rapidly (tens of minutes) in aqueous conditions, offering a narrow time‐window for labeling proteins expressed on the membranes of live cells. Thus, as cysteine conjugation will mostly occur in the first hour after addition of the maleimide–PTL, this process will label only the receptors located at the membrane during that time. This could be both a curse and a blessing (see Box 1). Lastly, the use of maleimide–PTLs requires ectopic expression of the modified, cysteine‐substituted protein. Despite the benefit of genetic‐targeting (e.g., Fig 2B, a single highlighted cell), expression of ectopic genes can result in unwarranted overexpression of the receptors and their promiscuous cellular localizations. With these advantages and limitations in mind, below we describe several families of neuronal receptors and channels rendered photosensitive by either azobenzene‐based PCLs or PTLs. We provide several examples where PCLs and PTLs target the same receptor, with the intent to showcase the alternatives the approaches offer, such as remaining under physiological conditions or enabling genetic‐targeting of the tool. We regret that owing to space constraints, we cannot list all available examples.
Box 1:In need of answers.
Labeling efficiency—Whereas naturally occurring photoreceptors are found at the membrane at a 1:1 stoichiometry with their chromophores, synthetic optogenetics tools are not. Following expression of modified receptors and incubation with the relevant PTL, it is difficult to determine the number of modified receptors that have been labeled by the PTL. Labeling efficiency will determine the effectiveness of the tool. The extent of labeling can be approximated by equating the photocurrent with the maximal ligand‐induced current. However, this method does not comply with photoswitches that exhibit partial agonism (e.g., GluAzo 131). As a result, methods to increase labeling efficiency, means to measure it, and alternative labeling schemes are still under development.
Narrow labeling time—Maleimides hydrolyze rapidly (tens of minutes). This presents the user with a narrow time‐window, during which the photoswitch would need to be applied as close as possible to the site expressing the receptors. For prolonged in vivo experiments, this is limiting as the photoswitch may need to be reapplied during the course of the experiment to maintain a constant amount of labeled receptors. This is cumbersome and possibly damaging, as it entails multiple applications of the photoswitch. Photoswitches with better stability would broaden the labeling time‐window and would also allow the user to apply them at a larger distance from the site of expression. This type of photoswitch could also be slowly and steadily administered to the brain via an implemented cannula, lessening the burden of multiple injections.
Solubility—Azobenzene are lipophilic compounds, typically solubilized in DMSO prior their application onto the preparation 180. Even more challenging are azobenzenes further decorated with lipophilic groups (such as fatty acids, e.g., 181). Therefore, photoswitches with improved solubility are still under pursuit 182.
Near‐UV absorption—Most azobenzene‐based photoswitches absorb near‐UV (< 380 nm, see Fig 1B), to isomerize from –trans to –cis, wavelengths that may be harmful to cells. This justifies the synthesis of red‐shifted photoswitches. Indeed, several red‐shifted photoswitches have been synthesized (e.g., MAG460 171), though this shift comes at the expense of bistability, resulting in the spontaneous relaxation of the photoswitch back to –trans. This may lower the photostationary population of the –cis isomers and would require longer or repetitive illumination to obtain continuous photoresponses. Therefore, better red‐shifted photoswitches that maintain excellent switching behavior are necessary.
Broad absorption—Azobenzenes exhibit broad absorption, in particular the –cis isomer (Fig 1B 55). This complicates the use of photoswitches with additional optical tools (such as fluorescent proteins and probes), because the photoswitch will be isomerized back to –trans during the imaging of the probes. Thus, narrower absorbing photoswitches are needed to achieve all‐optical interrogation of cells (see 183).
Clinical use—Whereas several research groups have shown the utility of photoswitches in vivo (e.g., fish and rodents), there are still questions pertaining to the safety of the use of azobenzene‐based photoswitches. This concern stems from their poor solubility and metabolic stability, both of which may lead to potential long‐term toxicity.
Availability—Most photoswitches are not commercially available, rather are typically obtained directly from the research groups. There are few exceptions, such as several MAG variants sold by Aspira Scientific (www.aspirasci.com). The lack of a chemical depository hinders the broad dissemination of the photoswitches. Unlike DNA depositories (e.g., Addgene), the synthesis process is much more expensive and laborious.
GABA receptors
GABA is the major inhibitory neurotransmitter of the brain. It binds to ionotropic GABAA or metabotropic GABAB receptors found at the membrane of neurons. When activated, GABAA receptors open and negatively charged chloride ions flow into the cell and inhibit neuronal firing 87. The importance and variety of these receptors, and lack of specific pharmacology, has motivated several groups to tackle activation or antagonism of these receptors by light.
A couple of examples have shown that GABAA receptors can be photoregulated by azobenzene‐based PCLs employing propofols, compounds known to potentiate GABAA currents, denoted azo‐propofols 88, 89. For example, in the report by Stein et al, azo‐propofol in –trans potentiates the GABA‐induced currents by almost twofold, and this effect is fully reversed by near‐UV light. Notably, azo‐propofols interact with unmodified GABAA receptors; thus, with restricted light illumination, these enable to map the localization of the receptors within neurons.
Examples of tethered photoswitches used with modified GABAA receptors include a large toolbox of azobenzene‐based PTLs containing various ligands as headgroups, namely muscimol, GABA, or its guanidinium analogs 90, 91. Their mechanism of action is postulated to resemble that of MAQ and SPARK (see above and Fig 1E), wherein the PTL is attached to an introduced cysteine on the receptor and the azobenzene modulates the pharmacophore's ability to physically reach its site of action on the receptor when in one conformation, but not when in the other. Notably, these photoswitches, and their cognate‐modified GABAA subunits, were the first to be used in mammalian brains in vivo 91, demonstrating the viability of this approach for manipulating receptors in living brains of behaving animals.
Noteworthy, the metabotropic GABAB receptor family has not been addressed to date by the technique, though other PTL‐gated GPCRs have been described (see below). This is unfortunate, as light‐gated GABAB receptors could help unravel their unique molecular roles at the post‐synapse, where they are suggested to modulate the function of ionotropic glutamate receptors, for example by reducing calcium permeability through NMDA receptors 92. Other GABA‐binding proteins have also been the target of light‐aficionados. The most abundant GABA transporter in the brain, mGAT1, was recently made photoantagonizable via PCLs containing tiagabine, a potent mGAT1 inhibitor 93. We note this, although beyond the scope of this review, as this is the first time that the synthetic optogenetic approach was applied on membrane transporters. Attractively, there are numerous other photochromic agents described in the literature that could, in principle, target GABA‐binding proteins, but to date remain untested 94. These are ready for the picking by laboratories interested in studying GABA‐mediated synaptic processes by light.
Glutamate receptors
On the other side of the spectrum, there is glutamate. Glutamate is the major excitatory neurotransmitter that activates members of the glutamate‐receptor superfamily, which includes 18 ionotropic receptors 95 and eight GPCRs 96. These receptors are expressed at pre‐, post‐, and extra‐synaptic regions, regulate essential processes in the brain, spinal cord, and peripheral nervous system, and are implicated in a myriad of diseases 97, 98. A large repertoire of pharmacological agents has been developed to probe the roles of glutamate receptors, but these agents attend to but a few receptor subtypes 95.
To address some of the limitations of pharmacological agents and of other methods used to study glutamate receptors, numerous synthetic tools have been developed, with the light‐gated kainate receptor—LiGluR—as the prominent representative of this group (e.g., 99, 100, 101, 102, 103, 104, 105, 106, 107, 108). LiGluR tethers L‐MAG (maleimide–azobenzene with a 4'L stereochemistry glutamate) by a cysteine introduced into the receptor's LBD (Fig 1E) 106. As noted above, this residue was located by analyzing the structures of several LBDs of kainate receptors (e.g., Fig 3 and 109). In particular, it is located at the surface of the protein and next to the opening of the LBD. To note, here the photoswitch does not interact directly with the pore of the receptor (as in SPARK, compare Fig 1D and E), but rather with allosteric elements of the receptor, explicitly the LBD. When bound at this site, the elongated –trans form of MAG does not allow the glutamate headgroup to reach into the glutamate‐binding pocket located deep within the LBD. Isomerization to –cis by near‐UV light propels the glutamate end into this groove, enabling LBD closure and, subsequently, channel opening (Fig 1E). Green‐light illumination isomerizes L‐MAG back to –trans, pulling the glutamate away from the receptor and triggering channel closure. LiGluR was initially expressed in neurons for the purpose of driving neuronal excitability in vitro 105 and extended to in vivo studies more recently 103, further validating the technique's applicability in the live mammalian brain. LiGluR was also used to study the desensitization process of the kainate receptor, showing the utility of the tool for biophysical studies of the receptor 110. In the degenerating retina, LiGluR was expressed in RGCs where, following in vivo injections of L‐MAG into the retina, the now light‐responsive LiGluR restored light sensitivity to the retina 100, 102. LiGluR has also been expressed in non‐neuronal cells, namely astrocytes, to stimulate glutamate exocytosis owing to its relatively high Ca2+‐permeability 104.
Figure 3. Crystal structure of the LBD of a kainate receptor bound to a photoswitch.

(A) Surface representation of GluK2‐LBD dimers. One LBD (orange) is bound to domoate (not shown), whereas the second LBD (blue) is in complex with GluAzo (magenta), a specific iGluR5 and ‐6 PCL (structure is from PDB: 4H8I). The structure shows a closed LBD conformation. (B) Front view of the LBD with the tip of the azobenzene tail protruding from the LBD. (C) Transparent view of the dimer, depicting the partially embedded GluAzo photoswitch within the LBD (dashed line) and the exit tunnel through which the azobenzene protrudes. (D) Front view of the LBD (GluAzo removed).
We have recently described photoswitchable NMDA receptors (LiGluNs) 111. We created a small library of GluN‐subunits bearing cysteine mutations to tether L‐MAGs, with a similar engineering logic as described for LiGluR, culminating in several photoswitchable NMDAR subunits. This process allowed us to photoagonize and photoantagonize the NMDA current 52, 111. The difference in activity was obtained by tethering L‐MAG at different locations at the LBD of the subunit. At one position, when in –cis, the glutamate headgroup could properly dock within the glutamate‐binding pocket resulting in proper clamshell closure and channel opening. However, when the photoswitch was tethered to another residue, even if only one amino acid away, the isomerization of L‐MAG would incorrectly position the glutamate headgroup in the pocket, leading to the obstruction of clamshell closure and channel opening. This obstruction persisted in the presence of soluble agonists, acting as a non‐competitive antagonist 111. We have also used the glutamate‐based L‐MAG photoswitch to photoantagonize the glycine‐binding GluN1 subunit. In this instance, the glutamate moiety of L‐MAG acts as an antagonist when positioned in the glycine‐binding pocket, as it prevents the binding of the native ligand glycine. These examples display the versatility of the method and demonstrate that a single photoswitch can be used with multiple subunits of the glutamate‐receptor family, even if those bind a different ligand than the one used in the photoswitch (e.g., glutamate and glycine).
To obtain modest expression levels of LiGluNs at the PM, we have taken advantage of the fact that GluN2 subunits are retained at the ER, unless bound to the GluN1 subunit. In this manner, the expression levels of LiGluN2 subunits are effectively controlled by the availability of the endogenous pool of GluN1 subunits in the neuron.
A unique example of a light‐gated ionotropic glutamate receptor has been described by Janovjak et al 112. The authors generated HyLighter (short for hyperpolarizing light‐gated glutamate receptor), a chimeric receptor containing the LBD of the mammalian iGluR6 and the membrane‐spanning domains (including the pore) of sGluR0, a prokaryotic homolog of mammalian iGluRs from cyanobacteria 113. The interesting feature of sGluR0 is that, unlike all other glutamate receptors, it holds the signature of the selectivity filter of K+ channels (GYG motif 114) and, thereby, solely conducts K+ ions. Thus, when HyLighter was bound to L‐MAG, near‐UV illumination would open the channel and hyperpolarize the neuron due to K+‐ion outflow.
Multiple light‐gated metabotropic glutamate receptors (LimGluRs) have been engineered 115, 116, 117, 118, 119. Levitz et al show that photocontrol over their LimGluR version requires 4'D stereochemistry of MAG, as opposed to 4'L (as for LiGluR and LiGluN). Broichhagen et al show that LimGluRs could support the fusion of a SNAP‐tag 120 to tether a benzylguanine‐based photoswitch, denoted BGAG 121. Whereas the SNAP‐tagging strategy overcomes several limitations of the maleimide chemistry, namely its short viable lifetime and potential off‐target cysteines conjugation (see Box 1), it requires the incorporation of a large protein domain (SNAP, ~20 kDa) into the receptor, which might be more detrimental to the protein's function than a single amino acid substitution.
Photoswitchable tethered ligands, though extremely useful, require genetically modified channels that need to be ectopically expressed (Fig 2B, plasmid). Overexpression of ion channels may limit their usage in certain contexts and preparations (see Box 1). To bypass this drawback, and still make use of PTLs, Izquierdo‐Serra et al devised a method to tether photoswitches to specific endogenous glutamate receptors without the necessity of cysteine mutagenesis. This was achieved by synthesizing a series of new photoswitches—targeted covalent photoswitches (TCPs)—containing a short‐lived, highly reactive anchoring group, such as epoxide and N‐hydroxysuccinimide esters 122. These anchoring groups covalently react with lysine residues exposed on the protein's surface. The ligand headgroup of the photoswitch affords a kinetically controlled site‐selective conjugation specifically in the vicinity of the LBD, ensuring target specificity (by affinity labeling 123). Synonymously, there are photoswitchable affinity labels (PALs), which are conceptually identical to TCPs (some PALs even include epoxide as do TCPs) in the sense that they are also tethered to native channels by affinity labeling 124, 125.
In sharp contrast, several soluble PCLs have been engineered to selectively target ionotropic glutamate receptors. For instance, GluAzo is a photoagonizing PCL specific to iGluR5 and ‐6 (Fig 3) 126. Laprell et al 127 designed a similar caged photoswitch to non‐specifically activate all types of NMDA receptors, termed ATG. Lastly, several potent PCLs that photoregulate AMPA receptors by a similar “uncaging” mechanism have also been synthesized, for example, ATA and ShuBQX‐3 128, 129. Importantly, while the ON photoswitching of PCLs can be compared to the photolysis of “conventional” caged compounds, PCLs can be turned OFF reversibly, contrasting the irreversible photolysis of caged drugs (see 130).
Recently, the Trauner and Schiefner groups succeeded in crystalizing a kainate receptor's LBD bound to the GluAzo photoswitch (Fig 3) 131. The structures show that the interactions of the glutamate moiety of GluAzo with its binding pocket are essentially the same as with soluble glutamate. In addition, the structure highlights how the LBD can assume a closed conformation with the photoswitch by allowing the azobenzene part of the molecule to protrude from the LBD via an exit tunnel only when in –trans (Fig 3A and B). Thus, because this cavity cannot accommodate the –cis isomer, it explains why only trans‐GluAzo can induce channel opening. Whereas soluble glutamate is almost entirely buried within the LBD after clamshell closure, GluAzo is not and results in a slightly more open LBD conformation (Fig 3C and D). This further elucidates why GluAzo acts as a partial agonist, as the degree of LBD closure correlates with the extent of channel activation 132, 133. Together, the structures validate many assumptions made in the design of light‐gated glutamate receptors and should further assist in the design of better kainate receptor photoswitches, as well as of photoswitches for other glutamate receptors.
Potassium channels
The K+ channel family is one of the largest ion channel families and the most widely distributed among organisms, consisting of more than 50 genes, found in both excitable and non‐excitable cells 114. Their roles are diverse (e.g., they control heart rate, insulin secretion, electrolyte transport, and cell volume regulation), but with respect to neuronal physiology, K+ channels are fundamentally responsible for setting membrane potential, decreasing excitability, and shaping action potentials 134, 135.
K+ channels can be divided into several subfamilies, namely voltage‐gated, calcium‐gated, inward rectifying, and two‐pore (2P) channels 136. Many of these have been targeted by PCLs or subjected to genetic manipulation to tether PTLs. In general, most synthetic optogenetic tools based on K+ channels have been developed for hyperpolarizing neuronal membranes, with a few exceptions. As briefly mentioned above, SPARK is a genetically modified channel based on the Drosophila Shaker potassium channel, belonging to the voltage‐gated family of channels (KV). SPARK, when bound to MAQ, was initially designed to hyperpolarize neurons (H‐SPARK) 77. However, SPARK was also further mutated and converted into a non‐selective cation channel (as opposed to the exclusive K+ conductance of Shaker) to depolarize neurons (D‐SPARK) 137. Similarly, several other potassium channels were designed to tether MAQ or MAQ‐like PTLs to control channel gating and membrane excitability, such as Kv1.3, Kv3.1, Kv7.2, and the Ca2+‐activated SK2 channel (Fig 2) 125. This long list of modified light‐gated K+ channels employing the same photoswitch, and likely photogating mechanism (i.e., channel block by the QA moiety), demonstrates once more the transferability of the photoswitch between members of the same family. Moreover, the MAQ photoswitch was even applied to photogate other ion channels (e.g., Ca2+ and Na+ channels 138). This is possible with photoswitches that are based on pharmacophores with non‐specific and broad activity such as QX‐314. Together, these demonstrations display the versatility of the technique.
The Kramer group (UC Berkeley) has extensively explored the photoregulation of endogenous K+ channels by a large repertoire of PCLs and PALs (124, 139, 140, 141, for an extensive list see 138). Of the many examples, we would like to highlight one interesting case involving a photoswitch denoted AAQ (acrylamide–azobenzene–QA). AAQ includes an acrylamide tethering group intended to react (to tether the photoswitch) with native extracellular residues located near the pore of the channels 124. Indeed, Fortin et al found that AAQ photoregulated the activity of K+ channels, presumably from the extracellular side of the membrane, meriting its classification as a PAL. Surprisingly, it was later discovered that the hydrophobic acrylamide group facilitated permeation of AAQ into the cell, before it could react with external residues (Fig 4A) 139. Thus, AAQ is a membrane‐permeable PCL and its photoregulation mechanism involves its entry into the inner vestibule of the channel when in –trans, but not when in –cis. Once in the vestibule, the QA headgroup exerts its block (Fig 4A). Though given lemons, the group was able to make lemonade from the AAQ misfortune by using these observations for the development of new hydrophobic photoswitches (e.g., BzAQ, PrAQ) that acted from the intracellular side of the membrane to block voltage‐gated potassium channels, but also voltage‐gated calcium and sodium channels 138, 139.
Figure 4. Intracellular photoswitchable blockers.
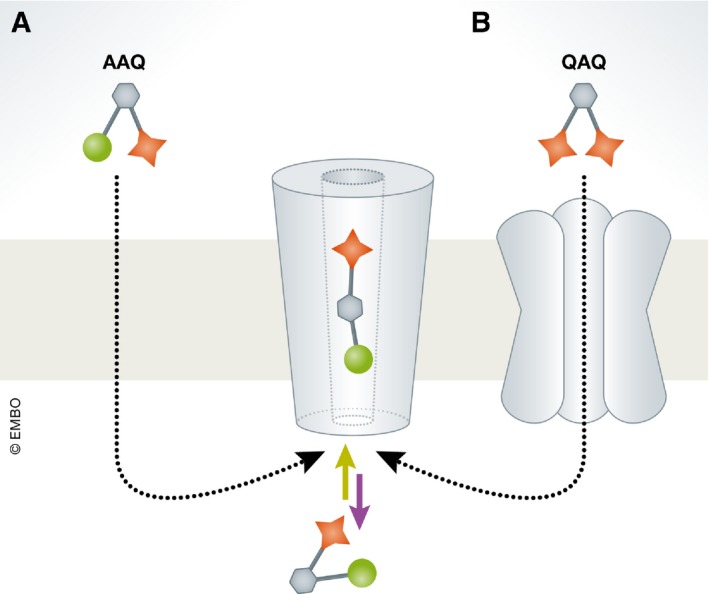
(A) AAQ (acrylamide–azobenzene–QA) crosses the membrane owing to its lipophilic acrylamide group, can enter the inner vestibule of the channel, and exert its block when in –trans. (B) The blocking mechanism of QAQ (QA–azobenzene–QA) resembles that of AAQ. However, QAQ cannot cross the membrane and needs to travel through pore‐dilated channels (e.g., TRPV1) to enter the cell.
Finally, 2P K+ channels and inward rectifiers (Kir) have not escaped the attention of photoengineers. Briefly, to photocontrol endogenous 2P‐TREK channels with the MAQ PTL, Sandoz et al devised a conditional expression scheme. To this end, the authors created a conditional TREK subunit bearing a cysteine and a truncated carboxy‐terminus (ΔCT), required for ER export of the subunit when intact. Thus, only when the ΔCT‐subunit dimerized with a wt subunit (endogenously present in the ER of the neuron) could the heteromeric channel complex exit the ER and reach the PM 142, 143. To open the GIRK channel, belonging to the family of inward rectifiers 144, the Trauner group designed a PCL denoted LOGO 145, 146. LOGO is an interesting PCL consisting of an azobenzene‐based photoswitch containing VU0259369, a potent and selective GIRK1 channel activator 147. Whereas GIRK channels are uniquely opened by the binding of Gβγ molecules derived from activated Gαi‐coupled GPCRs 148, VUO259369, and hence LOGO, can open the GIRK1‐containing channels even when the G‐protein cycle is disrupted by PTX. This photoswitch can thereby bypass the need to activate GPCRs to elucidate the gating mechanisms of the GIRK channel in neurons.
P2X receptors
P2X receptors are trimeric, ATP‐binding cation channels 149. With regard to the brain, P2X receptors are implicated in several diseases, such as neuropathic pain 150. To photogate P2X receptors, the team of Grutter has employed a unique strategy consisting of using charge to induce channel opening, reminiscent of the strategy employed by Kocer et al 83. To this end, the authors have synthesized a maleimide–azobenzene‐based PTL comprising positively charged trimethyl ammonium derivatives at the end of the molecule 151. When the PTL was tethered to cysteines at different TM domains, light‐dependent isomerization induced changes in the local charge that was sensed by local residues of the TMs and induced channel opening or closing, depending on the tethering location. With the use of this approach, the authors describe a unique ATP‐independent gating mechanism of P2X receptors. Accordingly, the authors suggest the name of opto‐gating to describe this process, as the photoswitchable reagents do not include the ATP ligand, nor target any particular active or known allosteric sites of the receptors 151. The same group next developed a new type of photoswitch they name photoswitchable tweezers (PXs). PXs are closely related to PTLs, but instead of tethering a single cysteine residue, they tether two cysteine residues resembling molecular rulers 152. However, unlike the latter, PXs are capable of pulling or pushing on gating elements of the receptor during isomerization (Fig 1J) 153. With these, the authors induce forceps‐like motion to open the pore of the P2X channels, resembling the motion of a camera's iris.
Nicotinic acetylcholine receptors
Acetylcholine (ACh) is an important neurotransmitter in both central and peripheral nervous systems (CNS and PNS, respectively). Its actions are mediated by two types of receptors—the G‐protein‐coupled muscarinic receptor (mAChR) and the nicotinic AChRs (nAChRs) 154. nAChRs function as non‐selective, excitatory cation channels with roles in the sympathetic and parasympathetic nervous system, and in skeletal muscle, they mediate contraction. In the brain, however, their roles are much less understood. They are found throughout the neuron's membrane with no obvious concentration at synaptic junctions. In fact, a large proportion of receptors can be found at nerve terminals. These receptors are considered neuromodulatory agents for neurotransmitter release and are implicated in numerous diseases 154, 155.
As noted above, the history of synthetic optogenetics intimately involves these receptors (e.g., PCL‐Bis‐Q 65, 70 and PTL‐Q‐Br 70, 156 and see above). However, Bis‐Q was found to be a muscle‐type photoagonist 157, and Q‐Br could not be targeted to defined subunits, as it conjugated native cysteines located next to the pore of the channel, extant in several subunits 70. Present studies have now elaborated on these earlier reports and produced several light‐gated nAChRs via azobenzene‐based PCLs and PTLs. Azocholine (azobenzene‐choline) is a photoagonizing PCL that targets and activates α7‐type nAChRs 157. Two genetically modified β subunits (2 and 4) have been engineered to covalently bind the photoactivating PTL, MAACh (maleimide–azobenzene–acylcholine), or the deactivating PTL, MAHoCh (maleimide–azobenzene–homocholine). The latter enable to photocontrol unique β2/4‐containing nAChRs receptors, denoted LinAChRs 158.
Next‐generation photoswitches
Spatially confine photomanipulation of light‐gated receptors located deep within scattering tissue is best achieved by 2PE 159. Whereas 2PE is readily used for photolysis of caged compounds 160, 161, 162 or for the photoactivation of photoreceptor‐based optogenetic tools (e.g., 163, 164, 165, 166, 167, 168), the activation of synthetic optogenetic tools by 2PE is far behind. This stems from the azobenzene's poor 2P absorption properties, owing to the molecule's symmetry 169. Among the first to tackle this issue was the group of Gorostiza Pau. In order to increase the absorption cross section of the L‐MAG photoswitch, the authors modified it to increase its push–pull characteristics or introduced light‐sensitive antennae to sensitize the isomerization process by resonance. These modifications yielded MAG2p and MAGA2p, respectively 170. The authors then used 2PE to photoactivate LiGluR in cultured neurons and astrocytes. Comparably, we have used a red‐shifted MAG photoswitch, denoted L‐ and D‐MAG460 171, to photoactivate LiGluR or LimGluRs, respectively, in neurons 101. Additional 2P‐compatible photoswitches have also been reported (e.g., 119, 127), showing that the field is steadily transitioning in the direction of 2PE, which is more suitable for in vivo applications. Notably, the transition from a 2PE‐incompatible to 2PE‐compatible photoswitch requires the design of an entirely new photoswitch by methods as described above. This may result in an altered or non‐functional photoswitch. Therefore, as the number of 2PE‐incompatible photoswitches exceeds, by far, that of compatible ones, it will take some time before we see photoswitches with revisited 2PE‐compatible design.
Bringing synthetic optogenetics to the clinic
Given that the use of photoswitches for clinical applications depends on the ability to deliver light into the tissue, it is not surprising that most of the translational progress made with synthetic optogenetics has mainly focused on the visual system 100, 102, 172, 173, 174. Collectively, these reports show that a variety of photoswitches and modified receptors can endow surviving neurons of the degenerating retina with sensitivity to environmental light and partially restore visual function to blind mice (see review in 175). However, there are still several hurdles to pass before this treatment transitions from bench to bedside, such as toxicity and solubility issues (see Box 1).
Another potentially relevant clinical application of photoswitches pertains to chronic pain. The Kramer group has developed a cell‐impermeant PCL named QAQ, containing two QA groups spanning the azobenzene. QAQ enables photosilencing of neurons by non‐specifically inhibiting several voltage‐gated channels (NaV, KV, and CaV channels 176). Interestingly, QAQ exerts its block from the intracellular side of the membrane. To reach the intracellular, QAQ must enter the cells via a membrane conduit, such as the pore‐dilated TRPV1 channel—the major heat sensors in nociceptor neurons—that allows permeation of large and charged molecules such as QAQ (Fig 4B). Because pathological pain is characterized by hyperactivity of nociceptor neurons (which may also exhibit increased activity of TRPV1), QAQ can thereby selectively enter hyperactive nociceptors to silence them. While the pore dilation mechanism is currently under debate 177, this mechanism has been established for a lidocaine‐related anesthetic, QX‐314 178.
As a therapy for cancer, a new class of photoswitches—photostatins—has recently emerged. These PCLs bind to and interfere with microtubule dynamics, inhibit mitosis, and promote apoptosis 179. These photoswitches are based on combretastatin A‐4, a potential chemotherapeutic drug in clinical trials. The photoswitch consists of a fragmented drug, whose parts span the azobenzene‐core. Following isomerization to –cis, the two fragments are combined, yielding the final active form of the drug (Fig 1I).
Conclusion
Synthetic optogenetics was once considered a highly specialized technique, benefitting but a handful of laboratories. Today, however, the technique has become more approachable, judging by the growing number of laboratories across the world employing and developing the technique. To date, the variety of tools makes it possible to explore the role and signaling mechanisms of numerous receptors subtypes, in their natural environment, in vitro as well as in vivo. Nevertheless, there are still several hurdles to cross before synthetic optogenetics becomes widely accepted and clinically used (see Box 1). Luckily, major steps have been taken in that direction, with better photoswitches and gating mechanisms increasingly appearing in the literature.
It is important to recognize that whereas photoreceptor‐based optogenetic tools are unsurpassed for controlling mechanisms such as cellular excitability, synthetic optogenetic tools are more suited for controlling native receptor function or for modulating endogenous signaling mechanisms. In fact, the two techniques are highly complementary. For example, ChR2 and LiGluNs could be used concomitantly at the synapse to induce LTP. In such an instance, photoactivation of ChR2 would be required to depolarize the synapse and relieve the Mg2+‐block from LiGluNs. Then, precisely timed photoactivation of LiGluN would induce robust Ca2 +‐entry, leading to LTP at the synapse.
Nevertheless, despite the numerous photoswitches produced, synthetic optogenetics is still not as broadly employed by the neuroscience community, especially when compared with opsin‐based optogenetic tools. One of the main reasons for this situation is the lack of commercial availability or absence of a chemical depository (but see exceptions in Box 1). It is unlikely that chemical companies will start synthesizing photoswitches for several reasons: the arduous and expensive synthesis process, low demand, therefore narrow profit margin, and patent issues. Secondly, though a chemical depository would certainly help for research groups to gain access to the compounds, such a chemical bank would need to eventually synthesize the photoswitches in‐house, which is expensive and thus highly unlikely. To date, the most common practice is to directly obtain photoswitches from the developing groups, despite potential conflicts of interests or competitions. In fact, as far as we are aware, most research groups are open to distribute their photoswitches and/or start collaborations.
Taken together, we expect the technique to be further perfected and optimized and hope to see many more exciting developments in the near future, such as novel light‐gated receptors, multiphoton‐compatible switches applied in vivo, and perhaps even acoustic and magneto‐switches.
Conflict of interest
The authors declare that they have no conflict of interest.
EMBO Reports (2017) 18: 677–692
See the Glossary for abbreviations used in this article.
References
- 1. Luscher C, Isaac JT (2009) The synapse: center stage for many brain diseases. J Physiol 587: 727–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14: 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Newcomer JW, Farber NB, Olney JW (2000) NMDA receptor function, memory, and brain aging. Dialogues Clin Neurosci 2: 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J, Yu S, Fu Y, Li X (2014) Synaptic proteins and receptors defects in autism spectrum disorders. Front Cell Neurosci 8: 276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Spronsen M, Hoogenraad CC (2010) Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep 10: 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lepeta K, Lourenco MV, Schweitzer BC, Martino Adami PV, Banerjee P, Catuara‐Solarz S, de La Fuente Revenga M, Guillem AM, Haidar M, Ijomone OM et al (2016) Synaptopathies: synaptic dysfunction in neurological disorders – A review from students to students. J Neurochem 138: 785–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petrini EM, Barberis A (2014) Methods for the study of synaptic receptor functional properties. Methods Mol Biol 1183: 117–141 [DOI] [PubMed] [Google Scholar]
- 8. Restituito S, Ziff EB (2006) Methods for uncovering the mechanisms of AMPA receptor trafficking. In The dynamic synapse: molecular methods in ionotropic receptor biology, Kittler JT, Moss SJ. (eds), Boca Raton, FL: CRC Press/Taylor & Francis; [PubMed] [Google Scholar]
- 9. Ohno M, Frankland PW, Chen AP, Costa RM, Silva AJ (2001) Inducible, pharmacogenetic approaches to the study of learning and memory. Nat Neurosci 4: 1238–1243 [DOI] [PubMed] [Google Scholar]
- 10. Chen C, Tonegawa S (1997) Molecular genetic analysis of synaptic plasticity, activity‐dependent neural development, learning, and memory in the mammalian brain. Annu Rev Neurosci 20: 157–184 [DOI] [PubMed] [Google Scholar]
- 11. Brandon EP, Idzerda RL, McKnight GS (1995) Knockouts. Targeting the mouse genome: a compendium of knockouts (Part I). Curr Biol 5: 625–634 [DOI] [PubMed] [Google Scholar]
- 12. Gouaux E (2004) Structure and function of AMPA receptors. J Physiol 554: 249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartlett GJ, Todd AE, Thornton JM (2003) Inferring protein function from structure. Methods Biochem Anal 44: 387–407 [PubMed] [Google Scholar]
- 14. O'Sullivan GJ, O'Tuathaigh CM, Clifford JJ, O'Meara GF, Croke DT, Waddington JL (2006) Potential and limitations of genetic manipulation in animals. Drug Discov Today Technol 3: 173–180 [DOI] [PubMed] [Google Scholar]
- 15. Talevi A (2015) Multi‐target pharmacology: possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front Pharmacol 6: 205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall TC (1971) The limitations of molecular pharmacology. J Clin Pharmacol New Drugs 11: 322 [PubMed] [Google Scholar]
- 17. Thornton JM, Todd AE, Milburn D, Borkakoti N, Orengo CA (2000) From structure to function: approaches and limitations. Nat Struct Biol 7(Suppl): 991–994 [DOI] [PubMed] [Google Scholar]
- 18. Deisseroth K (2015) Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci 18: 1213–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hegemann P, Nagel G (2013) From channelrhodopsins to optogenetics. EMBO Mol Med 5: 173–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tye KM, Deisseroth K (2012) Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci 13: 251–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Horst MA, Hellingwerf KJ (2004) Photoreceptor proteins, “star actors of modern times”: a review of the functional dynamics in the structure of representative members of six different photoreceptor families. Acc Chem Res 37: 13–20 [DOI] [PubMed] [Google Scholar]
- 22. Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E (2003) Channelrhodopsin‐2, a directly light‐gated cation‐selective membrane channel. Proc Natl Acad Sci USA 100: 13940–13945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K (2005) Millisecond‐timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268 [DOI] [PubMed] [Google Scholar]
- 24. Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH (2006) Ectopic expression of a microbial‐type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 50: 23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishizuka T, Kakuda M, Araki R, Yawo H (2006) Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light‐gated channels. Neurosci Res 54: 85–94 [DOI] [PubMed] [Google Scholar]
- 26. Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S (2005) Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci USA 102: 17816–17821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A (2005) Light activation of channelrhodopsin‐2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 15: 2279–2284 [DOI] [PubMed] [Google Scholar]
- 28. Forrest AR, Kawaji H, Rehli M, Baillie JK, de Hoon MJ, Haberle V, Lassmann T, Kulakovskiy IV, Lizio M, Itoh M et al (2014) A promoter‐level mammalian expression atlas. Nature 507: 462–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathes T (2016) Natural resources for optogenetic tools. Methods Mol Biol 1408: 19–36 [DOI] [PubMed] [Google Scholar]
- 30. Polstein LR, Gersbach CA (2015) A light‐inducible CRISPR‐Cas9 system for control of endogenous gene activation. Nat Chem Biol 11: 198–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levskaya A, Weiner OD, Lim WA, Voigt CA (2009) Spatiotemporal control of cell signalling using a light‐switchable protein interaction. Nature 461: 997–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tischer D, Weiner OD (2014) Illuminating cell signalling with optogenetic tools. Nat Rev Mol Cell Biol 15: 551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zemelman BV, Lee GA, Ng M, Miesenbock G (2002) Selective photostimulation of genetically chARGed neurons. Neuron 33: 15–22 [DOI] [PubMed] [Google Scholar]
- 34. Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P (2002) Channelrhodopsin‐1: a light‐gated proton channel in green algae. Science 296: 2395–2398 [DOI] [PubMed] [Google Scholar]
- 35. Dugue GP, Akemann W, Knopfel T (2012) A comprehensive concept of optogenetics. Prog Brain Res 196: 1–28 [DOI] [PubMed] [Google Scholar]
- 36. Govorunova EG, Cunha SR, Sineshchekov OA, Spudich JL (2016) Anion channelrhodopsins for inhibitory cardiac optogenetics. Sci Rep 6: 33530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prigge M, Schneider F, Tsunoda SP, Shilyansky C, Wietek J, Deisseroth K, Hegemann P (2012) Color‐tuned channelrhodopsins for multiwavelength optogenetics. J Biol Chem 287: 31804–31812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nussinovitch U, Gepstein L (2015) Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat Biotechnol 33: 750–754 [DOI] [PubMed] [Google Scholar]
- 39. Xie YF, Jackson MF, Macdonald JF (2013) Optogenetics and synaptic plasticity. Acta Pharmacol Sin 34: 1381–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malyshev A, Goz R, LoTurco JJ, Volgushev M (2015) Advantages and limitations of the use of optogenetic approach in studying fast‐scale spike encoding. PLoS One 10: e0122286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahn M, Prigge M, Ron S, Levy R, Yizhar O (2016) Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat Neurosci 19: 554–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perny M, Muri L, Dawson H, Kleinlogel S (2016) Chronic activation of the D156A point mutant of Channelrhodopsin‐2 signals apoptotic cell death: the good and the bad. Cell Death Dis 7: e2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malyshev AY, Roshchin MV, Smirnova GR, Dolgikh DA, Balaban PM, Ostrovsky MA (2017) Chloride conducting light activated channel GtACR2 can produce both cessation of firing and generation of action potentials in cortical neurons in response to light. Neurosci Lett 640: 76–80 [DOI] [PubMed] [Google Scholar]
- 44. Herman AM, Huang L, Murphey DK, Garcia I, Arenkiel BR (2014) Cell type‐specific and time‐dependent light exposure contribute to silencing in neurons expressing Channelrhodopsin‐2. Elife 3: e01481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schoenenberger P, Scharer YP, Oertner TG (2011) Channelrhodopsin as a tool to investigate synaptic transmission and plasticity. Exp Physiol 96: 34–39 [DOI] [PubMed] [Google Scholar]
- 46. Zhang YP, Oertner TG (2007) Optical induction of synaptic plasticity using a light‐sensitive channel. Nat Methods 4: 139–141 [DOI] [PubMed] [Google Scholar]
- 47. Marek KW, Davis GW (2002) Transgenically encoded protein photoinactivation (FlAsH‐FALI): acute inactivation of synaptotagmin I. Neuron 36: 805–813 [DOI] [PubMed] [Google Scholar]
- 48. Rost BR, Schneider F, Grauel MK, Wozny C, Bentz CG, Blessing A, Rosenmund T, Jentsch TJ, Schmitz D, Hegemann P et al (2015) Optogenetic acidification of synaptic vesicles and lysosomes. Nat Neurosci 18: 1845–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kramer RH, Mourot A, Adesnik H (2013) Optogenetic pharmacology for control of native neuronal signaling proteins. Nat Neurosci 16: 816–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Szobota S, Isacoff EY (2010) Optical control of neuronal activity. Annu Rev Biophys 39: 329–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reiner A, Isacoff EY (2014) Photoswitching of cell surface receptors using tethered ligands. Methods Mol Biol 1148: 45–68 [DOI] [PubMed] [Google Scholar]
- 52. Berlin S, Isacoff EY (2017) Optical control of glutamate receptors of the NMDA‐kind in mammalian neurons, with the use of photoswitchable ligands In Biochemical approaches to glutamatergic neurotransmission Chapter 7, Springer. (in press) [Google Scholar]
- 53. Kennis JT, Mathes T (2013) Molecular eyes: proteins that transform light into biological information. Interface Focus 3: 20130005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Grobner G, Burnett IJ, Glaubitz C, Choi G, Mason AJ, Watts A (2000) Observations of light‐induced structural changes of retinal within rhodopsin. Nature 405: 810–813 [DOI] [PubMed] [Google Scholar]
- 55. Szymanski W, Beierle JM, Kistemaker HA, Velema WA, Feringa BL (2013) Reversible photocontrol of biological systems by the incorporation of molecular photoswitches. Chem Rev 113: 6114–6178 [DOI] [PubMed] [Google Scholar]
- 56. Beharry AA, Woolley GA (2011) Azobenzene photoswitches for biomolecules. Chem Soc Rev 40: 4422–4437 [DOI] [PubMed] [Google Scholar]
- 57. Kaufman H, Vratsanos SM, Erlanger BF (1968) Photoregulation of an enzymic process by means of a light‐sensitive ligand. Science 162: 1487–1489 [DOI] [PubMed] [Google Scholar]
- 58. Bieth J, Wassermann N, Vratsanos SM, Erlanger BF (1970) Photoregulation of biological activity by photochromic reagents, IV. A model for diurnal variation of enzymic activity. Proc Natl Acad Sci USA 66: 850–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bieth J, Vratsanos SM, Wassermann N, Erlanger BF (1969) Photoregulation of biological activity by photocromic reagents. II. Inhibitors of acetylcholinesterase. Proc Natl Acad Sci USA 64: 1103–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wainberg MA, Erlanger BF (1971) Investigation of the active center of trypsin using photochromic substrates. Biochemistry 10: 3816–3819 [DOI] [PubMed] [Google Scholar]
- 61. Montagnoli G, Monti S, Nannicini L, Felicioli R (1976) Azoaldolase photosensitivity. Photochem Photobiol 23: 29–32 [DOI] [PubMed] [Google Scholar]
- 62. Montagnoli G (1974) Azoaldolase: a photochromic enzyme. Acta Vitaminol Enzymol 28: 268–275 [PubMed] [Google Scholar]
- 63. Hug DH (1978) The activation of enzymes with light In Photochemical and photobiological reviews, Smith KC. (ed.), Vol. 3, pp 1–33. Boston, MA: Springer US; [Google Scholar]
- 64. Deal WJ, Erlanger BF, Nachmansohn D (1969) Photoregulation of biological activity by photochromic reagents. 3. Photoregulation of bioelectricity by acetylcholine receptor inhibitors. Proc Natl Acad Sci USA 64: 1230–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lester HA, Chang HW (1977) Response of acetylcholine receptors to rapid photochemically produced increases in agonist concentration. Nature 266: 373–374 [DOI] [PubMed] [Google Scholar]
- 66. Lester HA, Krouse ME, Nass MM, Wassermann NH, Erlanger BF (1979) Light‐activated drug confirms a mechanism of ion channel blockade. Nature 280: 509–510 [DOI] [PubMed] [Google Scholar]
- 67. Chabala LD, Lester HA (1986) Activation of acetylcholine receptor channels by covalently bound agonists in cultured rat myoballs. J Physiol 379: 83–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chabala LD, Gurney AM, Lester HA (1986) Dose‐response of acetylcholine receptor channels opened by a flash‐activated agonist in voltage‐clamped rat myoballs. J Physiol 371: 407–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lester HA, Chabala LD, Gurney AM, Sheridan RE (1986) Experiments with photoisomerizable molecules at nicotinic acetylcholine receptors in cells and membrane patches from rat muscle. Soc Gen Physiol Ser 40: 447–462 [PubMed] [Google Scholar]
- 70. Bartels E, Wassermann NH, Erlanger BF (1971) Photochromic activators of the acetylcholine receptor. Proc Natl Acad Sci USA 68: 1820–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Namba K, Suzuki S (1975) Photo‐control of enzyme activity with a photochromic spiropyran compound: modification of α‐amylase with spiropyran compound. Chem Lett 9: 947–950 [Google Scholar]
- 72. Karube I, Nakamoto Y, Namba K, Suzuki S (1976) Photocontrol of urease‐collagen membrane activity. Biochim Biophys Acta 429: 975–981 [DOI] [PubMed] [Google Scholar]
- 73. Lester HA, Nass MM, Krouse ME, Nerbonne JM, Wassermann NH, Erlanger BF (1980) Electrophysiological experiments with photoisomerizable cholinergic compounds: review and progress report. Ann N Y Acad Sci 346: 475–490 [DOI] [PubMed] [Google Scholar]
- 74. Lester HA, Nerbonne JM (1982) Physiological and pharmacological manipulations with light flashes. Annu Rev Biophys Bioeng 11: 151–175 [DOI] [PubMed] [Google Scholar]
- 75. Erlanger BF (1976) Photoregulation of biologically active macromolecules. Annu Rev Biochem 45: 257–283 [DOI] [PubMed] [Google Scholar]
- 76. Brownlee C (2006) Gateways to collaboration. ACS Chem Biol 1: 10–13 [DOI] [PubMed] [Google Scholar]
- 77. Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH (2004) Light‐activated ion channels for remote control of neuronal firing. Nat Neurosci 7: 1381–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Adamantidis A, Arber S, Bains JS, Bamberg E, Bonci A, Buzsaki G, Cardin JA, Costa RM, Dan Y, Goda Y et al (2015) Optogenetics: 10 years after ChR2 in neurons–views from the community. Nat Neurosci 18: 1202–1212 [DOI] [PubMed] [Google Scholar]
- 79. Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280: 69–77 [DOI] [PubMed] [Google Scholar]
- 80. Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R (2002) The open pore conformation of potassium channels. Nature 417: 523–526 [DOI] [PubMed] [Google Scholar]
- 81. Clapham DE (2003) Symmetry, selectivity, and the 2003 Nobel Prize. Cell 115: 641–646 [DOI] [PubMed] [Google Scholar]
- 82. Folgering JH, Kuiper JM, de Vries AH, Engberts JB, Poolman B (2004) Lipid‐mediated light activation of a mechanosensitive channel of large conductance. Langmuir 20: 6985–6987 [DOI] [PubMed] [Google Scholar]
- 83. Kocer A, Walko M, Meijberg W, Feringa BL (2005) A light‐actuated nanovalve derived from a channel protein. Science 309: 755–758 [DOI] [PubMed] [Google Scholar]
- 84. Yoshimura K, Batiza A, Schroeder M, Blount P, Kung C (1999) Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys J 77: 1960–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Spicer CD, Davis BG (2014) Selective chemical protein modification. Nat Commun 5: 4740 [DOI] [PubMed] [Google Scholar]
- 86. Fodje MN, Al‐Karadaghi S (2002) Occurrence, conformational features and amino acid propensities for the pi‐helix. Protein Eng 15: 353–358 [DOI] [PubMed] [Google Scholar]
- 87. Raimondo JV, Richards BA, Woodin MA (2016) Neuronal chloride and excitability ‐ the big impact of small changes. Curr Opin Neurobiol 43: 35–42 [DOI] [PubMed] [Google Scholar]
- 88. Stein M, Middendorp SJ, Carta V, Pejo E, Raines DE, Forman SA, Sigel E, Trauner D (2012) Azo‐propofols: photochromic potentiators of GABA(A) receptors. Angew Chem Int Ed Engl 51: 10500–10504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yue L, Pawlowski M, Dellal SS, Xie A, Feng F, Otis TS, Bruzik KS, Qian H, Pepperberg DR (2012) Robust photoregulation of GABA(A) receptors by allosteric modulation with a propofol analogue. Nat Commun 3: 1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lin WC, Davenport CM, Mourot A, Vytla D, Smith CM, Medeiros KA, Chambers JJ, Kramer RH (2014) Engineering a light‐regulated GABAA receptor for optical control of neural inhibition. ACS Chem Biol 9: 1414–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lin WC, Tsai MC, Davenport CM, Smith CM, Veit J, Wilson NM, Adesnik H, Kramer RH (2015) A Comprehensive optogenetic pharmacology toolkit for in vivo control of GABA(A) receptors and synaptic inhibition. Neuron 88: 879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chalifoux JR, Carter AG (2011) GABAB receptor modulation of synaptic function. Curr Opin Neurobiol 21: 339–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Quandt G, Hofner G, Pabel J, Dine J, Eder M, Wanner KT (2014) First photoswitchable neurotransmitter transporter inhibitor: light‐induced control of gamma‐aminobutyric acid transporter 1 (GAT1) activity in mouse brain. J Med Chem 57: 6809–6821 [DOI] [PubMed] [Google Scholar]
- 94. Feliciano M, Vytla D, Medeiros KA, Chambers JJ (2010) The GABA(A) receptor as a target for photochromic molecules. Bioorg Med Chem 18: 7731–7738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62: 405–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Niswender CM, Conn PJ (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50: 295–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bowie D (2008) Ionotropic glutamate receptors & CNS disorders. CNS Neurol Disord Drug Targets 7: 129–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14: 383–400 [DOI] [PubMed] [Google Scholar]
- 99. Baier H, Scott EK (2009) Genetic and optical targeting of neural circuits and behavior–zebrafish in the spotlight. Curr Opin Neurobiol 19: 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Caporale N, Kolstad KD, Lee T, Tochitsky I, Dalkara D, Trauner D, Kramer R, Dan Y, Isacoff EY, Flannery JG (2011) LiGluR restores visual responses in rodent models of inherited blindness. Mol Ther 19: 1212–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Carroll EC, Berlin S, Levitz J, Kienzler MA, Yuan Z, Madsen D, Larsen DS, Isacoff EY (2015) Two‐photon brightness of azobenzene photoswitches designed for glutamate receptor optogenetics. Proc Natl Acad Sci USA 112: E776–E785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gaub BM, Berry MH, Holt AE, Reiner A, Kienzler MA, Dolgova N, Nikonov S, Aguirre GD, Beltran WA, Flannery JG et al (2014) Restoration of visual function by expression of a light‐gated mammalian ion channel in retinal ganglion cells or ON‐bipolar cells. Proc Natl Acad Sci USA 111: E5574–E5583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Levitz J, Popescu AT, Reiner A, Isacoff EY (2016) A toolkit for orthogonal and in vivo optical manipulation of ionotropic glutamate receptors. Front Mol Neurosci 9: 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li D, Herault K, Isacoff EY, Oheim M, Ropert N (2012) Optogenetic activation of LiGluR‐expressing astrocytes evokes anion channel‐mediated glutamate release. J Physiol 590: 855–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, Volgraf M, Numano R, Aaron HL et al (2007) Remote control of neuronal activity with a light‐gated glutamate receptor. Neuron 54: 535–545 [DOI] [PubMed] [Google Scholar]
- 106. Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D (2006) Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol 2: 47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang S, Szobota S, Wang Y, Volgraf M, Liu Z, Sun C, Trauner D, Isacoff EY, Zhang X (2007) All optical interface for parallel, remote, and spatiotemporal control of neuronal activity. Nano Lett 7: 3859–3863 [DOI] [PubMed] [Google Scholar]
- 108. Numano R, Szobota S, Lau AY, Gorostiza P, Volgraf M, Roux B, Trauner D, Isacoff EY (2009) Nanosculpting reversed wavelength sensitivity into a photoswitchable iGluR. Proc Natl Acad Sci USA 106: 6814–6819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mayer ML (2005) Crystal structures of the GluR5 and GluR6 ligand binding cores: molecular mechanisms underlying kainate receptor selectivity. Neuron 45: 539–552 [DOI] [PubMed] [Google Scholar]
- 110. Reiner A, Isacoff EY (2014) Tethered ligands reveal glutamate receptor desensitization depends on subunit occupancy. Nat Chem Biol 10: 273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Berlin S, Szobota S, Reiner A, Carroll EC, Kienzler MA, Guyon A, Xiao T, Tauner D, Isacoff EY (2016) A family of photoswitchable NMDA receptors. Elife 5: e12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Janovjak H, Szobota S, Wyart C, Trauner D, Isacoff EY (2010) A light‐gated, potassium‐selective glutamate receptor for the optical inhibition of neuronal firing. Nat Neurosci 13: 1027–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen GQ, Cui C, Mayer ML, Gouaux E (1999) Functional characterization of a potassium‐selective prokaryotic glutamate receptor. Nature 402: 817–821 [DOI] [PubMed] [Google Scholar]
- 114. MacKinnon R (2003) Potassium channels. FEBS Lett 555: 62–65 [DOI] [PubMed] [Google Scholar]
- 115. Levitz J, Pantoja C, Gaub B, Janovjak H, Reiner A, Hoagland A, Schoppik D, Kane B, Stawski P, Schier AF et al (2013) Optical control of metabotropic glutamate receptors. Nat Neurosci 16: 507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rovira X, Trapero A, Pittolo S, Zussy C, Faucherre A, Jopling C, Giraldo J, Pin JP, Gorostiza P, Goudet C et al (2016) OptoGluNAM4.1, a photoswitchable allosteric antagonist for real‐time control of mGlu4 receptor activity. Cell Chem Biol 23: 929–934 [DOI] [PubMed] [Google Scholar]
- 117. Dalton JA, Lans I, Rovira X, Malhaire F, Gomez‐Santacana X, Pittolo S, Gorostiza P, Llebaria A, Goudet C, Pin JP et al (2016) Shining light on an mGlu5 photoswitchable NAM: a theoretical perspective. Curr Neuropharmacol 14: 441–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pittolo S, Gomez‐Santacana X, Eckelt K, Rovira X, Dalton J, Goudet C, Pin JP, Llobet A, Giraldo J, Llebaria A et al (2014) An allosteric modulator to control endogenous G protein‐coupled receptors with light. Nat Chem Biol 10: 813–815 [DOI] [PubMed] [Google Scholar]
- 119. Gascon‐Moya M, Pejoan A, Izquierdo‐Serra M, Pittolo S, Cabre G, Hernando J, Alibes R, Gorostiza P, Busque F (2015) An optimized glutamate receptor photoswitch with sensitized azobenzene isomerization. J Org Chem 80: 9915–9925 [DOI] [PubMed] [Google Scholar]
- 120. Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K (2003) A general method for the covalent labeling of fusion proteins with small molecules in vivo . Nat Biotechnol 21: 86–89 [DOI] [PubMed] [Google Scholar]
- 121. Broichhagen J, Damijonaitis A, Levitz J, Sokol KR, Leippe P, Konrad D, Isacoff EY, Trauner D (2015) Orthogonal optical control of a G protein‐coupled receptor with a SNAP‐tethered photochromic ligand. ACS Cent Sci 1: 383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Izquierdo‐Serra M, Bautista‐Barrufet A, Trapero A, Garrido‐Charles A, Diaz‐Tahoces A, Camarero N, Pittolo S, Valbuena S, Perez‐Jimenez A, Gay M et al (2016) Optical control of endogenous receptors and cellular excitability using targeted covalent photoswitches. Nat Commun 7: 12221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wofsy L, Metzger H, Singer SJ (1962) Affinity labeling‐a general method for labeling the active sites of antibody and enzyme molecules. Biochemistry 1: 1031–1039 [DOI] [PubMed] [Google Scholar]
- 124. Fortin DL, Banghart MR, Dunn TW, Borges K, Wagenaar DA, Gaudry Q, Karakossian MH, Otis TS, Kristan WB, Trauner D et al (2008) Photochemical control of endogenous ion channels and cellular excitability. Nat Methods 5: 331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fortin DL, Dunn TW, Fedorchak A, Allen D, Montpetit R, Banghart MR, Trauner D, Adelman JP, Kramer RH (2011) Optogenetic photochemical control of designer K+ channels in mammalian neurons. J Neurophysiol 106: 488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Volgraf M, Gorostiza P, Szobota S, Helix MR, Isacoff EY, Trauner D (2007) Reversibly caged glutamate: a photochromic agonist of ionotropic glutamate receptors. J Am Chem Soc 129: 260–261 [DOI] [PubMed] [Google Scholar]
- 127. Laprell L, Repak E, Franckevicius V, Hartrampf F, Terhag J, Hollmann M, Sumser M, Rebola N, DiGregorio DA, Trauner D (2015) Optical control of NMDA receptors with a diffusible photoswitch. Nat Commun 6: 8076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Stawski P, Sumser M, Trauner D (2012) A photochromic agonist of AMPA receptors. Angew Chem Int Ed Engl 51: 5748–5751 [DOI] [PubMed] [Google Scholar]
- 129. Barber DM, Liu SA, Gottschling K, Sumser M, Hollmann M, Trauner D (2017) Optical control of AMPA receptors using a photoswitchable quinoxaline‐2,3‐dione antagonist. Chem Sci 8: 611–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ellis‐Davies GC (2007) Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat Methods 4: 619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Reiter A, Skerra A, Trauner D, Schiefner A (2013) A photoswitchable neurotransmitter analogue bound to its receptor. Biochemistry 52: 8972–8974 [DOI] [PubMed] [Google Scholar]
- 132. Armstrong N, Gouaux E (2000) Mechanisms for activation and antagonism of an AMPA‐sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron 28: 165–181 [DOI] [PubMed] [Google Scholar]
- 133. Jin R, Banke TG, Mayer ML, Traynelis SF, Gouaux E (2003) Structural basis for partial agonist action at ionotropic glutamate receptors. Nat Neurosci 6: 803–810 [DOI] [PubMed] [Google Scholar]
- 134. Shieh CC, Coghlan M, Sullivan JP, Gopalakrishnan M (2000) Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacol Rev 52: 557–594 [PubMed] [Google Scholar]
- 135. Hille B (2001) Ion channels of excitable membranes, 3rd edn Sunderland, MA: Sinauer Associates Inc; [Google Scholar]
- 136. Choe S (2002) Potassium channel structures. Nat Rev Neurosci 3: 115–121 [DOI] [PubMed] [Google Scholar]
- 137. Chambers JJ, Banghart MR, Trauner D, Kramer RH (2006) Light‐induced depolarization of neurons using a modified Shaker K(+) channel and a molecular photoswitch. J Neurophysiol 96: 2792–2796 [DOI] [PubMed] [Google Scholar]
- 138. Mourot A, Tochitsky I, Kramer RH (2013) Light at the end of the channel: optical manipulation of intrinsic neuronal excitability with chemical photoswitches. Front Mol Neurosci 6: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Banghart MR, Mourot A, Fortin DL, Yao JZ, Kramer RH, Trauner D (2009) Photochromic blockers of voltage‐gated potassium channels. Angew Chem Int Ed Engl 48: 9097–9101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mourot A, Kienzler MA, Banghart MR, Fehrentz T, Huber FM, Stein M, Kramer RH, Trauner D (2011) Tuning photochromic ion channel blockers. ACS Chem Neurosci 2: 536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Fehrentz T, Kuttruff CA, Huber FM, Kienzler MA, Mayer P, Trauner D (2012) Exploring the pharmacology and action spectra of photochromic open‐channel blockers. ChemBioChem 13: 1746–1749 [DOI] [PubMed] [Google Scholar]
- 142. Sandoz G, Levitz J (2013) Optogenetic techniques for the study of native potassium channels. Front Mol Neurosci 6: 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sandoz G, Levitz J, Kramer RH, Isacoff EY (2012) Optical control of endogenous proteins with a photoswitchable conditional subunit reveals a role for TREK1 in GABA(B) signaling. Neuron 74: 1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Dascal N, Kahanovitch U (2015) The roles of Gbetagamma and Galpha in gating and regulation of GIRK channels. Int Rev Neurobiol 123: 27–85 [DOI] [PubMed] [Google Scholar]
- 145. Barber DM, Schonberger M, Burgstaller J, Levitz J, Weaver CD, Isacoff EY, Baier H, Trauner D (2016) Optical control of neuronal activity using a light‐operated GIRK channel opener (LOGO). Chem Sci 7: 2347–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Trads JB, Burgstaller J, Laprell L, Konrad DB, de la Osa de la Rosa L, Weaver CD, Baier H, Trauner D, Barber DM (2016) Optical control of GIRK channels using visible light. Org Biomol Chem 15: 76–81 [DOI] [PubMed] [Google Scholar]
- 147. Kaufmann K, Romaine I, Days E, Pascual C, Malik A, Yang L, Zou B, Du Y, Sliwoski G, Morrison RD et al (2013) ML297 (VU0456810), the first potent and selective activator of the GIRK potassium channel, displays antiepileptic properties in mice. ACS Chem Neurosci 4: 1278–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yakubovich D, Berlin S, Kahanovitch U, Rubinstein M, Farhy‐Tselnicker I, Styr B, Keren‐Raifman T, Dessauer CW, Dascal N (2015) A quantitative model of the GIRK1/2 channel reveals that its basal and evoked activities are controlled by unequal stoichiometry of Galpha and Gbetagamma. PLoS Comput Biol 11: e1004598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Khakh BS, North RA (2012) Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron 76: 51–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Burnstock G, Nistri A, Khakh BS, Giniatullin R (2014) ATP‐gated P2X receptors in health and disease. Front Cell Neurosci 8: 204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lemoine D, Habermacher C, Martz A, Mery PF, Bouquier N, Diverchy F, Taly A, Rassendren F, Specht A, Grutter T (2013) Optical control of an ion channel gate. Proc Natl Acad Sci USA 110: 20813–20818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Mruk K, Kobertz WR (2015) Bioreactive tethers. Adv Exp Med Biol 869: 77–100 [DOI] [PubMed] [Google Scholar]
- 153. Habermacher C, Martz A, Calimet N, Lemoine D, Peverini L, Specht A, Cecchini M, Grutter T (2016) Photo‐switchable tweezers illuminate pore‐opening motions of an ATP‐gated P2X ion channel. Elife 5: e11050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP (2009) Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov 8: 733–750 [DOI] [PubMed] [Google Scholar]
- 155. Picciotto MR, Higley MJ, Mineur YS (2012) Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76: 116–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lester HA, Krouse ME, Nass MM, Wassermann NH, Erlanger BF (1980) A covalently bound photoisomerizable agonist: comparison with reversibly bound agonists at Electrophorus electroplaques. J Gen Physiol 75: 207–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Damijonaitis A, Broichhagen J, Urushima T, Hull K, Nagpal J, Laprell L, Schonberger M, Woodmansee DH, Rafiq A, Sumser MP et al (2015) AzoCholine enables optical control of alpha 7 nicotinic acetylcholine receptors in neural networks. ACS Chem Neurosci 6: 701–707 [DOI] [PubMed] [Google Scholar]
- 158. Tochitsky I, Banghart MR, Mourot A, Yao JZ, Gaub B, Kramer RH, Trauner D (2012) Optochemical control of genetically engineered neuronal nicotinic acetylcholine receptors. Nat Chem 4: 105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Denk W, Strickler JH, Webb WW (1990) Two‐photon laser scanning fluorescence microscopy. Science 248: 73–76 [DOI] [PubMed] [Google Scholar]
- 160. Svoboda K, Yasuda R (2006) Principles of two‐photon excitation microscopy and its applications to neuroscience. Neuron 50: 823–839 [DOI] [PubMed] [Google Scholar]
- 161. Ellis‐Davies GC (2008) Neurobiology with caged calcium. Chem Rev 108: 1603–1613 [DOI] [PubMed] [Google Scholar]
- 162. Olson JP, Kwon HB, Takasaki KT, Chiu CQ, Higley MJ, Sabatini BL, Ellis‐Davies GC (2013) Optically selective two‐photon uncaging of glutamate at 900 nm. J Am Chem Soc 135: 5954–5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Prakash R, Yizhar O, Grewe B, Ramakrishnan C, Wang N, Goshen I, Packer AM, Peterka DS, Yuste R, Schnitzer MJ et al (2012) Two‐photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat Methods 9: 1171–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Papagiakoumou E, Anselmi F, Begue A, de Sars V, Gluckstad J, Isacoff EY, Emiliani V (2010) Scanless two‐photon excitation of channelrhodopsin‐2. Nat Methods 7: 848–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Rickgauer JP, Tank DW (2009) Two‐photon excitation of channelrhodopsin‐2 at saturation. Proc Natl Acad Sci USA 106: 15025–15030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Mohanty SK, Reinscheid RK, Liu X, Okamura N, Krasieva TB, Berns MW (2008) In‐depth activation of channelrhodopsin 2‐sensitized excitable cells with high spatial resolution using two‐photon excitation with a near‐infrared laser microbeam. Biophys J 95: 3916–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Packer AM, Peterka DS, Hirtz JJ, Prakash R, Deisseroth K, Yuste R (2012) Two‐photon optogenetics of dendritic spines and neural circuits. Nat Methods 9: 1202–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Oron D, Papagiakoumou E, Anselmi F, Emiliani V (2012) Two‐photon optogenetics. Prog Brain Res 196: 119–143 [DOI] [PubMed] [Google Scholar]
- 169. De Boni L, Misoguti L, Zilio SC, Mendonca CR (2005) Degenerate two‐photon absorption spectra in azoaromatic compounds. ChemPhysChem 6: 1121–1125 [DOI] [PubMed] [Google Scholar]
- 170. Izquierdo‐Serra M, Gascon‐Moya M, Hirtz JJ, Pittolo S, Poskanzer KE, Ferrer E, Alibes R, Busque F, Yuste R, Hernando J et al (2014) Two‐photon neuronal and astrocytic stimulation with azobenzene‐based photoswitches. J Am Chem Soc 136: 8693–8701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Kienzler MA, Reiner A, Trautman E, Yoo S, Trauner D, Isacoff EY (2013) A red‐shifted, fast‐relaxing azobenzene photoswitch for visible light control of an ionotropic glutamate receptor. J Am Chem Soc 135: 17683–17686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Tochitsky I, Helft Z, Meseguer V, Fletcher RB, Vessey KA, Telias M, Denlinger B, Malis J, Fletcher EL, Kramer RH (2016) How azobenzene photoswitches restore visual responses to the blind retina. Neuron 92: 100–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Tochitsky I, Polosukhina A, Degtyar VE, Gallerani N, Smith CM, Friedman A, Van Gelder RN, Trauner D, Kaufer D, Kramer RH (2014) Restoring visual function to blind mice with a photoswitch that exploits electrophysiological remodeling of retinal ganglion cells. Neuron 81: 800–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Polosukhina A, Litt J, Tochitsky I, Nemargut J, Sychev Y, De Kouchkovsky I, Huang T, Borges K, Trauner D, Van Gelder RN et al (2012) Photochemical restoration of visual responses in blind mice. Neuron 75: 271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Tochitsky I, Kramer RH (2015) Optopharmacological tools for restoring visual function in degenerative retinal diseases. Curr Opin Neurobiol 34: 74–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Mourot A, Fehrentz T, Le Feuvre Y, Smith CM, Herold C, Dalkara D, Nagy F, Trauner D, Kramer RH (2012) Rapid optical control of nociception with an ion‐channel photoswitch. Nat Methods 9: 396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bean BP (2015) Pore dilation reconsidered. Nat Neurosci 18: 1534–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. McCleskey EW (2007) Neuroscience: a local route to pain relief. Nature 449: 545–546 [DOI] [PubMed] [Google Scholar]
- 179. Borowiak M, Nahaboo W, Reynders M, Nekolla K, Jalinot P, Hasserodt J, Rehberg M, Delattre M, Zahler S, Vollmar A et al (2015) Photoswitchable inhibitors of microtubule dynamics optically control mitosis and cell death. Cell 162: 403–411 [DOI] [PubMed] [Google Scholar]
- 180. Broichhagen J, Trauner D (2014) The in vivo chemistry of photoswitched tethered ligands. Curr Opin Chem Biol 21: 121–127 [DOI] [PubMed] [Google Scholar]
- 181. Frank JA, Moroni M, Moshourab R, Sumser M, Lewin GR, Trauner D (2015) Photoswitchable fatty acids enable optical control of TRPV1. Nat Commun 6: 7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Broichhagen J, Frank JA, Trauner D (2015) A roadmap to success in photopharmacology. Acc Chem Res 48: 1947–1960 [DOI] [PubMed] [Google Scholar]
- 183. Emiliani V, Cohen AE, Deisseroth K, Häusser M (2015) All‐optical interrogation of neural circuits. J Neurosci 35: 13917–13926 [DOI] [PMC free article] [PubMed] [Google Scholar]


