Abstract
Purpose
To provide sex‐stratified normative data on retinal thickness and study the relationship with sex, age and refractive status.
Methods
Population‐based study including 2617 women and 1891 men, aged 38–87 (mean 61 ± 8) years, without diabetes, glaucoma and retinal diseases, and spherical equivalent refraction (SER) within ±6 dioptres. Retinal thickness was measured with optical coherence tomography (spectral domain Cirrus HD‐OCT).
Results
Women had thinner retina than men. Retinal thickness was significantly associated with refraction, where mean change in retinal thickness per 1 D increase in SER was −1.3 (0.2) μm in the fovea, 0.7 (0.1) μm in the pericentral ring and 1.4 (0.1) μm in the peripheral ring. In the fovea, there was a non‐monotonic curved relationship between retinal thickness and age in both sexes with a maximum at about 60 years (p < 0.001). In the pericentral ring, the mean reduction in retinal thickness per 10‐year increase was 2.7 (0.3) μm in women and 4.0 (0.4) μm in men and corresponding results in the peripheral ring were 2.3 (0.3) μm in women and 2.6 (0.4) μm in men. In both regions, there was evidence for a nonlinear pattern with an increased rate of change with higher age. There was a significant interaction between sex and age for retinal thickness of the pericentral ring (p = 0.041).
Conclusion
Women had thinner retina than men, and thickness varied with refractive status. Retinal thickness was associated with age in all macular regions, and the rate of change in retinal thickness varied at different ages.
Keywords: age, epidemiology, optical coherence tomography, population based, refraction, retina, sex
Introduction
Optical coherence tomography (OCT) is a non‐invasive imaging technology, widely used in clinical practice to evaluate retinal thickness and the presence of structural changes in retinal diseases. Normative data from OCT software are not publicly available. Further, available OCT devices differ in segmentation algorithms, so measurements are not directly comparable between instruments (Giani et al. 2010; Pierro et al. 2010; Tan et al. 2015).
Most studies on retinal thickness from OCT have been small and partly clinically based, while a few larger scale population‐based studies have been published recently (Duan et al. 2010; Tariq et al. 2011; Gupta et al. 2013; Myers et al. 2015). There is good evidence that women have thinner retina than men (Kelty et al. 2008; Eriksson & Alm 2009; Duan et al. 2010; Ooto et al. 2010; Song et al. 2010; Adhi et al. 2012; Gupta et al. 2013; Myers et al. 2015). High age has been found to be associated with thinner retina in the peripheral macula in most studies while results considering the foveal thickness as well as change with age in younger adults have been inconsistent (Duan et al. 2010; Ooto et al. 2010, 2011; Song et al. 2010; Adhi et al. 2012; Demirkaya et al. 2013; Gupta et al. 2013; Myers et al. 2015). Refractive status and axial length seem to affect retinal thickness in the macula (Lam et al. 2007; Duan et al. 2010; Song et al. 2010; Gupta et al. 2013; Myers et al. 2015). Studies have shown differences in retinal thickness between different ethnic populations (Kelty et al. 2008; Girkin et al. 2011; Tariq et al. 2011).
Reliable normative data are important in both scientific and clinical settings. We aimed to investigate the influence of sex, age and refraction on the retinal thickness of the macula in persons without retinal diseases in a large population‐based study of women and men with a broad age span.
Subjects and Methods
Study sample
The Tromsø Eye Study is a substudy of the Tromsø Study, a population‐based multipurpose longitudinal study from the municipality of Tromsø, Norway. Participants were identified through the Population Registry of Norway, and the study selection criterion has been a combination of total and random samples of birth cohorts of the inhabitants of Tromsø, with details published elsewhere (Jacobsen et al. 2012; Bertelsen et al. 2013). The Tromsø Eye Study 1 was conducted in 2007–2008 within the sixth survey of the Tromsø Study. A total of 7306 persons attended (attendance rate of 64%) and 91% reported Caucasian ethnicity. Optical coherence tomography (OCT) scan of at least one eye was carried out in 6279 participants. We excluded participants with glaucoma and/or diabetes (n = 828). We further excluded eyes with OCT scans of low quality or with pathology on scans, eyes with high refractive error (spherical equivalent refraction) >+6.0 dioptres (D) or <−6.0 D, and eyes with retinal vascular occlusion evaluated from fundus photo, leaving 2617 women and 1891 men who were included in the study (Fig. S1). The study adhered to the tenets of the Declaration of Helsinki and was approved by the Data Inspectorate of Norway and Regional Committee for Medical and Health Research Ethics.
Procedures
Refraction was measured by Nidek AR 660A auto refractor (Nidek CO., LTD., Gamagori, Japan). Spherical equivalent refraction (SER) was calculated as spherical power plus half the cylindrical power in dioptres. Both pupils were dilated with tropicamide 0.5% (Chauvin Pharmaceuticals Ltd., Kingston Upon Thames, Surrey, England). Five‐field 45‐degree retinal colour photographs were taken on both eyes with a Visucam PRONM (CZM) digital retinal camera. Optical coherence tomography scans were taken of both eyes with the spectral domain (SD) Cirrus HD‐OCT model 4000 (Carl Zeiss Meditec (CZM), Jena, Germany), using the standard ‘512 × 128 macular cube’ scan protocol. cirrus hd‐oct research browser v.5.0 was used to evaluate scans (Bertelsen et al. 2013). A manual evaluation was performed to validate the automated lines for internal limiting membrane (ILM) and retinal pigment epithelium (RPE; segmentation line is drawn in the middle of the RPE band; Giani et al. 2010; Tan et al. 2015), and if scans were centred allowing measures of all nine sectors of the ETDRS (Early Treatment Diabetic Retinopathy Study) grid (Fig. 1). The presence of pathology on OCT within the whole macular cube was manually evaluated in regard to drusen, pigment epithelial detachment, epiretinal fibrosis, oedema, atrophy of RPE or retina, vitreomacular traction and/or macular hole. Automated measurements of average retinal thickness between ILM and RPE of nine subfields of the ETDRS grid (Fig. 1), macular volume of the total ETDRS grid and OCT scan signal strength were exported for analyses, all algorithms were supplied by CZM. Good quality scan was defined as scan satisfying (I) no defects or errors in the automated lines for ILM and RPE and (II) well centred and (III) signal strength ≥5.
Figure 1.
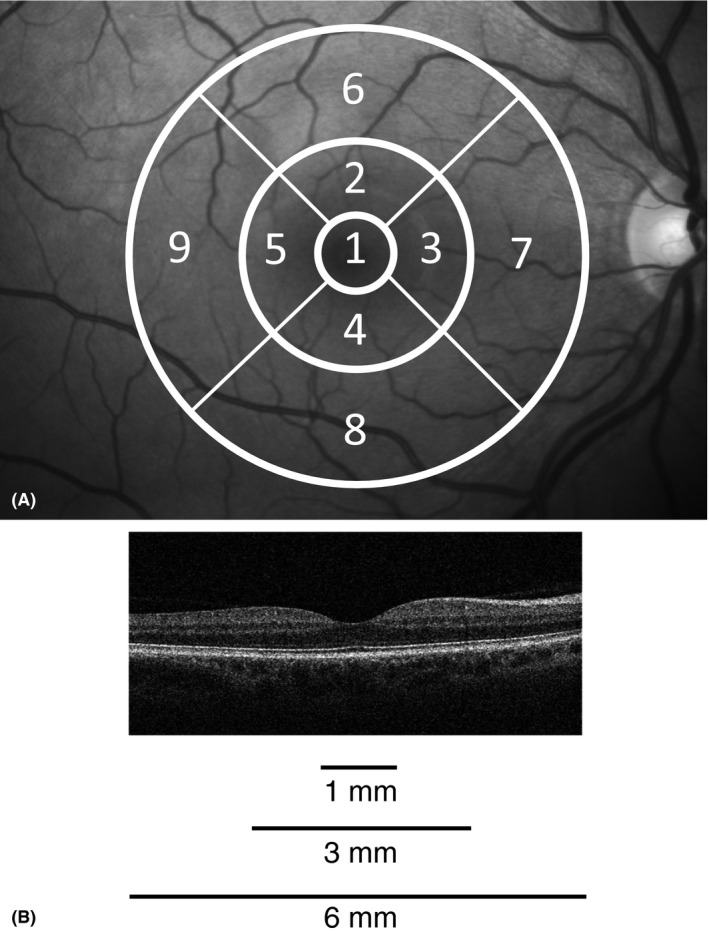
Optical coherence tomography (OCT) scan and the nine sectors of the ETDRS grid. (A) The nine sectors of the ETDRS grid (right eye). Fovea (sector 1), pericentral ring (sectors 2–5) and peripheral ring (sectors 6–9). (B) Representative horizontal spectral domain Cirrus HD‐OCT (Carl Zeiss Meditec) scan through the fovea from the right eye of a healthy subject.
Data on prevalent disease and cardiovascular risk factors were collected from questionnaires, laboratory testing and physical examinations, and details are given elsewhere (Jacobsen et al. 2012; von Hanno et al. 2014). The presence of glaucoma was based on self‐report from questionnaires. Diabetes was defined as self‐reported diabetes and/or use of diabetes medication and/or HbA1c ≥6.5%. Non‐fasting blood samples were obtained by venipuncture, and laboratory measurements were performed at the University Hospital of North Norway. HbA1c was measured in EDTA whole blood by high‐performance liquid chromatography (HPLC) using an automated analyser (Variant II, Bio‐Rad Laboratories Inc., Hercules, CA, USA).
Statistical analyses
stata/mp 14.0 for Mac (StataCorp, College Station, TX, USA) was used for all statistical analyses. p‐Values <0.05 were considered significant. Descriptive statistics were calculated for participant characteristics. Correlation between thickness measurements between right and left eyes was assessed with Pearson's correlation tests, and mean values of retinal thickness and refraction of both eyes were used when both eyes were available. Retinal thickness in nine sectors, the average of the four inner subfields (pericentral ring), the average of the four outer subfields (peripheral ring; Fig. 1) and retinal volume of the ETDRS grid were summarized and tested for gender difference in linear regression adjusted for age. Measurements were summarized in age groups <50 years, ≥80 years and 5 years age groups in between, stratified by sex, and tested for linear trend over age by linear regression. From inspection of age‐stratified thickness measurements we suspected a nonlinear relationship with age, we thus performed sex‐stratified fractional polynomial regression (mfp, Stata), which allows for nonlinear relationships (Royston et al. 1999; Royston & Sauerbrei 2008). Models, adjusted for refraction, were fitted by first (FP1, monotonic, linear/nonlinear) and second (FP2, non‐monotonic, nonlinear) degree fractional polynomials, with powers from the set φ = (−2, −1, −1/2, 0, 1/2, 1, 2, 3), giving in total 44 different models. The best model was chosen according to Royston & Sauerbrei (2008). The p‐value in FP2‐models was obtained by log‐likelihood test statistics comparing the model with the null model (variable not included). Associations, based on best (chosen) model, were graphically shown as fitted line. Regression residual plots from models, including smoothed residuals, were explored to evaluate model fit (Royston & Sauerbrei 2011). We performed sensitivity analyses on the part of the population aged 50–80 years to evaluate whether model choice was driven by the youngest and/or oldest age groups. Association with refraction was analysed by linear regression adjusted for sex and age, as there was no evidence of nonlinear relationship. Media opacities may influence retinal thickness measures (Darma et al. 2015) and we performed sensitivity analyses by restricting analyses to scans with signal strength ≥8 to see whether that affected the association with age and/or refraction. Explained variance, R 2, was calculated for each sex‐stratified model and a supplemental model including both sexes, and separate contribution from age, refraction and sex was evaluated by a R 2‐like statistics (Royston & Sauerbrei 2008). Post hoc we investigated multiplicative interaction between age and sex and age and refraction (difference between women and men in shape and/or slope of association) by entering the cross‐products in the regression models.
Results
Characteristics of study participants are presented in Table S1. Thickness measurements of right and left eyes were highly correlated, with correlation coefficients in the range 0.86–0.94 (all p < 0.001) for corresponding nine sectors, pericentral ring and peripheral ring. Compared to those excluded, persons included were younger and had lower blood pressure, HbA1c and SER and higher OCT signal strength, while other differences were only slight (Table S1).
Age‐stratified thickness and volume measures with linear trends are given for women (Table 1) and men (Table 2). Women had thinner retina in the fovea and pericentral ring than men; while in the peripheral ring, women had thinner retina in the temporal sector only (Table 3). In both the pericentral and peripheral ring, the nasal sector was thickest, followed by the superior, inferior and temporal sector. These regional patterns were similar in women and men.
Table 1.
Macular thickness and volume in healthy eyes in women, by age groups. Tromsø Eye Study 2007–2008
| Age groups (years) | Linear trenda | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| < 50 | 50–55 | 55–60 | 60–65 | 65–70 | 70–75 | 75–80 | ≥ 80 | p | |
| Number of subjects | 129 | 446 | 436 | 792 | 402 | 229 | 137 | 46 | 2617 |
| Macular thickness (μm) | |||||||||
| Fovea (1 mm diameter) | 259.5 (17.0) | 259.6 (20.5) | 261.9 (20.6) | 261.3 (19.5) | 259.5 (20.6) | 257.3 (20.0) | 254.7 (18.5) | 251.9 (21.2) | 0.002 |
| Pericentral ring (3 mm diameter) | |||||||||
| Nasal | 327.6 (14.1) | 326.9 (15.5) | 327.7 (14.4) | 325.8 (14.4) | 323.4 (15.4) | 320.4 (14.4) | 317.9 (13.8) | 315.7 (16.5) | <0.001 |
| Temporal | 312.1 (13.2) | 311.2 (14.2) | 313.0 (13.9) | 311.7 (13.3) | 309.8 (14.4) | 307.9 (13.6) | 305.8 (13.2) | 304.4 (15.9) | <0.001 |
| Superior | 324.6 (14.5) | 324.2 (15.1) | 324.8 (13.9) | 323.4 (13.7) | 320.8 (15.0) | 318.0 (14.2) | 315.0 (13.9) | 312.1 (18.1) | <0.001 |
| Inferior | 321.6 (13.8) | 320.5 (15.1) | 321.6 (14.5) | 319.7 (14.1) | 317.8 (15.0) | 315.0 (13.4) | 312.9 (14.0) | 311.4 (15.5) | <0.001 |
| Average | 321.5 (13.5) | 320.7 (14.5) | 321.7 (13.7) | 320.1 (13.5) | 318.0 (14.5) | 315.3 (13.4) | 312.9 (13.3) | 310.9 (16.0) | <0.001 |
| Peripheral ring (6 mm diameter) | |||||||||
| Nasal | 295.4 (15.5) | 294.6 (15.4) | 294.1 (14.5) | 291.7 (14.5) | 289.5 (15.7) | 285.9 (13.9) | 283.9 (15.0) | 282.7 (16.6) | <0.001 |
| Temporal | 259.7 (12.8) | 259.8 (12.4) | 260.9 (12.3) | 259.6 (12.2) | 258.5 (12.9) | 258.3 (11.5) | 256.9 (12.9) | 257.2 (13.7) | 0.002 |
| Superior | 277.8 (14.1) | 277.9 (13.5) | 277.8 (12.9) | 276.4 (12.5) | 274.7 (13.9) | 273.1 (12.8) | 271.2 (14.6) | 270.8 (15.5) | <0.001 |
| Inferior | 267.9 (13.1) | 267.4 (14.0) | 268.2 (13.1) | 266.0 (13.4) | 264.7 (14.0) | 263.2 (12.5) | 261.6 (13.4) | 260.8 (13.8) | <0.001 |
| Average | 275.2 (13.1) | 274.9 (13.1) | 275.2 (12.5) | 273.4 (12.3) | 271.8 (13.4) | 270.1 (11.7) | 268.4 (13.1) | 267.9 (14.0) | <0.001 |
| Macular volume (mm3) | |||||||||
| ETDRS grid | 8.048 (0.356) | 8.038 (0.363) | 8.053 (0.343) | 8.004 (0.341) | 7.956 (0.371) | 7.900 (0.327) | 7.847 (0.355) | 7.822 (0.397) | <0.001 |
Numbers are presented as mean (standard deviation).
Linear regression, age treated as continuous variable.
Table 2.
Macular thickness and volume in healthy eyes in men, by age groups. Tromsø Eye Study 2007–2008
| Age groups (years) | Linear trenda | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| < 50 | 50–55 | 55–60 | 60–65 | 65–70 | 70–75 | 75–80 | ≥ 80 | p | |
| Number of subjects | 59 | 336 | 369 | 548 | 244 | 199 | 102 | 34 | 1891 |
| Macular thickness (μm) | |||||||||
| Fovea (1 mm diameter) | 271.1 (18.3) | 273.9 (21.5) | 276.4 (19.4) | 276.5 (19.4) | 273.6 (20.9) | 269.7 (20.5) | 267.2 (22.3) | 267.9 (20.1) | 0.001 |
| Pericentral ring (3 mm diameter) | |||||||||
| Nasal | 333.0 (15.0) | 334.2 (16.2) | 333.0 (15.7) | 331.6 (14.9) | 327.6 (14.6) | 323.8 (16.0) | 321.5 (16.3) | 323.6 (14.7) | <0.001 |
| Temporal | 316.7 (15.1) | 318.8 (15.4) | 318.1 (15.2) | 318.0 (14.3) | 315.7 (14.5) | 311.5 (14.4) | 308.8 (16.0) | 309.5 (14.8) | <0.001 |
| Superior | 328.8 (15.0) | 328.8 (16.0) | 328.0 (15.6) | 327.3 (14.5) | 323.7 (15.0) | 319.6 (15.9) | 315.4 (15.8) | 317.5 (15.6) | <0.001 |
| Inferior | 325.7 (14.0) | 326.3 (15.6) | 325.1 (15.6) | 324.0 (14.8) | 321.0 (14.5) | 317.4 (15.3) | 314.8 (16.6) | 314.9 (15.7) | <0.001 |
| Average | 326.1 (14.3) | 327.0 (15.3) | 326.0 (15.1) | 325.2 (14.2) | 322.0 (14.2) | 318.1 (15.0) | 315.2 (15.7) | 316.4 (14.7) | <0.001 |
| Peripheral ring (6 mm diameter) | |||||||||
| Nasal | 294.8 (14.9) | 295.0 (15.9) | 293.9 (15.6) | 292.6 (15.2) | 288.9 (14.4) | 286.2 (15.3) | 282.6 (16.0) | 285.3 (16.8) | <0.001 |
| Temporal | 263.4 (11.9) | 264.2 (12.8) | 263.4 (12.7) | 263.8 (12.2) | 263.1 (12.4) | 260.9 (12.2) | 258.2 (12.8) | 258.3 (14.0) | <0.001 |
| Superior | 276.7 (12.6) | 277.1 (13.5) | 276.6 (13.4) | 276.5 (13.2) | 274.3 (13.4) | 272.4 (13.5) | 268.9 (14.0) | 269.2 (16.7) | <0.001 |
| Inferior | 267.4 (11.3) | 267.4 (13.8) | 267.3 (13.6) | 266.3 (12.9) | 264.8 (13.0) | 263.3 (13.0) | 261.0 (13.8) | 260.0 (16.0) | <0.001 |
| Average | 275.6 (11.8) | 275.9 (13.2) | 275.3 (13.1) | 274.8 (12.6) | 272.8 (12.5) | 270.7 (12.8) | 267.7 (13.1) | 268.2 (15.2) | <0.001 |
| Macular volume (mm3) | |||||||||
| ETDRS grid | 8.096 (0.331) | 8.111 (0.372) | 8.093 (0.366) | 8.078 (0.352) | 8.011 (0.350) | 7.940 (0.361) | 7.857 (0.375) | 7.876 (0.416) | <0.001 |
Numbers are presented as mean (standard deviation).
Linear regression, age treated as continuous variable.
Table 3.
Macular thickness and volume in healthy eyes, total and by sex and association with refraction. Tromsø Eye Study 2007–2008
| Sex | Refraction | |||||
|---|---|---|---|---|---|---|
| Totala | Womena | Mena | pb | β‐coeff (SE)c | p | |
| Number of subjects | 4508 | 2617 | 1891 | 4352 | ||
| Macular thickness (μm) | ||||||
| Fovea (1 mm diameter) | 265.9 (21.4) | 259.9 (20.0) | 274.1 (20.4) | <0.001 | −1.30 (0.17) | <0.001 |
| Pericentral ring (3 mm diameter) | ||||||
| Nasal | 327.2 (15.6) | 325.0 (15.0) | 330.3 (15.9) | <0.001 | 0.59 (0.13) | <0.001 |
| Temporal | 313.2 (14.7) | 310.8 (13.9) | 316.5 (15.1) | <0.001 | 0.55 (0.12) | <0.001 |
| Superior | 323.7 (15.2) | 322.3 (14.6) | 325.6 (15.8) | <0.001 | 0.86 (0.13) | <0.001 |
| Inferior | 320.7 (15.1) | 319.0 (14.6) | 322.9 (15.6) | <0.001 | 0.93 (0.13) | <0.001 |
| Average | 321.2 (14.7) | 319.3 (14.1) | 323.9 (15.1) | <0.001 | 0.73 (0.12) | <0.001 |
| Peripheral ring (6 mm diameter) | ||||||
| Nasal | 291.4 (15.5) | 291.4 (15.3) | 291.5 (15.8) | 0.61 | 1.11 (0.13) | <0.001 |
| Temporal | 260.9 (12.6) | 259.4 (12.4) | 263.0 (12.6) | <0.001 | 1.62 (0.10) | <0.001 |
| Superior | 275.8 (13.5) | 276.0 (13.4) | 275.4 (13.6) | 0.13 | 1.37 (0.11) | <0.001 |
| Inferior | 265.9 (13.5) | 265.9 (13.6) | 265.8 (13.4) | 0.89 | 1.47 (0.11) | <0.001 |
| Average | 273.5 (12.9) | 273.2 (12.8) | 273.9 (13.0) | 0.04 | 1.39 (0.11) | <0.001 |
| Macular volume (mm3) | ||||||
| ETDRS grid | 8.016 (0.362) | 7.993 (0.356) | 8.049 (0.368) | <0.001 | 0.033 (0.003) | <0.001 |
Numbers are presented as mean (standard deviation).
Linear regression, adjusted for age.
Difference, β‐coefficient (standard error), in thickness/volume pr. One dioptre increase in spherical equivalent refraction, linear regression adjusted for age and sex.
Retinal thickness of the fovea was nonlinearly associated with age, with a gradual increase by age to a maximum at about 60 years, followed by a steeper decline with increasing age (Fig. 2, panels A and D). The vertex occurred slightly earlier in men than in women, but this difference was not significant (p for interaction 0.6). Residual plots and sensitivity analyses supported the curved relationship (not shown).
Figure 2.
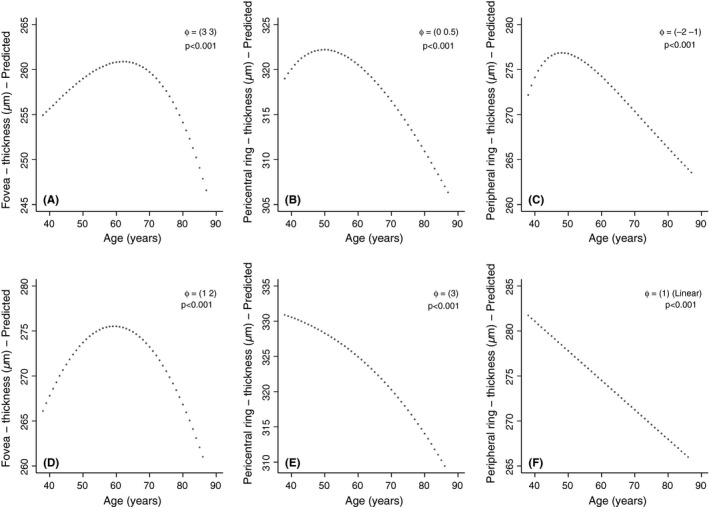
Predicted relationship between age and retinal thickness in the fovea, pericentral and peripheral ring of macula, by use of fractional polynomial multiple linear regression. Women are displayed in panels A–C and men in panels D–F, p = level of significance, φ = powers of model. φ = (a): First‐degree fractional polynomial (FP1) with the power a (describes a monotonic function). φ = (a b): Second‐degree fractional polynomial (FP2) with powers a and b (describes a non‐monotonic function). Models are adjusted for spherical equivalent refraction, and graphs centred at the sex‐specific median. Tromsø Eye Study 2007–2008.
In the pericentral and peripheral ring, the retinal thickness declined with age in both women and men (Tables 1 and 2). In the pericentral ring, the mean reduction in retinal thickness per 10 years was 2.7 (0.3) μm in women and 4.0 (0.4) μm in men and corresponding results in the peripheral ring were 2.3 (0.3) μm in women and 2.6 (0.4) μm in men. In women, the association between retinal thickness in the peripheral ring and age followed a nearly linear pattern with age above 60 years, corresponding to men (Fig. 2, panels C and F). Still there was evidence for a nonlinear pattern (Fig. 2, panels B, C and E), with an increased rate of change with higher age. The tendency of a maximum at lower ages (e.g. non‐monotonic relationship) seen in women was less robust, where results in the peripheral ring were dependent on measurements from the youngest age groups (results not shown). There was a significant interaction between sex and age for retinal thickness of the pericentral ring (p = 0.041), while not the peripheral ring (p = 0.7).
There was a significant association between retinal thickness and refraction. Higher SER was associated with thinner fovea and thicker retina in the pericentral and peripheral ring in both women and men. There was no significant interaction between sex and refraction in association with the thickness in either region (p > 0.3 for all); thus, results are shown for women and men together (Table 3).
Restricting analyses to scans with superior signal strength (≥8; N = 3627), the association with age and refraction remained (results not shown). The non‐monotonic curvilinear association with age in the fovea remained in both women and men. In the pericentral and peripheral ring, the curvilinear pattern with age was diminuated, in women the association changed to monotonic curved (FP1 3) in both, while in men the curved relationship in the pericentral ring changed to linear.
Thickness measures of the fovea had more variance than of the pericentral and the peripheral ring (Tables 1 and 2). Sex, age and refraction contributed most to the explained variance in the foveal thickness (13%), where sex was clearly the most important (Table S2).
Discussion
Our results provide sex‐stratified normative data of spectral‐domain optical coherence tomography (SD‐OCT) macular thickness in an adult and old population. The results reflect a non‐diabetic general population without retinal diseases in the age range 38–87 (mean 61 ± 8) years and we investigated the relationship with sex, age and refraction.
There are ethnic variances in macular retinal thickness, with Caucasians found to have thicker macular retina than African Americans and several ethnic Asian populations, even when accounted for refractive error (Kelty et al. 2008; Girkin et al. 2011; Tariq et al. 2011). Further, OCT measurements are not directly comparable between devices, as they differ in segmentation algorithms (Giani et al. 2010; Pierro et al. 2010; Tan et al. 2015). The SD Cirrus HD‐OCT software draws the outer limitation in the middle of the RPE band, while other algorithms draw the outer limitation at the upper border of the RPE band or at the RPE–Bruch's membrane complex. This is probably why our thickness measures, despite similar ethnic composition, were slightly lower than those in the Beaver Dam study, as they measured between ILM and Bruch's membrane (Myers et al. 2015). The Singapore Chinese Eye Study used the same OCT device as ours, implying that the slightly thinner measures probably are due to the ethnic component (Gupta et al. 2013). In the Handan study, thickness measures were considerably lower than in our study, probably due to both the ethnic component and the OCT device (CZM Stratus OCT), which draws the outer segmentation line at the interface of the inner and outer segments of the photoreceptors (Duan et al. 2010; Giani et al. 2010).
Age is an important risk factor for several retinal diseases. Our population covers an age range before diseases normally appear, which may be important to understand the age‐related changes eventually leading to disease. We found that retinal thickness was associated with age in all macular areas. The non‐monotonic curved pattern with age was a robust finding for foveal thickness, not driven by potential error from media opacity or outliers in lower age groups where number of participants was low, especially in men. The decline in retinal thickness in the highest ages seen in all regions is possibly reflecting atrophic changes with age, while the thickening of fovea in the lower ages must reflect other ageing processes, possibly accumulation of extra‐ or intracellular debris (Ardeljan & Chan 2013; Zealley & de Grey 2013).
The thickening of the fovea in middle age corresponds with findings from the Handan study (Duan et al. 2010). In the Beaver Dam population, there was no significant association between age and retinal thickness in the fovea, which may be explained by the older population (Myers et al. 2015). Studies that include segmentation on individual layers have been conflicting whether retinal thickness in the fovea changes with age. The study of Ooto et al. (2011) (n = 256) found increased retinal thickness of the fovea with significantly increased thickness of both photoreceptor outer segments and outer plexiform and nuclear layer by higher age, while the study of Demirkaya et al. (2013) (n = 120) found reduction in the retinal outer segment layer by higher age and no change in the other layers of the fovea. The segmentation algorithm of CZM Cirrus OCT draws the automated line of the outer retinal limit in the middle of the RPE; thus, changes in RPE may have contributed to our results. Still, the Handan study supports that our finding of foveal thickening in the lower age groups is due to increase in the retinal tissue, as their outer segmentation line was actually within the photoreceptor layer (Duan et al. 2010). Although not a significant finding, it is interesting to note that the vertex of the foveal thickness appeared slightly later in women than in men, which may reflect a slight delay in ageing changes in women as seen with cardiovascular disease appearing later in women than in men (Roger et al. 2012).
Women had thinner retina in the fovea and pericentral ring than men and was in accordance with results from different ethnic populations (Duan et al. 2010; Song et al. 2010; Adhi et al. 2012; Gupta et al. 2013; Myers et al. 2015). In the peripheral ring, women had thinner retina than men only in the temporal part. Ooto et al. (2010, 2011) reported similar results, which they related to the different distribution of RNFL between sectors, with thickest RNFL in the nasal, superior and inferior parts of the peripheral ring. As women had thicker RNFL than men, in these sectors this outweighed that other retinal layers were thinner than in men. A few studies on children have found that boys have thicker macula than girls, while several have found no gender difference (Molnar et al. 2015; Pérez‐García et al. 2016). This may indicate a change during adolescence and/or adulthood, possibly due to hormonal influence.
Retinal thickness was associated with refraction in both women and men, with lower SER associated with thicker retina in the fovea and thinner retina in the pericentral and peripheral ring. This mainly corresponds with results from other studies considering axial length, refraction and macular thickness (Lam et al. 2007; Duan et al. 2010; Gupta et al. 2013; Myers et al. 2015). Myopization correlates with axial elongation, and in vivo studies have shown a significantly lower photoreceptor packing density in myopic eyes, indicating a stretching and thus thinning of the myopic retina corresponding to our findings in the pericentral and peripheral ring (Grosvenor & Scott 1993; Chui et al. 2008). The mechanism for foveal thickening with higher degree of myopia is unclear. Wu et al. (2008) suggested that increased axial length could result in a stretching and flattening tendency of the ILM, resulting in an elevation of the fovea. A possible explanation by poorer fixation with high myopia as suggested by Lim et al. (2005) has been negated in studies that confirmed central fixation in each participant (Lam et al. 2007; Wu et al. 2008). Ocular magnification affects lateral measurements on OCT images (Sanchez‐Cano et al. 2008). This will affect the ETDRS grid, with more of the margin zone of the pericentral ring included in the foveal zone with higher myopia. Still this is not the whole explanation as studies have shown the same correlation with increased central fovea (foveola) thickness in myopia (Lam et al. 2007; Wu et al. 2008).
An important limitation of this study is the cross‐sectional design, which prohibits inferences about temporal and causal relationships. Our study provides data from a high number of subjects of both sexes, and the population‐based design and the relatively high attendance rate are important means to control for selection bias. Study size is particularly important when exploring nonlinear associations, and our quite wide age range, ranging from middle to older age, is an important strength of our study, while we may not infer about age‐related changes in earlier adulthood and childhood.
Supporting information
Figure S1. Flow chart of participants with optical coherence tomography (OCT) in healthy eyes in the Tromsø Eye Study 2007–08.
Table S1. Characteristics of included and excluded participants.
Table S2. Explained variance (R 2) of macular thickness in healthy eyes.
The North Norway Regional Health Authority. The funding organization had no role in the design or conduct of this research.
References
- Adhi M, Aziz S, Muhammad K & Adhi MI (2012): Macular thickness by age and gender in healthy eyes using spectral domain optical coherence tomography. PLoS ONE 7: e37638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeljan D & Chan CC (2013): Aging is not a disease: distinguishing age‐related macular degeneration from aging. Prog Retin Eye Res 37: 68–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen G, Erke MG, von Hanno T, Mathiesen EB, Peto T, Sjølie AK & Njølstad I (2013): The Tromsø Eye Study: study design, methodology and results on visual acuity and refractive errors. Acta Ophthalmol 91: 635–642. [DOI] [PubMed] [Google Scholar]
- Chui TY, Song H & Burns SA (2008): Individual variations in human cone photoreceptor packing density: variations with refractive error. Invest Ophthalmol Vis Sci 49: 4679–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darma S, Kok PH, van den Berg TJ et al. (2015): Optical density filters modeling media opacities cause decreased SD‐OCT retinal layer thickness measurements with inter‐ and intra‐individual variation. Acta Ophthalmol 93: 355–361. [DOI] [PubMed] [Google Scholar]
- Demirkaya N, van Dijk HW, van Schuppen SM, Abràmoff MD, Garvin MK, Sonka M, Schlingemann RO & Verbraak FD (2013): Effect of age on individual retinal layer thickness in normal eyes as measured with spectral‐domain optical coherence tomography. Invest Ophthalmol Vis Sci 54: 4934–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan XR, Liang YB, Friedman DS et al. (2010): Normal macular thickness measurements using optical coherence tomography in healthy eyes of adult Chinese persons: the Handan Eye Study. Ophthalmology 117: 1585–1594. [DOI] [PubMed] [Google Scholar]
- Eriksson U & Alm A (2009): Macular thickness decreases with age in normal eyes: a study on the macular thickness map protocol in the Stratus OCT. Br J Ophthalmol 93: 1448–1452. [DOI] [PubMed] [Google Scholar]
- Giani A, Cigada M, Choudhry N et al. (2010): Reproducibility of retinal thickness measurements on normal and pathologic eyes by different optical coherence tomography instruments. Am J Ophthalmol 150: 815–824. [DOI] [PubMed] [Google Scholar]
- Girkin CA, McGwin G, Sinai MJ et al. (2011): Variation in optic nerve and macular structure with age and race with spectral‐domain optical coherence tomography. Ophthalmology 118: 2403–2408. [DOI] [PubMed] [Google Scholar]
- Grosvenor T & Scott R (1993): Three‐year changes in refraction and its components in youth‐onset and early adult‐onset myopia. Optom Vis Sci 70: 677–683. [DOI] [PubMed] [Google Scholar]
- Gupta P, Sidhartha E, Tham YC et al. (2013): Determinants of macular thickness using spectral domain optical coherence tomography in healthy eyes: the Singapore Chinese Eye study. Invest Ophthalmol Vis Sci 54: 7968–7976. [DOI] [PubMed] [Google Scholar]
- von Hanno T, Bertelsen G, Sjølie AK & Mathiesen EB (2014): Retinal vascular calibres are significantly associated with cardiovascular risk factors: the Tromsø Eye Study. Acta Ophthalmol 92: 40–46. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T & Njølstad I (2012): Cohort profile: the Tromso Study. Int J Epidemiol 41: 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelty PJ, Payne JF, Trivedi RH, Kelty J, Bowie EM & Burger BM (2008): Macular thickness assessment in healthy eyes based on ethnicity using Stratus OCT optical coherence tomography. Invest Ophthalmol Vis Sci 49: 2668–2672. [DOI] [PubMed] [Google Scholar]
- Lam DS, Leung KS, Mohamed S et al. (2007): Regional variations in the relationship between macular thickness measurements and myopia. Invest Ophthalmol Vis Sci 48: 376–382. [DOI] [PubMed] [Google Scholar]
- Lim MC, Hoh ST, Foster PJ, Lim TH, Chew SJ, Seah SK & Aung T (2005): Use of optical coherence tomography to assess variations in macular retinal thickness in myopia. Invest Ophthalmol Vis Sci 46: 974–978. [DOI] [PubMed] [Google Scholar]
- Molnar A, Holmström G & Larsson E (2015): Macular thickness assessed with spectral domain OCT in a population‐based study of children: normative data, repeatability and reproducibility and comparison with time domain OCT. Acta Ophthalmol 93: 470–475. [DOI] [PubMed] [Google Scholar]
- Myers CE, Klein BE, Meuer SM et al. (2015): Retinal thickness measured by spectral‐domain optical coherence tomography in eyes without retinal abnormalities: the Beaver Dam Eye Study. Am J Ophthalmol 159: 445–456. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooto S, Hangai M, Sakamoto A et al. (2010): Three‐dimensional profile of macular retinal thickness in normal Japanese eyes. Invest Ophthalmol Vis Sci 51: 465–473. [DOI] [PubMed] [Google Scholar]
- Ooto S, Hangai M, Tomidokoro A et al. (2011): Effects of age, sex, and axial length on the three‐dimensional profile of normal macular layer structures. Invest Ophthalmol Vis Sci 52: 8769–8779. [DOI] [PubMed] [Google Scholar]
- Pérez‐García D, Ibañez‐Alperte J, Remón L, Cristóbal JÁ, Sanchez‐Cano A & Pinilla I (2016): Study of spectral‐domain optical coherence tomography in children: normal values and influence of age, sex, and refractive status. Eur J Ophthalmol 26: 135–141. [DOI] [PubMed] [Google Scholar]
- Pierro L, Giatsidis SM, Mantovani E & Gagliardi M (2010): Macular thickness interoperator and intraoperator reproducibility in healthy eyes using 7 optical coherence tomography instruments. Am J Ophthalmol 150: 199–204. e1. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd‐Jones DM et al. (2012): Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 125: e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P & Sauerbrei W (2008): MFP: multivariable model‐building with fractional polynomials In: Royston P. & Sauerbrei W. (eds). Multivariable model‐building: a pragmatic approach to regression analysis based on fractional polynomials for modelling continuous variables. Hoboken, NJ: John Wiley & Sons; 115–150. [Google Scholar]
- Royston P & Sauerbrei W (2011): Multivariable model‐building: a pragmatic approach to regression analysis based on fractional polynomials for modelling continuous variables. Stata ado‐files. Available at: https://portal.uni-freiburg.de/imbi/Royston-Sauerbrei-book/index.html. Updated Feb 28, 2011. (Accessed on 15 Dec 2015).
- Royston P, Ambler G & Sauerbrei W (1999): The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 28: 964–974. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Cano A, Baraibar B, Pablo LE & Honrubia FM (2008): Magnification characteristics of the Optical Coherence Tomograph STRATUS OCT 3000. Ophthalmic Physiol Opt 28: 21–28. [DOI] [PubMed] [Google Scholar]
- Song WK, Lee SC, Lee ES, Kim CY & Kim SS (2010): Macular thickness variations with sex, age, and axial length in healthy subjects: a spectral domain‐optical coherence tomography study. Invest Ophthalmol Vis Sci 51: 3913–3918. [DOI] [PubMed] [Google Scholar]
- Tan CS, Chan JC, Cheong KX, Ngo WK & Sadda SR (2015): Comparison of retinal thicknesses measured using swept‐source and spectral‐domain optical coherence tomography devices. Ophthalmic Surg Lasers Imaging Retina 46: 172–179. [DOI] [PubMed] [Google Scholar]
- Tariq YM, Li H, Burlutsky G & Mitchell P (2011): Ethnic differences in macular thickness. Clin Exp Ophthalmol 39: 893–898. [DOI] [PubMed] [Google Scholar]
- Wu PC, Chen YJ, Chen CH, Chen YH, Shin SJ, Yang HJ & Kuo HK (2008): Assessment of macular retinal thickness and volume in normal eyes and highly myopic eyes with third‐generation optical coherence tomography. Eye (Lond) 22: 551–555. [DOI] [PubMed] [Google Scholar]
- Zealley B & de Grey AD (2013): Strategies for engineered negligible senescence. Gerontology 59: 183–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart of participants with optical coherence tomography (OCT) in healthy eyes in the Tromsø Eye Study 2007–08.
Table S1. Characteristics of included and excluded participants.
Table S2. Explained variance (R 2) of macular thickness in healthy eyes.


