Abstract
Aims
To describe the relative health and economic outcomes associated with different second‐line therapeutic approaches to manage glycaemia in older type 2 diabetes patients requiring escalation from metformin monotherapy.
Materials and methods
The Clinical Practice Research Datalink database was used to inform a retrospective observational cohort study of patients with type 2 diabetes treated with metformin monotherapy requiring escalation (addition or switch) to a second‐line oral regimen from January 1, 2008 to December 31, 2014. Primary outcomes included time to first event (any event, myocardial infarction [MI], stroke, or composite of MI/stroke [major adverse cardiovascular event; MACE]) and total event rate. The health economic consequences associated with the choice of second‐line treatment in older patients were assessed using the CORE Diabetes Model.
Results
A total of 10 484 patients were included; the majority escalated to second‐line treatment with metformin + sulphonylurea (SU; 42%) or switched to SU monotherapy (28%). In multivariate adjusted analyses, total event rates for MACE with metformin + dipeptidyl peptidase‐4 (DPP‐4) inhibitor were significantly lower than with metformin + SU (0.61, 95% confidence interval [CI] 0.39‐0.98), driven by a lower MI rate in the metformin + DPP‐4 inhibitor group (0.52, 95% CI 0.27‐0.99). Economic analyses estimated that metformin + DPP‐4 inhibitor treatment was associated with the largest gain in health benefit, and cost‐effectiveness ratios were favourable (<£30 000 per quality‐adjusted life‐year) for all second‐line treatment scenarios.
Conclusions
With respect to treatment choice, data from the present study support the notion of prescribing beyond metformin + SU, as alternative regimens have been shown to be associated with reduced outcomes risk and value for money.
Keywords: management, metformin, older patients, second‐line, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes is the most common metabolic disorder in the older adult population and imposes considerable burden on patient health, life expectancy and associated quality of life. The appropriate management of older patients is imperative, given the rising prevalence of type 2 diabetes and that older patients account for the majority of newly diagnosed cases.1 Diabetes‐related vascular complications, such as cardiovascular disease, visual loss and foot disease, increase the risk of disability significantly. The care of older people with diabetes is also complicated by the presence of comorbidities, frailty and the frequent use of medicines that together pose various management challenges for the clinical team.1, 2, 3, 4 Recent guidance advocates an individualized approach to glycaemia management, reflecting the physical, psychological and social challenges in providing appropriate care to this patient group.1, 5, 6
Evidence from randomized controlled trials is emerging as to the feasibility and benefit of treating older patients to individualized glycated haemoglobin (HbA1c) targets.7 This is complementary to the findings of the key intensive glucose control trials, that were suggestive of clinical benefit from tight glycaemic control in older patients with short duration of diabetes, low HbA1c levels or a low number of comorbidities.2, 4, 7, 8 There remains a paucity of data, however, on the outcomes of alternative approaches to glycaemic management in the older patient,1 and a need to establish whether the benefits of tight glycaemic control suggested by existing and recent trials are observed in routine clinical practice. Previously, Morgan et al. addressed the question, “What next after metformin?”, with a retrospective evaluation of the outcomes associated with second‐line glucose‐lowering therapies amongst type 2 diabetes patients of all ages in UK clinical practice.9 They found that pioglitazone, a thiazolidinedione (TZD), was associated with superior clinical outcomes compared with sulphonylurea (SU) when added to metformin, and that SU monotherapy resulted in the worst outcomes.9 Prescribing beyond SU therapy in older patients is also justified in view of the unnecessary high risk of hypoglycaemia in this population10; however, whilst this evidence is certainly useful, it does not specifically comment on the utility of managing glycaemia in older patients in terms of superiority of clinical outcomes, or whether alternative therapeutic approaches to glycaemia management represent value for money.
Although SU added to metformin has previously been described as the most cost‐effective prescribing alternative after metformin monotherapy failure,11 there is a requirement to demonstrate the cost‐effectiveness of prescribing beyond SU at second‐line, particularly amongst older patients. Considering this, the present study sought to provide evidence that can inform the utility, from clinical and cost‐effectiveness perspectives, associated with different therapeutic approaches to manage glycaemia in older patients with type 2 diabetes. Given that metformin is the most commonly prescribed first‐line glucose‐lowering agent in this age group, we conducted the present retrospective observational study, with economic assessment, in older patients failing metformin monotherapy who escalated to second‐line therapy. The regimens examined in the study included SU, dipeptidyl peptidase‐4 (DPP‐4) inhibitors, and TZD‐based therapies, as these are advocated in current UK clinical guidelines12 and were the most commonly prescribed second‐line agents in a large UK primary care database: the Clinical Practice Research Datalink (CPRD),13 formerly the General Practice Research Database (GPRD). The CPRD database was used in the present study as it represents a source of real‐world clinical data on elderly patients with type 2 diabetes, a population not typically captured in randomized controlled trials.
2. MATERIALS AND METHODS
2.1. Data source
The CPRD database was established in 1987, and contains data for ~11.3 million individuals registered with selected general practitioners (GPs) in the UK.14 The CPRD has been the source of many observational studies, including research on diabetes and antidiabetic therapies.15, 16 In the present analysis, patient‐level data were extracted from the CPRD database to obtain patient demographic and lifestyle information, as well as information on medical diagnoses, symptoms, referrals, hospitalizations, deaths and prescriptions, for each patient. Prescriptions are generated directly within the system, and contain the name of the preparation, instructions for use, route of administration, dose and number of tablets for each entry. The recorded information on drug exposure and diagnoses has repeatedly been validated and proven to be of high quality.17, 18
2.2. Study design
The study was conducted retrospectively for a cohort of patients with type 2 diabetes who were treated with metformin monotherapy and required therapy escalation (addition or switch) to a second‐line regimen between January 1, 2008 and December 31, 2014 (index date was defined as date of second‐line therapy initiation). The baseline data period was defined appropriately for study variables, as either the quarter prior to the index date or the 12‐month pre‐index period, for all patients. Linear interpolation was performed between 2 observations (quarterly measurements) for each covariate at the patient level, to reduce the degree of missing data in study variables.
Inclusion criteria were: age ≥65 years at index date; diagnosis of type 2 diabetes (based on one or more prescriptions of oral antidiabetic drug [OAD]); receiving metformin first‐line monotherapy for at least 180 days; and escalation (addition or switch) from metformin monotherapy to a non‐injectable second‐line regimen within 180 days of ceasing the first‐line regimen. Exclusion criteria were: type 1 diabetes (via inclusion criteria); receiving blood glucose‐lowering therapy (other than metformin) prior to index date; diagnosis of polycystic ovary syndrome prior to index date; and malignant disease at any time point prior to the index date or during the follow‐up period. Infrequently observed second‐line regimens accounting for <1% of the total observed patients were excluded.
Patient subgroups were defined according to choice of second‐line regimen, overall prevalent (history of complication pre‐index date) and incident case analysis (no history of complication pre‐index date), and minimum patient follow‐up (exposure time) post index date. The Independent Scientific Advisory Committee (ISAC) approved the study for the Medicines and Healthcare products Regulatory Agency (MHRA) database research (Protocol 15_062R).
2.3. Study outcomes
The primary outcomes evaluated included time to first event and total event rates, based on patient follow‐up post index date. Time to first event included any diabetes‐related complication (termed “any event”; see Table 2 for complication types and ISAC Protocol 15_062R for associated read codes [available on request]), myocardial infarction (MI), stroke, or a composite of MI and stroke (termed major adverse cardiovascular event [MACE]). Time to first event was evaluated using descriptive analyses and adjusted Cox proportional hazards models, based on the time from index date to first event (diabetes‐related complication) or censorship (end of study, loss to follow‐up or death). Total event rates were analysed as unadjusted event incidence per 1000 person‐years and in adjusted Poisson regression models. Secondary outcomes included the analysis of change in HbA1c and weight for each patient from baseline to 12 months post index date. Any patients with <12 months follow‐up were not included in this analysis.
Table 2.
Total observed event rates per 1000 person‐years (by second‐line regimen)
| Event incidence, mean (rate per 1000 person‐years) | All eligibleN = 10 484 | Second‐line regimen | |||||
|---|---|---|---|---|---|---|---|
| DPP‐4 inhibitor N = 676 | Metformin + DPP‐4 inhibitor N = 1463 | SUN = 2921 | Metformin + SUN = 4451 | TZDN = 268 | Metformin + TZDN = 705 | ||
| Amputation | 38 (1.27) | 0 (0.00) | 6 (1.76) | 15 (1.84) | 13 (0.99) | 0 (0.00) | 4 (1.39) |
| Blindness | 9 (0.30) | 0 (0.00) | 3 (0.88) | 3 (0.37) | 3 (0.23) | 0 (0.00) | 0 (0.00) |
| CHF | 495 (16.54) | 9 (7.06) | 54 (15.85) | 171 (20.92) | 21 (16.76) | 8 (7.85) | 32 (11.13) |
| IHD | 242 (8.09) | 12 (9.42) | 14 (4.11) | 86 (10.52) | 98 (7.43) | 7 (6.87) | 25 (8.70) |
| MI | 231 (7.72) | 11 (8.63) | 12 (3.52) | 81 (9.91) | 97 (7.36) | 6 (5.89) | 24 (8.35) |
| Nephropathy | 15 (0.50) | 1 (0.78) | 1 (0.29) | 4 (0.49) | 9 (0.68) | 0 (0.00) | 2 (0.70) |
| Neuropathy | 27 (0.90) | 2 (1.57) | 2 (0.59) | 5 (0.61) | 16 (1.21) | 0 (0.00) | 0 (0.00) |
| Renal failure | 111 (3.71) | 8 (6.28) | 8 (2.35) | 46 (5.63) | 40 (3.03) | 1 (0.98) | 8 (2.78) |
| Retinopathy | 1935 (64.65) | 91 (71.43) | 229 (67.24) | 502 (61.42) | 827 (62.73) | 89 (87.33) | 197 (68.52) |
| Stroke | 158 (5.28) | 8 (6.28) | 15 (4.40) | 47 (5.75) | 73 (5.54) | 3 (2.94) | 12 (4.17) |
| Ulcer | 18 (0.60) | 0 (0.00) | 3 (0.88) | 5 (0.61) | 7 (0.53) | 0 (0.00) | 3 (1.04) |
| MACE | 389 (13.00) | 19 (14.91) | 27 (7.93) | 128 (15.66) | 170 (12.89) | 9 (8.83) | 36 (12.52) |
| Mortality | 861 (28.77) | 28 (21.98) | 55 (16.15) | 347 (42.46) | 361 (27.38) | 17 (16.68) | 53 (18.43) |
| Any event | 3279 (109.55) | 142 (111.46) | 347 (101.88) | 965 (118.07) | 1404 (106.50) | 114 (111.86) | 307 (106.78) |
| Patient time post index date, years | 2.44 (1.82) | 1.63 (1.36) | 2.02 (1.52) | 2.38 (1.80) | 2.54 (1.84) | 3.26 (1.97) | 3.44 (1.97) |
Abbreviations: CHF, congestive heart failure; IHD, ischaemic heart disease; PVD, peripheral vascular disease.
2.4. Statistical analyses
For baseline patient and treatment characteristics, descriptive analyses for continuous variables (number of patients, mean, standard deviation [SD], median, minimum and maximum values, 25th and 75th percentiles) and categorical variables (the number and proportions of patients) were reported. Statistical significance of between‐group comparisons was estimated using chi‐squared tests (categorical variables) and analysis of variance (continuous variables), to determine significant differences at the 5% level of testing. Multivariate analyses were based on a general‐specific selection methodology, with covariates excluded at the 5% level of statistical significance. Overall model fit was determined by goodness of fit statistics, for example, the R‐squared statistic and likelihood ratio test, or the Akaike Information Criterion and Bayesian Information Criterion, as appropriate. The following covariates in multivariate regression models were included if P < .05: baseline measurements for gender, smoking status, HbA1c, history of diabetes‐related events, metformin monotherapy exposure, number of GP visits pre‐index date, duration of diabetes, blood pressure (systolic and diastolic), cholesterol (total, LDL and HDL), urine albumin, estimated glomerular filtration rate, white blood cell count, heart rate, serum creatinine, and concomitant use of lipid‐lowering or antihypertensive therapy.
Hazard ratios were used to compare outcome risk for each second‐line regimen with SU (the most commonly observed index regimen). Data were reported for “all cases, incident and prevalent”, “prevalent cases” and “incident cases”, based on patient event history prior to the index date.
2.5. Economic analyses
The health and economic consequences associated with the choice of second‐line treatment in older patients were assessed using the CORE Diabetes Model (CDM).19 Cost‐effectiveness was evaluated in two‐ways: (1) by assessing the cost‐effectiveness of alternative regimens (incremental cost per quality‐adjusted life‐year (QALY) gained with metformin + SU compared with metformin + DPP‐4 inhibitor and metformin + TZD), and (2) by assessing the cost‐effectiveness of prescribing choice; that is, was the decision to prescribe a DPP‐4 inhibitor, SU or TZD in combination with and after metformin monotherapy a cost‐effective choice (with each group acting as their own control arm). This analysis assumed the perspective of payers and clinicians who are interested in the clinical and economic value of prescribing based on real‐world clinical practice, to assess whether the right patients are receiving the right treatment, and the value for money associated with different therapy escalation choices.
The base case analysis was used to evaluate the relative cost‐effectiveness of the most common second‐line dual therapy regimens (metformin + SU, metformin + TZD, metformin + DPP‐4 inhibitor). In the base case analysis, patients in each arm could switch to rescue therapy when HbA1c reached ~7.5% (which corresponded to a model horizon of ~5 years post initiation of second‐line regimen). In a scenario analysis, the switch to rescue therapy was assumed to occur when HbA1c returned to the baseline value of the respective cohort modelled. Rescue therapy was modelled as a switch to NPH insulin, based on data from a mixed‐treatment comparison meta‐analysis of patients with type 2 diabetes.20
In a separate analysis, the cost‐effectiveness of the decision to prescribe a DPP‐4 inhibitor or SU or TZD after and in combination with metformin therapy was evaluated, by comparing each treatment cohort with itself (and assuming no treatment effects applied to the comparator arm). Baseline profiles and treatment effects were obtained from the observational data. Costs and QALYs, sourced from UK literature, were discounted at 3.5% annually, and the model was run over a lifetime horizon (up to 50 years) without escalation to rescue therapy. A lifetime perspective is appropriate when estimating the relative costs and benefits of alternative treatment regimens because diabetes is a lifelong (chronic) condition, and its costs and health outcomes are expected to vary between treatment strategies over a lifetime. The UK payer perspective was adopted. Where data relating to clinical effectiveness and additional input variables were not available, default CDM values were used.
Probabilistic sensitivity analyses were conducted, taking a non‐parametric bootstrapping approach, and sampling values from distributions around the means of input variables in the model. Analyses of HbA1c trajectories over a 5‐year period post index date were undertaken to inform the economic analysis. Observed HbA1c trajectories were compared with a default CDM approach, which assumes a gradual progression in HbA1c after an observed change in HbA1c from baseline to 12 months. Implications of these alternative trajectories were evaluated, as time to or threshold for therapy escalation can be an important determinant of the relative cost‐effectiveness of treatments.21 A description of the model, model input variables and assumptions are reported in Appendix S1,Tables S1 and S2.
3. RESULTS
3.1. Baseline patient characteristics
Based on prescription records within the CPRD database, a total of 514 734 patients were identified as having type 2 diabetes. Of these, a total of 10 484 patients were considered eligible for inclusion in the present study (Figure S1 in Appendix S1). At baseline, patients had a mean age of 73 years, HbA1c of 8.3%, disease duration of 6 years and body weight of 87 kg. Approximately 56% of the cohort were men, 12% had a history of MACE, and 13% had a history of diabetic retinopathy. After metformin monotherapy failure, the majority of patients (42%) had an SU added to metformin (metformin + SU), or were switched to SU monotherapy (28%). Far fewer patients were escalated (added or switched) to a DPP‐4 inhibitor or a TZD‐based regimen (20% and 9%, respectively). Baseline characteristics of the cohort are summarized in Table 1.
Table 1.
Baseline patient characteristics
| Measurements | All eligible | Second‐line regimen | |||||
|---|---|---|---|---|---|---|---|
| DPP‐4 inhibitor | Metformin + DPP‐4 inhibitor | SU | Metformin + SU | TZD | Metformin + TZD | ||
| N = 10 484 | N = 676 | N = 1463 | N = 2921 | N = 4451 | N = 268 | N = 705 | |
| Demographic | |||||||
| Male, n (%) | 5911 (56.4) | 383 (56.7) | 820 (56.0) | 1598 (54.7) | 2548 (57.2) | 142 (53.0) | 420 (59.6) |
| Non‐smoker, n (%) | 2091 (19.9) | 125 (18.5) | 296 (20.2) | 613 (21.0) | 866 (19.5) | 49 (18.3) | 142 (20.1) |
| Current smoker, n (%) | 1419 (13.5) | 102 (15.1) | 211 (14.4) | 385 (13.2) | 581 (13.1) | 43 (16.0) | 97 (13.8) |
| Former smoker, n (%) | 6974 (66.5) | 449 (66.4) | 956 (65.3) | 1923 (65.8) | 3004 (67.5) | 176 (65.7) | 466 (66.1) |
| Clinical, Mean (SD) | |||||||
| Age, years | 72.85 (6.15) | 72.60 (6.21) | 71.85 (5.81) | 73.86 (6.60) | 72.71 (5.98) | 72.80 (6.12) | 71.87 (5.24) |
| HbA1c, % | 8.25 (1.35) | 8.13 (1.23) | 8.28 (1.17) | 8.06 (1.40) | 8.46 (1.37) | 7.73 (1.23) | 7.88 (1.28) |
| Duration of diabetes, years | 6.05 (4.08) | 6.11 (4.23) | 6.14 (4.08) | 6.04 (4.10) | 5.91 (4.02) | 6.02 (3.82) | 6.79 (4.35) |
| Body weight, kg | 86.63 (17.51) | 89.58 (19.71) | 90.65 (17.84) | 84.05 (17.62) | 86.16 (16.65) | 87.56 (18.83) | 88.71 (16.85) |
| BMI, kg/m2 | 30.89 (5.50) | 31.68 (6.15) | 32.36 (5.66) | 30.20 (5.47) | 30.62 (5.26) | 31.44 (5.96) | 31.49 (5.26) |
| SBP, mm Hg | 136.01 (14.50) | 134.75 (14.36) | 135.66 (13.28) | 135.94 (15.34) | 136.05 (14.35) | 137.65 (13.55) | 137.35 (14.59) |
| DBP, mm Hg | 75.20 (8.77) | 75.20 (8.35) | 75.45 (8.42) | 74.63 (9.23) | 75.35 (8.63) | 75.16 (8.91) | 76.05 (8.60) |
| Total cholesterol, mmol/L | 4.17 (0.94) | 4.20 (0.93) | 4.13 (0.95) | 4.22 (0.99) | 4.14 (0.91) | 4.29 (0.93) | 4.14 (0.90) |
| HDL cholesterol, mmol/L | 1.22 (0.34) | 1.21 (0.31) | 1.21 (0.32) | 1.24 (0.35) | 1.21 (0.34) | 1.26 (0.32) | 1.23 (0.33) |
| LDL cholesterol, mmol/L | 2.07 (0.77) | 2.12 (0.79) | 2.01 (0.75) | 2.12 (0.80) | 2.05 (0.74) | 2.22 (0.82) | 2.06 (0.79) |
| Urine albumin, mg/L | 25.38 (34.21) | 22.20 (26.38) | 25.83 (33.83) | 28.56 (37.89) | 24.00 (33.44) | 24.28 (28.62) | 23.65 (31.77) |
| eGFR, mL/min/m2 | 63.39 (17.83) | 60.04 (20.11) | 66.21 (15.68) | 57.55 (20.53) | 66.12 (15.69) | 63.34 (17.26) | 67.57 (13.80) |
| White blood cell count, cells × 109/L | 7.76 (2.36) | 7.61 (2.05) | 7.80 (1.90) | 7.88 (3.04) | 7.78 (2.11) | 7.27 (1.94) | 7.33 (1.87) |
| Heart rate, bpm | 76.49 (13.59) | 76.25 (13.72) | 76.36 (12.88) | 76.53 (13.56) | 76.82 (13.99) | 72.62 (11.68) | 76.13 (13.14) |
| Serum creatinine, mg/dL | 1.03 (0.34) | 1.05 (0.40) | 0.96 (0.25) | 1.13 (0.44) | 0.98 (0.26) | 1.05 (0.38) | 1.00 (0.25) |
| Event history 1 , n (%) | |||||||
| MACE | 1309 (12.5) | 96 (14.2) | 168 (11.5) | 400 (13.7) | 561 (12.6) | 23 (8.6) | 61 (8.7) |
| CHF | 559 (5.3) | 35 (5.2) | 76 (5.2) | 222 (7.6) | 204 (4.6) | 7 (2.6) | 15 (2.1) |
| IHD | 1030 (9.8) | 74 (10.9) | 124 (8.5) | 325 (11.1) | 440 (9.9) | 19 (7.1) | 48 (6.8) |
| MI | 960 (9.2) | 72 (10.7) | 120 (8.2) | 295 (10.1) | 416 (9.3) | 15 (5.6) | 42 (6.0) |
| Stroke | 349 (3.3) | 24 (3.6) | 48 (3.3) | 105 (3.6) | 145 (3.3) | 8 (3.0) | 19 (2.7) |
| Amputation | 87 (0.8) | 3 (0.4) | 10 (0.7) | 25 (0.9) | 40 (0.9) | 3 (1.1) | 6 (0.9) |
| Blindness | 34 (0.3) | 3 (0.4) | 4 (0.3) | 9 (0.3) | 17 (0.4) | 1 (0.4) | 0 (0) |
| Renal failure | 126 (1.2) | 12 (1.8) | 6 (0.4) | 72 (2.5) | 29 (0.7) | 6 (2.2) | 1 (0.1) |
| Ulcer | 12 (0.1) | 0 (0) | 1 (0.1) | 5 (0.2) | 5 (0.1) | 0 (0) | 1 (0.1) |
| Diabetic nephropathy | 9 (0.1) | 0 (0) | 0 (0) | 4 (0.1) | 5 (0.1) | 0 (0) | 0 (0) |
| Diabetic neuropathy | 42 (0.4) | 0 (0) | 4 (0.3) | 17 (0.6) | 19 (0.4) | 0 (0) | 2 (0.3) |
| Diabetic retinopathy | 1385 (13.2) | 99 (14.6) | 173 (11.8) | 407 (13.9) | 578 (13.0) | 34 (12.7) | 94 (13.3) |
| Atrial fibrillation | 352 (3.4) | 31 (4.6) | 58 (4.0) | 97 (3.3) | 148 (3.3) | 5 (1.9) | 13 (1.8) |
| PVD | 530 (5.1) | 35 (5.2) | 78 (5.3) | 166 (5.7) | 212 (4.8) | 5 (1.9) | 34 (4.8) |
| History of dementia | 155 (1.5) | 12 (1.8) | 13 (0.9) | 60 (2.1) | 64 (1.4) | 4 (1.5) | 2 (0.3) |
| Treatment‐related | |||||||
| Mean (SD) metformin monotherapy exposure, y | 3.99 (2.80) | 4.04 (2.93) | 4.05 (2.84) | 3.85 (2.84) | 3.93 (2.71) | 4.30 (2.91) | 4.63 (2.90) |
| Mean (SD) GP visits pre‐index | 19.55 (12.13) | 20.65 (12.87) | 18.42 (11.32) | 21.46 (13.23) | 19.03 (11.73) | 17.73 (10.89) | 16.92 (9.83) |
| Lipid‐lowering therapy 2 , n (%) | 8626 (82.3) | 558 (82.5) | 1250 (85.4) | 2313 (79.2) | 3685 (82.8) | 215 (80.2) | 605 (85.8) |
| Anti‐hypertensive therapy, n (%) | 8591 (81.9) | 559 (82.7) | 1223 (83.6) | 2415 (82.7) | 3589 (80.6) | 223 (83.2) | 582 (82.6) |
| Patient time post index date, years | 2.44 (1.82) | 1.63 (1.36) | 2.02 (1.52) | 2.38 (1.80) | 2.54 (1.84) | 3.26 (1.97) | 3.44 (1.97) |
Abbreviations: CHF, congestive heart failure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; IHD, ischaemic heart disease; PVD, peripheral vascular disease; SBP, systolic blood pressure.
Any history of an event in the pre‐index period.
Concomitant.
3.2. Time to first event analysis
Hazard ratios for time to first MI, stroke, MACE or any event were not statistically significant for any alternative regimen compared with metformin + SU. Trends in incident and prevalent cases were similar (in patients with and without a history of the complication; Figure 1 and Table S3 in Appendix S1). For all‐cause mortality, when compared with metformin + SU, hazard ratios were higher for SU monotherapy (hazard ratios >1; P < .05 in unadjusted and age‐ and sex‐adjusted analyses); in comparison, all other non‐SU‐containing regimens had a lower risk of all‐cause mortality (hazard ratios <1) across analyses when compared with metformin + SU (Figure S2 in Appendix S1). In full multivariate analyses, TZD alone was associated with a significant reduction in all‐cause mortality compared with metformin + SU (Figure S2 in Appendix S1). Patient factors that were significant in the analysis of time to first event included baseline age, comorbidities, body mass index (BMI), anti‐hypertensive and lipid‐lowering therapy use, and male gender. An adjusted analysis of time to first event, stratified by total patient follow‐up time post index date, demonstrated no statistically significant differences in hazards for alternative regimens compared with metformin + SU (Figure S3 in Appendix S1).
Figure 1.
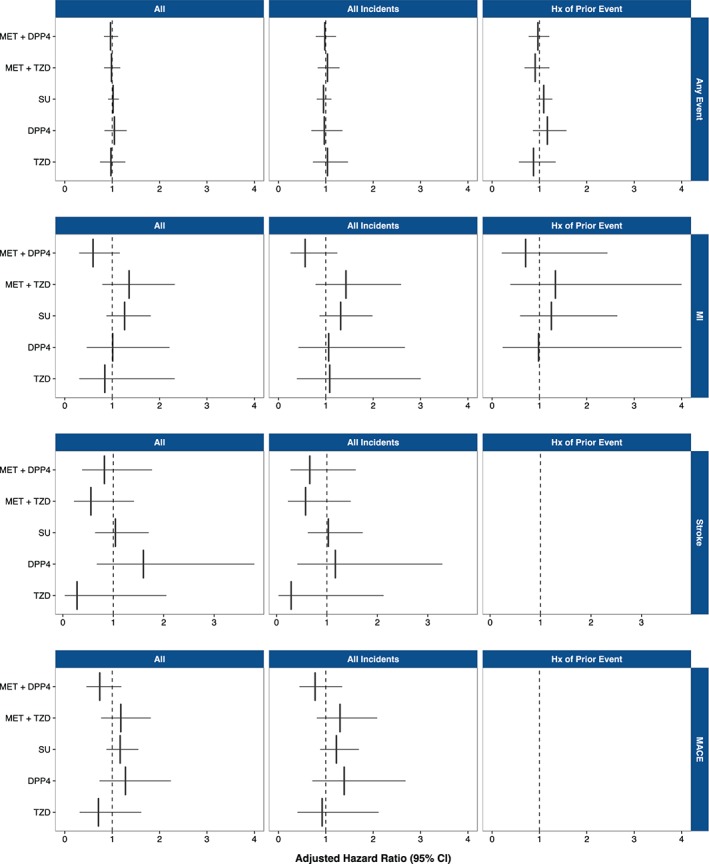
Multivariate adjusted time to first event hazard ratios, 1 = metformin (MET) + SU (by regimen and event type) for all, incident and prevalent cases. Abbreviations: Hx, history of.
3.3. Total event analysis
Over a mean follow‐up time of 2.44 years (maximum 7 years), a total of 3279 complications were observed across patients escalating to a second‐line regimen (Table 2). The SU monotherapy cohort was associated with the highest event rate per 1000 person‐years (118.07/1000 person‐years) compared with an overall event rate of 109.55/1000 person‐years. The lowest event rate among the choice of second‐line regimens was metformin + DPP‐4 inhibitor, with an overall event rate of 101.88/1000 person‐years. The cohorts associated with the lowest event rate for the combined endpoint of MACE were metformin + DPP‐4 inhibitor (7.93/1000 person‐years) and TZD monotherapy (8.83/1000 person‐years), compared with an overall event rate for MACE across regimens of 13.00/1000 person‐years. The highest event rate for MACE was observed for SU monotherapy with an overall event rate of 15.66/1000 person‐years.
A total of 861 out of 10 484 patients died during the follow‐up period, with an overall rate of 28.77/1000 person‐years. Death rates were higher for metformin + SU (27.38/1000 person‐years) and SU monotherapy (42.46/1000 person‐years), compared with other non‐SU‐containing regimens. The regimens with the lowest death rates were metformin + DPP‐4 inhibitor and TZD monotherapy which were associated with 16.15 and 16.68 deaths per 1000 person‐years, respectively.
In multivariate adjusted analyses, total event rates per 1000 person‐years for metformin + DPP‐4 inhibitor were significantly lower compared with metformin + SU for MACE (incidence rate ratio 0.61, 95% CI 0.39‐0.98; Figure S4 and Table S5 in Appendix S1). In all cases, this was driven by a lower event rate for MI in the metformin + DPP‐4 inhibitor group (incidence rate ratio 0.52, 95% CI 0.27‐0.99). This finding was significant in the all‐case analysis, with a trend towards a hazard ratio <1 in the incident‐only and prevalent‐only analyses. Total event rates were not significantly different for remaining regimens compared with metformin + SU. Patient factors that were significant in the analysis of total event incidence included baseline age, comorbidities, systolic blood pressure, total cholesterol, anti‐hypertensive and lipid‐lowering therapy use, GP visits, and male gender.
A multivariate analysis of total events, stratified by total patient follow‐up time post index date, demonstrated a statistically significant lower total event incidence (incidence rate ratio <1) for metformin + DPP‐4 inhibitor compared with metformin + SU for patients with ≥1 (borderline significant), 2 and 3 years follow‐up (Figure S5 in Appendix S1). The incident rates between regimens for MI, stroke and MACE, stratified by follow‐up time, were not statistically significant.
3.4. Analysis of HbA1c and weight
Analysis of the secondary outcomes (change in HbA1c and weight from baseline to 12 months) suggested that weight increased overall and for all treatment cohorts except metformin + DPP‐4 inhibitor and DPP‐4 inhibitor monotherapy, which were associated with significant reductions in weight (−1.21 and −1.06 kg, respectively; P < .001 vs other regimens; Table S4 in Appendix S1). The metformin + SU cohort was associated with the greatest reduction in HbA1c (−1.02%), whilst the metformin + DPP‐4 inhibitor cohort was associated with larger reductions in HbA1c than metformin + TZD (−0.76% vs −0.57%; P = .044), TZD monotherapy (−0.76% vs −0.46%; P = .024) and DPP‐4 inhibitor monotherapy (−0.76% vs −0.54%; P = .044).
Analysis of HbA1c trajectories over a 5‐year period post index date (undertaken to inform the economic analysis) for the observed and assumed (CDM default) approaches showed that the observed trajectories for different second‐line regimens converge after the initial 12‐month change, at ~7.62% at year 5 (baseline adjusted for comparability). Conversely, an assumed gradual progression implied an average HbA1c value of 8.44% at year 5 (Figure S6 in Appendix S1).
3.5. Economic analyses
The first economic analysis compared the cost‐effectiveness of the most commonly observed dual therapy second‐line treatment regimens (Table 3). In this analysis, metformin + DPP‐4 inhibitor was associated with the largest gain in health benefit (QALYs) but at an incremental cost compared with metformin + SU and metformin + TZD. The costs per QALY gained for metformin + DPP‐4 inhibitor were £15 343 versus metformin + TZD and £18 680 versus metformin + SU, which are within conventional thresholds for cost‐effectiveness in the UK of between £20 000 and £30 000 per QALY. The probability that metformin + DPP‐4 inhibitor was cost‐effective at the £30 000 threshold was 57% and 62% compared with metformin +SU and metformin + TZD, respectively. Similar results were observed in a scenario analysis, where the switch to rescue therapy was assumed to occur once baseline HbA1c had returned to baseline values.
Table 3.
Economic analysis comparing the cost‐effectiveness of alternative second‐line regimens
| Base case analysis: switch to RT at 5 years post initiation second‐line (~7.5%) | Scenario analysis: switch to RT at baseline HbA1c | |||||
|---|---|---|---|---|---|---|
| Metformin + SU | Metformin + TZD | Metformin + DPP‐4 inhibitor | Metformin + SU | Metformin + TZD | Metformin + DPP‐4 inhibitor | |
| Absolute results | ||||||
| Total costs, £ | 22 960 | 22 788 | 24 057 | 20 975 | 22 013 | 23 105 |
| Total QALYs | 5.58 | 5.55 | 5.64 | 5.53 | 5.56 | 5.63 |
| Total life‐years | 8.35 | 8.25 | 8.33 | 8.34 | 8.27 | 8.34 |
| Incremental results (versus metformin + DPP‐4 inhibitor) | ||||||
| Costs, £ | 1097 | 1269 | 2131 | 1092 | ||
| QALYs | 0.06 | 0.08 | 0.10 | 0.07 | ||
| Life years | −0.02 | 0.07 | −0.01 | 0.06 | ||
| Cost‐effectiveness | ||||||
| Cost/QALY, £ | 18 680 | 15 343 | 20 587 | 15 947 | ||
| Probability CE (%) at: £20k, £30k | 51, 57 | 57, 62 | 49, 61 | 53, 59 | ||
Abbreviations: CE, cost‐effective; k, thousand; RT, rescue therapy.
The second economic analysis was used to evaluate the cost‐effectiveness of the prescribing or treatment decision (Table 4). In this analysis, all second‐line regimens were associated with favourable cost‐effectiveness ratios: metformin + DPP‐4 inhibitor and metformin + SU had similar cost per QALY estimates of £21 318 and £17 640, respectively; and metformin + TZD was cost‐saving with QALY gains attributable to its cost and effect profile when compared against itself. The probability that metformin + DPP‐4 inhibitor was cost‐effective at the £30 000 threshold was 61%, 54% for metformin + SU, and 74% for metformin + TZD.
Table 4.
Economic analysis comparing the cost‐effectiveness of prescribing choice
| Control | Metformin + SU | Control | Metformin + TZD | Control | Metformin + DPP‐4 inhibitor | |
|---|---|---|---|---|---|---|
| Absolute results | ||||||
| Total costs, £ | 19 228 | 19 507 | 18 550 | 18 345 | 18 599 | 21 289 |
| Total QALYs | 5.34 | 5.36 | 5.73 | 5.81 | 5.48 | 5.61 |
| Total life years | 7.98 | 8.15 | 8.54 | 8.63 | 8.25 | 8.39 |
| Incremental results (versus control) | ||||||
| Costs, £ | 279 | −205 | 2690 | |||
| QALYs | 0.02 | 0.07 | 0.13 | |||
| Life years | 0.17 | 0.09 | 0.15 | |||
| Cost‐effectiveness | ||||||
| Cost/QALY, £ | 17 640 | −2787 | 21 318 | |||
| Probability CE (%) at: £20k, £30k | 51, 54 | 75, 74 | 48, 61 | |||
Abbreviations: CE, cost‐effective; k: thousand.
“Control” is the same profile as intervention arm but no treatment effects.
4. DISCUSSION
Despite the rising prevalence of type 2 diabetes within an aging population, there is a paucity of clinical and economic data to inform on the utility of therapeutic strategies to manage older patients who have failed first‐line metformin monotherapy. The present real‐world observational study and economic assessment is one of the first to assess the clinical and cost‐effectiveness of second‐line antidiabetic therapies in older patients, by characterizing the nature and outcomes of 10 484 individuals treated in UK clinical practice. Findings from this research challenge the notion that there is a lack of evidence for prescribing beyond SU after metformin monotherapy failure, suggesting that agents of the DPP‐4 inhibitor class, and TZDs to a lesser extent, are associated with improvements in some clinical outcomes. In particular, the metformin + DPP‐4 inhibitor group had the lowest overall event rate, clinically meaningful HbA1c reductions, a statistically lower risk of MACE (driven by a lower risk of MI) compared with metformin + SU, and lower risks for all‐cause mortality compared with metformin + SU; it was the only treatment to reduce body weight, and was cost‐effective compared with metformin + SU and metformin + TZD at conventional thresholds.
In subgroup analyses, second‐line regimens were compared with metformin + SU for: (1) different minimum follow‐up periods post index date, to explore the idea that a minimum number of follow‐up years are required to observe the effects of different glucose‐lowering agents on macrovascular event rates, and (2) patients with a history of an event to mimic “high‐risk” patients, such as those with a previous MI or stroke. In these analyses, the metformin + DPP‐4 inhibitor group was associated with lower time to first event and total event incidence for longer follow‐up periods (≥3 years) and a lower total event incidence for MI and MACE when analysing incidence and prevalence cases together (with a trend towards lower incidence when incidence/prevalence groups were analysed separately). In these subgroup analyses, across all regimens, “all” patients tended to have a higher (non‐significant) risk compared with strata of increasingly longer follow‐up who had a trend towards reduced risk. This apparent discrepancy may be explained by the non‐exhaustive nature of the reported stratified analyses. Data pertaining to stratified duration analyses (≥1‐3 years) do not capture patients with follow‐up <1 year, and in these patients, rate ratios were similar to the “all” patient data. This may be evidence of a survivorship effect, in that patients with longer observed follow‐up tended to have lower event risk across all regimens. Conversely, the data may suggest that a minimum follow‐up period is required to observe significant differences in event outcomes across alternative glucose‐lowering regimens. In the present study, the only group associated with a statistically significant reduction in event risk as follow‐up time increased was the metformin + DPP‐4 inhibitor group.
Previous database studies have reported improved clinical outcomes with DPP‐4 inhibitor‐based regimens, compared with other approaches of glycaemic management for type 2 diabetes.22 The present study demonstrated similar clinical benefits of metformin + DPP‐4 inhibitor specifically amongst older patients, and accompanying economic analyses confirmed the cost‐effectiveness of the clinical decision to prescribe a DPP‐4 inhibitor regimen after metformin in this population. Similarly, the decision to prescribe an SU or TZD after metformin was associated with some improvements in clinical outcomes and was a cost‐effective prescribing decision. These data are important to payers and clinicians who are interested in the clinical and economic value of prescribing; based on data from this study and real‐world clinical practice, the value for money associated with different therapy escalation choices was established for DPP‐4 inhibitor, SU and TZD add‐on to metformin regimens. These findings may be related to patient attributes and event outcomes, since it was demonstrated that patient age, number of comorbidities prior to second‐line therapy, anti‐hypertensive and lipid‐lowering therapy use, total cholesterol and male gender were associated with the largest significant hazards (≥1) in time to first event and/or total event rate analyses.
In the economic analyses, life years were marginally lower for metformin + DPP‐4 inhibitor and metformin + TZD compared with metformin + SU. This may be attributable to the greater HbA1c reduction observed with metformin + SU compared with other regimens evaluated; however, metformin + SU was additionally associated with increased BMI and hypoglycaemia incidence, with these effects not fully reflected in life‐year predictions. By contrast, once quality of life effects were added to life‐year estimates, metformin + DPP‐4 inhibitor treatment had the greatest numerical QALY gain compared with metformin + SU and metformin + TZD treatment. Furthermore, whilst metformin + SU had a higher life‐year estimate in economic analyses, the opposite was observed in the observational study where non‐SU regimens tended to have lower mortality. This discrepancy may be explained by the nature of the economic analyses, which are based on longer‐term (lifetime) predictions and are subject to uncertainties surrounding model‐based extrapolations and assumptions. The observed mortality estimates reflect the shorter term (observational study) and adjustments for differences in patient characteristics (multivariate analyses) that are not fully captured in the economic results.
While these data provide an evidence‐linked approach to therapy escalation following metformin monotherapy failure in older people with type 2 diabetes, there are limitations to this methodological approach and the interpretation of findings. Observational studies that stratify outcomes by therapy type are potentially subject to the bias of “confounding by indication”, such that any observed patterns within the data are a function of the patient phenotype, and the patient phenotype is the reason for prescription of a specific therapy. Furthermore, not all relevant confounding factors are captured in the CPRD database (such as educational and professional status); thus, predicting causality between treatment and outcome can be difficult from these types of data. The approach used to minimize the impact of confounding in the present study was to select a homogenous cohort (patients on metformin monotherapy requiring therapy change), and to evaluate primary and secondary outcomes using stratification and statistical adjustment as the principle mechanisms of accounting for the effect of differences in patient type and prescribing choice on study outcomes. As part of this approach, regression models were fitted to the data to estimate within‐ and between‐group differences in outcomes, adjusting for the influence of observed covariates at baseline. It is acknowledged that statistical adjustment for observed covariates within and between strata is unlikely to account for all sources of variation; thus, adjusted estimates are reported with explicit reference to these potential limitations.
There are several strengths and limitations associated with the use of routinely collected data such as those contained in the CPRD database. Given the current lack of randomized controlled trial data informing on the efficacy of second‐line therapies in older patients with type 2 diabetes, the greatest strength of the CPRD database in the context of the present study was that it provided informative real‐world data on the population of interest. Variables including weight, smoking status and HbA1c were also likely to be well captured after the introduction of the Quality Outcomes Framework in April 2004. The index data period was subsequently defined from 2008, to mitigate the risk of poor data recording prior to 2004, and to maximize both the observational window and the number of patients eligible for inclusion in the study. This additionally served to strengthen inferences from the research and to capture contemporary clinical practice, given that DPP‐4 inhibitors were first introduced in the UK in April 2007. The calendar year of second‐line therapy initiation was considered to account for any temporal effects associated with therapy initiation, but was found to be non‐significant. Although the duration of diabetes derived from the CRPD database is an estimation of the true value, and factors relating to lifestyle modifications are not routinely captured, it is unlikely that any biases arising from these limitations would confound the results for any particular drug class. Furthermore, hypoglycaemia was not included as a study variable based on an a priori expectation that hypoglycaemia would be poorly recorded in the CPRD database, and even if adequately captured, is self‐reported and therefore could not be confirmed via blood measurements to obtain a reliable assessment.
The objective of the present study was not to inform on the entirety of clinical guidelines or their development, but rather to generate evidence that supports informed treatment decisions for older patients with type 2 diabetes. Specifically, data from the present study may be useful in supporting guideline development relating to second‐line oral therapy choices in the older patient. Other classes of injectable and non‐injectable agents are certainly relevant to clinical practice and guidelines; however, the prescribing volume of these agents was low in the CPRD database. Subsequently, outcomes after prescribing agents including glucagon‐like peptide‐1 (GLP‐1) antagonists and sodium‐glucose co‐transporter‐2 inhibitors, were not evaluated in the present analysis. Low volume prescribing for such agents is not unexpected, since the use of SU, TZD and DPP‐4 inhibitors is more strongly advocated within UK‐based guidelines in patients requiring escalation from metformin monotherapy.12 Furthermore, had adequate data for these agents been available in the CPRD database, based on current UK guidelines, the inclusion of such agents may have introduced significant prescribing bias that could not be controlled for. For example, the type of patient being initiated on a GLP‐1 antagonist would reflect prescribing conditions related to weight and comorbidity profiles, and, in the case of insulins, only patients with a very high HbA1c level may be initiated on insulin at second‐line therapy.
An important aim of the present research was to provide data that inform future guideline development and clinical practice in the management of type 2 diabetes in the older patient. Data arising from the present study may best inform the choice between second‐line oral therapies, particularly SU, TZD or DPP‐4 inhibitor regimens. In this context, a linked evidence approach was used to assess both clinical and economic outcomes of older patients following alternative management approaches, based on comprehensive data from routine primary care practice. For clinicians choosing between alternative treatment regimens for their patients after metformin failure, data from the present study have identified a number of phenotypic characteristics predictive of increased outcome risk independent of treatment, including age, male gender, and number of comorbidities. Furthermore, with respect to treatment choice, these data support the notion of prescribing beyond metformin + SU, since alternative regimens to metformin + SU were associated with reduced outcomes risk and value for money. These findings may therefore have important implications for future clinical guidelines and clinical practice.
Supporting information
Appendix S 1. XXX.
ACKNOWLEDGMENTS
The authors thank Daniel Sugrue, Samantha Webster and Karina Hamilton (HEOR Ltd) for writing services in the development of this manuscript.
Conflict of interest
J. G., P. M., M. E. and A. S. have served as consultants to and received research funding from Takeda Development Centre Europe Ltd in relation to this study. J. P. is an employee of Takeda Development Centre Europe Ltd.
Author contributions
J. G. and P. M. were involved in the design, conduct/data collection, analysis and writing of the manuscript. J. P., M. E. and A. S. were involved in the design, analysis and writing of the manuscript.
Gordon J, McEwan P, Evans M, Puelles J and Sinclair A. Managing glycaemia in older people with type 2 diabetes: A retrospective, primary care‐based cohort study, with economic assessment of patient outcomes. Diabetes Obes Metab. 2017;19:644–653. https://doi.org/10.1111/dom.12867
Funding information The study was funded by an unrestricted grant from Takeda Development Centre Europe Ltd.
REFERENCES
- 1. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650‐2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129‐139. [DOI] [PubMed] [Google Scholar]
- 3. van Dieren S, Czernichow S, Chalmers J, et al. Weight changes and their predictors amongst 11 140 patients with type 2 diabetes in the ADVANCE trial. Diabetes Obes Metab. 2012;14(5):464‐469. [DOI] [PubMed] [Google Scholar]
- 4. The Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545‐2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sinclair AJ, Paolisso G, Castro M, Bourdel‐Marchasson I, Gadsby R, Mañas LR. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab. 2011;37:S27‐S38. [DOI] [PubMed] [Google Scholar]
- 6. International Diabetes Federation . IDF global guideline for managing older people with type 2 diabetes. 2013. http://www.idf.org/guidelines-older-people-type-2-diabetes. Accessed November 5, 2015.
- 7. Strain WD, Lukashevich V, Kothny W, Hoellinger M‐J, Paldánius PM. Individualised treatment targets for elderly patients with type 2 diabetes using vildagliptin add‐on or lone therapy (INTERVAL): a 24 week, randomised, double‐blind, placebo‐controlled study. Lancet. 2013;382(9890):409‐416. [DOI] [PubMed] [Google Scholar]
- 8. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560‐2572. [DOI] [PubMed] [Google Scholar]
- 9. Morgan CL, Poole CD, Evans M, Barnett AH, Jenkins‐Jones S, Currie CJ. What next after metformin? A retrospective evaluation of the outcome of second‐line, glucose‐lowering therapies in people with type 2 diabetes. J Clin Endocrinol Metab. 2012;97(12):4605‐4612. [DOI] [PubMed] [Google Scholar]
- 10. Sinclair AJ. Special considerations in older adults with diabetes: meeting the challenge. Diabetes Spectrum. 2006;19(4):229‐233. [Google Scholar]
- 11. Klarenbach S, Cameron C, Singh S, Ur E. Cost‐effectiveness of second‐line antihyperglycemic therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin. Can Med Assoc J. 2011;183(16):E1213‐E1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Institute for Health and Care Excellence . Type 2 diabetes in adults: management. NICE guideline [NG28]. 2015. https://www.nice.org.uk/guidance/ng28. Accessed 27 October, 2016.
- 13. Medicines & Healthcare Products Regulation Agency . The clinical practice research datalink (CPRD). 2015. https://www.cprd.com/home/. Accessed October 5, 2015.
- 14. Wood L, Martinez C. The general practice research database. Drug Saf. 2004;27(12):871‐881. [DOI] [PubMed] [Google Scholar]
- 15. Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med. 2008;168(8):820‐825. [DOI] [PubMed] [Google Scholar]
- 16. Brauchli YB, Jick SS, Curtin F, Meier CR. Association between use of thiazolidinediones or other oral antidiabetics and psoriasis: a population based case‐control study. J Am Acad Dermatol. 2008;58(3):421‐429. [DOI] [PubMed] [Google Scholar]
- 17. Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the general practice research database: a systematic review. Br J Gen Pract. 2010;60(572):e128‐e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long‐term clinical outcomes, costs and costeffectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision‐making. Curr Med Res Opin. 2004;20(S1):S5‐S26. [DOI] [PubMed] [Google Scholar]
- 20. McIntosh B, Cameron C, Singh SR, Yu C, Dolovich L, Houlden R. Choice of therapy in patients with type 2 diabetes inadequately controlled with metformin and a sulphonylurea: a systematic review and mixed‐treatment comparison meta‐analysis. Open Med. 2012;6(2):e62‐e74. [PMC free article] [PubMed] [Google Scholar]
- 21. McEwan P, Gordon J, Evans M, Ward T, Bennett H, Bergenheim K. Estimating cost‐effectiveness in type 2 diabetes: the impact of treatment guidelines and therapy duration. Med Decis Making. 2015;35:660–670. [DOI] [PubMed] [Google Scholar]
- 22. Mathieu C, Barnett A, Brath H, et al. Effectiveness and tolerability of second‐line therapy with vildagliptin vs. other oral agents in type 2 diabetes: a real‐life worldwide observational study (EDGE). Int J Clin Pract. 2013;67(10):947‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S 1. XXX.


