Abstract
Aims
This trial consisted of a 24‐week multicentre, randomized, double‐blind, double‐dummy, active‐controlled study and a 52‐week open label extension study to assess the efficacy and safety of evogliptin, a novel dipeptidyl peptidase‐4 inhibitor, compared to sitagliptin in patients with type 2 diabetes who have inadequate glycaemic control with metformin alone.
Methods
Adult patients with type 2 diabetes mellitus (N = 222) with HbA1c 6.5% to 11% who were receiving stable doses of metformin (≥1000 mg/d) were randomized 1:1 to add‐on evogliptin 5 mg (N = 112) or sitagliptin 100 mg (N = 110) once daily for 24 weeks. The primary efficacy analysis consisted of a comparison of the change from baseline HbA1c at week 24. Non‐inferiority was concluded if the upper limit of the 2‐sided 95% confidence interval for the HbA1c difference between treatments was <0.35%.
Results
Mean changes in HbA1c following addition of evogliptin or sitagliptin were −0.59% and −0.65%, respectively. The between‐group difference was 0.06% (2‐sided 95% confidence interval, −0.10 to 0.22), demonstrating non‐inferiority. After the 52‐week treatment, evogliptin caused a persistently decreased level of HbA1c (−0.44% ± 0.65%, P < .0001). In general, both treatments were well tolerated, with incidences and types of adverse events comparable between the two groups. Hypoglycaemic events, mostly mild, were reported in 0.9% of patients treated with evogliptin and in 2.8% of patients treated with sitagliptin for 24 weeks.
Conclusions
Evogliptin 5 mg added to metformin therapy effectively improved glycaemic control and was non‐inferior to sitagliptin and well tolerated in patients with type 2 diabetes mellitus that was inadequately controlled by metformin alone.
Keywords: combination therapy, DPP‐4 inhibitor, evogliptin, metformin, sitagliptin, type 2 diabetes mellitus
1. INTRODUCTION
Dipeptidyl peptidase 4 (DPP‐4) inhibitors comprise a relatively novel pharmacological class of glucose‐lowering agents that inhibit the degradation of glucagon‐like peptide 1 (GLP‐1) and cause modest elevations in circulating GLP‐1 levels. They do not cause hypoglycaemia unless used in combination with sulfonylureas or insulin and they also do not cause weight gain.1 Therefore, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) have published consensus guidelines for pharmacotherapy to control hyperglycaemia in type 2 diabetes, recommending DPP‐4 inhibitors as second‐ or first‐line agents in specific situations. Likewise, the American Association of Clinical Endocrinologists and the American College of Endocrinology have published a consensus statement recommending DPP‐4 inhibitors as an acceptable alternative to metformin as initial therapy.2, 3 Moreover, DPP‐4 inhibitors are currently widely used as second line treatment for patients with type 2 diabetes (T2DM).4
Evogliptin is a novel antidiabetic agent that potently and selectively inhibits DPP‐4. Namyi Gu et al. reported the pharmacokinetic and pharmacodynamic profiles of evogliptin in healthy subjects.5 The peak plasma concentration of evogliptin was reached within 4 to 5 hours. The inhibition of DPP‐4 activity (>80%) was sustained for over 24 hours, and it provided an increase in postprandial active GLP‐1 levels, 1.5‐ to 2.4‐fold. As a result, evogliptin reduced postprandial glucose by 20% to 35% compared to placebo.5 And another study reported the pharmacokinetic and pharmacodynamic profiles of evogliptin in renal impairment; the plasma concentration of evogliptin increased 1.98 times in severe renal impairment, but within a therapeutic window.6 Drug administration in patients with T2DM is usually performed over extended periods of time; therefore, an evaluation of the safety and efficacy of these drugs over a long term is extremely important. However, there was no more than 12 weeks of follow‐up for the study with evogliptin. Therefore, we conducted this phase III, randomized, double‐blind, active‐controlled, parallel‐group, multi‐centre study to determine the efficacy and safety of evogliptin compared to sitagliptin for 24 weeks in Korean patients with T2DM, and we also determined the efficacy and safety of evogliptin over an extension period of 52 weeks.
2. METHODS
This study consisted of a randomized, double‐blind, active‐controlled, parallel‐group, multi‐centre, dose‐confirmatory study. The study was conducted at 24 university hospitals throughout Korea between May 2013 and November 2014 (ClinicalTrials.gov Identifier: NCT02949193). The study was conducted in accordance with the principles of Good Clinical Practice, and it was approved by the appropriate institutional review boards and regulatory agencies. All patients provided written informed consent prior to participation.
2.1. Participants
Participants eligible for the study were patients aged 18 years and older with T2DM who recently had experienced inadequate glycaemic control (6.5% ≤ HbA1c < 11.0%) with metformin monotherapy for more than 12 weeks and metformin ≥ 1000 mg daily for more than 6 weeks. The additional inclusion criterion was 20.0 kg/m2 ≤ body mass index ≤ 40.0 kg/m2. Exclusion criteria included type 1 diabetes mellitus, secondary diabetes or gestational diabetes; acute myocardial infarction or stroke within 6 months; NYHA class III to IV congestive heart failure, liver cirrhosis, gallbladder disease, acromegaly, asthma, allergic dermatitis at screening; a history of coronary bypass surgery or gastrointestinal tract resection surgery; specific medication (a potent inducer or inhibitor of cytochrome P4503A4); history of insulin or glucagon‐like peptide‐1 analogue treatment in the preceding 6 months (except temporary insulin treatment for less than 2 weeks), history of use of thiazolidinediones or DPP‐4 inhibitors in the preceding 6 months, history of alcohol or drug abuse in the preceding 2 months, being pregnant or nursing or suspected of being pregnant, or history of participation in other clinical studies in the preceding 2 months. Other exclusion criteria included fasting plasma glucose (FPG) ≥ 15 mmol/L, alanine aminotransferase (ALT) or aspartate aminotransferase (AST) ≥ 2.5 times the upper limit of the normal range, creatine phosphokinase (CPK) ≥ 2.5 times the upper limit of normal with chest pain or dyspnea, serum creatinine >132.60 µmol/L in men and >123.76 µmol/L in women, clinically significant thyroid‐stimulating hormone (TSH) values outside the normal range or fasting triglycerides >4.52 mmol/L at screening.
2.2. Study design
After a 2‐week screening period, eligible and consenting participants underwent baseline evaluation and randomized sampling stratified according to HbA1c (using an 8.5% cut‐off) into 2 parallel groups (evogliptin 5 mg and sitagliptin 100 mg; 1:1 matching). During the 24‐week treatment period after randomization, participants visited the investigational site at weeks 6, 12, 18 and 24 (Figure 1). Those participants, who completed the 24‐week treatment period, gave their consent to receive another extended 28 weeks of open label treatment with 5 mg evogliptin qd. In the extended study, patients visited the site every 7 weeks (at weeks 31, 38, 45 and 52). During the 2‐week screening and throughout the study period, patients received the same dose of metformin as monotherapy (≥1000 mg daily). During the study, patients who did not meet progressively stricter glycaemic goals started rescue therapy with glimepiride. The glycaemic rescue criteria were set according to US Food and Drug Administration (FDA) guidelines.7 Specifically, goals included an FPG level >15.0 mmol/L between randomization and week 6, an FPG level >13.3 mmol/L from week 6 through week 12 or an FPG level >11.1 mmol/L from week 12 through week 52. At every visit, the patient's weight was measured and vital signs, including systolic and diastolic blood pressure and pulse rate, were checked. Safety was assessed by recording adverse events (AEs), physical examinations, haematology, serum chemistry and urinalysis from a local laboratory at every visit and ECG monitoring at weeks 0, 24 and 52. Efficacy was evaluated by measuring levels of HbA1c, FPG, fasting lipid parameters (total cholesterol, low density lipoprotein cholesterol [LDL‐C], high density lipoprotein cholesterol [HDL‐C], triglyceride [TG], free fatty acid [FFA]), fasting insulin and C‐peptide from a central laboratory. The homeostasis model assessment of β‐cell function (HOMA‐β), the homeostasis model assessment of insulin resistance (HOMA‐IR) and the quantitative insulin sensitivity check index (QUICKI) were used to estimate the degree of ß‐cell function and insulin resistance from fasting glucose and insulin concentrations. Insulin resistance (IR) was estimated with HOMA‐IR, calculated using the following formula: (fasting insulin [μU/mL] × fasting glucose [mmol/L])/22.5 and HOMA‐β was calculated using the following formula: (20 × fasting insulin [μU/mL])/(fasting glucose [mmol/L] − 3.5).8 QUICKI was calculated using the following formula: 1/(log [fasting insulin (μU/mL)] + log [fasting glucose (mg/dL)]).9 In this study, mean daily glucose (MDG) was derived from the mean value of 7‐point glucose measurements (before and 2 hours after breakfast, lunch and dinner, and before bedtime).
Figure 1.
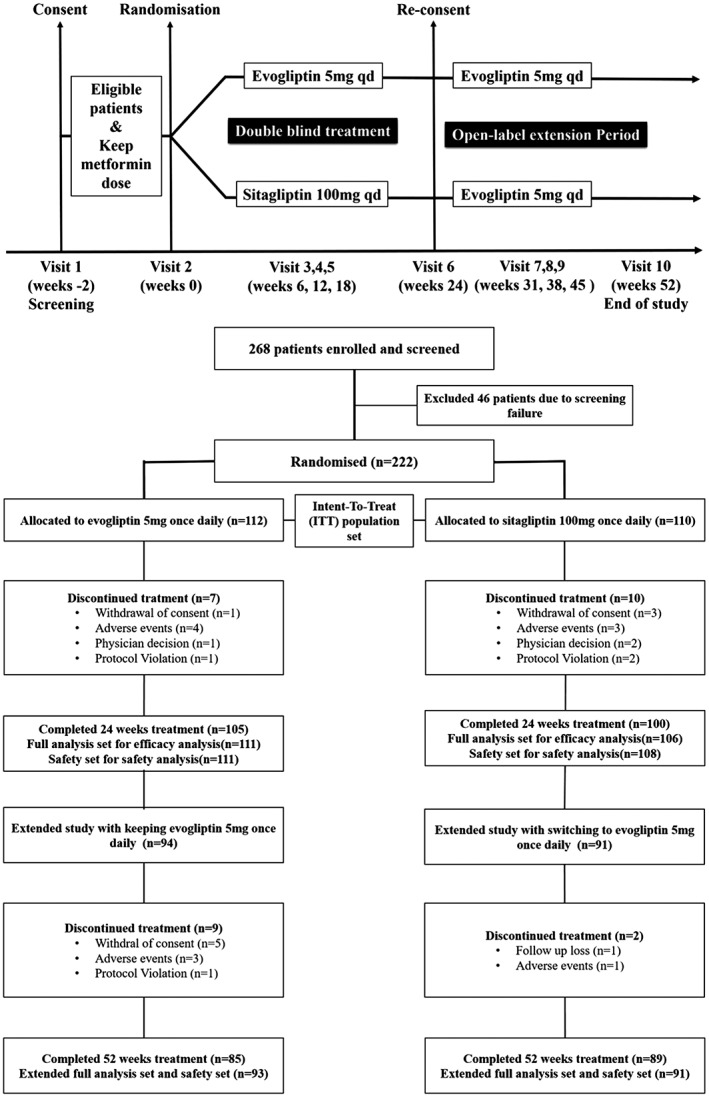
Study scheme and trial profile.
2.3. Study endpoints
The primary efficacy endpoint of this study was change in HbAlc level from baseline to week 24. The secondary efficacy endpoints were change in HbAlc level from baseline to week 52, the HbA1c response rate (HbA1c < 6.5%), rescue therapy rate, changes in FPG, fasting lipid parameters (total cholesterol, LDL‐C, HDL‐C, TG, FFA), body weight, fasting insulin, C‐peptide, HOMA‐β, HOMA‐IR, QUICKI and MDG at week 24 and week 52. Safety and tolerability were evaluated throughout the study up to week 52 through an interview, physical examinations, monitoring of vital signs, safety‐related laboratory measurements (including serum chemistry, haematology and urinalysis) and 12‐lead ECG. Adverse events were monitored and evaluated for intensity (severity), duration, outcome and relationship to the study drug. The incidence of hypoglycaemia was assessed by reviewing patient data for signs and symptoms of hypoglycaemia, in addition to reviewing the self‐monitoring of blood glucose (SMBG) data. Hypoglycaemic events were classified through a report of a Workgroup of the American Diabetes Association and the Endocrine Society.10
Severe hypoglycaemia is defined as an event requiring the assistance of another person to actively administer carbohydrates, glucagon or take other corrective actions. Documented symptomatic hypoglycaemia is defined as an event during which typical symptoms of hypoglycaemia are accompanied by a measured plasma glucose concentration ≤3.9 mmol/L. Asymptomatic hypoglycaemia is not accompanied by the typical symptoms of hypoglycaemia, but has a measured plasma glucose concentration ≤3.9 mmol/L. Probable symptomatic hypoglycaemia is defined as an event during which symptoms typical of hypoglycaemia are not accompanied by a plasma glucose determination. Relative hypoglycaemia is defined as an event during which the person with diabetes reports any of the typical symptoms of hypoglycaemia with a measured plasma glucose concentration >3.9 mmol/L, but approaching that level.
2.4. Statistical analysis
The primary objective of the parent study (week 0‐24) was to demonstrate the non‐inferiority of evogliptin 5 mg once daily vs sitagliptin 100 mg once daily in reducing HbA1c levels from baseline until week 24 (non‐inferiority margin δ = 0.35%; if the upper boundary of the 2‐sided 95% confidence interval (CI) for the mean difference between evogliptin and sitagliptin was less than the pre‐specified non‐inferiority margin, evogliptin was assessed as being non‐inferior to sitagliptin). A sample size of 104 patients per group was estimated to provide 80% power to prove the non‐inferiority between evogliptin and sitagliptin. We assumed an approximate 20% dropout rate (ie, patients who did not complete 24 weeks of treatment). Therefore, a total of 260 patients (in a 1:1 allocation ratio to receive evogliptin 5 mg once daily and sitagliptin 100 mg once daily) were planned to be randomized. Efficacy analyses were based on the full analysis set population, which consisted of all randomized patients who received at least one dose of the study medication and who had a baseline measurement and at least one post‐baseline measurement. ANCOVA was used to compare the primary endpoint (change in HbAlc level) between treatment groups as a sensitivity analysis. In this analysis, stratification factors (site, baseline HbA1c) were set as fixed effects.
Demographic and baseline characteristic data for the 2 groups are shown as the mean, standard deviation, median, minimum and maximum values for continuous variables, or frequencies and percentages for categorical variables. For the other efficacy endpoints (FPG, body weight, lipid parameters, HOMA‐β, HOMA‐IR, insulin and C‐peptide) 2 sample t‐tests or Wilcoxon rank sum tests were used to compare changes between the two groups. Tolerability and safety data are summarized, using descriptive statistics, and the purpose of the extended study (weeks 24‐52) was to evaluate tolerability and safety. Therefore, we conducted a descriptive analysis with extended data. Two‐sided P values < .05 were considered to be statistically significant, and all statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, North Carolina).
3. RESULTS
3.1. Patient disposition
Although 260 patients in 2 groups (evogliptin group, 130 patients; sitagliptin group, 130 patients) were planned for randomization, 222 patients (112 patients in the evogliptin group and 110 patients in the sitagliptin group; Intention‐To‐Treat [ITT]) population set) were actually randomized. From this total of 222 patients, 105 patients (93.8%, evogliptin group) and 100 patients (90.9%, sitagliptin group) completed 24 weeks of treatment (Figure 1). From among these 205 patients who completed the 24‐week study, 185 patients (94 patients in the evogliptin group and 91 patients in the sitagliptin group) agreed to the terms of participation in the extended study. Finally, 174 patients (85 patients in the evogliptin group and 89 patients in the sitagliptin group) completed the extended study with evogliptin 5 mg once daily from week 24 to week 52.
In this study, we analysed efficacy in 217 patients (111 patients in the evogliptin group and 106 patients in the sitagliptin group; full analysis set, FAS) who had baseline and post‐baseline values of the primary efficacy end‐point and also in 184 patients (93 patients in the evogliptin/evogliptin group and 91 patients in the sitagliptin/evogliptin group; extended full analysis set, E‐FAS) who agreed to participate in the extended study and had post‐baseline values of the efficacy end‐point after 24 weeks (Figure 1). We also analysed safety in 219 patients (111 patients in the evogliptin group and 108 patients in the sitagliptin group; safety set) and in 184 patients (93 patients in the evogliptin/evogliptin group and 91 patients in the sitagliptin/evogliptin group; extended safety set) who received at least 1 dose of the study drug and underwent 1 more data capture for safety of the study drugs (Figure 1).
Demographic information and baseline characteristics of the ITT population enrolled in this clinical trial are summarized in Table 1. Mean age of the study subjects was 57.5 ± 9.3 years, and 103 subjects (46.4%) were men. There were no significant differences in the subjects’ baseline characteristics (Table 1).
Table 1.
Baseline characteristics of study participants according to treatment group (intent‐to‐treat population set)
| Characteristic | Evogliptin (N = 112) | Sitagliptin (N = 110) | Total (N = 222) |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 57.6 ± 9.4 | 57.3 ± 9.3 | 57.5 ± 9.3 |
| Median, minimum, maximum | 58.0, 34.0, 86.0 | 57.0, 34.0, 77.0 | 57.0, 34.0, 86.0 |
| ≤34 | 2(1.8) | 1(0.9) | 3(1.4) |
| 35‐44 | 8(7.1) | 8(7.3) | 16(7.2) |
| 45‐54 | 30(26.8) | 37(33.6) | 67(30.2) |
| 55‐64 | 44(39.3) | 35(31.8) | 79(35.6) |
| 65‐74 | 26(23.2) | 27(24.5) | 53(23.9) |
| 75‐84 | 1(0.9) | 2(1.8) | 3(1.4) |
| ≥85 | 1(0.9) | 0(0.0) | 1(0.5) |
| Sex (n) | |||
| Men (%) | 51(45.5) | 52(47.3) | 103(46.4) |
| Women (%) | 61(54.5) | 58(52.7) | 119(53.6) |
| Body weight (kg) | |||
| Mean ± SD | 67.5 ± 13.0 | 66.1 ± 10.3 | 66.8 ± 11.8 |
| Median, minimum, maximum | 65.3, 44.7, 130.1 | 66.0, 47.0, 96.5 | 65.5, 44.7, 130.1 |
| Height (cm) | |||
| Mean ± SD | 162.1 ± 8.5 | 161.2 ± 8.4 | 161.7 ± 8.5 |
| Median, minimum, maximum | 161.0, 144.0, 187.0 | 159.0, 147.0, 180.0 | 161.0, 144.0, 187.0 |
| BMI (kg/m2) | |||
| Mean ± SD | 25.6 ± 3.7 | 25.3 ± 2.7 | 25.5 ± 3.2 |
| Median, minimum, maximum | 25.2, 19.2, 40.1 | 25.1, 20.1, 33.6 | 25.1, 19.2, 40.1 |
| ≤25 kg/m2 (%) | 55(49.1) | 54(49.1) | 109(49.1) |
| >25 kg/m2 (%) | 57(50.9) | 56(50.9) | 113(50.9) |
| Duration of diabetes (years) | |||
| Mean ± SD | 8.5 ± 5.5 | 7.9 ± 4.9 | 8.2 ± 5.2 |
| Median, minimum, maximum | 8.0, 1.0, 35.0 | 7.0, 1.0, 24.0 | 7.5, 1.0, 35.0 |
| HbA1c (%) | |||
| Mean ± SD | 7.44 ± 0.73 | 7.44 ± 0.73 | 7.44 ± 0.73 |
| Median, minimum, maximum | 7.26, 6.31, 9.51 | 7.34, 6.41, 9.98 | 7.29, 6.31, 9.98 |
| <8.5% (%) | 97(86.6) | 99(90.0) | 196(88.3) |
| ≥8.5% (%) | 15(13.4) | 11(10.0) | 26(11.7) |
3.2. Efficacy
Mean baseline HbA1c values of the evogliptin and sitagliptin groups were 7.42% ± 0.71% and 7.45% ± 0.74%, respectively, and the difference was not statistically significant (P = .9201). Decreases in HbA1c value were observed in the evogliptin and sitagliptin groups until 12 weeks, after which HbA1c values were sustained until 24 weeks (Figure 2). Evogliptin and sitagliptin decreased the HbA1c level after treatment during 24 weeks (−0.59% ± 0.61% and −0.65% ± 0.61%, respectively; all P < .0001) (Table 2). The difference in HbA1c change from baseline to week 24 between the evogliptin and sitagliptin groups was 0.06 (95% confidence interval [CI], −0.10 to 0.22) (Table 2). The upper 95% CI limit (0.22%) was less than the prespecified non‐inferiority margin, 0.35%, which confirmed the non‐inferiority of evogliptin to sitagliptin (Table 2). After the 52‐week treatment perod, evogliptin caused a persistent decrease in the level of HbA1c (−0.44 ± 0.65%, P < .0001) although there was a slight increase in HbA1c level from week 24 to week 52 (0.15 ± 0.42%, P = .0045) (Figure S1, Supporting Information). After 24 weeks, a similar proportion of patients reached the HbA1c target of <6.5% in each treatment group; 38.68% (41/106) of sitagliptin‐treated patients achieved an HbA1c level <6.5% and 29.73% (33/111) of evogliptin‐treated patients achieved an HbA1c level <6.5% (P = .1645, derived from the chi‐square test) (Table 2). Among patients with an HbA1c level ≥6.5% at week 24, a similar proportion of patients reached the HbA1c target of <6.5% in each treatment group at week 52; 12.73% (7/55) of the sitagliptin/evogliptin‐treated patients achieved an HbA1c level <6.5% and 15.38% (10/65) of evogliptin/evogliptin‐treated patients achieved an HbA1c level <6.5% (P = .6775, derived from a chi‐square test). Evogliptin and sitagliptin decreased FPG (−0.60 ± 1.11 mmol/L and −0.59 ± 1.47 mmol/L, respectively; all P < .0001) and MDG (−0.92 ± 1.60 mmol/L and −1.30 ± 1.71 mmol/L, respectively; all P < .0001) levels from baseline through week 24. However, the change in FPG and MDG levels from baseline to week 24 in the 2 medication groups did not show a statistically significant difference (P = .7155 and P = .1062, respectively). After 52 weeks, the evogliptin/evogliptin group exhibited a persistent decrease in FPG (−0.38 ± 1.19 mmol/L; P = .0048) and MDG (−1.00 ± 1.69 mmol/L; P < .0001).
Figure 2.
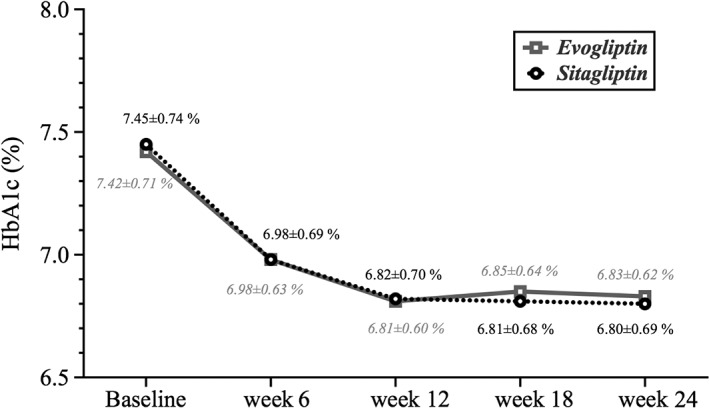
Changes in mean glycated haemoglobin (HbA1c) level from baseline to week 24.
Table 2.
Baseline, results at week 24 and changes in results from baseline for HbA1c, fasting plasma glucose, mean daily glucose and HOMA‐β
| Variable | Evogliptin | Sitagliptin | P value | ||
|---|---|---|---|---|---|
| (N = 111) | (N = 106) | ||||
| HbA1c (%) | |||||
| Baseline | |||||
| Mean ± SD | 7.42 ± 0.71 | 7.45 ± 0.74 | |||
| Median, minimum, maximum | 7.25, 6.31, 9.42 | 7.34, 6.41, 9.98 | |||
| At week 24 | |||||
| Mean ± SD | 6.83 ± 0.62 | 6.80 ± 0.69 | |||
| Median, minimum, maximum | 6.77, 5.68, 9.68 | 6.61, 5.60, 9.68 | |||
| Change from baseline to week 24 (Mean ± SD) | −0.59 ± 0.61 | −0.65 ± 0.61 | P = .4771 (mean difference from sitagliptin and evogliptin; 95% CI, 0.06 [−0.10, 0.22]) | ||
| P value for mean difference from baseline to week 24 | <0.0001 1 | <0.0001 1 | |||
| HbA1c response rate | |||||
| At week 24 | |||||
| <6.5% | 33(29.73%) | 41(38.68%) | .1645 2 | ||
| ≥6.5% | 78(70.27%) | 65(61.32%) | |||
| Fasting plasma glucose (mmol/L) | |||||
| Baseline | |||||
| Mean ± SD | 7.36 ± 1.41 | 7.41 ± 1.46 | |||
| Median, minimum, maximum | 6.99, 4.94, 12.15 | 7.10, 5.11, 13.43 | |||
| At week 24 | |||||
| Mean ± SD | 6.76 ± 1.26 | 6.82 ± 1.56 | |||
| Median, minimum, maximum | 6.55, 4.50, 12.04 | 6.44, 4.66, 13.10 | |||
| Change from baseline to week 24 (Mean ± SD) | −0.60 ± 1.11 | −0.59 ± 1.47 | .7155 3 | ||
| P value for mean difference from baseline to week 24 | <0.0001 1 | <0.0001 1 | |||
| Mean daily glucose (mmol/L) | |||||
| Baseline | |||||
| Mean ± SD | 97 | 9.18 ± 1.98 | 93 | 9.46 ± 2.06 | |
| Median, minimum, maximum | 8.72, 5.94, 15.71 | 9.24, 5.34, 17.67 | |||
| At week 24 | |||||
| Mean ± SD | 87 | 8.24 ± 1.39 | 81 | 8.25 ± 1.71 | |
| Median, minimum, maximum | 8.07, 6.08, 12.44 | 7.72, 5.90, 15.86 | |||
| Change from baseline to week 24 (Mean ± SD) | 81 | −0.92 ± 1.60 | 75 | −1.30 ± 1.71 | .1062 3 |
| P value for mean difference from baseline to week 24 | <0.0001 1 | <0.0001 1 | |||
| HOMA‐β (%) | |||||
| Baseline | |||||
| Mean ± SD | 110 | 48.34 ± 28.06, | 106 | 53.18 ± 34.89 | |
| Median, minimum, maximum | 41.19, 12.00, 179.32 | 45.14, 13.47, 237.75 | |||
| At week 24 | |||||
| Mean ± SD | 110 | 59.58 ± 33.18 | 104 | 64.54 ± 38.59 | |
| Median, minimum, maximum | 50.27, 7.83, 193.43 | 55.33, 13.62, 277.71 | |||
| Change from baseline to week 24 (Mean ± SD) | 109 | 11.36 ± 27.92 | 104 | 11.82 ± 29.43 | |
| P value for mean difference from baseline to week 24 | <0.0001 1 | <0.0001 1 | 0.3791 3 |
P values were derived from Wilcoxon signed rank test.
P values were derived from chi‐square test.
P values were derived from Wilcoxon rank sum test.
A subgroup analysis of HbA1c and FPG was carried out for HbA1c levels (≥8.5% or <8.5%), sex (men or women), age (<65 or ≥65 years) and body mass index (≤25 or >25 kg/m2) at screening, and treatment effects for evogliptin and sitagliptin were not different among all subgroups. The use of rescue medication was minimal, with no use of rescue medication in both the evogliptin and sitagliptin groups from baseline to week 24 and with 2 patients receiving rescue medication in the evogliptin/evogliptin group from week 24 to week 52.
β‐cell function, as assessed by the HOMA‐β score, improved in both medication groups at week 24. After 24 weeks, the HOMA‐β had increased by 11.36% ± 27.92% (baseline vs week 24; P < .0001) in the evogliptin group and by 11.82% ± 29.43% (baseline vs week 24; P < .0001) in the sitagliptin group, but the change in the 2 medication groups was not different (P = .3791). Evogliptin/evogliptin treatment consistently improved β‐cell function during 52 weeks (mean change from baseline to week 52, 9.21% ± 26.09%; P = .0026). Unlike HOMA‐β, change in fasting insulin, c‐peptide, HOMA‐IR and QUICKI after 24 weeks was not statistically significant in the evogliptin group (Table S1, Supporting Information). HOMA‐IR and QUICKI showed statistically significant changes in the sitagliptin group (P = .0364 and P = .0094, respectively). Comparing changes from baseline to week 24 between treatment groups, there was no statistically significant difference in HOMA‐ß, HOMA‐IR and QUICKI.
All fasting lipid parameters, which included total cholesterol, LDL‐C, HDL‐C, TG and FFA, did not change significantly after 24 weeks of evogliptin treatment nor after 52 weeks of evogliptin/evogliptin treatment (Table S1, Supporting Information). The sitagliptin group showed a statistically significant change in FFA from baseline to week 24 (P = .0066) but change in total cholesterol, LDL‐C, HDL‐C and TG, was not statistically significant in the sitagliptin group. Inter‐group comparison of the change from baseline to week 24 revealed that none of the fasting lipid parameters exhibited any statistically significant difference.
After 24 weeks of treatment, mean body weight change from baseline in the evogliptin and sitagliptin groups was −0.14 ± 1.81 kg (P = .4583) and −0.05 ± 1.97 kg (P = .8493), respectively, and it had not changed significantly. After 52 weeks of treatment with evogliptin/evogliptin, body weight change was −0.12 ± 1.90 kg (P = .9691).
3.3. Tolerability and safety
In the parent study, incidences of AE were not significantly different between the 2 groups (P = .4337; chi‐square test) (Table 3). There were 84 adverse events (AEs) among 50 patients (45.0%) in the evogliptin treatment group (n = 111) and 76 AEs among 43 patients (39.8%) in the sitagliptin treatment group (n = 108). Adverse drug reactions (ADRs), which might have a causal relationship with sitagliptin or evogliptin, were observed more frequently in the evogliptin treatment group (22 ADRs in 14 patients [12.6%] in the evogliptin group vs 6 ADRs in 4 patients [3.7%] in the sitagliptin group; P = .0164]. However, these were mostly mild ADRs, with 2 cases of moderate ADRs (1 case in each treatment arm) and no case of severe ADR. Seven serious AEs occurred in 6 patients (5.6%) in the sitagliptin group (lumbar vertebral fracture, atrioventricular block complete, prostate cancer, benign prostatic hyperplasia, back pain, preauricular cyst and spontaneous abortion) and 4 serious AEs occurred in 4 patients (3.6%) in the evogliptin group (post‐traumatic neck syndrome, unstable angina, vestibular disorder and colitis). Among these, there were no serious ADRs. The incidences of serious AE was not significantly different between the 2 groups of the parent study (P = .5346; Fisher's exact test). During the extended study (week 24 to week 52) there were no serious ADRs.
Table 3.
Summary of clinical AEs and hypoglycaemic events
| From week 24 to week 52 | Evogliptin | Sitagliptin | ||||
|---|---|---|---|---|---|---|
| Subjects with event (%) [95% CI] | Event | Subjects with event (%) [95% CI] | Event | |||
| N = 111 | N = 108 | |||||
| Adverse event | 50(45.0) [35.6, 54.8] | Mild | 75 | 43(39.8) [30.5, 49.7] | Mild | 58 |
| Moderate | 7 | Moderate | 15 | |||
| Severe | 2 | Severe | 3 | |||
| Total | 84 | Total | 76 | |||
| Adverse drug reaction | 14(12.6) [7.1, 20.3] | Mild | 21 | 4(3.7) [1.0, 9.2] | Mild | 5 |
| Moderate | 1 | Moderate | 1 | |||
| Severe | 0 | Severe | 0 | |||
| Total | 22 | Total | 6 | |||
| Serious adverse event | 4(3.6) [1.0, 9.0] | Mild | 1 | 6(5.6) [2.1, 11.7] | Mild | 2 |
| Moderate | 1 | Moderate | 2 | |||
| Severe | 2 | Severe | 3 | |||
| Total | 4 | Total | 7 | |||
| Serious adverse drug reaction | 0(0.0) [0.0, 3.3] | 0 | 0(0.0) [0.0, 3.4] | 0 | ||
| Hypoglycaemia | 1(0.9) [0.0, 4.9] | 1 | 3(2.8) [0.6, 7.9] | 7 | ||
| From week 24 to week 52 | Evogliptin/Evogliptin | Sitagliptin/Evogliptin | ||||
|---|---|---|---|---|---|---|
| Subjects with event (%) [95% CI] | Event | Subjects with event (%) [95% CI] | Event | |||
| N = 93 | N = 91 | |||||
| Adverse event | 27(29.0) [20.1, 39.4] | Mild | 40 | 46(50.5) [39.9, 61.2] | Mild | 80 |
| Moderate | 10 | Moderate | 5 | |||
| Severe | 1 | Severe | 2 | |||
| Total | 51 | Total | 87 | |||
| Adverse drug reaction | 4(4.3) [1.2, 10.6] | Mild | 4 | 3(3.3) [0.7, 9.3] | Mild | 5 |
| Moderate | 0 | Moderate | 0 | |||
| Severe | 0 | Severe | 0 | |||
| Total | 4 | Total | 5 | |||
| Serious adverse event | 4(4.3) [1.2, 10.6] | Mild | 0 | 4(4.4) [1.2, 10.9] | Mild | 0 |
| Moderate | 7 | Moderate | 0 | |||
| Severe | 1 | Severe | 0 | |||
| Total | 8 | Total | 0 | |||
| Serious adverse drug reaction | 0(0.0) [0.0, 3.9] | 0 | 0(0.0) [0.0, 4.0] | 0 | ||
| Hypoglycaemia | 1(1.1) [0.0, 5.8] | 1 | 0(0.0) [0.0, 4.0] | 0 | ||
In the parent study, one hypoglycaemic event occurred in 1 case (0.9%) in the evogliptin group and 7 hypoglycaemia events occurred in 3 patients (2.8%) in the sitagliptin group (P = .3648; Fisher's exact test) (Table 3). One hypoglycaemic event in the evogliptin treatment group was asymptomatic hypoglycaemia, which was also observed in 3 cases in the sitagliptin treatment group (P = .6181; Fisher's exact test). Documented symptomatic hypoglycaemia and probable symptomatic hypoglycaemia were observed in 3 cases and in 1 case in the sitagliptin treatment group, respectively (P = .2421 and P = .4932, respectively; Fisher's exact test). The causal relationship of all hypoglycaemic events with sitagliptin or evogliptin was “probably not” or “definitely not” and the outcome was found to be “recovered without sequelae.”
4. DISCUSSION
In this phase III, randomized, double‐blind, active‐controlled study we proved that adding evogliptin or sitagliptin for inadequately controlled T2DM patients being treated with stable metformin therapy would accomplish a similar improvement in glycaemic control. With respect to HbA1c reduction, evogliptin, when added to metformin, is not inferior to sitagliptin when added to metformin, and the 2 treatment groups similarly achieved an HbA1c level < 6.5% (evogliptin, 29.73% vs sitagliptin, 38.68%; P = .1645) and FPG reduction (evogliptin, −0.60 ± 1.11 mmol/L vs sitagliptin, −0.59 ± 1.47 mmol/L; P = .7155) after 24 weeks. The results obtained in our study are compatible with those of previous studies, as well as with our expectations. In a previous phase II study, evogliptin resulted in statistically significant reductions in HbA1c compared to the placebo group after a 12‐week treatment period. Twelve weeks of treatment with evogliptin 5 mg once daily decreased the HbA1c level by −0.57% (95% CI, −0.86, −0.29; P < .0001), which is similar to the decrease with use of other DPP‐4 inhibitors (sitagliptin 100 mg, −0.55% and vildagliptin 100 mg, −0.53%).11, 12, 13 In an 18‐week non‐inferiority trial comparing the efficacy of saxagliptin 5 mg once daily and sitagliptin 100 mg once daily, the adjusted mean changes in HbA1c level were −0.52% and −0.62% in the saxagliptin and sitagliptin groups, respectively, in inadequately controlled T2DM patients treated with metformin alone.14 In our study, mean changes in HbA1c after 24 weeks were −0.59% (evogliptin) and −0.65% (sitagliptin). Although the mean change in HbA1c, FPG and MDG levels from week 24 to week 52 was slightly increased (0.15%, 0.19 mmol/L, 0.05 mmol/L, respectively, in the evogliptin/evogliptin group), the improvement in HbA1c, FPG and MDG levels caused by evogliptin was sustained until week 52 (−0.44%, −0.38 mmol/L, −1.00 mmol/L, respectively).
Pancreatic β‐cell dysfunction is one of the primary mechanisms of T2DM pathogenesis.15 Therefore, the modalities used to improve β‐cell function are a critical aspect of T2DM management. In our study, β‐cell function was estimated by HOMA‐β, and evogliptin improved HOMA‐β by 11.36% ± 27.92% (P < .0001) at week 24 and it was not different from the improvement with sitagliptin (11.82% ± 29.43%, P < .0001). A recent meta‐analysis of 27 double‐blind, randomized, controlled trials with sitagliptin and saxagliptin, linagliptin, alogliptin showed a similar result; a DPP‐4 inhibitor, when added to metformin, increased HOMA‐β by 10.21% (95% CI, 7.73‐12.69).16 In a previous clinical study with evogliptin, insulin secretory function, assessed by the insulinogenic index and post‐OGTT C‐peptide AUC0–2h, was significantly improved at 12 weeks,10 but the effect of evogliptin on human β‐cell function was not clear after 12 weeks. In our study, evogliptin improved HOMA‐β (9.21% ± 26.09%, P = .0026) up to week 52. Several studies have suggested that DPP‐4 inhibitors increased β‐cell mass via increased β‐cell neogenesis and decreased β‐cell apoptosis in young rodents.17, 18, 19 Evogliptin treatment in streptozotocin‐treated mice increased the volume density of β‐cells and the number of replicating β‐cells.20 Evogliptin also induces Pdx‐1 expression in small β‐cells, indicating neogenesis, and some insulin‐positive islet cells and ductal cells are seen.20 However, another study reported that vildagliptin treatment in mature rodents failed to increase the β‐cell mass.21 Moreover, in a study in humans, 3‐ or 12‐month treatment periods with vildagliptin or sitagliptin increased the capacity for insulin secretion during treatment, but failed to preserve the increased capacity of insulin secretion after a 2‐week drug washout period.22, 23, 24 Our study showed the possible long‐term effects of evogliptin on β‐cell function in humans, but additional data with a longer follow‐up and drug washout are needed.25 In the present study, mean changes in HOMA‐IR from baseline to 24 weeks showed that evoglipitin did not have a statistically significant effect on insulin resistance (−0.17 ± 1.72, P = .1233). In a previous study among Korean T2DM patients, another DPP‐4 inhibitor (vildagliptin) had similar effects when compared to pioglitazone.26
We first verified the tolerability and safety of a daily dose of evogliptin 5 mg up to week 52. The incidence of AEs was similar to that with use of sitagliptin, which is one of the most widely prescribed DPP‐4 inhibitors. The frequency of serious AEs was very low with use of evogliptin and sitagliptin, and both treatment groups showed no serious ADRs. Many conventional glucose‐lowering agents commonly result in weight gain,27 but treatment with evogliptin for 52 weeks resulted in a stable body weight (mean change, −0.12 ± 1.90; P = .9691). Also, the incidence of a hypoglycaemic event during the 24‐week period was very low in each treatment group (evogliptin, 0.9% vs sitagliptin, 2.8%), and there was no case of severe hypoglycaemia. In particular, the evogliptin group had only 1 case of asymptomatic hypoglycaemia during the 24‐week period.
In conclusion, this study showed that 5 mg of evogliptin exhibits non‐inferiority to sitagliptin when added to metformin therapy. This study also showed that adding evogliptin in patients with inadequately controlled T2DM who are being treated with metformin alone is effective in lowering HbA1c and is generally well tolerated and safe until week 52.
Supporting information
Figure S1. Changes in the mean glycated haemoglobin (HbA1c) level from baseline to week 52.
Table S1. Baseline, week 24 and changes in results from baseline for secondary outcomes.
ACKNOWLEDGMENTS
We thank the other investigators for their cooperation in this study. The full list of other investigators is as follows: J. G. Kang (Hallym University Sacred Heart Hospital, Hallym University School of Medicine, Gyeonggi‐do, Republic of Korea), G. P. Koh (Jeju National University School of Medicine, Jeju, Korea), D. M. Kim (Hallym University, Seoul, Republic of Korea), S. R. Kim (The Catholic University of Korea, Seoul, Korea), Y. S. Kim (Inha University School of Medicine, Incheon, Korea), K. Y. Park (College of Medicine, Konyang University, Daejeon, Korea), J‐Y. Park (Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea), Y. A. Sung (Ewha Womans University School of Medicine, Seoul, Republic of Korea), K. H. Song (The Catholic University of Korea, Seoul, South Korea) K. J. Ahn (Kyung Hee University School of Medicine, Seoul, Republic of Korea), M. K. Lee (Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.), I.‐K. Lee (Kyungpook National University School of Medicine Daegu, South Korea), J. H. Lee (Myongji Hosipital, Goyang, Republic of Korea), H. W. Lee (Yeungnam University, Daegu, Republic of Korea), S. Lim (Seoul National University Bundang Hospital, Seongnam, Korea), B. S. Cha (Yonsei University College of Medicine, Seoul, Korea), and K. M. Choi (Korea University, Seoul, Republic of Korea).
Conflict of interest
Dong‐Min Hwang is an employee of Dong‐A ST Co., Ltd. The other authors have no conflicts of interest related to this work.
Author contributions
Sang‐Mo Hong, Cheol‐Young Park, Kyung Ah Han, Chang Beom Lee, Choon Hee Chung, Kun‐Ho Yoon, Ji‐Oh Mok, Kyong Soo Park, and Sung‐Woo Park designed and performed the clinical study; Sang‐Mo Hong, Cheol‐Young Park, Dong‐Min Hwang, and Sung‐Woo Park analyzed the data; Sang‐Mo Hong, Cheol‐Young Park and Sung‐Woo Park drafted and finalized the full manuscript; Kyung Ah Han, Chang Beom Lee, Choon Hee Chung, Kun‐Ho Yoon, Ji‐Oh Mok, and Kyong Soo Park reviewed and revised the manuscript.
Hong S‐M, Park C‐Y, Hwang D‐M, Han KA, Lee CB, Chung CH, Yoon K‐H, Mok J‐O, Park KS and Park S‐W. Efficacy and safety of adding evogliptin versus sitagliptin for metformin‐treated patients with type 2 diabetes: A 24‐week randomized, controlled trial with open label extension. Diabetes Obes Metab. 2017;19:654–663. https://doi.org/10.1111/dom.12870
Funding information This study was supported by Dong‐A ST Co., Ltd, Seoul, Republic of Korea. The sponsor participated in the study design, data collection and analysis of the data. The sponsor had no role in writing the manuscript and in the decision to submit the manuscript for publication.
REFERENCES
- 1. Deacon CF, Lebovitz HE. Comparative review of dipeptidyl peptidase‐4 inhibitors and sulphonylureas. Diabetes Obes Metab. 2016;18:333–347. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association. Approaches to glycemic treatment. Diabetes Care. 2016;39(suppl 1):S52–S59. [DOI] [PubMed] [Google Scholar]
- 3. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 executive summary. Endocr Pract. 2016;22:84–113. [DOI] [PubMed] [Google Scholar]
- 4. Schulte JM, Rothaus CS, Adler JN. Clinical decisions. Management of type 2 diabetes‐‐polling results. N Engl J Med. 2014;370:e2. [DOI] [PubMed] [Google Scholar]
- 5. Gu N, Park MK, Kim TE, et al. Multiple‐dose pharmacokinetics and pharmacodynamics of evogliptin (DA‐1229), a novel dipeptidyl peptidase IV inhibitor, in healthy volunteers. Drug Des Devel Ther. 2014;8:1709–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oh J, Kim AH, Lee S, et al. Effects of renal impairment on the pharmacokinetics and pharmacodynamics of a novel dipeptidyl peptidase‐4 inhibitor, evogliptin (DA‐1229). Diabetes Obes Metab. 2017;19:294–298. doi: 10.1111/dom.12813. [DOI] [PubMed] [Google Scholar]
- 7. Food and Drug Administration . Guidance for Industry: Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. US Department of Health and Human Services; 2008. http://www.fda.gov/downloads/drugs/guidancecomplianceregu‐ latoryinformation/guidances/ucm071627.pdf. Accessed February 7, 2017. [Google Scholar]
- 8. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 9. Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. [DOI] [PubMed] [Google Scholar]
- 10. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jung CH, Park CY, Ahn KJ, et al. A randomized, double‐blind, placebo‐controlled, phase II clinical trial to investigate the efficacy and safety of oral DA‐1229 in patients with type 2 diabetes mellitus who have inadequate glycaemic control with diet and exercise. Diabetes Metab Res Rev. 2015;31:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanefeld M, Herman GA, Wu M, et al. Once‐daily sitagliptin, a dipeptidyl peptidase‐4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin. 2007;23:1329–1339. [DOI] [PubMed] [Google Scholar]
- 13. Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase‐4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab. 2005;7:692–698. [DOI] [PubMed] [Google Scholar]
- 14. Scheen AJ, Charpentier G, Ostgren CJ, Hellqvist A, Gause‐Nilsson I. Efficacy and safety of saxagliptin in combination with metformin compared with sitagliptin in combination with metformin in adult patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2010;26:540–549. [DOI] [PubMed] [Google Scholar]
- 15. Chon S, Gautier JF. An update on the effect of incretin‐based therapies on beta‐cell function and mass. Diabetes Metab J. 2016;40:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao W, Wang Q, Yu S. Efficacy, safety and impact on beta‐cell function of dipeptidyl peptidase‐4 inhibitors plus metformin combination therapy in patients with type 2 diabetes and the difference between Asians and Caucasians: a meta‐analysis. J Endocrinol Invest. 2016;39:1061–1074. [DOI] [PubMed] [Google Scholar]
- 17. van Genugten RE, van Raalte DH, Diamant M. Dipeptidyl peptidase‐4 inhibitors and preservation of pancreatic islet‐cell function: a critical appraisal of the evidence. Diabetes Obes Metab. 2012;14:101–111. [DOI] [PubMed] [Google Scholar]
- 18. Hamamoto S, Kanda Y, Shimoda M, et al. Vildagliptin preserves the mass and function of pancreatic beta cells via the developmental regulation and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetes Obes Metab. 2013;15:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah P, Ardestani A, Dharmadhikari G, et al. The DPP‐4 inhibitor linagliptin restores beta‐cell function and survival in human isolated islets through GLP‐1 stabilization. J Clin Endocrinol Metab. 2013;98:E1163–E1172. [DOI] [PubMed] [Google Scholar]
- 20. Cho JM, Jang HW, Cheon H, et al. A novel dipeptidyl peptidase IV inhibitor DA‐1229 ameliorates streptozotocin‐induced diabetes by increasing beta‐cell replication and neogenesis. Diabetes Res Clin Pract. 2011;91:72–79. [DOI] [PubMed] [Google Scholar]
- 21. Omar BA, Vikman J, Winzell MS, et al. Enhanced beta cell function and anti‐inflammatory effect after chronic treatment with the dipeptidyl peptidase‐4 inhibitor vildagliptin in an advanced‐aged diet‐induced obesity mouse model. Diabetologia. 2013;56:1752–1760. [DOI] [PubMed] [Google Scholar]
- 22. Foley JE, Bunck MC, Moller‐Goede DL, et al. Beta cell function following 1 year vildagliptin or placebo treatment and after 12 week washout in drug‐naive patients with type 2 diabetes and mild hyperglycaemia: a randomised controlled trial. Diabetologia. 2011;54:1985–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. D'Alessio DA, Denney AM, Hermiller LM, et al. Treatment with the dipeptidyl peptidase‐4 inhibitor vildagliptin improves fasting islet‐cell function in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aaboe K, Knop FK, Vilsboll T, et al. Twelve weeks treatment with the DPP‐4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non‐glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2010;12:323–333. [DOI] [PubMed] [Google Scholar]
- 25. Ahren B, Foley JE. Improved glucose regulation in type 2 diabetic patients with DPP‐4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia. 2016;59:907–917. [DOI] [PubMed] [Google Scholar]
- 26. Kim JH, Kim SS, Baek HS, et al. Comparison of vildagliptin and pioglitazone in Korean patients with type 2 diabetes inadequately controlled with metformin. Diabetes Metab J. 2016;40:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Dieren S, Czernichow S, Chalmers J, et al. Weight changes and their predictors amongst 11 140 patients with type 2 diabetes in the ADVANCE trial. Diabetes Obes Metab. 2012;14:464–469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Changes in the mean glycated haemoglobin (HbA1c) level from baseline to week 52.
Table S1. Baseline, week 24 and changes in results from baseline for secondary outcomes.


