Abstract.
This study aimed to determine whether a reduction in radiation dose was found for percutaneous coronary interventional (PCI) patients using a cardiac interventional x-ray system with state-of-the-art image enhancement and x-ray optimization, compared to the current generation x-ray system, and to determine the corresponding impact on clinical image quality. Patient procedure dose area product (DAP) and fluoroscopy duration of 131 PCI patient cases from each x-ray system were compared using a Wilcoxon test on median values. Significant reductions in patient dose () were found for the new system with no significant change in fluoroscopy duration (); procedure DAP reduced by 64%, fluoroscopy DAP by 51%, and “cine” acquisition DAP by 76%. The image quality of 15 patient angiograms from each x-ray system (30 total) was scored by 75 clinical professionals on a continuous scale for the ability to determine the presence and severity of stenotic lesions; image quality scores were analyzed using a two-sample -test. Image quality was reduced by 9% () for the new x-ray system. This demonstrates a substantial reduction in patient dose, from acquisition more than fluoroscopy imaging, with slightly reduced image quality, for the new x-ray system compared to the current generation system.
Keywords: radiation dose, image quality, x-ray imaging, percutaneous coronary interventions, observer study
1. Introduction
X-ray imaging systems that provide images in real-time are essential for the diagnosis and treatment of coronary heart disease. In angiography, cardiologists use live, high-quality acquired image sequences of the coronary arteries for diagnosis during percutaneous coronary interventional (PCI) procedures. If an arterial narrowing restricts blood flow, the patient is treated via image-guided angioplasty in which interventional devices such as guide wires, balloons, and stents are manipulated using lower quality x-ray imaging known as fluoroscopy. The quality of the images must be sufficient to enable safe and effective diagnosis and treatment. However, image quality is related to the amount of radiation used to capture the image,1 and radiation dose must be kept “as low as reasonably practicable (ALARP).”
Exposure to x-rays can be harmful, and radiation doses from interventional cardiac procedures are the highest of any routine medical procedure.2 Deterministic effects occur from radiation doses exceeding a threshold; these include skin burns and hair loss among patients (threshold absorbed dose of 2 Gy)3–6 and cataracts to the eye lens of interventional cardiologists (occupational threshold dose of ).7–9 Stochastic effects, with no specific threshold dose,10 result from damaged DNA, causing long-term genetic defects and cancers; this is generally more of a concern for pediatric than adult patients.11 In 2014, over 96,000 interventional cardiac procedures were performed at 118 centers in the UK; by contrast, in 2002 there were 50,000 procedures at 62 centeres,12 illustrating the rise in the number of these procedures and associated rising risk. Given the increasingly ageing population, these numbers will likely continue to increase. As equipment continues to advance, longer, more complicated cases are undertaken.
Digital image processing plays an increasingly significant role in diagnostic radiology, enhancing displayed images using algorithms to reduce the visual impression of noise and enhance anatomic structures that are clinically relevant. Here the image quality is improved irrespective of the radiation dose, i.e., there is no corresponding increase in radiation dose, which is usually inherent in improved x-ray image quality. As a result, image enhancement may lead to a reduction in the amount of dose required to produce a clinically acceptable image. As computing power increases, faster, more complex enhancement algorithms are being used. This is particularly beneficial for cardiac interventional x-ray imaging, where real-time images are required. Each manufacturer has its own unique algorithms that adapt to image content in real-time, with clinical task-specific enhancement. Philips Healthcare’s most recent interventional x-ray system, AlluraClarity (Philips Healthcare, The Netherlands) has ClarityIQ image enhancement with real-time image noise reduction algorithms that, in combination with anatomy-specific x-ray optimization, promise to reduce patient dose.13 With this option, both the radiographic settings used to capture images and the computer processing applied to the images are different from the current generation interventional x-ray system by the manufacturer. Studies have shown a patient dose reduction from this system upgrade in neuroradiology14,15 and other digital subtraction angiography (DSA) applications,16 cardiac interventional,17–20 and electrophysiology (EP) procedures.21 However, a statistically robust investigation of corresponding changes in clinical image quality for PCI patients, using a range of projection angles, has yet to be published; such a comprehensive assessment of both radiation dose and image quality is crucial for establishing a thorough understanding of a new x-ray system and its impact on clinical practice.
An AlluraClarity (hereafter Clarity) system was installed in Yorkshire Heart Centre, where six cardiac catheter labs are in clinical operation. PCI procedures, specifically, were chosen for this study because their procedural radiation doses are among the highest.22–24 This study’s primary aims were to investigate if the Clarity system significantly reduces radiation dose to PCI patients and to determine the corresponding impact on patient image quality, compared to the current generation system. Secondary aims were to assess the dose reduction in fluoroscopy and acquisition modes separately and to determine if there was a significant difference in procedural duration between the two x-ray systems.
2. Materials and Methods
The study comprised two components: an analysis of radiation dose and an assessment of clinical image quality. Both components were completed in two phases: a pilot experiment to provide data for power calculations and then the main investigation. Two of the six cardiac catheter labs in the center were included in the study—the newly installed Clarity FD10 lab and an Allura Xper FD10 lab (Philips Healthcare, The Netherlands), which was already in use, primarily for PCI procedures, as the reference lab for comparison.
This observational study collected patient doses from hospital IT system records, and images were collected from the picture archive and communications system (PACS). Practitioners were not aware of the study and so performed the intervention as per typical practice. Both labs were generally fully booked for clinical use. All data were anonymized by removing personally identifiable information.
The imaging modes (fluoroscopy and “cine” acquisition) used during PCI procedures in the two labs had the manufacturer’s default settings; i.e., no adjustments had been made since installation to tailor the settings to the needs of this particular hospital. Due to the proprietary nature of the commercial image processing algorithm, details on how it operates can only be found in manufacturer-provided documentation.25
2.1. Radiation Dose
For the pilot study, 555 PCI patient cases were collected from the reference lab to use for a sample size calculation. For the main study, patient procedure dose details were recorded for 131 PCI patients from the study lab and 131 patients from the reference lab. Details recorded were dose area product (DAP) for fluoroscopy and acquisition, total procedure DAP, and total fluoroscopy duration.
2.2. Image Quality
Image sequences from randomly selected PCI patient procedures from the study and reference labs were collected, and DICOM headers were extracted for relevant metadata. Fifteen angiograms from each lab were selected from this database to include left and right coronary arteries captured at a range of projection angles. Only one angiogram was chosen from any given patient. All angiograms were acquired at 15 frames per second. A broad range of patient body habitus were represented for each group; body mass indices (BMI) of the patients ranged from 26 to for the study lab and 22 to for the reference lab, with means 31 and , respectively, and no significant difference () between groups. Patient condition, or case complexity, was also varied within each group; the number of stents in the angiograms ranged from zero to three for the study lab and zero to two for the reference lab, with means 0.6 and 0.7, respectively, and no significant difference (). These datasets were compared using a Wilcoxon test. The two groups of angiograms were independently scored on a continuous scale in a blind observer study. The observers were familiarized with the scoring software prior to beginning the image quality assessment. The two end points were “unsatisfactory” (0) and “exceeds requirements” (1) with the midpoint “acceptable” (0.5), as shown in Fig. 1. Observers were asked to focus on overall level of diagnostic image quality, completing the sentence “To determine the presence and severity of stenotic lesions, the image quality is…” All angiograms were 512 by 512 pixels at 8 bit depth, displayed at using MATLAB 2013b (The Mathworks Inc, Natick). Bespoke software with a graphical user interface (GUI) was designed in MATLAB® specifically to execute this observer study. The angiograms were shown to observers in a random order, which differed for each observer; they looped continuously until the observer clicked anywhere on the continuous scale, then the next angiogram was shown. Ratings for each angiogram were automatically translated into quantitative scores between zero and one for statistical analysis.
Fig. 1.
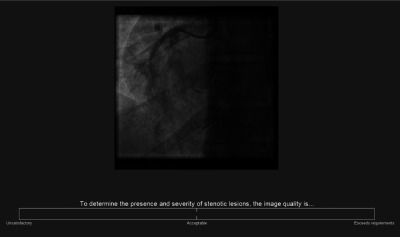
The observer study GUI showing a single frame from the start of an angiogram.
A pilot study was performed to power the observer study. Three medical imaging experts with 10, 22, and 27 years’ experience viewed the angiograms on a RadiForce RX340 medical grade monitor (EIZO Corporation, Ishikawa, Japan) away, in a room with slightly dimmed lighting (as a radiology reporting room). The observer study was approved by the University of Leeds Research Ethics Committee. Observer recruitment took place in Leeds and Nottingham NHS Trust Hospitals and the British Cardiovascular Society annual cardiology meeting exhibition hall. Volunteer observers were blinded to the purpose of the study; they were provided with a participant information sheet and signed a participant consent form; the forms were not linked to results; hence the data were anonymous. The information listed below was collected from each observer. Images were scored using an Eonis MDRC-2224 BL clinical display unit (Barco, Brussels, Belgium); Leeds participants used a Radiforce RX340 monitor. Both monitors were DICOM-calibrated.
-
•
Clinical profession (choice of nine categories);
-
•
Number of years of experience (free text);
-
•
Whether they view cardiac images in their daily work (yes/no).
2.3. Statistical Analysis
Patient procedure DAPs from the pilot dose data were used to calculate the sample size required to test for a 30% difference in dose between the two labs at a 5% significance level with 90% power in the main study. A Wilcoxon test, specifically the ranksum function in MATLAB® 2013b, was used to compare median DAP and fluoroscopy duration from the two labs.
A sample size calculation was performed using the image quality pilot study results to determine how many observations would be required for a 30% difference in image quality scores, with 80% power at a 5% significance level. The image quality statistical analysis was conducted in R 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria) with the continuous image quality scores analyzed using a two sample -test and a boxplot. Since the same observers viewed both sets of 15 images, the observer characteristics were not a variable when comparing the two labs, allowing these tests to be conducted. Multilevel models were used to investigate the effects of observer characteristics and observer study setup on scores, using the lme4 package in R. The outcome of interest was the continuous image quality score, with independent predictors including which x-ray system was used, the clinical profession and number of years’ experience of the observers, and whether they view cardiac images in their daily work. Background lighting, indicated by location of the observer experiment, and the clinical monitor used were individually added to the model as fixed effects. The observer ID was added as a random effect, since observers each scored 30 images and it is expected that observers will score similarly to themselves, yet differently to others.
3. Results
3.1. Radiation Dose
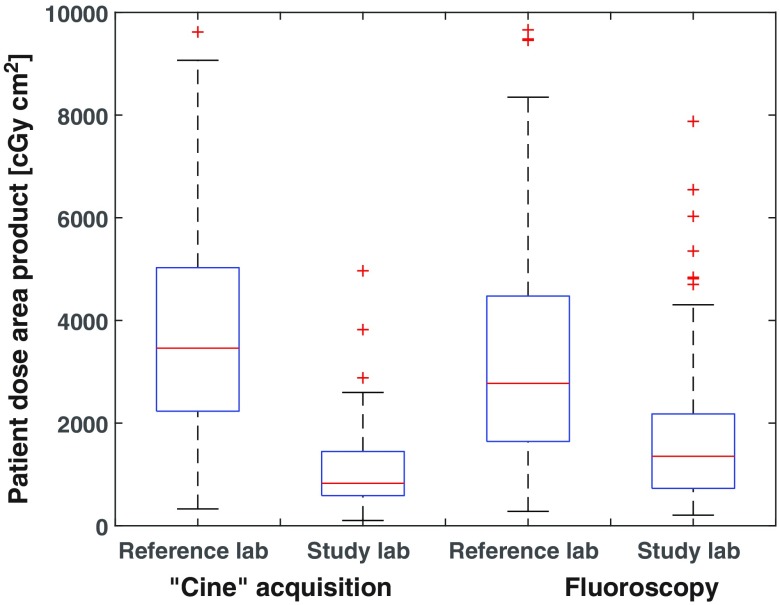
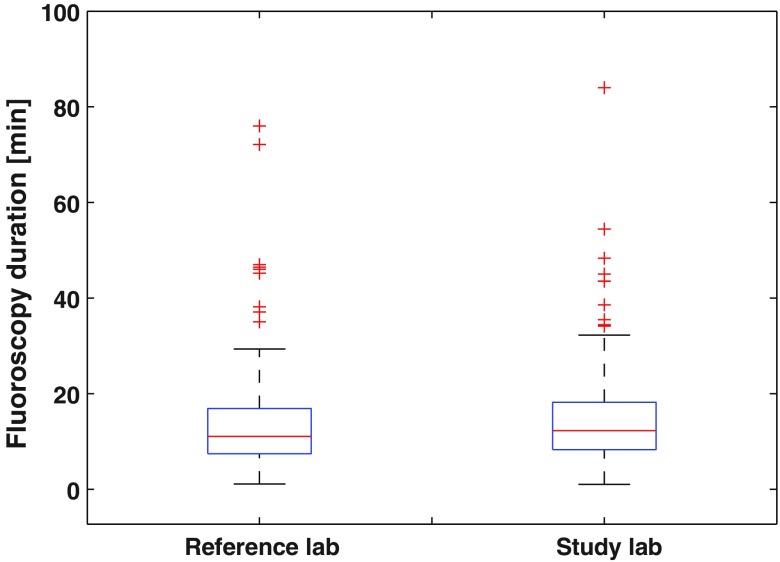
Sample size calculations showed that a minimum of 100 patients from each of two labs were required for comparison of dose. Boxplots are shown in Figs. 2 and 3 for DAP and fluoroscopy duration, respectively, for the 131 cases—more than the 100 required. Median total patient procedure doses were 2292 and from the study and reference labs, respectively, showing the study lab to be 64% lower. Median acquisition DAPs were 827 and from the study and reference labs, respectively, showing a 76% reduction. Fluoroscopy median DAPs were 1354 and from the study and reference labs, respectively, showing a 51% reduction. The Wilcoxon test showed strong statistically significant differences in medians for both fluoroscopy and acquisition patient doses at the 5% significance level ( in both cases). Median fluoroscopy durations were 12:29 (min:sec) and 11:09 for the study and reference labs, respectively, showing no statistically significant difference () between the two labs.
Fig. 2.
Box plots for acquisition and fluoroscopy dose; median values are shown with first and third quartiles as boxes, minimum and maximum values, and outliers as plus signs.
Fig. 3.
Box plots for fluoroscopy duration; median values are shown with first and third quartiles as boxes, minimum and maximum values, and outliers as plus signs.
3.2. Image Quality
The sample size calculation showed that 61 observers would be required in the main image quality study; 75 observers (60 at the conference and 15 in hospital viewing rooms) participated, hence more than the 61 required. Observer professions are shown in Table 1, with 50 observers classed as specialists, categorized by their knowledge of, or experience with, the heart and/or angiography. Fifty-four observers viewed cardiac images in their daily work. The average number of years of experience was nine, ranging from 0 to 37 years.
Table 1.
Number of observers in each clinical profession.
| Specialists | |
| Interventional cardiologist | 9 |
| Cardiology registrar | 20 |
| Other cardiology | 14 |
| Radiographer |
7 |
| Nonspecialists | |
| Nurse practitioner | 2 |
| Nurse (other) | 4 |
| Student | 4 |
| Medical physicist | 4 |
| Other | 11 |
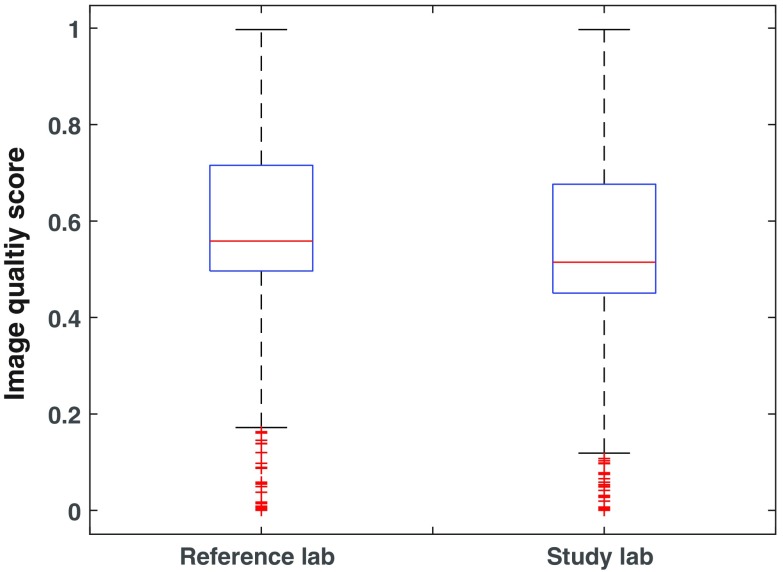
Associated with the large dose reduction, there was a small reduction in image quality, with median scores of 0.51 and 0.56 (Fig. 4) from the study and reference labs, respectively, showing a difference of 9% (). The image quality scores covered the entire scale, ranging from 0 to 1 for both labs. A larger proportion of low image quality scores were assigned to the reference lab than to the study lab, and more high scores were assigned to the study lab than the reference lab, with the transition in the middle of the continuous scale. For the 0.4 to 0.5 range of scores, there were more assigned to the reference lab, and for the 0.5 to 0.6 range of scores, there were more assigned to the study lab. For the reference and study labs, respectively, 13% and 10% of scores were between 0.2 and 0.4, with 24% and 29% between 0.6 and 0.8. There were 11% and 13% of the reference and study lab scores, respectively, assigned to the very high quality scores ranging 0.8 to 1.0.
Fig. 4.
Box plots for the continuous image quality scores; median values are shown with first and third quartiles as boxes, minimum and maximum values, and outliers as plus signs.
Multilevel modeling using the lme4 package in R showed that observers who view cardiac images in their daily work, who were classed as a specialist, or who had more years’ experience did not impact the image quality scores, nor did the observer study location (background lighting) or the clinical monitor used.
4. Discussion
When optimizing an x-ray imaging system, it is important that a robust assessment of clinical image quality and radiation dose is made. Both aspects were covered by appropriately powered experiments in this case. The image quality study involved 75 observers from a large number of institutions, 29 of which would be expected to be involved in decision making in clinical care based on imaging. Subsequent analysis of the scores revealed that the remaining observers, from related disciplines, did not score differently from this core group; this has been found previously for observers from related disciplines using a differing observer study design.26 Different coronary vessels, patient body habitus and condition, and image (C-arm) projection angles were included in the 15 angiograms from each x-ray system; no significant difference was found between the patient body habitus or condition of the two sets of angiograms; therefore, neither factor would have created bias toward one set over the other.
These results have important implications for PCI patients and personnel because changes in x-ray settings that allow for lower reported DAPs will also allow for lower radiation exposures to patients, the interventional cardiologists performing the procedures, and other personnel near the x-ray beam.27 These exposures refer to both entrance surface and absorbed dose, which may cause deterministic and stochastic effects, respectively (see Sec. 1). Since this was a retrospective study, DAP—the standard dose metric used for reporting and dose audits—was the only dose metric available. Until recently, concern for long-term damaging effects of radiation from PCI has typically been directed at patients only;28,29 however, more focus has been directed to personnel with groups such as the Organization for Occupational Radiation Safety in Interventional Fluoroscopy. Cardiologists may begin clinical practice as young as their early 30s,30 increasing the risk of radiation-induced cancer during their lifetime. Women are at slightly higher risk of stochastic effects than men,10 and the number of female cardiologists is rising.30 In 2016, in an effort to address the rising concern for exposure to interventional cardiologists, the British Institute of Radiology introduced an online resource for cardiologists to learn about ionizing radiation,31 as radiologists are expected to do during their training.
While there are standard protocols for image quality assessment in routine quality assurance tests, these tests do not assess clinical image quality. Any observer experiment that attempts to do so contains a number of compromises in design; the experimental design depends on the clinical context and practicalities of the study, and there is considerable variety in the approaches taken in such experiments.32–35 This study utilized a large number of observers; the number of angiograms reviewed per observer was comparatively small to assure the time for observers to complete the study was not too onerous, therefore increasing observer participation rates. The number of angiograms assessed was small enough to achieve this yet sufficient to provide a representative sample from each lab. The broad range and number of observers reduced the chances of a number of potential observer biases; recruiting observers predominantly from one hospital, for example, where one of the x-ray systems was in use but not the other, may have led to a greater preference for that x-ray system due to familiarity.
Reference images were not provided for the observers to allow the observers to use the grading scale as they saw fit. While it would have been possible to provide very good or very poor angiograms for reference, this would have added little value, as observers would surely recognize such images. Providing a reference “acceptable” image could have effectively altered observers’ scores by changing their opinion of acceptability and potentially influencing the results and thus conclusions. Before starting each observer study, the observer was made familiar with the scoring software; observers assessed a small number of sequences from the study (randomly selected, as in the real study) until they were comfortable with the scoring task. These scores were discarded, allowing the observers to settle into a consistent scoring method before beginning the observer study.
The scoring task presented to the observers in the image quality assessment (see Fig. 1) was focused on the most important aspect of the angiogram—was it good enough for use? In terms of overall image quality, an angiogram may not be good enough for use because it is too noisy, blurred, or lacks contrast, for example, and it would have been possible to ask a wider range of questions about individual image quality characteristics. The issue here is the interrelation between such characteristics; for example, a noisy image may be acceptable if it contains high contrast presentation of the arteries and unacceptable if the contrast is lower. A greater number of scoring tasks may have been of interest to investigate why observers felt a given angiogram was of better or worse overall quality; however, this would have taken additional time for the observers. Moreover, it was not the primary concern of this study, i.e., it would not have answered the research question. Finally, the single scoring task presented was selected because it reflects the clinical task performed in the given PCI setting. Care must be taken when generalizing the findings of this study, however, as the results may not reflect a different clinical task (for instance EP procedures where fluoroscopy quality is paramount), nor may they represent the full range of PCI-related purposes (for instance, assessing stent deployment or arterial wall dissection).
Most, but not all, of the image quality scores for both systems were just above the midpoint, which indicated diagnostic acceptability, yet all of the angiograms were used during a patient procedure. This may be explained by the observers being presented only one angiogram from a case, therefore not having the additional imaging or contextual information and not being aware of the patient case background.36
Past studies have been published comparing Philips’ Clarity with Xper systems (the two compared in this study), investigating vascular DSA and cardiac interventional imaging applications. For patient dose comparisons, although study methodologies vary, results are in general agreement, reporting a 50% to 75% reduction in dose.14–19,21 A similar Toshiba upgrade (PureBrain, Toshiba Medical Systems Corporation, Shimoishigami, Japan) was evaluated for pediatric cardiac interventional patients, reporting a 50% dose reduction.37
Robust comparisons of image quality in coronary interventional imaging are not so common. Some of the dose comparison studies did not perform any comparison of image quality18 or used an inappropriate surrogate measure (e.g., duration of imaging).21 When image quality was compared, the number of observers was often limited and from a single institution. Ten Cate et al.19 used a paired comparison of images obtained in a single projection and reported an 85% preference for the Clarity system. The study contained 234 observations with only six observers, compared to 2250 in this study, with no mention of a power calculation upon which to base the sample size. Moreover, the statistical analysis did not take into account correlations in the observations (e.g., repeated images). Eloot et al.17 reported no change in resolution, contrast, or overall image quality and a reduction in noise levels on the Clarity system. Once more, the number of observers was limited (four), all from the same institution. The statistical analysis used was inappropriate for the analysis of ordinal data (assigning a numeric value to categories and taking the mean) and did not take account of correlations in the observations;38 again, no power calculation was reported.17
There were some limitations to this study. Fluoroscopy duration was compared to assure any changes in dose were from the difference in interventional labs, not from a difference in x-ray duration (for example, due to a difference in case complexity between the two groups). However, the number of acquisition image frames was not included in this analysis because it was not accurately recorded; in the hospital database, it was impossible to differentiate an acquisition sequence from a fluoroscopy loop that was saved as per good radiological practice. Some of the image quality assessments took place in the exhibition hall of a conference, and therefore the ambient lighting was not dimmed as it would be in a radiology reporting room. However, dimmed lighting is not used in the local cardiac catheter labs, as reported elsewhere.39 Moreover, for each observer, both sets of angiograms were viewed under the same lighting conditions, and therefore lighting was not a variable between the two sets of angiograms. Image quality scores were not significantly different between the differing observer study surroundings, i.e., both background lighting and the clinical monitor used.
This comparison of radiation dose and image quality showed that the new Philips Clarity interventional x-ray imaging system enabled substantial reductions in patient dose with a small reduction in image quality compared to the current generation Xper interventional x-ray system. The reduction in acquisition dose was more substantial than the reduction in fluoroscopy dose. The Clarity system has two key differences in acquisition settings compared to the Xper system. The first is the change in x-ray settings—increased spectral x-ray beam filtration and decreased peak tube voltage and reduced image detector dose request. The second is the state-of-the-art digital image enhancement algorithm, which includes improved spatial and temporal filtering for noise reduction.14 It would be interesting to study the effect of these two changes on image quality independently, investigating to what extent the loss in image quality due to the reduced dose can be recovered by the computer image enhancement. This would provide further insight on the results from this study, to understand which change to the x-ray system is most responsible for the large reduction in dose and small reduction in image quality.
Acknowledgments
The research group would like to acknowledge Michael Lupton, lead radiographer in the catheter labs at Leeds General Infirmary, for his enthusiasm and support on this project. We would like to acknowledge Sjirk Boon from Philips Healthcare for providing anonymised patient procedure data from the imaging system. This research was funded by Philips Healthcare (the Netherlands); Andrew Davies held a research grant from Philips Healthcare during this research.
Biographies
Amber J. Gislason-Lee is a medical physicist based at the University of Leeds, United Kingdom. She received her BSc degree from the University of Winnipeg, Canada, and her MSc degree in medical physics from the University of Leeds. Her research has included pediatric dose optimization in diagnostic radiology and quantifying performance of cardiac flat-panel detector-based interventional x-ray imaging systems.
Claire Keeble is a biostatistician based in the Division of Epidemiology and Biostatistics at the University of Leeds. Her work focuses on the development and application of statistical methods for medical research, including the analysis of data relating to image quality and radiation dose in medical imaging, and the survival of subgroups of the population following primary percutaneous coronary intervention.
Daniel Egleston graduated from Bristol University with an MSci degree in physics with astrophysics in 2015, after undertaking a final-year project analyzing computational simulations of radiotherapy treatments. He worked as a summer student at Leeds University in 2014, importing and cataloguing image sequences from x-ray imaging devices for this study, and returned the following summer to write a program emulating pixel noise in imaging devices. He currently holds a graduate position at BAE Systems Applied Intelligence.
Josephine Bexon is a final-year physics student studying at the University of Surrey. She has a very keen interest in medical physics and is aiming to go into the sector following her graduation. She recently partook in a year’s placement with the Regional Radiation Protection Service located in Guildford, where she worked on projects such as image quality in mammography systems and analysis of staff eye dose in radiology departments.
Stephen M. Kengyelics received his MSc degree in physics from the University of Leeds, United Kingdom, in 1997. He received his BEng degree in electrical and electronic engineering from the University of Plymouth in 1991. His research has included quantifying the performance of medical x-ray image detectors and the application of machine vision to interventional cardiac x-ray imaging.
Andrew G. Davies is a lecturer in medical imaging at the University of Leeds, United Kingdom, where he received his MSc degree in medicine in 1995 and his BSc degree in computer science in 1990. His research interests include image quality, system performance, and optimization of medical x-ray imaging systems.
Disclosures
This research was funded by Philips Healthcare (the Netherlands); Andrew Davies held a research grant from Philips Healthcare during this research; no other authors have any potential conflict of interest to disclose. No authors have any relevant financial interests in this study.
References
- 1.Bushberg J., et al. , The Essential Physics of Medical Imaging, 2nd ed., Wilkins, Philadelphia: (2001). [Google Scholar]
- 2.Chida K., et al. , “Radiation dose of interventional radiology system using a flat-panel detector,” Am. J. Roentgenol. 193(6), 1680–1685 (2009). 10.2214/AJR.09.2747 [DOI] [PubMed] [Google Scholar]
- 3.Vlietstra R. E., et al. , “Radiation burns as a severe complication of fluoroscopically guided cardiological interventions,” J. Interventional Cardiol. 17(3), 131–142 (2004). 10.1111/j.1540-8183.2004.09885.x [DOI] [PubMed] [Google Scholar]
- 4.Frazier T. H., et al. , “Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked,” Arch. Dermatol. 143(5), 637–640 (2007). 10.1001/archderm.143.5.637 [DOI] [PubMed] [Google Scholar]
- 5.Henry M. F., et al. , “Fluoroscopy-induced chronic radiation dermatitis: a report of three cases,” Dermatol. Online J. 15(1), 3 (2009). [PubMed] [Google Scholar]
- 6.Balter S., et al. , “Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair,” Radiology 254(2), 326–341 (2010). 10.1148/radiol.2542082312 [DOI] [PubMed] [Google Scholar]
- 7.Kleiman N. J., “Radiation cataract,” Ann. ICRP 41(3–4), 80–97 (2012). 10.1016/j.icrp.2012.06.018 [DOI] [PubMed] [Google Scholar]
- 8.Jacob S., et al. , “Interventional cardiologists and risk of radiation-induced cataract: results of a French multicenter observational study,” Int. J. Cardiol. 167(5), 1843–1847 (2013). 10.1016/j.ijcard.2012.04.124 [DOI] [PubMed] [Google Scholar]
- 9.Vano E., et al. , “Patient radiation dose management in the follow-up of potential skin injuries in neuroradiology,” Am. J. Neuroradiol. 34(2), 277–282 (2013). 10.3174/ajnr.A3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Commission on Radiation Protection, “ICRP publication 103,” Ann ICRP, 2007, http://148.184.174.31/about-nrc/regulatory/rulemaking/potential-rulemaking/opt-revise/icrp-pub-103-free-extract.pdf (February 2009). [DOI] [PubMed]
- 11.Andreassi M. G., et al. , “Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease,” Eur. Heart J. 27(22), 2703–2708 (2006). 10.1093/eurheartj/ehl014 [DOI] [PubMed] [Google Scholar]
- 12.BCIS, “BCIS audit returns adult interventional procedures,” Basingstoke, 2015, http://www.bcis.org.uk/pages/page_box_contents.asp?pageid=678&navcatid=25 (January 2016).
- 13.Philips Healthcare, “ClarityIQ technology,” Best, Netherlands, 2012, http://www.healthcare.philips.com/main/products/interventional_xray/Product/alluraclarity/documents.wpd (December 2012).
- 14.Söderman M., et al. , “Image noise reduction algorithm for digital subtraction angiography: clinical results,” Radiology 269(2), 553–560 (2013). 10.1148/radiol.13121262 [DOI] [PubMed] [Google Scholar]
- 15.Söderman M., et al. , “Radiation dose in neuroangiography using image noise reduction technology: a population study based on 614 patients,” Neuroradiology 55(11), 1365–1372 (2013). 10.1007/s00234-013-1276-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Racadio J., et al. , “Significant dose reduction for pediatric digital subtraction angiography without impairing image quality: preclinical study in a piglet model,” Am. J. Roentgenol. 203(4), 904–908 (2014). 10.2214/AJR.13.12170 [DOI] [PubMed] [Google Scholar]
- 17.Eloot L., et al. , “Novel x-ray imaging technology enables significant patient dose reduction in interventional cardiology while maintaining diagnostic image quality,” Catheterization Cardiovasc. Interv. 86(5), E205–E212 (2015). 10.1002/ccd.25913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura S., et al. , “Patient radiation dose reduction using an x-ray imaging noise reduction technology for cardiac angiography and intervention,” Heart Vessels 31(5), 655–663 (2016). 10.1007/s00380-015-0667-z [DOI] [PubMed] [Google Scholar]
- 19.Ten Cate T., et al. , “Novel x-ray image noise reduction technology reduces patient radiation dose while maintaining image quality in coronary angiography,” Neth. Heart J. 23(11), 525–530 (2015). 10.1007/s12471-015-0742-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gislason-Lee A. J., et al. , “Does new image enhancement technology provide a substantial radiation dose reduction for patients in percutaneous coronary interventional procedures?” in RSNA e-Poster, RSNA, Chicago: (2014). [Google Scholar]
- 21.Dekker L. R. C., et al. , “New image processing and noise reduction technology allows reduction of radiation exposure in complex electrophysiologic interventions while maintaining optimal image quality: a randomized clinical trial,” Heart Rhythm 10(11), 1678–1682 (2013). 10.1016/j.hrthm.2013.08.018 [DOI] [PubMed] [Google Scholar]
- 22.Vano E., et al. , “Skin radiation injuries in patients following repeated coronary angioplasty procedures,” Br. J. Radiol. 74(887), 1023–1031 (2001). 10.1259/bjr.74.887.741023 [DOI] [PubMed] [Google Scholar]
- 23.Betsou S., et al. , “Patient radiation doses during cardiac catheterization procedures,” Br. J. Radiol. 71(846), 634–639 (1998). 10.1259/bjr.71.846.9849387 [DOI] [PubMed] [Google Scholar]
- 24.Cusma J. T., et al. , “Real-time measurement of radiation exposure to patients during diagnostic coronary angiography and percutaneous interventional procedures,” J. Am. Coll. Cardiol. 33(2), 427–435 (1999). 10.1016/S0735-1097(98)00591-9 [DOI] [PubMed] [Google Scholar]
- 25.Philips Healthcare, “Making the difference with Philips live image guidance helping everyone benefit from AlluraClarity,” 2014, http://incenter.medical.philips.com/doclib/enc/11506928/Allura_Clarity_family_brochure_NON_US_CANADA_452299102261_LR.pdf?func=doc.Fetch&nodeid=11506928 (November 2014).
- 26.Gislason-Lee A. J., et al. , “How much image noise can be added in cardiac x-ray imaging without loss in perceived image quality?” J. Electron. Imaging 24(5), 051006 (2015). 10.1117/1.JEI.24.5.051006 [DOI] [Google Scholar]
- 27.Kim K., et al. , “Occupational radiation doses to operators performing fluoroscopically-guided procedures,” Health Phys. 103(1), 80–99 (2012). 10.1097/HP.0b013e31824dae76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuipers G., et al. , “Radiation exposure during percutaneous coronary interventions and coronary angiograms performed by the radial compared with the femoral route,” JACC: Cardiovasc. Interventions 5(7), 752–757 (2012). 10.1016/j.jcin.2012.03.020 [DOI] [PubMed] [Google Scholar]
- 29.Picano E., Vano E., “The radiation issue in cardiology: the time for action is now,” Cardiovasc. Ultrasound 9(1), 35 (2011). 10.1186/1476-7120-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Royal College of Physicians, “British Cardiovascular Society,” http://www.bcs.com/exhibit/exhibitor_details.asp?ExhibitorId=1867 (September 2015).
- 31.Hiles P. A., “Education and training in RP for cardiologists—a BIR initiative,” in British Institute of Radiology Annual Congress Proc., London, United Kingdom, p. 4 (2015). [Google Scholar]
- 32.Båth M., “Evaluating imaging systems: practical applications,” Radiat. Prot. Dosim. 139(1–3), 26–36 (2010). 10.1093/rpd/ncq007 [DOI] [PubMed] [Google Scholar]
- 33.Dobbins J. T., III, “Image quality metrics for digital systems,” in Beutel J., Kundel H., Van Metter R., Eds., Handbook of Medical Imaging Vol I: Medical Physics and Psychophysics, SPIE Press, Bellingham, Washington: (2000). [Google Scholar]
- 34.Metz C. E., “Fundamental ROC analysis,” in Beutel J., Kundel H., Van Metter R., Eds., Handbook of Medical Imaging Vol I: Medical Physics and Psychophysics, SPIE Press, Bellingham, Washington: (2000). [Google Scholar]
- 35.Krupinski E. A., “Practical applications of perceptual research,” in Beutel J., Kundel H., Van Metter R., Eds., Handbook of Medical Imaging Vol I: Medical Physics and Psychophysics, pp. 895–929, SPIE, Bellingham, USA: (2000). [Google Scholar]
- 36.Samuelson F., Abbey C., He X., “Comparing diagnostic image reading performance in laboratory and clinical studies,” in Medical Image Perception Conf. XVI, Ghent, Krupinski E., et al., Eds., p. 39, Medical Image Perception Society, Belgium: (2015). [Google Scholar]
- 37.Sawdy J. M., et al. , “Use of a dose-dependent follow-up protocol and mechanisms to reduce patients and staff radiation exposure in congenital and structural interventions,” Catheterization Cardiovasc. Interv. 78(1), 136–142 (2011). 10.1002/ccd.v78.1 [DOI] [PubMed] [Google Scholar]
- 38.Keeble C., et al. , “Methods for the analysis of ordinal response data in medical image quality assessment,” Br. J. Radiol. 89(1063), 20160094 (2016). 10.1259/bjr.20160094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balter S., “A preliminary investigation of ambient light in the interventional fluoroscopy laboratory,” Proc. SPIE 5749, 348 (2005). 10.1117/12.593701 [DOI] [Google Scholar]