Abstract
Jozic et al. describe mechanisms of glucocorticoid (GC) downregulation of wound healing by interaction with the membrane bound GC receptor, followed by stimulation of β-catenin and c-myc pathways. Targeting the membrane bound GC receptor or the recently discovered interaction of GC with mineralocorticoid receptors may counteract negative effects of GC on the skin barrier and potentially could serve as a remedy for age-related skin atrophy.
Novel mechanisms of glucocorticoid activity during wound healing
The investigative team led by Dr Tomic Canic reports that glucocorticoids (GC), through activation of nongenomic GC signal transduction pathways in epidermal keratinocytes, inhibit wound healing (Jozic et al., 2017). Although inhibitory effects of GC on wound healing were expected, data concerning the mechanism of action are highly novel. Specifically, through a series of elegant experiments, the authors demonstrated that dexamethasone, a synthetic GC, after binding to membrane bound GC receptors, stimulates the phospholipase C/protein kinase C signaling system, with inhibition of glycogen synthase kinase 3β, leading to the activation of β-catenin and c-myc (Jozic et al., 2017). A final phenotypic consequence of this signaling circuitry is the inhibition of keratinocyte migration and wound closure (Jozic et al., 2017). Because these findings are likely to have wide clinical and scientific implications, they have been analyzed in a wide context, below.
Skin as a stress organ: GC disturb and regulate local homeostasis
Skin, together with the subcutaneous fat, called by some authors the hypodermis and considered to be an integral part of the integument, is not only a target of stress hormones but also has the capability of generating them and inducing their central production through sophisticated systems initiated by cutaneous stressors (reviewed in Slominski et al., 2012). A main GC in humans, cortisol, is released predominantly by the adrenal cortex, representing the final step of the hypothalamo-pituitary-adrenal (HPA) axis (main body response to sustained stress). Cortisol counteracts stressinduced damage under optimal homeostasis (called eustasis), and it exerts an array of pleiotropic activities in central and peripheral organs (Chrousos, 2009). A deficiency or excess of cortisol may have harmful effects, called allostasis (cacostasis) (Chrousos, 2009). Skin also has the capacity to produce GC, which can be controlled by local hormonal signals, including the cutaneous HPA axis— sHPA (Slominski et al., 2013;Slominski and Mihm, 1996), and its levels can be regulated by local activating or inactivating GC enzymes (reviewed in Slominski et al., 2015 Fig. 1). Recent findings indicate that systemic GC levels can also be regulated in stressed skin through HPA-dependent or -independent mechanisms (Skobowiat et al., 2017;Skobowiat and Slominski, 2015). In the skin during eustasis, endogenous or systemic GC should generate optimal homeostatic activities for local HPA axis elements and their regulators such as cytokines, because their effects would be harmful during cacostasis (allostasis). Examples of deficient homeostatic activity during cutaneous cacostasis include inflammatory states such as psoriasis, allergic reactions, and autoimmune processes. On the other hand, excessive cacostasis for GC would include inhibition of epidermal barrier function (Aberg et al., 2007), inhibition of wound healing (reported and reviewed in Jozic et al., 2017), and immunosuppression, which might lead to infection. Excessive allostatic activities of GC in skin will make an organism vulnerable to infection, damage by external physicochemical agents, and a loss of water and energy. A possible explanation for this dichotomy in the homeostatic activities of GC may be found in the theory that proposes the integumental origin of the HPA axis (Slominski, 2007). This theory proposes that cytokines, corticotropin releasing factor, and related peptides such as urocortins and proopiomelanocortin-derived peptides act as builders of the barrier and protective functions of the skin, with GC acting as terminators of these responses. Interestingly, recent findings have shown that induction of keratinocyte differentiation can regulate the expression of sHPA elements (Wierzbicka et al., 2017), whereas earlier studies have shown that corticotropin releasing factor acts as a cytokine by inhibiting proliferation and inducing differentiation of keratinocytes (Slominski et al., 2006). Thus, expression and activity of the sHPA axis is connected tightly with the mechanisms that regulate epidermal barrier function.
Figure 1. Influence of GC on renewal or regeneration of the epidermal barrier.
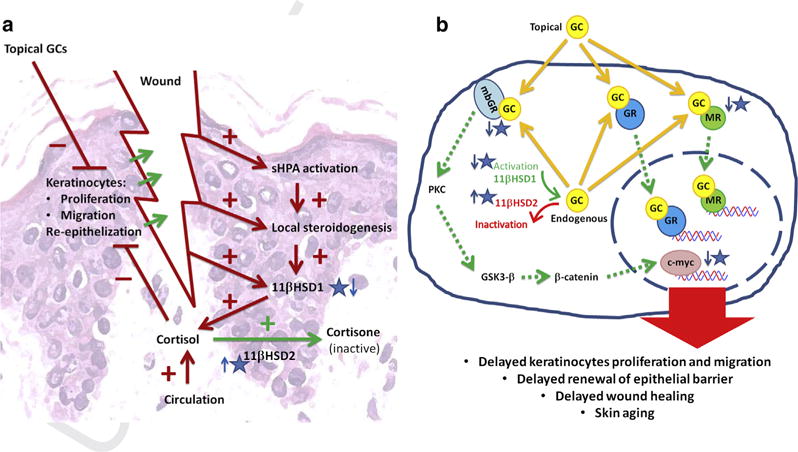
(a) Cortisol, when applied topically, produced endogenously, or derived from the circulation, impairs wound healing by inhibiting keratinocyte proliferation and migration. Damage to the epidermal barrier as well as infection would activate the skin equivalent of the HPA axis (sHPA), and local steroidogenesis, as well, would stimulate 11 βHSD1. Inhibition of elements of sHPA, 11 βHSD1 activity, and stimulation of 11 βHSD2 activity are the potential pharmacological targets for therapy of difficult-to-heal wounds, especially in patients receiving GC. (b) In keratinocytes, GC were shown to activate the classic nuclear (GR) receptor, as well as the recently described membrane bound GR (mbGR) (Jozic et al., 2017) and the mineralocorticoid receptor (MR) (Maubec et al., 2015). This novel pathway, triggered by binding of GC to mbGR, leads to activation of PKC, downregulation of GSK3-β, followed by stimulation of β-catenin and finally c-myc. Secondly, MR binds GC with equal strength as its natural ligand aldosterone; thus, excess GC results in constant activation of the receptor. Recent studies show that deactivation of mbGR or MR may prevent skin atrophy in patients treated with GC. Increased production of endogenous GC (cortisol) as a result of stimulation of sHPA and/or overexpression of 11 βHSD1 may also lead to delayed reepithelialization in wound healing, as well as age-related skin atrophy. Thus, the pathway of synthesis (steroidogenesis) and cortisol inactivation by 11 βHSD2 are potential targets for novel therapies. 11 βHSD1, 11 β-hydroxysteroid dehydrogenase type 1; 11 βHSD2, 11 β-hydroxysteroid dehydrogenase type 2; GC, glucocorticoid; GR, GC receptor; HPA, hypothalamo-pituitary-adrenal; PKC, protein kinase C.
GC and the skin: clinical and theoretical implications
In the clinic, GC are used widely to treat skin inflammatory diseases. For example, in psoriasis, they not only downregulate immune responses but also attenuate hyperproliferation of keratinocytes. Unfortunately, prolonged exposure to GC leads to skin atrophy, weakens barrier function, and attenuates immune responses as well as inhibiting wound healing. Such excess activity can be defined as cacostasis (allostasis). Such negative processes should not occur during eustasis when skin levels of GC are regulated precisely, by their endogenous production and inactivation (reviewed in Slominski et al., 2012, 2013). Their action in the skin is determined by interaction with corresponding nuclear receptors, predominantly GC receptors (Lu et al., 2006), membrane bound GC receptors (reviewed in Jozic et al., 2017), and mineralocorticoid receptors (Maubec et al., 2015), which may also include membrane bound mineralocorticoid receptors, because rapid nongenomic activities of steroids and secosteroids, acting on cell membrane receptors, are well documented. Thus, modulation of these receptor activities, using selective antagonists, biased agonists, or reverse agonists, may reduce, or even eliminate, the side effects of GC. For example, it has already been documented that the use of mineralocorticoid receptor antagonists can attenuate GC-induced epidermal atrophy (Maubec et al., 2015). We believe that the future of skin pharmacology lies in educated development of agonists and antagonists that act selectively at either nuclear or membrane bound steroid receptors Fig. 1. The work by Jozic et al. (2017) suggests that targeting the membrane bound GC receptor by antagonists or reverse agonists may lead to efficient management of wound healing.
Synthesis and development of new concepts
It is clear that the eustatic activities of steroids in skin will not only depend on their local concentrations, secondary to their endogenous production/inactivation regulated by a cutaneous neuroendocrine system, but also on the availability of corresponding receptors, coupled to relevant signal transduction pathways Fig. 1. In case of their deficiency or excessive external supply, leading to cacostasis (allostasis), eustasis can be restored, not only by regulation of their local production (through, e.g., the action of the local HPA axis) or regulation of 11 β-hydroxysteroid dehydrogenase type 1 or type 2 enzyme activities, but also by positive or negative regulation of the availability of the corresponding receptors Fig. 1 Thus, multiple pathways securing the cutaneous GC eustasis can be pharmacologically or nonpharmacologically (e.g., by ultraviolet radiation) regulated Fig. 1 Identification of already existing or the discovery of new chemical molecules that selectively regulate this circuitry represents a rewarding challenge for skin researchers Fig. 1.
Clinical Implications.
Pharmacological regulation of local glucocorticoid levels can improve wound healing.
Downregulation of membrane bound glucocorticoid or mineralocorticoid receptors can improve wound healing.
Agonist/antagonist actions on steroid receptors represent future therapies in skin pharmacology.
Acknowledgments
Per journal’s regulations we are restricted to limited number of references; however, many original papers are cited in the listed references and reviews. We acknowledge a partial support by grants R21AR066505, 1R01AR056666, and 2R01AR052190 from NIH to ATS, and N402 662840 from the Polish Ministry of Science and Higher Education to MAZ.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339–49. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–81. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Jozic I, Vukelic S, Stojadinovic O, Liang L, Ramirez HA, Pastar I, et al. Stress signals, mediated by membranous glucocorticoid receptor, activate PLC/PKC/GSK-3beta/betacatenin pathway to inhibit wound closure. J Invest Dermatol. 2017 doi: 10.1016/j.jid.2016.11.036. http://dx.doi.org/10.1016/j.jid.2016.11.036. [DOI] [PMC free article] [PubMed]
- Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, et al. International Union of Pharmacology LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782–97. doi: 10.1124/pr.58.4.9. [DOI] [PubMed] [Google Scholar]
- Maubec E, Laouenan C, Deschamps L, Nguyen VT, Scheer-Senyarich I, Wackenheim-Jacobs AC, et al. Topical mineralocorticoid receptor blockade limits glucocorticoid-induced epidermal atrophy in human skin. J Invest Dermatol. 2015;135:1781–9. doi: 10.1038/jid.2015.44. [DOI] [PubMed] [Google Scholar]
- Skobowiat C, Postlethwaite AE, Slominski AT. Skin exposure to ultraviolet B rapidly activates systemic neuroendocrine and immunosuppressive responses. Photochem Photobiol. 2016 doi: 10.1111/php.12642. http://dx.doi.org/10.1111/php.12642. [DOI] [PMC free article] [PubMed]
- Skobowiat C, Slominski AT. UVB activates hypothalamic-pituitary-adrenal axis in C57BL/6 mice. J Invest Dermatol. 2015;135:1638–48. doi: 10.1038/jid.2014.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. J Clin Invest. 2007;117:3166–9. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Mihm MC. Potential mechanism of skin response to stress. Int J Dermatol. 1996;35:849–51. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006;206:780–91. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015;103:72–88. doi: 10.1016/j.steroids.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v.vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34:827–84. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka JM, Zmijewski MA, Antoniewicz J, Sobjanek M, Slominski AT. Differentiation of keratinocytes modulates skin HPA analog. J Cell Physiol. 2017;232:154–66. doi: 10.1002/jcp.25400. [DOI] [PubMed] [Google Scholar]


