Abstract
This work investigates the effect of combining physical and chemical gelation processes in a biopolymer blend: chitosan and tilapia fish gelatin. Chemical (C) gels are obtained by cross-linking with the microbial enzyme transglutaminase at 37 °C. Hybrid physical-co-chemical (PC) gels are cross-linked at 21 °C, below gelatin gelation temperature. These protocols provide two microenvironments for the gelation process: in C gels, both gelatin and chitosan are present as single strands; in PC gels, cross-linking proceeds within a transient physical gel of gelatin, filled by chitosan strands. The chitosan/gelatin chemical networks generated in PC gels show a consistently higher shear modulus than pure C gels; they are also less turbid than their C gels counterparts, suggesting a more homogeneous network. Finally, chitosan enhances the gels' shear modulus in all gels. Proliferation assays show that MC3T3 cells proliferate in these mixed, hybrid gels and better so on PC gels than in C mixed gels.
Introduction
Hydrogels obtained from the chemical or physical association of macromolecules are an important area of material science, due to the wide array of design possibilities and countless biomedical applications, for instance as skin substitutes, adhesives, or drug delivery matrices.[1–5] Through a careful choice of the macromolecules, the molecular associations that maintain the hydrogel 3D network can be selected and tuned. Biopolymers, inherently more suited for medical applications than their synthetic counterparts, and often available in vast amounts from cheap, renewable sources, have become a great focus of interest.[1, 4] However, due to their physical and chemical complexity, the properties of natural polymers, such as gelatin and chitosan used in this study, remain poorly understood.
Gelatin is the denatured form of the most abundant animal protein, collagen.[6, 7] In water, above its melting temperature, gelatin exists as flexible single polypeptide strands.[8–10] Below the gelation temperature, gelatin undergoes a reversible sol-gel transition, acquiring a triple-helix conformation similar to native collagen.[11–13] Accordingly, a thermo-reversible transient physical network is formed. Due to its characteristic structural and mechanical properties, gelatin has attracted a great deal of scientific interest,[11, 14–17] in particular for biomedical applications, as it presents useful biological properties: it is biodegradable and biocompatible, and favours cell adhesion and function.[3, 18–21] For health and religious reasons, gelatin extracted from other sources than mammalian have attracted increasing interest.[14] The main difference between mammalian and fish gelatin is the gelation temperature, which tends to be lower for the fish variants, due to the lower proline and hydroxyproline content of fish gelatin.[22]
Technological products are often a combination of many different components. Combining more than one natural polymer can produce synergistic effects to help tune the system and improve the final properties.[23] Chitosan, obtained from the partial deacetylation of chitin, has a molecular structure very similar to the glycosaminoglycans, a major component of the extra-cellular matrix. Chitosan is known for its non-toxicity, biocompatibility, biodegradability, wound-healing and haemostatic capabilities,[24] making it a good candidate for biomedical applications.[2, 25, 26]
To combine gelatin and chitosan in a permanent chemical hydrogel, a cross-linker is required. Bacterial transglutaminase (mTGase) has been chosen in this work due to its biological compatibility. Transglutaminase (TGase, EC 2.3.2.13, amine-γ-glutamyl transferase), a calcium-dependent naturally occurring enzyme found in almost all living organisms, catalyses acyl-transferase reactions between the NH2 part of the γ-amine group of a glutamine residue and a primary amino group (e.g., lysine), resulting in an ϵ-(γ-glutamyl) lysine isopeptide bond (Scheme 1). In our systems, this could involve the glucosamine amine of chitosan and a glutamine residue from gelatin. Microbial transglutaminase (mTGase) offers the advantage of being calcium-independent.[27] The feasibility of using mTGase as a cross-linker for mixed biopolymer gels, including chitosan/gelatin gels, was demonstrated recently by Payne and co-workers.[28–30]
Scheme 1.
Enzymatic cross-linking mediated by transglutaminase.
The presence or concomitant growth of gelatin physical networks has been shown to improve the cross-linking process, imparting control on the final mechanical properties.[31–33] Djabourov and co-workers[32, 33] have studied the chemical gelation of gelatin induced by the cross-linker bisvinylsulfonyl-methane and the role of the physical network on this process. They reported a higher storage modulus (G′) for chemical gels obtained in the presence of the physical network. Recently, we reported an extensive investigation on the enzymatically-cross-linked gels of tilapia fish gelatin.[31, 34] A combination of rheology, small-angle neutron scattering and optical rotation revealed that the presence of the physical network drastically affects the rheological and microstructural properties of the gels. Those results suggest that the physical network may act as a template, by directing a more efficient distribution of the cross-links. Given the importance of gel elasticity in directing stem cell differentiation and expression,[35] utilizing hybrid gelation processes seems a very attractive approach to provide cheap and facile adjustment of the properties. Blending two biopolymers provides further control over the properties and may lead to synergistic effects; to our knowledge, hybrid gelation processes in mixtures of biopolymers have not been reported to date.
In this work, we explore the gelation kinetics and equilibrium rheological properties of mixed gels of chitosan and gelatin, where both biopolymers participate to the network. Our objective is to assess the impact of the chitosan/gelatin blend compared to the single-component gels (gelatin alone[31]), the effect of a hybrid gelation protocol on the network characteristics, and how these parameters affect cellular response, in view of developing these gels for tissue repair applications.[34]
2. Experimental Section
2.1. Materials
Chitosan, derived from crab shell with a minimum deacetylation degree of 85%, acetic acid (99%), anhydrous sodium acetate and tyrosinase from mushroom were purchased from Sigma-Aldrich. L-Lysine hydrochloride (BP386) was purchased from Fisher Bioreagents.
Chitosan molecular weight was found to be 900 kDa ± 5% determined by viscometric methods. The procedure has been described elsewhere.[36]
Tilapia fish skin gelatin (Type A, acidic extracted) was kindly donated by Rousselot (France). The average molecular weight was determined to be ca 36 kDa ± 12% using gel permeation chromatography (GPC; Smithers Rapra) with a melting temperature of ≈ 23 °C.
mTGase was purchased from N-Zyme BioTec GmbH (Darmstadt, Germany). mTGase has a specific activity of 1.6 Units mg−1 solid, a molecular weight of 38 kDa and a purity superior to 80% according to sodium dodecylsulfonate-polyacrylamide gel electrophoresis (SDS-PAGE), as indicated by the supplier. All compounds were used as received. Deionised water was used throughout the experiments.
2.2. Methods
2.2.1. Preparation of Solutions
Chitosan samples were prepared by solubilisation in 2.0 wt% aqueous solutions of acetic acid overnight. After complete dissolution, the pH was raised to 5 by adding suitable amounts of anhydrous sodium acetate, forming an acetate buffer (≈ 0.25 M acetic acid/0.55 M sodium acetate). This pH is still within the activity range of mTGase.[37] Chitosan/gelatin solutions were prepared by adding gelatin to the chitosan solutions and leaving the mixture to swell overnight at 4 °C. Before use, the samples were heated up to 37 °C for 30 min to ensure complete melting of tilapia fish gelatin and system homogenization.
mTGase solutions were prepared by solubilisation of the solid and used on the same day. On average, 30 µL of mTGase solution were added to 1.0 g of chitosan/gelatin solutions. mTGase concentrations of 10, 20, 30, and 40 U/ggelatin were studied.
In this work, the concentrations of biopolymers used were 0.10, 0.50, 1.0, and 2.0 wt% chitosan (Cs) and 10 wt% tilapia fish gelatin (Gel), in order to facilitate comparison with previous work conducted in our group, where pure tilapia gels were studied at 10 wt%.[31]
To produce the gels, three different gelation procedures were employed:
Physical gelation (P): For pure physical gels, Cs/Gel solutions were cooled for 2.0 h at 12.0 °C or 21.0 °C (below gelatin gelation temperature of ≈ 23 °C). In this case the gelatin physical gel is filled with chitosan macromolecules, as chitosan does not participate to the physical network and is present in the sol state.
Chemical gelation (C): Chemical gelation was performed at 37.0 °C, that is, above gelatin melting temperature. A fixed amount of mTGase, from 10 to 40 U/ggelatin, was added to Cs/Gel solutions, vortex-mixed and left to gel for 3.5 h before thermal deactivation of mTGase by a temperature jump to 70 °C. Both macromolecules are expected to be part of the chemical network.
Physical-co-chemical gelation (PC): Physical and chemical gelation were performed simultaneously. To achieve this, selected amounts of mTGase (10–40 U/ggelatin) were added to Cs/Gel solutions and the system was quickly cooled down to 21.0 °C (below gelation temperature). The gelation was conducted for 3.5 h, following which mTGase was thermally deactivated.
2.2.2. Rheology
All rheological measurements were conducted on a strain-controlled ARES rheometer (TA Instruments) equipped with a titanium parallel plate geometry (25.0 mm diameter). Temperature was controlled by a Peltier unit (± 0.1 °C). To prevent evaporation, a thin layer of low viscosity paraffin oil was applied on the edge of the plate. The tests were repeated 3 times with a standard deviation typically below 20%. The results presented in this work are examples of typical data obtained, not averages, except when indicated otherwise.
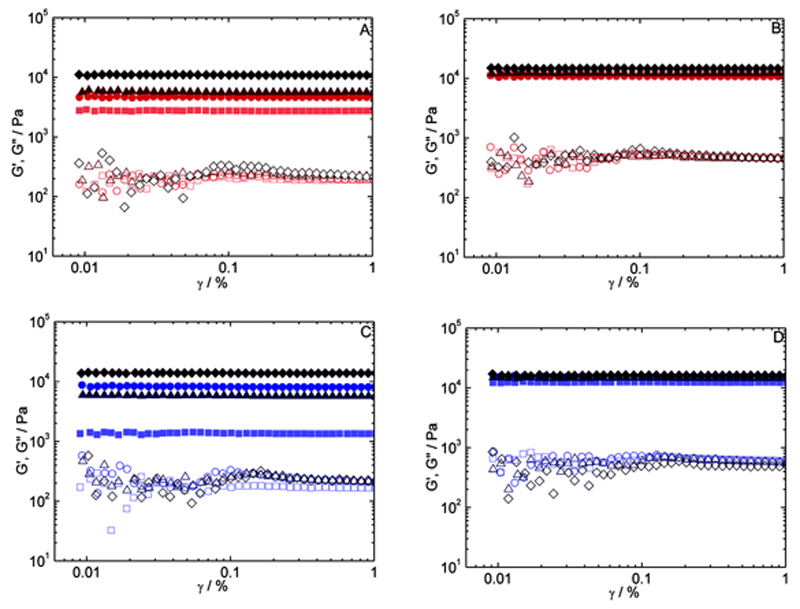
Oscillatory tests were performed as follow: time sweeps (curing curves), where both storage (G′) and loss (G″) moduli were followed against time at a fixed frequency (6.28 rad s−1) and strain amplitude (0.1%) for 210 min. The measurements were conducted at 37.0 °C (chemical gelation) and 21.0 °C (physical-co-chemical gelation and physical gelation). At the end of the curing experiments, the temperature was raised to 70.0 °C for 5 min in order to irreversibly deactivate the enzyme. On the same sample, a strain amplitude sweep was then conducted at 37.0 °C (pure chemical networks). After that, the samples were cooled down to 12.0 °C for 2.0 h and another strain amplitude sweep was acquired at 12.0 °C (both chemical and physical networks present). This was done in order to evaluate the regrowth of the physical network. Linear viscoelastic ranges were extracted from these amplitude sweeps (Figure 1). Amplitude sweeps were conducted at 6.28 rad s−1, covering the range from 0.010 to 1.0% strain. In no experiment was the limit of the linear viscoelastic range was reached.
Figure 1.
Shear strain amplitude sweep curves for chitosan 2.0%/gelatin 10% A,B) chemical gels and C,D) physical-co-chemical gels. Curves in (A,C) were measured at 37 °C, above the gelatin melting temperature. Curves in (B,D) were measured a 12 °C, below the gelatin gelation temperature, to grow the physical network. mTGase concentrations of 10 (▪), 20 (●), 30 (▴), and 40 (♦) U/ggelatin G′ are shown by filled symbols, and G″ by empty symbols.
2.2.3. Optical Rotation (OR)
OR measurements were performed on an Applied Photophysics Ltd (Leatherhead, UK) Chirascan spectrometer, equipped with a Quantum NorthWest TC125 Peltier unit. The instrument was flushed continuously with pure evaporated nitrogen throughout the measurements. Temperatures were measured directly with a thermocouple probe in the sample with an accuracy of ± 0.2 °C. All samples were measured at a wavelength of 436 nm. Chitosan/gelatin samples were measured at 37.0 °C in order to obtain the optical rotation angle for the gelatin single strand state, αcoil (10 wt%), in our experimental conditions. The gelation kinetics measurements were made directly after transferring about 300 µL of the chitosan/gelatin solution assayed (incubated at 37.0 °C) to an empty 1.00 mm cuvette placed in the spectrometer and pre-thermostated at the appropriate temperature. The gelatin solution in the Chirascan took approximately 2 min to reach the target temperature. The optical rotation angle calibration and correction has been described elsewhere.[34]
2.2.4. Turbidity Measurements
A Perkin-Elmer Lambda 2S spectrometer was used to acquire the transmittance spectra of the hydrogels studied. The turbidity data was taken at 700 nm. The hydrogels assayed were prepared directly in plastic UV cells and measured on the same day. PC gels were gelled at 21.0 °C and chemical gels at 37.0 °C both for 3.5 h before reaction quenching by thermal jump to 70 °C for 10 min. The samples were measured at room temperature, that is, ca. 25 °C. In the conditions studied, a physical network was not present in the samples. Solutions of Cs/Gel with 0 U/ggelatin were used as blank, assuming a 100% transmittance.
2.2.5. Cell Culture
MC3T3-E1 clone 4 osteoblasts cell line used in this work was obtained from American Tissue Culture Center (ATCC). Cells were cultured in 75 cm2 tissue culture flasks in α-modified eagle's medium (α-MEM) containing 10 vol% foetal bovine serum (FBS) with 1.0% L-glutamine, 1.0% penicillin/streptomycin (all Invitrogen, Paisley, UK) at 37 °C, 5% CO2, 95% humidified air. Medium was changed every 2 d. Care was taken to passage cells when sub-confluent. Upon 70% confluence, cells were detached using 0.02% EDTA/0.02% trypsin solution and split 1:10 into a new flask. Cells used for experiments were between passages 10 and 15.
Plate Preparation
Solutions of tilapia fish gelatin (10 wt%), chitosan (0.10 wt%)/tilapia fish gelatin (10 wt%) and chitosan (0.50 wt%)/tilapia fish gelatin (10 wt%) vortex mixed with the appropriated amount of mTGase (10, 20, 30 and 40 U/ggelatin) were transferred to 48-well plates and left to gel at 37.0 °C (chemical gels) and 21.0 °C (physical-co-chemical gels). After 2 h of gelation, the plates were heated to 70.0 °C for 10 min in order to irreversibly deactivate the mTGase. The plates were then sterilised by UV light irradiation for 24 h, and then rinsed with calcium/magnesium free Dulbeccos phosphate-buffered saline (D-PBS; Invitrogen, UK) until the pH reached the value of 6.9–7.0 measured by pH paper and confirmed with a pH meter at later point. The rinse process was standardized as 24 h per rinse, 5 rinses in total.
F-Actin Staining
ells were seeded on hydrogels and tissue culture plastic (TCP) at a density of 5 × 103 cells cm−2 (48-well plate) and cultured for 2 d. Cells were fixed with 3.7% aqueous formaldehyde (Sigma, UK) solution for 15 min at room temperature, permeabilised with 0.25 vol% Triton X-100 (BDH Laboratories, UK) solution in calcium/magnesium free D-PBS for 5 min, then stained with Alexa Fluor 568-conjugated phalloid toxin (1:300) (Molecular Probes, The Netherlands) for 30 min followed by 4′,6-diamidino-2-phenylindole hydrochloride (DAPI) for 5 min (1:10 000 in dH2O).
After incubation, fluorescence was visualized using an inverted Olympus BX-51 microscope equipped with an Olympus DP070 colour digital camera and Olympus DP Controller software (Olympus UK Ltd, UK).
Cell Proliferation
Cell proliferation was determined by absorbance in a 48-well plate assay using the tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS) (Promega, UK). Cells were seeded on tilapia fish gelatin and chitosan/tilapia fish gelatin hydrogels and TCP (positive control) at a density of 5 × 103 cells cm−2 and cultured for 4 h, 1 d, 7 d, and 14 d. Medium was replaced every two days, when applicable. At the end of each incubation period, the medium was also replaced and 30 μL of CellTiter 96 Aqueous One solution reagent (Promega) was added to 150 μL of medium/well. Cells were then incubated in the dark for 1 h at 37 °C. During this period MTS is metabolized by cells into a coloured aqueous soluble formazan product. The amount of formazan produced is proportional to the amount of cells present. The absorbance of the formazan compound was measured at 490 nm in a microplate spectrophotometer (Spectramax M5, Molecular Devices). Assays were carried out in technical triplicates.
3. Results
In this work, we used the microbial enzyme mTGase as a cross-linking agent. mTGase requires two different substrates, a primary free amine and a glutamine residue. Gelatin offers both binding sites. The free amine of the lysine residue is present at ca. 2.5% and the glutamine residue at around 6.9% in tilapia fish gelatin.[38] As indicated in the literature,[37, 39] chitosan free amines (from the deacetylated units) should be viable reaction sites for mTGase. The chitosan used in this work has a degree of deacetylation of 85%. Thus, the limiting step for the cross-linking reaction is the availability of glutamine residues, since chitosan should provide a large excess of free amines. Both macromolecules are positively charged, hence we do not expect the formation of a polyelectrolyte complex between chitosan and gelatin, as might be expected for a mixture of chitosan and alkaline-extracted gelatin (pI ca. 5) at the pH of this study (pH = 5).
For convenience, throughout this work, G′ values are identified as G′C, G′P and G′T, when referring to the storage modulus of the chemical network, physical network and total measured modulus, respectively.
3.1. Physical Gelation of Chitosan/Gelatin Mixtures
Figure 2 shows the time dependence of the storage (G′) and loss (G″) moduli for three systems: chitosan (1.0% and 2.0%)/gelatin (10%) and gelatin alone (10%) at 21.0 °C. As this temperature lies below the gelation temperature, the system undergoes a sol-gel transition, as shown by G′ and G″ build-up with time as a result of the partial refolding of gelatin single strands into triple-helices.[11, 13] The chitosan/gelatin system shows higher values of G′ and G″, reaching a G′P of 6.0 kPa against G′P = 1.7 kPa (at 210 min) for pure gelatin gels under the same conditions; this can be attributed to the large molecular weight of chitosan (900 kDa).
Figure 2.
Time sweep curves for gelatin 10% (●), chitosan 1.0%/gelatin 10% (mabi201300472-gra-0002) and chitosan 2.0%/gelatin 10% (▪) physical gels at a reaction temperature of 21 °C. G′ plots (filled symbols) are always above the corresponding G″ curves (lines).
3.1.1. Effect of Gel Composition
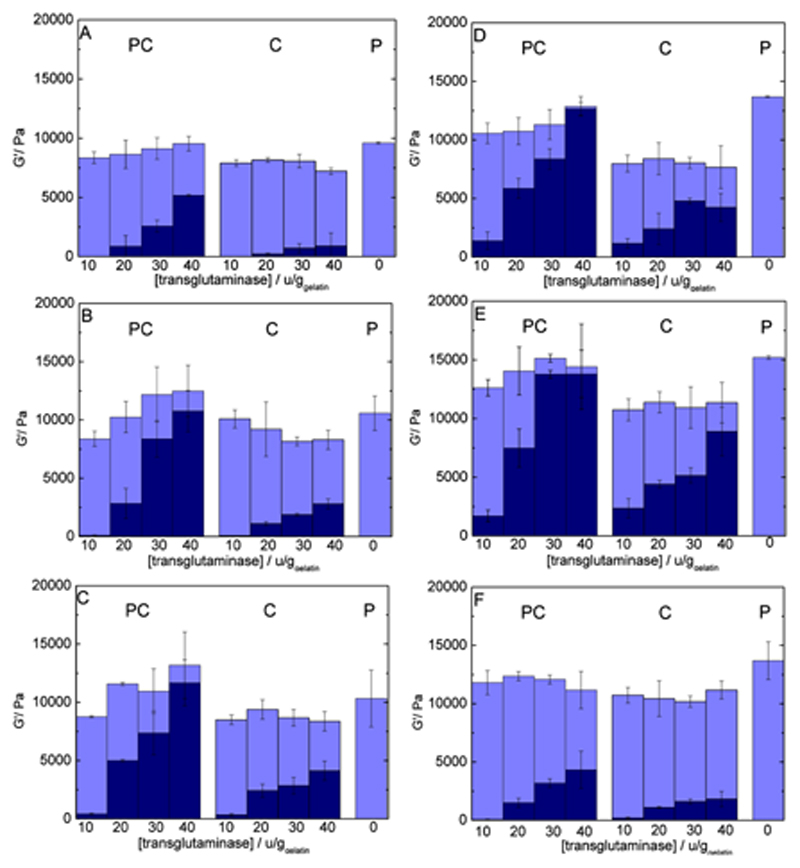
The final gel elasticity is clearly dependent on system composition. The influence of increasing gelatin concentration is visible, with G′P = 3.80 kPa for Cs2.0%/Gel5.0% and G′P = 31.0 kPa for Cs2.0%/Gel15% (wt%), measured at 12 °C (Supporting Information 1). Chitosan concentration – over the range studied here – impacts less on G′ values: with 0.10% chitosan (Cs0.10%/Gel10%), G′P reaches 10.6 kPa against G′P = 14.7 kPa for Cs2.0%/Gel10% (wt%) (Figure 3B,E). This reflects the determining role of gelatin triple-helix network on the rheological properties of the physical gels.
Figure 3.
Individual contribution of each network type to the overall shear modulus with varying enzyme (10 to 40 U/ggelatin) and chitosan concentration (0.10–2.0 wt%) and fixed gelatin concentration (10%). Light shades represent G′P, the contribution from the physical network extracted from data measured at 12 °C. Dark shades represent G′C, the chemical network measured at 37 °C. The total column height represents G′T, the gel's shear modulus measured at 12 °C. Chitosan amounts are as follow: A): 0%; B) 0.10%; C) 0.50%; D) 1.0%; E) 2.0%; F) 2.0% with added L-lysine (20 mg/gsolution).
3.1.2. OR
The sol-gel transition affects the optical rotation angle of the system since the signal is determined by the proportion of amino acids in a helical conformation. In the case of pure gelatin, this is reflected by increasingly negative values of the OR angle as the sol-gel transition progresses.[15, 31, 40] Above the melting temperature, the optical rotation angle of the mixture fluctuates between math formula = –233 ± 15 and math formula = −226 ± 15 mdegrees for Cs1.0%/Gel10% and Cs2.0%/Gel10%, respectively. As the temperature is decreased to 21.0 °C, the optical rotation angle becomes more negative, for instance, the OR angle for Cs1.0%/Gel10% physical gels is ca. math formula = –390 ± 10 mdegrees, reflecting the growth of triple-helices (Table 1). As the physical network does not reach a true equilibrium state[11] (helices continuously grow over time), the OR angle does not reach a real plateau value.[11, 31, 41, 42] Therefore, in the remainder of the paper OR angles are reported at 5000s (≈ 80 min) of gelation.
Table 1.
Optical rotation angle after simultaneous physical and chemical gelation (PC gels), and physical gelation following cross-linking (C gels) for chitosan (Cs)/gelatin (Gel) networks at varying enzyme concentration (10 to 40 U/ggelatin), given after 80 min of gelation.
| System type | mTGase [U/ggelatin] |
|
|
|---|---|---|---|
| Cs1.0%/Gel10% | Cs2.0%/Gel10% | ||
| Physico-co-chemical gel (PC) | 10 | -361 ± 3 | - |
| 20 | -347 ± 3 | -346 ± 4 | |
| 30 | -344 ± 3 | - | |
| 40 | -346 ± 5 | -338 ± 5 | |
| Chemical gel (C) | 10 | -420 ± 10 | - |
| 20 | -351 ± 3 | -360 ± 15 | |
| 30 | N/A | N/A | |
| 40 | N/A | N/A | |
| Physical gel at 12°C | 0 | -540 ± 15 | -510 ± 15 |
| Physical gel at 21°C | 0 | -390 ± 10 | -387 ± 10 |
| Solution at 37° | 0 | -233 ± 15 | -226 ± 15 |
3.2. Chemical Gelation of Chitosan/Gelatin Mixtures
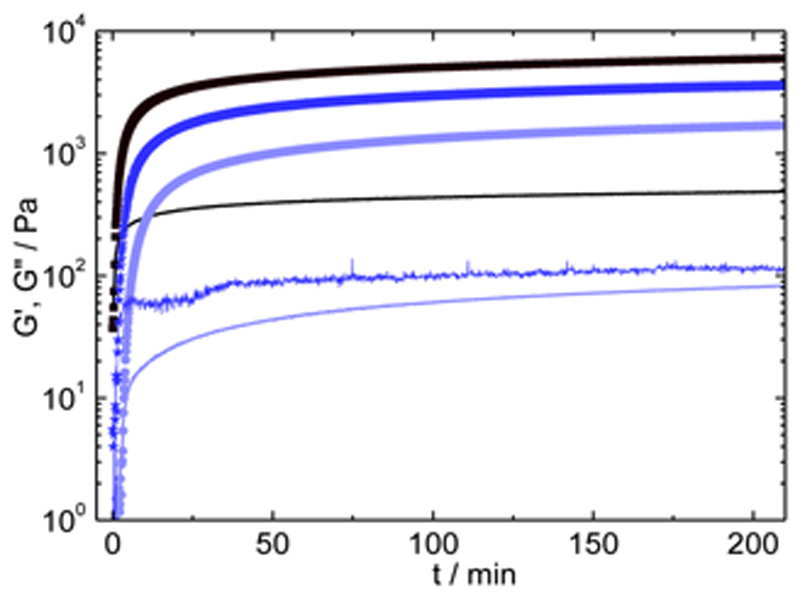
Time sweep measurements of the chemical gelation of chitosan 2.0%/gelatin 10% systems (37.0 °C) at different mTGase concentrations (10 to 40 U/ggelatin) are presented in Figure 4A. As a reference, the data from the pure physical gelation process are also shown (mixed gels at 21.0 °C, no mTGase present). Increasing mTGase concentration reduces gelation time, from ca. 18 min to 4 min. Chemical cross-linking is considerably slower than physical gelation: the gelation time for the physical process at 21.0 °C is ≈ 1 min (Figure 2), whereas the shortest gelation time measured for the chemical gelation is ca. 4 min at 40 U/ggelatin of mTGase (Figure 4A). G′C shows a maximum value of 10.8 kPa at 40 U/ggelatin (after 210 min of gelation at 37 °C).
Figure 4.
Time sweep curves for chitosan 2%/gelatin 10% A) chemical gelation at 37 °C and B) subsequent physical gelation at 12 °C; equally for C) physical-co-chemical gelation at 21 °C and D) subsequent physical gelation at 12 °C, with varying enzyme concentration. Solid lines represent chitosan 2%/gelatin 10% physical gels (no mTGase) at the reaction temperature. mTGase concentration of 10 (▪), 20 (●), 30 (▴) and 40 (♦) U/ggelatin, and G′ are shown by filled symbols are shown in increasingly darker shades, G″ by empty symbols (all plots for G′ are above the corresponding G″ plots).
3.2.1. Effect of Composition
Adding chitosan to the gels has a measurable impact: chitosan/gelatin chemical gels are considerably stronger than pure gelatin gels. On average, 10% gelatin at 40 U/ggelatin produces gels of G′C = 0.889 kPa (Figure 3A) compared to 8.88 kPa for Cs2%/Gel10% (Figure 3E) at the same enzyme concentration, a 10-fold increase. Even a relatively small addition of chitosan of 0.10% (40 U/ggelatin) results in an average G′C of 2.80 kPa (Figure 3B), nearly three times the value of pure gelatin gels under the same conditions.
3.2.2. Impact of Chemical Network on Helices Growth
After 210 min, the gelation process was interrupted by a temperature jump (70 °C for 5 min), which causes irreversible mTGase denaturation. Subsequent to this, the temperature was lowered to 12 °C for two hours and the build-up of the physical network within the confines of the chemical network was followed both by OR and rheology. The rheological data for Cs2.0%/Gel10% are presented in Figure 4B; the curves have been shifted in order to facilitate the comparison with the pure physical network (thin line). Namely, the value of the modulus of the first point (at the end of the chemical gelation) was subtracted from all following points, so that all curves start from G′P = 0 Pa at t = 0 min. The G′ values obtained for the physical networks grown within the chemical gels (G′P) decrease with increasing mTGase concentration (Figure 3B–E). At 10 U/ggelatin for instance, G′ is about 50% lower than the value obtained for the equivalent physical gel, which reflects the hindrance exerted by the chemical network on the chains, limiting the conformational changes required to form the triple-helix junctions. However, while it is clear that the chemical network interferes with the formation of the physical network, it does not totally prevent it, implying that the mesh size of the covalent network is large enough to accommodate the conformational changes of gelatin strands to form triple helices. A nearly linear inverse correlation exists between G′C and G′P (Supporting Information 2), showing that a larger extent of the chemical network correlates directly with a weaker physical network. This correlation has also been observed in pure gelatin systems.[31] Regarding the influence of chitosan, G′P values for Cs0.10%/Gel10% to Cs2.0%/Gel10% are about the same order of magnitude for a given mTGase concentration, except at 40 U/ggelatin,where G′P values show a significant concentration dependence (Figure 3B–E). This suggests that triple-helices formation is more sensitive to the extent of cross-linking, largely related to mTGase concentration, than to the presence of chitosan.
3.2.3. OR
Using OR, we can follow the change in the extent of helical conformation as the temperature is decreased from 37.0 °C to 12.0 °C in order to regrow the physical network, as done in Figure 4B. Starting from math formula = –233 ± 15 for Cs1.0%/Gel10% solutions at 37.0 °C, this value changes to math formula = –351 ± 3 mdegrees for Cs1%/Gel10% gels at 20 U/ggelatin, after chemical cross-linking and followed by physical gelation at 12.0 °C. Under the same conditions, we had obtained math formula = –540 ± 15 mdegrees (Table 1) for the pure physical gels. This implies that a significantly smaller amount of residues are in a helical conformation in the physical gels grown within the confines of the chemical gels, when compared to the pure physical gels, in agreement with the rheological data.
3.2.4. Turbidimetry: A Clue to Network Inhomogeneity
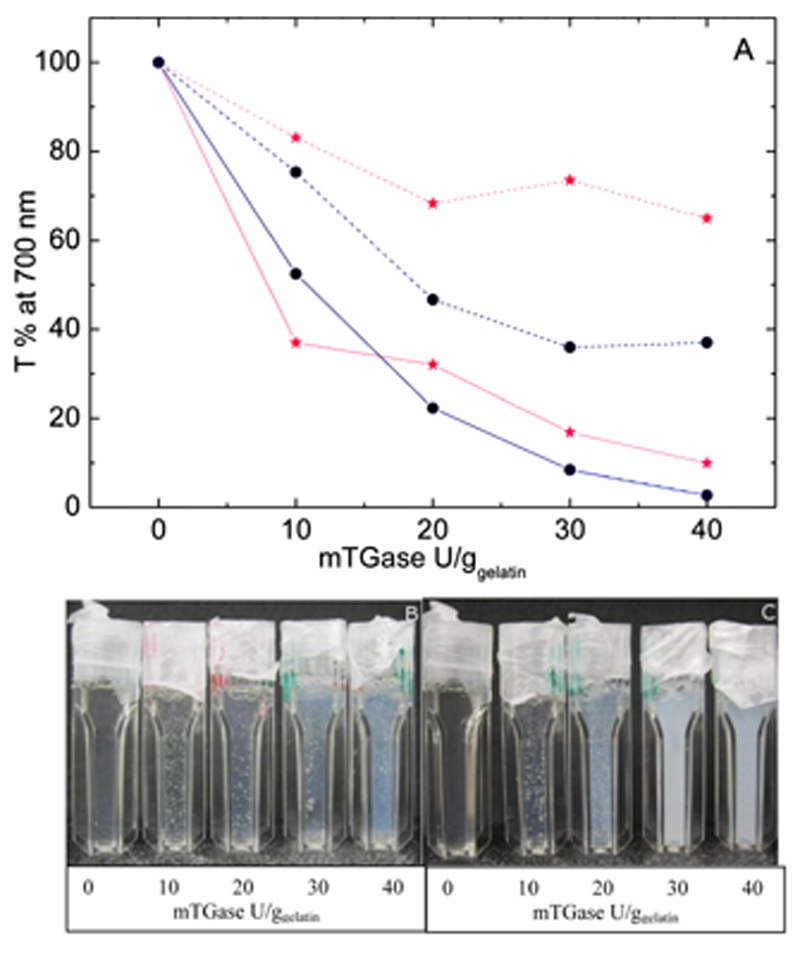
Turbidity measurements reveal that chemical gels are highly turbid, reflecting the presence of large domains scattering light, and therefore suggesting rather inhomogeneous networks. The turbidity (and thus inhomogeneity) increases with both mTGase and chitosan concentrations (Figure 5). For instance, 10 U/ggelatin Cs1.0%/Gel10% shows a transmittance of 75% against 53% when doubling the chitosan concentration (Cs2.0%/Gel10%) or 37% when increasing enzyme concentration to 40 U/ggelatin (Figure 5). Souguir and co-authors observed that for pure gelatin gels cross-linked by mTGase a concomitant build-up of both G′ and turbidity takes place as the enzymatic gelation proceeds.[43] They suggested that the turbidity arises from an increase of the local polymer concentration; as the cross-linking proceeds, the balance between osmotic pressure, which leads to coil expansion in a good solvent, and the elastic pressure, which leads to coil contraction, shifts towards coil contraction, increasing the polymer local concentration, which in turn increases the rate of gelation, leading to a positive feedback.[43]
Figure 5.
A) Transmittance values for physical-co-chemical (mabi201300472-gra-0002) and chemical gels (●) of Cs/Gel with varying enzyme concentration (0 to 40 U/ggelatin). Cs2.0%/Gel10% is represented by continuous lines and Cs1.0%/Gel10% by dashed lines. Photographs of B) typical physical-co-chemical and C) chemical gels (Cs1.0%/Gel10%) are shown below.
3.2.5. Cell Culture Studies
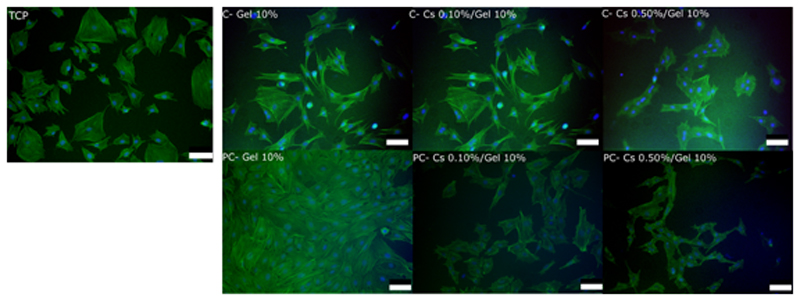
Cell spreading and morphology on gelatin and chitosan/gelatin chemical gels compared to TCP was evaluated via F-actin staining and found to be qualitatively similar among samples examined (Figure 6).
Figure 6.
Actin staining of MC3T3 cells cultured for 48 h on gelatin 10%, chitosan 0.10%/gelatin 10% and chitosan 0.50%/gelatin 10% (wt%) chemical and physical-co-chemical gels and TCP. Hydrogels were cross-linked with 20 U mTGase/ggelatin for 2 h at 37 °C (chemical) and 21 °C (physical-co-chemical). Nuclei: blue; F-actin: green. The scale bars represent 200 μm.
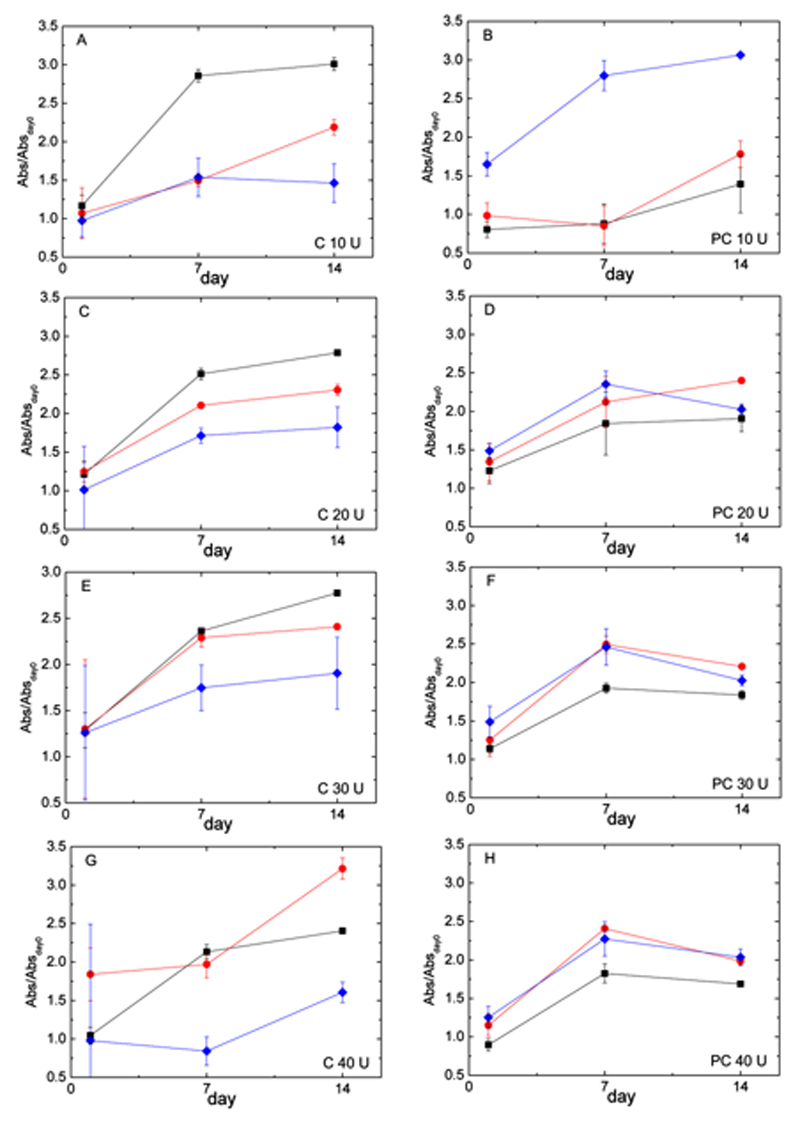
The capability of hydrogels to support cell proliferation was then evaluated through MTS assays. Three formulations were assessed: Gel10%, Cs0.10%/Gel10%, and Cs0.50%/Gel10% at 4 different mTGase concentrations (10 to 40 U/ggelatin), covering the following time frame: 4 h, 1 d, 7 d, and 14 d (Figure 7, left-hand side). Data show that cells proliferate on all gels up to 7 d, reaching a plateau between day 7 and 14 due to becoming confluent. Absorbance decreases with chitosan concentration in chitosan/gelatin chemical hydrogels for most of the samples (with the exception of Cs0.1%/Gel10% with 40 U/ggelatin of mTGase at day 1 and 14). This decrease in cell proliferation shows some correlation with turbidity levels, as chitosan strongly increases the turbidity in chemical gels (Figure 5), reflecting a higher structural inhomogeneity in the gels. The large error bars observed at day 1 are due the strong background signal from the gel itself. Due to the high heterogeneity of chemical gels, this background contribution is hard to subtract and increases as the gels become less homogenous (from low to high mTGase concentration). As the number of cells increases, the gel contribution becomes less relevant and the error bar decreases. In PC gels (Figure 7, right-hand side), the error bars at day 1 are much smaller, due to PC gels higher homogeneity.
Figure 7.
Cell proliferation of MC3T3 cells on hydrogels. Cells were cultured on Gel 10% (▪), Cs 0.10%/Gel 10% (●) and Cs 0.50%/Gel 10% (♦) (wt%). Data were collected at 1, 7 and 14 days using an MTS assay (normalized to day 0) for chemical (A, C, E, G) and physical-co-chemical hydrogels (B, D, F, H) at A,B) 10, C,D) 20, E,F) 30, and G,H) 40 U/ggelatin of transglutaminase. Values are mean ± SD.
The concentration of mTGase does not have a clear effect on cell proliferation. For chitosan/gelatin gels, the trend is a slight increase in cell proliferation with mTGase concentration; however, for Cs0.5%/Gel10% at 40 U/ggelatin a decrease is observed. For pure gelatin gels instead, the opposite seems true: after day 1, cell proliferation is slower as mTGase increases (Figure 7, Supporting Information 3B).
3.3. Physical-co-chemical (PC) Gelation of Chitosan/Gelatin Mixtures
3.3.1. Impact of Chemical Network on Helices Growth
For the physical-co-chemical gelation, carried out at 21.0 °C, that is, below the gelatin gelation temperature (≈ 23 °C), simultaneous formation of both types of networks occurs. As mentioned above, physical gelation is faster than chemical cross-linking. In addition, the lower temperature (21 °C vs 37 °C) slows down the enzymatic reaction.[31] Therefore, it is expected that a sizeable amount of physical junctions will have formed before the chemical network starts to impact on the build-up of the gels. In the chitosan/gelatin mixed systems, OR measurements give an average value of math formula = –390 ± 10 mdegrees for pure physical Cs/Gel (1.0%/10%) gels (21.0 °C); for physical-co-chemical gels under the same conditions, values of math formula = –361 ± 3 to –346 ± 5 mdegrees are obtained for 10 and 40 U/ggelatin of mTGase, respectively, and math formula = –233 ± 15 for the equivalent solutions (Table 1). This confirms a considerable build-up of the physical network during chemical cross-linking, as the OR values are comparable to the ones obtained for the pure physical gels. However, the formation of chemical bonds clearly prevents the system from reaching the same amount of network as the pure physical network.
3.3.2. Synergy of Hybrid Gels
Time sweep experiments of the physical-co-chemical gelation are presented in Figure 4C. The kinetics are dominated by the build-up of the physical network, with a gelation time below one minute, which is much faster than pure chemical gels (Figure 4A). The G′ values obtained for the physical-co-chemical gels are notably higher than the pure physical (Figure 4C) and the pure chemical gels (Figure 4A). This result, also reported for the pure gelatin gels,[31, 33] shows that the addition of the second biopolymer preserves the positive impact of the physical networks on the chemical cross-linking process, allowing us to combine both the synergy in composition (adding a second component) and the synergy resulting from the hybrid gelation, in order to tune the gels properties. With 40 U/ggelatin of mTGase for instance, the physical-co-chemical gels reach a final G′ value of 14.5 kPa (at 210 min); under the same conditions (21.0 °C), pure physical gels only reach G′ = 6.0 kPa, while chemical gels (37.0 °C) show a final G′ value of 10.8 kPa.
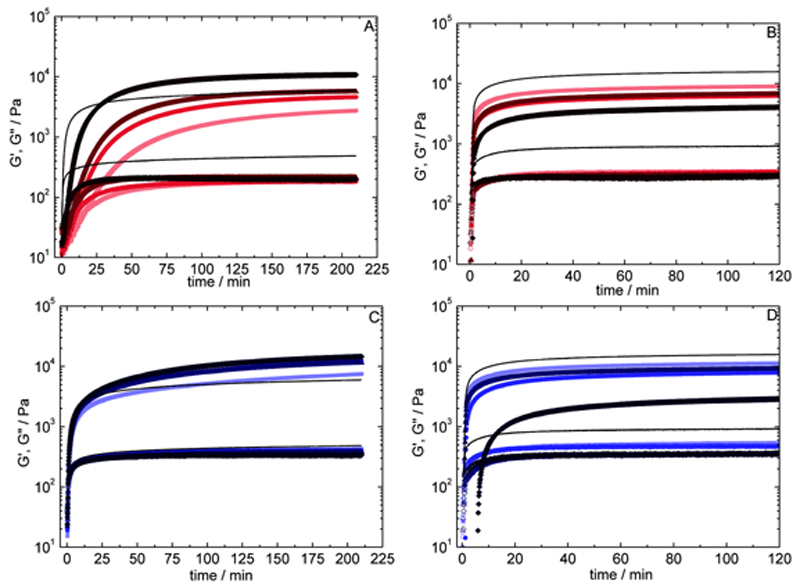
In order to compare the contribution arising from the chemical network in the overall gel, G′C, in both gelation processes (pure chemical vs. physical-co-chemical), the PC gels were brought to 37°C (Figure 1C), leaving only the covalent network in place. The G′C values from the PC gels are significantly higher than G′C from their pure chemical counterparts (Figure 1A). For instance, G′C values for Cs2.0%/Gel10% PC gels at 40 U/ggelatin are on average ca. 60% times larger than for the equivalent chemical gels (Figure 3E).
3.3.3. Impact of Chemical Network on Helices Growth
To evaluate how detrimental the presence of the chemical network is to the formation of the triple-helix network, the physical network, after melting at 37 °C, was then re-grown on top of the chemical scaffold at 12 °C (Figure 4D). The values are off-set to allow all curves to start from G′P = 0 at t=0, as described above. As previously observed for the pure chemical gels,[31] G′P decreases with an increase in mTGase concentration, which reflects the increasing hindrance of the covalent network (G′C) on the physical network (G′P). However, unlike the chemical gels (previous section), there is a clear effect of chitosan concentration on G′P at high enzyme concentrations: while at 10 and 20 U/ggelatin G′P values are rather insensitive to chitosan concentration, at 30 and 40 U/ggelatin, G′P steadily decreases as chitosan concentration increases, from 1.72 kPa for Cs0.10%/Gel10% to 0.613 Pa for Cs2.0%/Gel10% (Figure 3).
3.3.4. Turbidimetry: PC vs C Gels
The turbidity of the chemical scaffold in PC gels follows the same trend observed for the C gels: it increases with both mTGase and chitosan concentrations, reflecting the increasing inhomogeneity of the networks. However, the turbidity in PC gels is significantly lower than for the equivalent C gels (Figure 5). At 30 U/ggelatin, Cs1.0%/Gel10% PC and C gels show a transmittance of 73% vs 36% respectively; when doubling chitosan concentration in Cs2.0%/Gel10%, a 4-fold increase is observed: 17% vs. 4%, respectively. As mentioned earlier, Souguir and co-workers suggested that the inhomogeneous distribution of cross-links responsible for the turbidity arises from an interplay between the osmotic and elastic pressures. The former favours the expansion of the coils and the latter their contraction.[43] In chemical gels, the cross-linking process induces a local build-up of the elastic pressure, at the site of the enzyme binding, leading to a local increase in polymer concentration, thus increasing the reaction rate and creating a positive feedback, resulting in a higher concentration of cross-links in a localized area. In the case of PC gels, the gelatin's physical network is present before any measurable build-up of the chemical network occurs. This would impose a constant elastic pressure throughout the medium, which could off-set the local elastic pressure oscillation due to the enzymatic reaction. This, in principle, should diminish the efficiency of that positive feedback and reduce the gel inhomogeneity, thus the overall turbidity of the gels.
3.3.5. Cell Culture Studies
Cell culture experiments were carried at physiological temperature (37 °C). Therefore, in these experiments; the chemical network generated under PC conditions is evaluated, rather than the hybrid physical and chemical network. Cell spreading on gelatin, chitosan/gelatin gels and TCP was evaluated via F-actin staining and found to be qualitatively similar among the samples examined (Figure 6). Cell proliferation was also supported on all gels from day 1 to 7 (Figure 7). For PC gels, the presence of chitosan consistently improves cell proliferation when compared to the pure gelatin gels (Figure 7), opposite to what had been observed for pure chemical gels. Above 10U/ggelatin, the influence of mTGase concentration on cell proliferation is rather negligible.
4. Discussion
4.1. Chemical vs Physical-co-Chemical Gelation
The elasticity of gels plays a key-role in cell differentiation.[35] Thus, it is important to build gels that offer elasticity in a range that is physiologically relevant. It is also useful to be able to tune the gel elasticity without changing its chemical composition. As discussed below, by combining physical and chemical networks during the enzymatic cross-linking, we can not only increase the range of gel elasticity available for a fixed gel composition, but also increase the gel transparency without changing the formulation.
Figure 3 provides a summary of all the results by showing the averaged individual contributions from both types of networks, G′P and G′C, to the total gel modulus (G′T), for each gelation process (chemical or physical-co-chemical) and varying enzyme and chitosan concentration: the chemical contribution (G′C) arising from the covalent bonds catalysed by mTGase, and the physical contribution (G′P) from the triple-helix network grown on top of the existing chemical network.
For biomedical applications, G′C is the more relevant parameter, as the physical network would not be present at physiological temperature. Thus, in order to separate these two contributions, the gels obtained from both processes (C and PC) were studied above and below the gelatin gelation/melting temperature. At 37.0°C (above the melting temperature), only the chemical network exists; any physical contribution is lost as the gelatin chains are present as single strands (Figure 1A and 1C). At 12.0°C (below gelatin gelation temperature), gelatin chains re-organize themselves into triple-helices, generating the junction points of the physical network within the confines of the chemical network already present (Figure 1B and 1D). In other words, at 12.0°C, both physical and chemical contributions are present, whereas at 37.0 °C only the chemical contribution is present. Assuming that the two contributions are additive, the G′ values measured at 37.0°C (G′C) can be attributed to the chemical contribution, and the physical contribution (G′P) can be attributed to the difference between G′ at 12.0°C (G′T) and at 37.0 °C (G′C).
When comparing PC and C gels, the covalent network appears to be significantly stronger in the PC gels (G′C values are higher), and the difference between the two gelation processes is more marked as mTGase concentration increases (Figure 3), as we had also established for pure gelatin gels (Figure 3A).[31] For example, Cs0.10%/Gel10% in the presence of 10 U/ggelatin mTGase gives values of G′C for the PC and C gels of 0.096 kPa and 0.082 kPa, respectively. When increasing the enzyme concentration to 40 U/ggelatin, the same system gives G′C = 10.7 kPa and 2.8 kPa, respectively, for PC and C gels. Overall, at 10 U/ggelatin mTGase, the G′C values for the C gels are ca. 15% lower than their PC counterparts, compared to 75% lower at 40 U/ggelatin mTGase, on average (Figure 3B). Increasing the chitosan concentration to 2.0% reduces slightly the difference between PC and C processes. For a Cs2.0%/Gel10% sample gelled with 40 U/ggelatin mTGase, we obtained a G′C of 13.8 and 8.8 kPa, respectively, for PC and C. At this higher chitosan concentration, G′C values from the C gels are about 36% lower than PC gels at 40 U/ggelatin mTGase, compared to 75% at 0.10% chitosan (Figure 3E).
In a network, not every connection counts towards the overall elasticity. Some connections are ‘elastically active’: they contribute to creating and propagating the spanning cluster; instead, other connections do not participate to the overall elasticity: they are ‘elastically inactive’ (e.g., intramolecular bonds, closed loops). During the cross-linking reaction, the enzyme may generate both elastically active and inactive bonds. The ratio between the two types of bonds affects the final G′ value of the network, as only elastically active bonds are measured by the rheological experiments.
When comparing the G′C values of physical-co-chemical gels with their chemical counterparts (Figure 3), the G′C values from the physical-co-chemical gels were generally found to be higher, as described above. Similar to what had been observed for pure gelatin gels, the presence of the physical network leads to chemical bonding with better elastic efficiency.[31, 33] In other words, the triple-helices of the physical network influence the ratio between active and inactive bonds, possibly by modifying the spatial distribution of the gelatin reaction sites.[31, 33] In the sol state, the gelatin exists as single strands, the peptide backbone is relative flexible and the lysine –CH2CH2CH2CH2CONH3+ and glutamine –CH2CH2CONH2 side chains can access the mTGase enzyme binding sites. In the gel state, the formation of the triple-helix network restricts the access to the binding sites, for example, by burying them inside the triple-helices, or by increasing the rigidity of the macromolecular ensemble and thus keeping binding sites within the same macromolecule far apart.
The addition of a second component (chitosan), which is not part of the physical network, does not suppress this synergistic effect, but it does affect its intensity. It is indeed observed that, as the concentration of chitosan is increased from 0.10 to 2.0%, the difference between the G′C values measured from PC and C gels diminishes: at 40 U/ggelatin mTGase, the difference between G′C values for C gels and PC is 75%, 65% and 36% as the chitosan concentration increases from 0.10% to 0.50% to 2.0% (Figure 3).
The difference in turbidity between PC and C gels corroborates the finding of an improved spatial ordering induced by the physical network (Figure 5). The turbidity originates from the scattering of visible light by aggregates of appropriate size range (hundreds of nanometers), originating from a high local concentration of bonds. The spatial constraints imposed by the physical network seem to offset this local clustering of bonds. The increased turbidity observed with increasing mTGase concentration, both for PC and C gels, is likely to reflect the increasing size of aggregates cross-linked by the enzyme.[31, 34]
4.1.1. Insights from a Cross-linking ‘Quencher’
To bring more understanding into the mechanisms of gelation and in particular the issue of functional substrate accessibility, the effect of a cross-linking ‘quencher’ was studied by adding 20 mg/gsolution of L-lysine to both physical-co-chemical and chemical gels (Cs 2.0%/Gel 10%). L-Lysine is a substrate of mTGase and should form cross-links with glutamine residues, and is therefore expected to act as a network inhibitor. In the presence of a large excess of free lysine, a large reduction of the gels elasticity is therefore expected, independently of the gelation protocol. This is indeed what we observe (Figure 3F). However, quite remarkably, gelation in C gels is significantly more affected by the presence of L-lysine than in PC gels (Figure 3F). At 10 U/ggelatin, PC gels reached, on average, 88% of the values in the absence of lysine (1.5 kPa against 1.7 kPa) and C gels 47% of the original values (1.1 kPa against 2.3 kPa on average). Interestingly, increasing enzyme concentration leads to higher quenching of the cross-linking for both types of gels, however reduces the difference between PC and C processes: at 40 U/ggelatin mTGase, PC gels with free L-lysine reach 31% of the original values against 20% for the C gels. These results again highlight the positive impact of the triple-helices scaffold in guiding the cross-linking process: the spatial restrictions imposed by the physical network in PC gels must impact on the local concentration and localisation of reaction partners in such a way that it reduces the effect of a network inhibitor (lysine).
4.1.2. An Alternative Enzymatic Cross-Linker
In order to further tune the gels' mechanical properties, Cs/Gel were cross-linked by another enzyme, tyrosinase from mushroom (EC 1.14.18.1), and a mixture of both mTGase and tyrosinase. Payne and co-workers[30] had demonstrated the use of tyrosinase to gel mixed chitosan/porcine gelatin systems, however at substantially lower concentrations than reported herein (0.24%/chitosan/2% gelatin), yielding very weak gels (≈ 10.6 Pa). Using a similar approach, we were also able to obtain gels, however much weaker than those cross-linked with mTGase (SI. 4): with Cs1.0%/Gel10% gels at 1k U/gsolution of tyrosinase, PC gels reached G′C = 0.52 kPa and C gels G′C = 0.21 kPa, while equivalent gels with mTGase reached G′C = 12.6 kPa and G′C = 4.2 kPa for PC and C gels, respectively. Remarkably however, PC gels cross-linked with tyrosinase showed significantly higher G′C values than their C counterparts, demonstrating again, for a different cross-linker, the network-enhancing effect of the triple-helices. When using both enzymes in combination, Cs/Gel cross-linked with a mixture of mTGase and tyrosinase produced weaker gels than the gels obtained with mTGase only, showing a competitive effect rather than synergy between the two enzymes (SUpporting Information 4).
4.1.3. Cell Culture Studies
In cell studies with MC3T3 osteoblasts, it was found that the presence of chitosan positively affected cellular growth in PC gels, while the opposite result was obtained in C gels. By comparing MTS data, we observed that pure gelatin C gels (Figure 7A,C,E,G) showed consistently higher absorbance than gels containing chitosan. In PC gels (Figure 7B,D,F,H), the opposite was observed. PC gels also reached an overall maximum in the relative absorbance measured throughout all the formulations at day 7, at around 2.5, while C gels reached their overall maximum at day 14, with a higher fluctuation in the values (Figure 7). Overall, when compared to pure gelatin gels, PC gels show better cell proliferation in chitosan/gelatin gels than their C counterpart (Figure 7). With this yet limited set of data, we cannot pinpoint the exact origin of this effect, but it could be linked to the higher values of the modulus and better structural ordering of the gels. On-going small-angle neutron scattering measurements are expected to shed more light on the organisation of the gels at the nanoscale level, and in particular on the role of chitosan.
5. Conclusion
In this work, we explored the effect of blending two biopolymers (gelatin and chitosan) and combining two gelation processes (physical and chemical) on the rheological properties and cellular response of the gels. The two gelation protocols consisted of i) cross-linking with an enzyme, mainly: transglutaminase (mTGase), and to a lesser extent tyrosinase and a combination of both, in the sol state, where covalent bonds are expected to be created between both macromolecules present as individual, positively charged strands; and ii) physical-co-chemical gelation, undertaken in the gel state, where cross-linking proceeds within the confines of a transient physical network (gelatin triple-helices). Our results show that the two protocols substantially affect the final gel modulus—which is also strongly dependent on system composition (both mTGase and chitosan concentration)—and have a measurable impact on cellular growth. The physical-co-chemical protocol, for both enzymes used, produces gels with a higher shear modulus than the undisturbed cross-linking process (pure chemical gels) and the gels are structurally more homogeneous (as inferred from their higher transmittance). When adding an inhibitor of the cross-linking, in the form of free lysine, a significant reduction of the covalent network is obtained, however the quenching effect is substantially attenuated in PC gels compared to C gels.
Increasing amounts of chitosan result in gels with higher moduli, while slightly attenuating the difference between the two gelation processes. In addition, the presence of chitosan also increases the structural heterogeneity of the gels, to a larger extent in chemical gels than in physical-co-chemical gels. In terms of cellular response, MTS assays show that the presence of chitosan has a positive impact on physical-co-chemical gels, while it negatively affects cell proliferation in chemical gels.
The observed behaviour can be rationalized in terms of the ratio of intermolecular/intramolecular bonds and the spatial distribution of covalent bonds. Physical-co-chemical gelation probably favours intermolecular bonding, by bringing reaction partners closer together through the formation of triple-helices, leading to a more homogenous distribution throughout the system. This confirms what we had reported earlier for the one-component system (tilapia fish gelatin gels).[31, 34] The addition of a second component, chitosan, a much larger macromolecule with a large excess of one of the binding sites for mTGase, leads to a substantial improvement of the mechanical properties (higher shear modulus), without sacrificing the gain obtained from the synergistic combination of the physical and chemical gelation processes.
The gels reported here, made from a mixture of widely available biopolymers obtained from waste products, combined to an enzymatic process, provide a facile and cheap approach towards functional materials. The use of hybrid gelation processes to tune the elasticity is of interest both from a fundamental viewpoint—to understand the effect of competing or synergistic mechanisms of gelation—and also highly relevant from a practical standpoint: by exploiting the natural gelation of gelatin, can we improve the covalent cross-linkinG? Our work substantiates the benefits of combining physical and chemical processes, extending our previous findings to a mixture of biopolymers and to two enzymatic cross-linkers. We expect that these new results, and the type of systematic physical characterisation provided here, will contribute to providing a better understanding and therefore new avenues for the development of biopolymers-based materials.
Supplementary Material
Acknowledgements
This project was funded by a Leverhulme Trust research grant (F/07 040/AR). F.B. thanks the EPSRC (grant EP/F037902/1) for financial support. M.M.S. thanks ERC-SIG grant “Naturale” for funding. Dr. T. Bui is acknowledged for help with the optical rotation measurements. Dr. L. Kudsiova is acknowledged for helpful discussions about cellular behaviour.
Contributor Information
Dr. Marcelo A. da Silva, King’s College London, Institute of Pharmaceutical Science, 150 Stamford Street, London SE1 9NH, UK
Dr. Franziska Bode, King’s College London, Institute of Pharmaceutical Science, 150 Stamford Street, London SE1 9NH, UK
Dr. Alex F. Drake, Biomolecular Spectroscopy Centre, King’s College London, The Wolfson Wing, Hodgkin Building, London SE1 1UL, UK
Dr. Silvia Goldoni, Department of Materials, Department of Bioengineering and Institute for Biomedical Engineering, Imperial College London, Exhibition Road, London SW7 2AZ, UK
Prof. Molly M. Stevens, Department of Materials, Department of Bioengineering and Institute for Biomedical Engineering, Imperial College London, Exhibition Road, London SW7 2AZ, UK
Dr. Cécile A. Dreiss, King’s College London, Institute of Pharmaceutical Science, 150 Stamford Street, London SE1 9NH, UK
References
- 1.Langer R, David AT. Nature. 2004;428:487. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Fan H, Cui Y, Chen Y, Yao K, Goh JCH. Biomacromolecules. 2007;8:1446. doi: 10.1021/bm061025e. [DOI] [PubMed] [Google Scholar]
- 3.Silva SS, Mano JF, Reis RL. Crit Rev Biotechnol. 2010;30:200. doi: 10.3109/07388551.2010.505561. [DOI] [PubMed] [Google Scholar]
- 4.Vlierberghe SV, Dubruel P, Schacht E. Biomacromolecules. 2011;12:1387. doi: 10.1021/bm200083n. [DOI] [PubMed] [Google Scholar]
- 5.Place ES, Evans ND, Stevens MM. Nat Mater. 2009;8:457. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 6.Ward AG. J Appl Phys. 1954;5:85. [Google Scholar]
- 7.Shoulder MD, Raines RT. Annu Rev Biochem. 2009;78:929. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo L, Colby RH, Lusignan CP, Whitesides TH. Macromolecules. 2003;39:9999. [Google Scholar]
- 9.Siligardi G, Drake AF. Biopolymers. 1995;37:281. doi: 10.1002/bip.360370406. [DOI] [PubMed] [Google Scholar]
- 10.Drake AF, Siligardi G, Gibbons WA. Biophys Chem. 1988;31:143. doi: 10.1016/0301-4622(88)80019-x. [DOI] [PubMed] [Google Scholar]
- 11.Djabourov M. Contemp Phys. 1988;29:273. [Google Scholar]
- 12.Veis A. The Macromolecular Chemistry of Gelatin. Academic Press; London, U.K: 1964. [Google Scholar]
- 13.Ferry JD. Adv Protein Chem. 1948:1. doi: 10.1016/s0065-3233(08)60004-2. [DOI] [PubMed] [Google Scholar]
- 14.Karim AA, Bhat R. Food Hydrocolloids. 2009;23:563. [Google Scholar]
- 15.Gornall JL, Terentjev EM. Soft Matter. 2008;4:544. doi: 10.1039/b713075a. [DOI] [PubMed] [Google Scholar]
- 16.Nijenhuis KT. Adv Polym Sci. 1997;130:1. [Google Scholar]
- 17.Ross-Murphy SB. Polymer. 1992;33:2622. [Google Scholar]
- 18.Bigi A, Cojazzi G, Panzavolta S, Roveri N, Rubini K. Biomaterials. 2002;23:4827. doi: 10.1016/s0142-9612(02)00235-1. [DOI] [PubMed] [Google Scholar]
- 19.Kopecek J, Yang JY. Polym Int. 2007;56:1078. [Google Scholar]
- 20.Zhu Y, Tramper J. Trends Biotechnol. 2008;26:559. doi: 10.1016/j.tibtech.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Sha H, Fu X. J Controlled Release. 2010;142:149. doi: 10.1016/j.jconrel.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Eastoe JE. Biochem J. 1957;65:363. doi: 10.1042/bj0650363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harding SE, Hill SE, Mitchell JR. Biopolymer mixtures. Nottingham University Press; Nottingham, UK: 1995. [Google Scholar]
- 24.Khor E, Lim LY. Biomaterials. 2003;24:2339. doi: 10.1016/s0142-9612(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 25.Moura MJ, Fance H, Lima MP, Gil MH, Figuereiro MM. Biomacromolecules. 2011;12:3275. doi: 10.1021/bm200731x. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Y-H, Yang S-H, Su W-Y, Chen Y-C, Yang K-C, Cheng WT-K, Wu S-C, Lin F-H. Tissue Eng Part A. 2009;16:695. doi: 10.1089/ten.TEA.2009.0229. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Liu Y, Payne GF. J Adhesion. 2009;85:576. [Google Scholar]
- 28.Aberg CM, Chen T, Olumide A, Raghavan SR, Payne GF. J Agric Food Chem. 2004;52:788. doi: 10.1021/jf034626v. [DOI] [PubMed] [Google Scholar]
- 29.Chen T, Embree HD, Brown EM, Taylor MM, Payne GF. Biomaterials. 2003;24:2831. doi: 10.1016/s0142-9612(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 30.Chen T, Embree HD, Wu L-Q, Payne GF. Biopolymers. 2001;64:292. doi: 10.1002/bip.10196. [DOI] [PubMed] [Google Scholar]
- 31.Bode F, da Silva MA, Drake AF, Ross-Murphy SB, Dreiss CA. Biomacromolecules. 2011;12:3741. doi: 10.1021/bm2009894. [DOI] [PubMed] [Google Scholar]
- 32.Hellio-Serughetti D, Djabourov M. Langmuir. 2006;22:8509. doi: 10.1021/la060375j. [DOI] [PubMed] [Google Scholar]
- 33.Hellio-Serughetti D, Djabourov M. Langmuir. 2006;22:8516. doi: 10.1021/la0605384. [DOI] [PubMed] [Google Scholar]
- 34.Bode F, da Silva MA, Smith P, Lorenz CD, McCullen S, Stevens MM, Dreiss CA. Soft Matter. 2013;9:6986. doi: 10.1039/c3sm00125c. [DOI] [PubMed] [Google Scholar]
- 35.Engler AJ, Shamik S, Sweeney HL, Discher DE. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Bo S, Li S, Qin W. Int J Biol Macromol. 1991;13:281. doi: 10.1016/0141-8130(91)90027-r. [DOI] [PubMed] [Google Scholar]
- 37.Sang L-Y, Zhou X-H, Yun F, Zhang G-L. J Sci Food Agric. 2010;90:58. doi: 10.1002/jsfa.3779. [DOI] [PubMed] [Google Scholar]
- 38.Sarabia AI, Gómez-Guillén MC, Montero P. Food Chem. 2000;70:71. [Google Scholar]
- 39.Kolodziejska I, Piotrowska B, Bulge M, Tylingo R. Carbohydr Polym. 2006;65:404. [Google Scholar]
- 40.Giraudier S, Hellio D, Djabourov M, Larreta-Garde V. Biomacromolecules. 2004;5:1662. doi: 10.1021/bm049670d. [DOI] [PubMed] [Google Scholar]
- 41.Clark AH, Ross-Murphy SB. Advances in Polymer Science, Structural and mechanical properties of biopolymer gels. Vol. 83 Springer, Berlin Heidelberg; Germany: 1987. [Google Scholar]
- 42.Joly-Duhamel C, Hellio D, Djabourov M. Langmuir. 2002;18:7208. [Google Scholar]
- 43.Souguir H, Ronsin O, Larreta-Garde V, Narita T, Caroli C, Baumberger T. Soft Matter. 2012;8:3363. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.