Abstract
Puberty onset involves increased GnRH release due to decreased sensitivity to oestrogen (E)-negative feedback. Because GnRH neurones lack E receptor alpha, this pathway must contain interneurones. One likely candidate is KNDy neurones (kisspeptin, neurokinin B, dynorphin). Our overarching hypothesis was that the prepubertal hiatus in LH release involves reduced kisspeptin and/or heightened dynorphin input. We first tested the specific hypothesis that E would reduce kisspeptin-immunopositive cell numbers and increase dynorphin-immunopositive cell numbers. We found that kisspeptin cell numbers were higher in ovariectomised (OVX) lambs than OVX lambs treated with E (OVX+E) or those left ovary-intact. Very few arcuate dynorphin cells were identified in any group. Next, we hypothesized that central blockade of κ-opioid receptors (KOR) would increase LH secretion at a prepubertal (6 months), but not postpubertal (10 months) age. LH pulse frequency and mean LH increased during infusion of a KOR antagonist, nor-BNI, in OVX+E lambs at the prepubertal age, but not in the same lambs at the postpubertal age. We next hypothesized that E would increase KOR expression in GnRH neurones or alter synaptic input to KNDy neurones in prepubertal ewes. E treatment decreased the percentage of GnRH neurones coexpressing KOR (~68%) compared to OVX alone (~78%). No significant differences in synaptic contacts per cell between OVX and OVX+E groups were observed. While these initial data are consistent with dynorphin inhibiting pulsatile LH release prepubertally, additional work will be necessary to define the source and mechanisms of this inhibition.
Key Terms: Dynorphin, Kisspeptin, GnRH, Kappa Opioid Receptor, Ovine, Puberty
INTRODUCTION
Puberty is a complex series of events that is governed by activation of the hypothalamic-pituitary-gonadal axis. Increases in the pulsatile release of gonadotropin-releasing hormone (GnRH) are a prerequisite for the initiation of pubertal development and maintenance of normal reproductive functioning throughout adulthood. These changes in GnRH drive alterations in anterior pituitary secretion of the gonadotropins (LH and FSH) that are responsible for follicular maturation and gonadal steroid production. The neural mechanisms underlying the increase in rhythmic discharge of GnRH, and thus LH, that results in the acquisition of sexual maturation still remain unclear. During the prepubertal period of female sheep, the hypothalamus is highly sensitive to the inhibitory effects of small quantities of oestrogen (E) produced by the ovary, leading to infrequent pulsatile secretion of GnRH and LH. In sheep, as the animal grows and matures and photoperiod becomes permissive, the ability of E to inhibit pulsatile GnRH and LH secretion wanes, allowing for an increased frequency of GnRH, and thus LH, pulses. This in turn leads to heightened E secretion from developing follicles, and ultimately the LH surge and first ovulation (1). Importantly, GnRH neurones are devoid of oestrogen receptor alpha (ER-α), the receptor that mediates the negative feedback effects of E (2), indicating the involvement of intermediary neurones in this process. Recent evidence suggests that neurones in the arcuate nucleus (ARC) of the hypothalamus that coexpress kisspeptin, neurokinin B (NKB), and dynorphin (i.e. KNDy neurones) play a critical role in the elevation of pulsatile GnRH release that heralds the onset of puberty.
Most attention given to the role of KNDy neurones in the onset of puberty has focused on kisspeptin and NKB. Mutations in the kisspeptin receptor, Kiss1r (previously GPR54) (3), or NKB as well as its cognate receptor, NK3R (4), preclude pubertal development and lead to hypogonadotropic hypogonadism in humans (4,5). Further work showed that administration of kisspeptin stimulates GnRH and/or LH secretion prior to puberty in many species, including sheep (6). An agonist of NK3R, senktide, stimulates LH release in primates and sheep (7,8), but has varied effects on LH secretion in rodents that seem to be dependent upon steroid milieu (9). In sheep, GnRH neurones coexpress Kiss1r (10), but do not express NK3R (11), which suggests that the actions of kisspeptin, but not NKB, on GnRH secretion are direct. Some GnRH neurones express NK3R in rodents (12), but NKB is not thought to act directly on GnRH neurones in these species (13). Since KNDy neurones do express NK3R, it has been suggested that NKB influences GnRH secretion via autocrine or paracrine stimulation of kisspeptin release (14). Consistent with previous work and the fact that KNDy neurones express ER-α, our recent study reported that numbers of kisspeptin- and NKB-immunoreactive cells in the ARC increase after ovariectomy in prepubertal lambs (8), suggesting that sex steroids may directly regulate prepubertal kisspeptin and NKB expression. However, synaptic input to KNDy neurones has been shown to be influenced by gonadal steroids (15), raising the possibility that E may act indirectly to alter KNDy neurone function. Regardless of whether the influence is direct or indirect (or both), the increase in pulsatile GnRH and LH release that occurs in response to a reduction in E-negative feedback during pubertal development likely involves heightened input from kisspeptin and NKB.
Dynorphin, the third neuropeptide composing KNDy neurones, has been given much less attention. Dynorphin is an endogenous opioid peptide that selectively binds the κ-opioid receptor (KOR). Dynorphin has been implicated as a potential mediator of progesterone inhibition of GnRH release in adult rats (16) and sheep (17), but few studies have examined its role in puberty onset. An early study reported that injection of anti-dynorphin antibodies in the ARC of immature female rats increased serum LH levels (18). More recently, Nakahara et al. (19), demonstrated that administration of norbinaltorphimine (nor-BNI), a KOR antagonist, advanced first oestrus and increased LH pulse frequency in female rats (19). As an initial foray into this area in prepubertal ewe lambs, we formed the overarching hypothesis that reduced kisspeptin input and increased dynorphin input contributes to the suppression of GnRH, and thus, LH secretion in these animals. These experiments tested four specific hypotheses: 1) that numbers of kisspeptin-immunoreactive cells would be reduced, but that dynorphin-immunoreactive cell numbers would be greater, in the presence, versus the absence, of E in prepubertal lambs, 2) that blockade of KOR would increase LH secretion in ovariectomised (OVX) lambs given E that were of a prepubertal age, but would be without effect in lambs of a postpubertal age, 3) that KOR receptor coexpression would be evident in GnRH neurons prepubertally and that expression would be increased by E, and 4) that removal of E-negative feedback in prepubertal lambs is accompanied by an overall increase in synaptic input to KNDy neurones.
MATERIALS AND METHODS
Animals
Late February- to early March-born Suffolk ewe lambs were purchased from a local producer and housed indoors where they received a commercial premium alfalfa-timothy cube food ration (Triple Crown Nutrition, Inc., Wayzata, MN, crude protein ≥12%, crude fat ≥18%, crude fiber ≤32%) and had free access to water and mineral block supplement. Prepubertal experiments were performed in August at 5–6 months of age and postpubertal experiments were performed at 9–10 months of age in November. The average weight of the lambs was 37.4±1.1 kg (range of 34–40 kg) at 5–6 months of age. Animals were housed two per pen (6.75 ft x 6.75 ft) on raised flooring with a clear view of all other sheep. Indoor lighting simulated the natural changes in day length. Ovariectomies and neurosurgeries were performed under aseptic conditions. For both surgeries, animals were first anaesthetised by intravenous (IV) injection of ketamine (7 mg/kg) and midazolam (0.3 mg/kg), and then maintained on 3% isoflurane. Ovarian vasculature was ligated and the ovaries were removed via a midventral incision. For cannulation of the lateral ventricle, the head was positioned in a stereotaxic instrument (Kopf Instruments, Tujunga, CA) and a small hole (0.5 cm in diameter) was drilled 5 mm anterior and 4 mm lateral to bregma. The dura was exposed, cauterised, and a 16 gauge needle (with a reservoir containing sterile water) was lowered until water flowed into the ventricle. Radio-opaque dye (1.5 mL Iohexol (Patterson Veterinary, Devens, MA) was injected into the ventricle, a lateral X-ray was taken, and the dorsal-ventral postion of the needle adjusted so that it was centered in a dorsal-ventral direction within the ventricle. The needle was plugged and cemented in place, covered with a plastic cap using dental acrylic and the skin then sutured around the dental acrylic. Blood samples (3–4 mL) were collected by either venepuncture (Exp. 1) or jugular catheter (Exp. 2), placed in heparinised tubes, and plasma stored at −20 C until assayed for LH.
Oestrogen replacement was accomplished via subcutaneous insertion of a 1-cm long SILASTIC implant (inner diameter = 0.34 cm, outer diameter = 0.46 cm; Dow Corning Corp., Midland, MI) at the time of OVX that was left in place for the duration of the experiments. Progesterone replacement for the preliminary experiment done prior to Experiment 2 was accomplished by insertion of two intravaginal progesterone Controlled Internal Drug Release (CIDR) devices for at least 4 days before drug treatments. Previous work had shown that they produce circulating progesterone of about 2 ng/ml (17). All procedures were approved by the West Virginia University Animal Care and Use Committee and followed National Institutes of Health guidelines for use of animals in research.
Experiments
Experiment 1: Does E inhibit kisspeptin and/or increase dynorphin immunoreactive cell numbers in prepubertal lambs?
Prepubertal ewe lambs (6 months old) were randomly assigned to three treatment groups: ovary-intact (n=5), OVX (n=5), and OVX with E replacement (OVX+E; n=4). Ovary-intact animals did not undergo surgery or receive E implants. Two weeks following the time of OVX, blood samples were collected every 12 min for 4 h from all groups by jugular venepuncture. Immediately following the final blood sample, hypothalami were collected as previously described (20). Briefly, all sheep were heparinised (20,000 U) and euthanised using an intravenous overdose of sodium pentobarbital (Euthasol; Webster Veterinary, Devens, MA). Heads were removed and perfused via carotid arteries with 4 L of 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) containing 0.1% sodium nitrite. Blocks of tissue containing the preoptic area (POA) and hypothalamus were then removed and stored in 4% paraformaldehyde for 24 h at 4°C and transferred to 20% sucrose until sectioned. Frozen coronal sections (30 μm) were cut with a freezing microtome and stored in cryopreservative until the time of immunocytochemical staining.
For immunocytochemistry, three sections encompassing the middle to caudal ARC were selected for analysis of kisspeptin and dynorphin cell numbers. In addition, sections taken from adult ewes that were in the luteal phase of the oestrous cycle were used as positive controls for dynorphin immunostaining and concurrently assessed with lamb tissue. Dynorphin immunostaining was also examined in the POA and the paraventricular nucleus (PVN) to determine the specificity of the response to E within populations of dynorphin neurones.
On day 1 of the immunocytochemistry protocol, sections were washed 4×5 min in 0.1 M phosphate-buffered saline (PBS) to remove excess cryoprotectant and stored overnight at 4°C. On day 2, sections were washed 4×5 min in PBS then placed in 10% H2O2 for 10 min followed by 4×5 min washes in PBS. Tissue was then incubated for 1 h with 0.4% Triton X-100 (Sigma-Aldrich, St. Louis, MO) in 20% normal goat serum (NGS; Jackson Immunosearch Laboratories, Inc., West Grove, PA) for kisspeptin or 4% NGS for dynorphin, both made in PBS. These neurones were identified using primary antibodies raised in rabbits against kisspeptin (gift from A. Caraty) and dynorphin (Phoenix Pharmaceuticals, Inc., Burlingame, CA), that have been validated previously for use in sheep (21,22). Tissue sections were incubated with 1:50,000 kisspeptin antiserum or 1:10,000 dynorphin antiserum for 18 h at room temperature. On day 3, biotinylated goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA) at 1:500 and Vectastain ABC-elite (Vector Laboratories) at 1:500 were applied sequentially for 1 h each with 4×5-min washes of PBS between incubations. Sections were then placed in a 3,3′-diaminobenzidine tetrahydrochloride (DAB) solution (10 mg of DAB [Sigma-Aldrich] in 50 mL of PB with 20 μl of 30% H2O2 added just before the 10 min incubation). After 3×5-min washes in PB, sections were mounted on Superfrost microscope slides (Fisher Scientific, Pittsburgh, PA), dehydrated using a series of increasing alcohol baths, and coverslipped using Eukitt Mounting Reagent, Hawthorne, NY.
Experiment 2: Does dynorphin hold LH pulse frequency in check in prepubertal, but not post-pubertal ewe lambs?
A preliminary experiment was first performed in adult ewes to test the effectiveness of a dose of nor-BNI (Sigma-Aldrich, St. Louis, MO) on LH secretion. This initial dose was selected based on data from a study using adult goats (23). Adult OVX ewes (n=4) with a chronic guide tube in the lateral cerebroventricle were pretreated with a 1 cm long E implant and 2 CIDRs to mimic a luteal phase because nor-BNI is known to increase LH secretion in the presence of progesterone (24). Ewes were infused ICV with artificial cerebrospinal fluid (aCSF) for 3 h (300 μl/h) followed by ICV infusion of nor-BNI for 3 h (300 μl/h; 60 nmol/h). Battery-operated pumps strapped to the back of each ewe were used to infuse aCSF and nor-BNI through sterilised line and 22 gauge stainless steel tubing that extended to the tip of the needle in the lateral ventricle (25). At the end of the experiment, fixed tissue was collected and the location of each needle in the lateral ventricle confirmed histologically. Throughout, blood samples were collected every 12 minutes via jugular catheters. Since this dose of nor-BNI significantly increased (P < 0.04) LH pulse frequency from 1.0 ± 0.4 pulses/3 h to 2.3 ± 0.6 pulses/3 h in adult Suffolk ewes, we chose to use it in our study.
Next, prepubertal ewes (6 months old; n=6) were OVX, implanted with a 1-cm E implant and a guide cannula was placed in one lateral cerebroventricle. At least 14 days after surgery, ewe lambs were randomly assigned to receive either aCSF (26) as a control or nor-BNI. Animals were infused ICV (300 ul/h) with aCSF for 3 h or with 60 nmol/h of nor-BNI for 3 h. Blood samples were collected at 12 min intervals for 36 min prior to, as well as, for the subsequent 3 h infusion period by jugular catheter. One week later, the process was repeated using a crossover design so that each lamb received both treatments. At 10 months of age, a time when lambs are considered to be postpubertal, the experiment was repeated using a similar approach, only samples were collected for 4 h to more accurately assess an expectedly more rapid pulse frequency.
Experiment 3: Does E increase KOR colocalisation with GnRH in prepubertal lambs?
Tissue from Experiment 1 was used to determine whether E treatment in prepubertal lambs increases the percentage of GnRH neurones in the POA that coexpress KOR. Series of sections containing POA from either OVX (n=5) or OVX+E (n=5) lambs were processed for dual-label immunofluorescence using a biotinylated tyramide amplification procedure and analysed with confocal microscopy. First, sections were washed 4×5 min in 0.1 M PBS and stored overnight at 4°C. The following day, a heat-mediated antigen retrieval protocol was used as previously described (27). Sections were then washed 4×5 min in PBS and placed in 1% H2O2 for 10 min followed by 4×5 min washes in PBS. Tissue was then incubated for 1 h with 0.4% Triton X-100 (Fisher Scientific, Pittsburgh, PA) in 20% NGS made in PBS, followed by incubation for 17 h with monoclonal mouse anti-KOR (1:250; Santa Cruz Biotechnology, Inc., Dallas, TX) in 0.4% Triton X-100 and 4% NGS made in PBS. Following primary incubation, tissue sections were sequentially incubated for 1 h in biotinylated goat anti-mouse secondary antibody and Vectastain ABC-elite (1:500 each), for 10 min in biotinylated tyramide (TSA; 1:250 with 3% H2O2 made in PBS; PerkinElmer, Waltham, MA), and 30 min in Alexa 555-Streptavidin (1:100; Molecular Probes, Grand Island, NY) with 4x5 min PBS washes in-between each step. Next, sections were incubated for 17 h in rabbit anti-GnRH (1:400; Immunostar, Hudson, WI) with 0.4% Triton X-100 and 4% NGS made in PBS. The following day, sections were incubated for 30 min in Dylight 488 goat anti-rabbit secondary antibody (1:100; Thermo Scientific, Waltham, MA), mounted on Superfrost microscope slides (Fisher Scientific, Pittsburgh, PA), and coverslipped with Gelvatol, a mounting medium that contains anti-fading agent (1,4-diazabicyclo(2,2)octane (DABCO); 50 mg/ml).
Experiment 4: Does E increase synaptic input to dynorphin-containing neurones in prepubertal ewe lambs?
Tissue collected from Experiment 1 was used to determine whether synaptic input to dynorphin neurones changes with E treatment in prepubertal ewes using dual immunofluorescence and confocal microscopy. Because very few dynorphin neurones were evident in Experiment 1, kisspeptin was used to identify ARC dynorphin neurones since this population highly coexpresses kisspeptin, neurokinin B, and dynorphin (21). Following the same pre-incubation steps as in Experiment 1, tissue was incubated in a solution of PBS containing 0.4% Triton X-100 and 20% NGS for at least 1 h. Kisspeptin neurones were identified using the same primary antibody as in Exp. 1 (Millipore, Darmstadt, Germany, catalog number AB-9754) and synaptophysin was identified with a mouse monoclonal primary antibody (Sigma-Aldrich, St. Louis, MO) that has been validated previously for use in sheep (21,28). Sections were incubated with 1:2000 kisspeptin antiserum and 1:200 synaptophysin antibody for 16 h at room temperature. Differences in kisspeptin antiserum concentrations between this experiment and Experiment 1 reflect the differing approaches (DAB-based immunocytochemistry vs. immunofluorescence) and source of the antibody (a direct gift from Dr. Caraty vs. Millipore). On d 3, tissue sections were first washed 3x5 min in PBS and then incubated in goat anti-mouse DyLight green secondary antibody (Thermo Fisher Scientific, Waltham, MA) at 1:100 for 30 min. Sections and reagents with fluorescent substrates were covered to prevent light exposure from this point in the procedure onward. Sections were then washed 4x5 min in PBS and incubated in goat anti-rabbit Alexa 555 secondary antibody (Life Technologies, Carlsbad, CA) at 1:100 for 30 min. Sections were washed 4x5 min in PBS, mounted on Superfrost slides, and coverslipped using Gelvatol, and stored in the dark at 4 C.
Data analysis
Immunocytochemistry
For single antigen staining, cell bodies were identified by brown cytoplasmic staining and were counted manually using an Olympus AZ70 transmitted light microscope (Center Valley, PA) from sections of middle to caudal ARC, POA and PVN. Mean cell numbers/section for each neuroanatomical area in each ewe were averaged; there was no difference in cell numbers between the middle and caudal ARC, so these data were combined. Three sections that were 150 μm apart were used for each animal per hypothalamic region.
For sections processed for KOR and GnRH staining, images were captured using Nikon D-Eclipse C1 laser-scanning confocal system attached to a Nikon Eclipse E800 microscope (Nikon Corporation). Optical sections (1μm; 60x magnification) were captured through KOR- and GnRH-immunoreactive neurones (red and green, respectively) using EZ-C1 Gold version 3.80 software. Total number of single- and double-label GnRH-immunoreactive cells through POA of each animal were calculated and are reported as percentages ± SEM.
For kisspeptin/synaptophysin staining, analysis of the number of synaptophysin-positive close contacts (green) on kisspeptin neurones (red) was determined for 10 kisspeptin neurones/ewe from at least 3 different tissue sections ranging from middle to caudal ARC. Dual-immunofluorescent images were taken using a Zeiss LSM 510 laser scanning confocal system (Hornwood, NY) on a Zeiss Axio Image Z1 upright microscope with a Plan Apochromat 63x/1.4 oil objective. Confocal Z-stacks of optical sections were taken at 0.41-μm increments through each kisspeptin cell. The number of close contacts onto kisspeptin cell bodies was analysed using Zen Software from Zeiss. As contacts were counted through the entire Z-stack, markers were placed on each individual contact to ensure that no contacts were counted more than once. Orthogonal views were used to confirm that contacts were touching the cell in all planes.
Assays
LH concentrations were measured in duplicate by RIA as previously described (29) using reagents provided by the National Hormone and Peptide Program (Torrance, CA). Assays used 100 to 200 ul of plasma and sensitivity averaged 0.07 ng/tube (NIH S24) with intra- and interassay coefficients of variation being 12.7 and 18.2%, respectively.
Statistics
As in previous work, three criteria were used to determine a LH pulse: 1) a peak must occur within two samples of the previous nadir, 2) the amplitude must be greater than the sensitivity to the LH assay, and 3) the LH level at the peak must exceed the 95% confidence limits (based on overall assay variability) of the concentration at both the preceding and subsequent nadir (30). In Experiment 1, means for kisspeptin cell numbers, mean LH, and LH pulse amplitude (peak minus preceding nadir) from the three treatment groups were compared by one-way analysis of variance with endocrine condition as the main factor. Significant differences among groups were determined by a test of least significant differences. A Wilcoxon Mann-Whitney test was used to compare LH pulse frequency among treatment groups. In Experiment 2, mean LH and LH pulse amplitudes within age were compared by repeated measures analysis of variance and LH pulse frequency was compared by Friedman repeated measures ANOVA. In Experiment 3, group differences in the percentage of GnRH neurones expressing KOR were analysed via chi-square analysis. In Experiment 4, numbers of synaptophysin contacts per kisspeptin neurone were compared by t-test. Differences were considered to be statistically significant at P < 0.05.
RESULTS
Experiment 1: Does E inhibit kisspeptin and/or increase dynorphin immunoreactive cell numbers in prepubertal lambs?
Mean LH concentrations were significantly increased by OVX (6.28 ± 0.9 ng/mL) compared to animals in the ovary-intact or OVX+E groups (P < 0.0001), with mean LH concentrations being lower in OVX+E females (1.88 ± 0.1 ng/mL) than in ovary-intact females (2.84 ± 0.3 ng/mL) (P = 0.001). This finding was reflected by LH pulse frequencies that were significantly higher in OVX females (3.00 ± 0.4 pulses/4 h) compared to the ovary-intact (0.80 ± 0.3 pulses/4 h) (P = 0.01) and OVX+E groups (1.40 ± 0.7 pulses/4 h) (P = 0.05), although frequencies did not differ significantly between ovary-intact and OVX+E females (P = 0.58). We did not compare LH pulse amplitudes among groups as the absence of pulses in a number of the ovary-intact and OVX+E females precluded meaningful analysis.
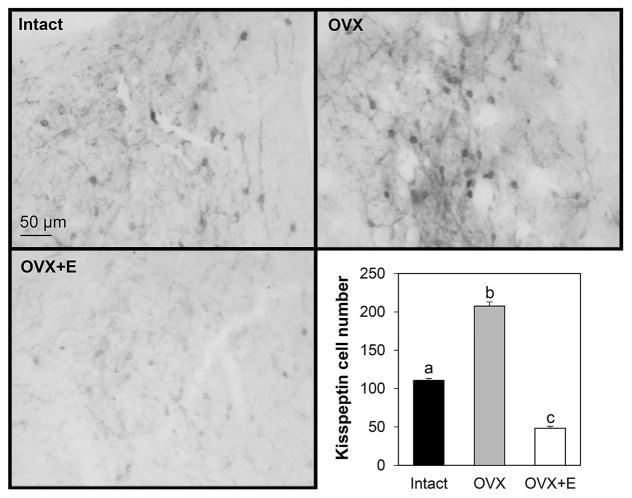
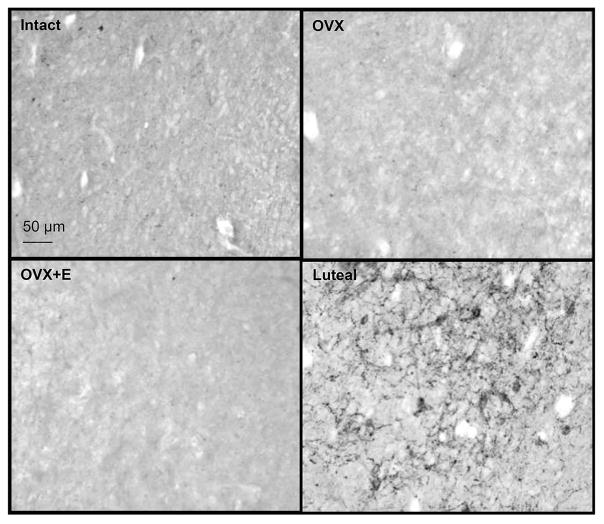
The number of kisspeptin-immunoreactive cells in the ARC was increased by OVX compared to either ovary-intact or OVX+E groups in prepubertal animals and immunoreactive cell numbers were lower in the OVX+E group compared to ovary-intact females (Fig. 1). Contrary to our hypothesis that dynorphin expression would be increased by E in prepubertal females compared to OVX females, very few dynorphin-immunoreactive cells were found in the ARC of any treatment group, even though cell bodies and fibres were readily evident in hypothalamic tissue concurrently assessed from adult ewes in the luteal phase of the oestrous cycle (Fig. 2). In addition, neurones exhibiting dynorphin staining were not observed in significant numbers within the POA, but they were readily evident in the PVN. However, cell numbers in the PVN did not differ among treatment groups: intact (323.6 ± 32.3 cells/section), OVX (247.5 ± 49.5 cells/section), OVX+E (318.6 ± 48.8 cells/section).
Figure 1.
Representative photomicrographs of kisspeptin immunostaining within the arcuate nucleus of prepubertal lambs that were ovary-intact (n=4), ovariectomised (OVX; n=5), or OVX and treated with an oestrogen implant (OVX+E; n=5). Mean±SEM kisspeptin cell numbers in the arcuate nucleus of female lambs from the three treatment groups are shown in bottom right panel. Differing letters denote significant differences (P < 0.05). Scale bar = 50 μm.
Figure 2.
Representative photomicrographs of dynorphin immunostaining within the arcuate nucleus of prepubertal lambs that were ovary-intact (n=4), ovariectomised (OVX; n=5), or OVX and treated with an oestrogen implant (OVX+E; n=5). Very few dynorphin-labelled cells were seen in any prepubertal group. A section of arcuate nucleus from an adult ewe taken during the luteal phase is also shown as a positive control for dynorphin immunostaining. Scale bar = 50 μm.
Experiment 2: Does dynorphin hold LH pulse frequency in check in prepubertal, but not post-pubertal ewe lambs?
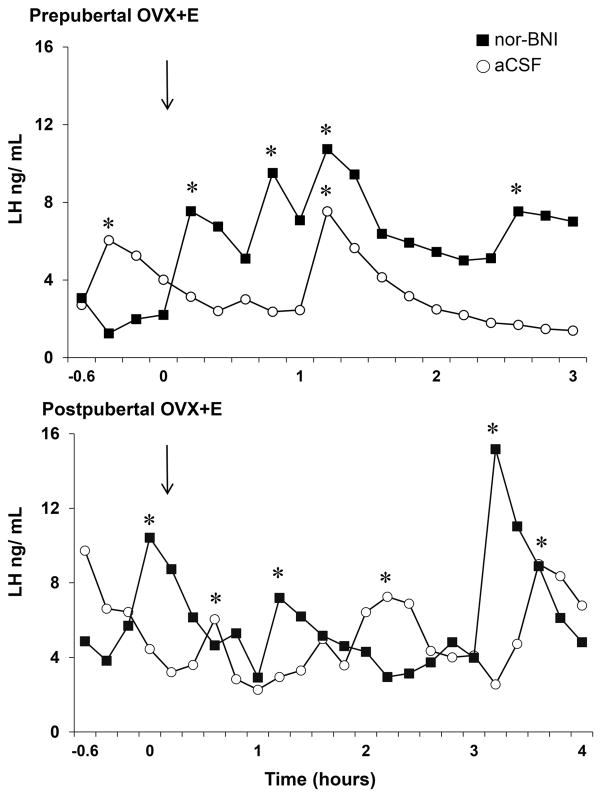
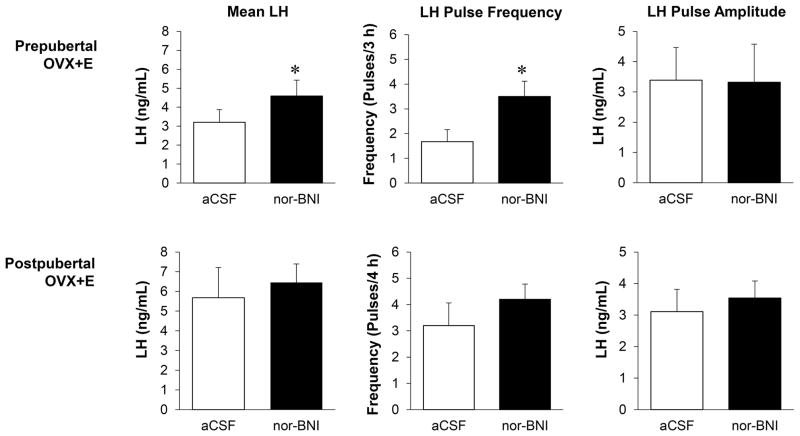
LH secretory profiles from representative animals of a prepubertal or postpubertal age after receiving either aCSF or nor-BNI are shown in Figure 3. Administration of nor-BNI significantly increased mean LH and LH pulse frequency compared to aCSF-controls in OVX+E females of a prepubertal age (Fig. 4A). In OVX+E females of a postpubertal age, there were no significant differences in mean LH, LH pulse frequency, or LH pulse amplitude between treatments (Fig. 4B). Note that in controls, both mean LH concentrations and LH pulse frequencies were approximately twice as high in the females at a postpubertal age than in the same females at a prepubertal age, but these data were not statistically analysed as this was not a pre-planned comparison.
Figure 3.
Representative secretory profiles of plasma LH during lateral cerebroventricular infusion of norbinaltorphimine (nor-BNI; black squares) or artificial cerebrospinal fluid (aCSF; white open circles) in prepubertal (top panel) and postpubertal (bottom panel) ewes that were ovariectomised and treated with oestrogen (OVX+E). Pulses are signified by asterisks. Time 0 equals the start of infusion and is designated by arrows.
Figure 4.
Mean±SEM for mean LH, LH pulse frequency (pulse/sampling period), and LH pulse amplitude for a single group of ovariectomised female lambs (n=6) that received oestrogen implants (OVX+E) at either a prepubertal (top panel) or postpubertal (bottom panel) age. Lambs received lateral cerebroventricle infusion of nor-BNI or aCSF. Asterisk indicates significant differences (P < 0.05).
Experiment 3: Does E increase KOR colocalisation with GnRH in prepubertal lambs?
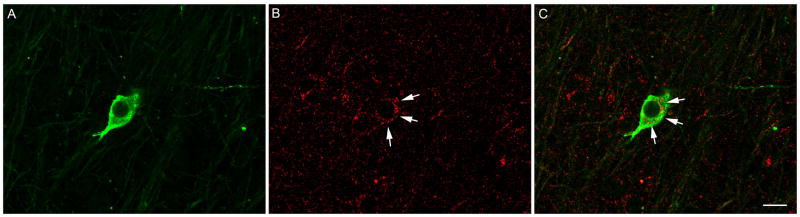
The percentage of GnRH-immunoreactive cells that coexpressed KOR was significantly decreased (P < 0.05) by E treatment: OVX+E (67.8 ± 2.9%) vs. OVX (77.7 ± 2.5%) in prepubertal ewes. This difference was not due to changes (P = 0.81) in total number of GnRH cells: OVX+E (11.2 ± 1.8 cells/section) vs. OVX (10.6 ± 1.6 cells/section). Moreover, prepubertal OVX ewes showed rates of KOR/GnRH colocalisation that were similar to that previously reported in adult luteal phases ewes (27). In addition to dual-labeled GnRH/KOR neurones, single-labeled GnRH- and KOR-immunoreactive neurones were readily discernible throughout the POA (Fig. 5).
Figure 5.
Representative confocal image of a 1 μm optical section showing a GnRH immunopositive neurone (green) in the preoptic area coexpressing immunofluorescence for KOR (red) in an OVX ewe. (A) GnRH alone, (B) KOR alone and (C) the merged image. White arrows indicate KOR immunoreactivity in cytoplasm surrounding nucleus of GnRH neurone. Scale bar = 20 μm.
Experiment 4: Does E increase synaptic input to dynorphin-containing neurones in prepubertal ewe lambs?
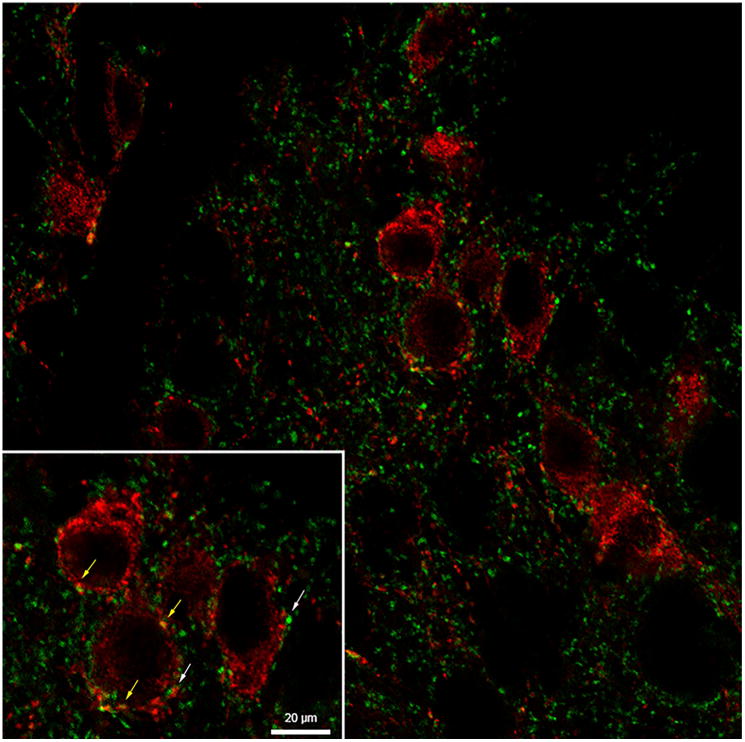
Synaptophysin-immunoreactive contacts onto kisspeptin-immunoreactive neurones were readily discernible (Fig. 6); however, the number of synaptophysin-positive close contacts per kisspeptin-immunoreactive neurone did not differ significantly among treatment groups (Table 1).
Figure 6.
Representative image of kisspeptin-immunopositive neurones (red) with synaptophysin-positive close-contacts (green) within the arcuate nucleus from an ovariectomized prepubertal lamb. Synaptophysin to kisspeptin contacts are denoted by white arrows and kisspeptin to kisspeptin contacts denoted by yellow arrows. Scale bar = 20 μm (inset).
Table 1.
Number (Mean±SEM) of synaptophysin close-contacts onto kisspeptin neurones (Kiss:SYP) and kisspeptin to kisspeptin (Kiss:SYP+Kiss) contacts in the arcuate of prepubertal female lambs
| Close-contacts |
Treatment
|
||
|---|---|---|---|
| Intact (n=4) | OVX (n=5) | OVX+E (n=5) | |
| Kiss:SYP | 25.9 ± 8.1 | 17.9 ± 4.1 | 20.1 ± 3.8 |
| Kiss:SYP+Kiss | 0.9 ± 0.5 | 1.1 ± 0.6 | 1.4 ± 0.6 |
DISCUSSION
The neural mechanisms underlying the increase in GnRH and LH pulse frequency that leads to puberty onset are not clear. This is particularly true for those inputs that may act as a brake on GnRH secretion during the prepubertal period. Herein we show that pharmacological blockade of the KOR in lambs of a prepubertal age increased pulsatile LH secretion, but was without effect in those same lambs at a postpubertal age. However, E did not increase immunopositive cell numbers in the ARC, nor were there large changes in synaptic input to KNDy neurones or KOR expression in GnRH neurones. Thus, while the present findings are consistent with the hypothesis that dynorphin inhibits pulsatile LH secretion prepubertally, the neural substrates responsible for this inhibition are not entirely clear.
We found in the current study that there was an increase in kisspeptin-immunoreactive cell numbers following OVX that could be blocked by E treatment, and that these changes were positively associated with changes in mean LH and LH pulse frequency. A very high percentage of KNDy neurones express ER-α, making them potential targets for direct effects of E on KNDy peptide expression. Indeed, treatment with E reduces both kisspeptin and NKB mRNA expression in rats, primates and adult ewes (31–34) within the ARC. However, prior to our work, no studies have been performed that specifically evaluate the influence of E on kisspeptin expression in prepubertal ewes. Our previous work used ovary-intact ewes and showed that increases in kisspeptin-immunoreactive cell numbers occurred in prepubertal, but not postpubertal, female sheep following OVX (8). Another study noted a change in the number of neurones expressing kisspeptin mRNA in the middle, but not rostral or caudal, ARC during pubertal development and that the number of cells expressing kisspeptin mRNA were positively correlated with LH pulse frequency (35). That study used OVX+E lambs and did not include OVX controls or ovary-intact animals, thus a specific role for E cannot be specifically ascertained. Our findings are consistent with the aforementioned work and suggest that changes in immunopositive kisspeptin cell numbers during puberty are primarily due to changes in effectiveness of E-negative feedback.
In contrast to our finding of changes in kisspeptin cell numbers, we saw very few dynorphin immunopositive cells in any treatment group. We do not believe this to be an issue with the antibody for several reasons. First, we observed abundant dynorphin-positive cells in ARC tissue from luteal phase ewes run concurrently with the prepubertal tissue. Also, we noted large numbers of dynorphin-positive neurones in the paraventricular nucleus from these same animals. Finally, we tried both increasingly concentrated dilutions of antibody as well as antigen retrieval on these tissues and still failed to detect very many dynorphin-positive neurons within the ARC. The role of dynorphin in the KNDy triad of neuropeptides has been suggested to be inhibitory (36) and our previous work showed that dynorphin was important in mediating progesterone negative feedback during the luteal phase of the oestrous cycle (17,20,24). Thus, our findings were contrary to our hypothesis that E would increase dynorphin immunopositive cell numbers compared to those of OVX counterparts. Although reports on the interactions of E specifically with dynorphin or its receptor are relatively few, endogenous opioid peptides do not appear to mediate E negative feedback in adult ewes (37). However, to our knowledge, this is the first study to examine specifically the effect of E on ARC dynorphin immunopositive cell numbers during the prepubertal period. Previous work in ovary-intact rats reported an increase in dynorphin-immunoreactive perikarya that began at postnatal day 20 and progressively increased until puberty (38), a change that is not consistent with an inhibitory action of dynorphin during this period. Due to the lack of dynorphin positive cells in the ARC, it may be useful in the future to examine dynorphin mRNA expression by in situ hybridization. However, mRNA levels (or changes therein) do not always reflect transcription and this approach shares some of the same limitations that immunocytochemistry possesses, i.e. mRNA levels are a balance between synthesis and degradation. There are several possible explanations for the lack of dynorphin immunoreactivity in the ARC at this stage of pubertal development. It may be that progesterone is necessary for dynorphin synthesis and involvement in regulating GnRH and LH secretion and thus dynorphin simply is not involved in regulating pulsatile GnRH, and thus LH, secretion during the E-dominated prepubertal period. This would imply a dissociation of the developmental trajectory of kisspeptin, NKB, and dynorphin in KNDy neurones as both kisspeptin and NKB are expressed and easily detectable prior to puberty onset. In this regard, examination of the abundance of these peptides at varying time points during development would be useful to address this potential explanation. This idea would be consistent with previous work reporting no significant effect of E on dynorphin cell numbers or KOR mRNA expression in rats (39), and that nor-BNI did not alter infusion on pulsatile LH release in young adult or prepubertal female rats (40,41). Alternatively, it may be that dynorphin is being synthesised, but quickly transported to its site of action, making dynorphin-positive cells difficult to detect. Finally, the sensitivity to dynorphin input at this stage of development may be elevated and, thus, very little is needed to inhibit GnRH/LH release, making cells difficult to detect. To address the latter two possibilities, a second experiment was performed which tested the hypothesis that blockade of KOR would stimulate pulsatile LH release in prepubertal OVX+E females.
Intracerebroventricular infusion of nor-BNI consistently and significantly increased pulsatile LH secretion in OVX+E females of a prepubertal age, but not in these same animals at a postpubertal age. Thus, while we failed to see any stimulatory effect of E on dynorphin-positive cell numbers in the ARC, POA, or PVN, our data derived from pharmacologically inhibiting KOR is consistent with an inhibitory action of dynorphin on pulsatile GnRH and LH secretion during the prepubertal period. This is in contrast to the abovementioned study of Grachev et al. (40), but in agreement with Nakahara et al., who reported that subcutaneous infusion of nor-BNI accelerated puberty onset by 4 days in female rats and significantly increased LH pulse frequency (19). Our findings are also consistent with an early report that administration of dynorphin anti-serum into the ARC increased LH secretion in prepubertal female rats (18). It should be noted that given the design of our study, we cannot definitively ascribe responsibility for the inhibition by dynorphin on pulsatile LH release solely to the effect of E as we did not examine the possibility that nor-BNI might elevate LH secretion in a steroid-independent manner in OVX females. It is possible that dynorphin “tone” on the system exists in the absence of E. In that regard, reports on the effect of nor-BNI in OVX females are mixed. Our previous work demonstrated that placing microimplants of nor-BNI within the arcuate nucleus of adult, OVX ewes resulted in an acceleration of pulse frequency (42). However, direct comparison of this work and the current work is problematic due to the differing animal models used (OVX vs. OVX+E) and the differing drug delivery approaches employed (intra-arcuate implants vs. icv infusion). One would presume that a microimplantation approach would deliver much higher local concentrations of the drug. Previous work had shown a change in GnRH pulse shape, but not frequency in adult OVX or OVX+E ewes in response to a less specific inhibitor, naloxone (37). To our knowledge, icv infusion of nor-BNI in adult OVX+E ewes has not been done. Wakabayashi et al. reported an increase in multi-unit activity in OVX goats within the area of the ARC during lateral cerebroventricle infusion of nor-BNI (23). In contrast, Mostari et al. (39) reported no effect on LH secretion in young, OVX adult female rats during third cerebroventricle infusion, but did report an increase in LH secretion in OVX+E females that was due to an increase in baseline LH secretion and not LH pulse frequency or amplitude. Interestingly, we did not see an effect of nor-BNI in the OVX+E females at what would be considered a postpubertal age, suggesting that the inhibition of GnRH secretion by endogenous dynorphin is lost as ewe lambs progress through puberty. This could possibly be explained by changes in KOR number. In one report in sheep, intravenous administration of naloxone, a relatively nonspecific opioid receptor antagonist, increased LH secretion in ovary-intact and OVX+E prepubertal female lambs, as well as postpubertal ewes (43), which is not in accord with the present results. Nor-BNI is highly selective for KOR (44), supporting the idea that DYN-KOR signalling could be the major inhibitory pathway in prepubertal animals. A role for μ-opioid receptors should not be ruled out, however.
While our data are consistent with the notion that dynorphin plays a role in the inhibition of GnRH, and thus LH, secretion during pubertal development, the source or locus of this potential inhibition remains unclear. Dynorphin neurones are located in several areas of the brain, including several nuclear areas of the hypothalamus and POA. We found an abundance of dynorphin-immunoreactive neurones within the PVN, but numbers of cells were not changed by E treatment. Dynorphin of ARC origin has been suggested to be important for GnRH pulse generation (36,45). Intra-ARC administration of a KOR agonist to young, adult OVX+E female rats decreased LH pulse frequency, but a KOR antagonist had no effect, although it blocked the inhibitory effect of senktide, a NK3R agonist, on LH secretion (39). In contrast, microimplantation of nor-BNI within the ARC in OVX ewes decreased LH inter-pulse interval (42) suggesting that perhaps important species differences exist. Thus, dynorphin could be acting in a paracrine or autocrine fashion to inhibit stimulatory output to GnRH neurones/axons by KNDy neurones. Interestingly, dynorphin has recently been shown to reduce spontaneous KNDy neurone electrical activity in tissue slices from mice, but again a KOR antagonist had no effect (46). However, only about 20% of ARC kisspeptin neurones in female (45), and only 6% in male (47), mice coexpress mRNA for KOR, raising the possibility that dynorphin may also impact GnRH/LH release via other means. Previous work reported a lack of KOR expression in GnRH neurones (48). However, recent data has suggested that approximately 75% and 95% of ovine GnRH neurones in the POA or medial basal hypothalamus, respectively, coexpress KOR, raising the possibility that dynorphin may directly modulate GnRH release (27). Herein we examined whether GnRH neurones of prepubertal ewe lambs coexpressed KOR and tested the hypothesis that the percentage of GnRH neurons expressing KOR would be increased by E. We did find that GnRH neurons in prepubertal ewes expressed KOR. However, in contrast to our hypothesis, we found that approximately 78% of GnRH neurones coexpress KOR of OVX prepubertal ewes and that E treatment significantly reduced the rate of colocalisation by approximately 10%. While this was a statistically significant reduction in expression, the difference is relatively small and it is difficult to see how such an E-induced decrease in KOR expression in GnRH neurons would play a significant role in dynorphin mediation of E-negative feedback in prepubertal lambs.
We found no significant difference in numbers of inputs amongst any of the treatment groups, suggesting that large changes in synaptic input do not occur in response to E during the prepubertal period in female sheep. This seems in contrast to work in rats where numbers of axosomatic synapses in randomly chosen ARC neurones have been reported to decrease prior to the gonadotropin surge, a change that is dependent upon the preovulatory increase in E (49,50). However, KNDy neurones were not specifically examined in those studies. A significant increase in synaptic input to KNDy neurones has been noted during the LH surge in comparison to the luteal phase in sheep (15), but the influence of E alone was not investigated. It should be noted that while our data suggest that a significant reorganization of synaptic input does not occur in response to E, a shift in the type of input (i.e. stimulatory vs. inhibitory) cannot be ruled out. NKB neurones express NK3R, suggesting the potential for autocrine or paracrine interactions within the ARC KNDy neurones (11,51). However, a reorganization of KNDy to KNDy innervation is unlikely because we found that neither the percentage of cells exhibiting kisspeptin-positive contacts on kisspeptin-positive cells nor the number of such contacts per cell changed with treatment.
In summary, we found that pulsatile LH release and ARC kisspeptin-immunoreactive cell numbers were negatively regulated by E. In contrast to the hypothesized E-induced increase in dynorphin-immunoreactive cell numbers, we found very few dynorphin-positive cells in the ARC of prepubertal female sheep that were either OVX, OVX+E, or ovary-intact. Nonetheless, our finding that blockade of KOR elevated LH secretion in OVX+E lambs that were of a prepubertal, but not postpubertal, age is consistent with a role for dynorphin in the prepubertal suppression of pulsatile LH release. However, because we administered nor-BNI via lateral ventricle infusion, one limitation of this work is that we cannot tell which population(s) of dynorphin neurones are involved in this apparent suppression. Thus, the possibility cannot be ruled out that dynorphin from KNDy neurones may not be involved, perhaps due to a different developmental trajectory than kisspeptin and NKB. Finally, we did not find significant changes in synaptic input to KNDy neurones in response to E during the prepubertal period or E-induced increases in the percentage of GnRH neurons expressing KOR. While our data are suggestive of a role for dynorphin in inhibiting pulsatile LH release prior to puberty, additional work will be necessary to determine the source of this apparent inhibition as well as the mechanisms involved.
Acknowledgments
We thank Dr. Miro Valent and Gail Nesselrod-Sager for their technical help, and Dr. Margaret Minch for her assistance with animal care. Imaging experiments and image analysis were performed in the West Virginia University Microscope Imaging Facility, which has been supported by the Mary Babb Randolph Cancer Center and National Institutes of Health (NIH) Grants P20 RR016440 and P30 RR032138. This work supported by USDA 2013-00896 (SMH) and by NIH Grant P20GM103434 to the West Virginia IDeA Network for Biomedical Research Excellence.
Footnotes
Disclosure Statement: The authors have nothing to disclose
References
- 1.Foster DL, Hileman SM. Chapter 31 - Puberty in the Sheep. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s Physiology of Reproduction (Fourth Edition) 4. San Diego: Academic Press; 2015. pp. 1441–1485. [Google Scholar]
- 2.Lehman MN, Ebling FJP, Moenter SM, Karsch FJ. Distribution of estrogen receptor-immunoreactive cells in the sheep brain. Endocrinology. 1993;133(2):876–886. doi: 10.1210/endo.133.2.8344223. [DOI] [PubMed] [Google Scholar]
- 3.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt Sa, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF, Aparicio SaJR, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 4.Topaloglu a K, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.d’Anglemont de Tassigny X, Colledge WH. The role of kisspeptin signaling in reproduction. Physiology (Bethesda) 2010;25(4):207–17. doi: 10.1152/physiol.00009.2010. [DOI] [PubMed] [Google Scholar]
- 7.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin Na, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nestor CC, Briscoe AMS, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology. 2012;153(6):2756–2765. doi: 10.1210/en.2011-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandoval-Guzmán T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026(2):307–312. doi: 10.1016/j.brainres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001–1012. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- 11.Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: Colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2010;22(1):1–12. doi: 10.1111/j.1365-2826.2009.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- 13.García-Galiano D, Van Ingen Schenau D, Leon S, Krajnc-Franken MAM, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, Van Noort PI, Pinilla L, Blomenröhr M, Tena-Sempere M. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153(1):316–328. doi: 10.1210/en.2011-1260. [DOI] [PubMed] [Google Scholar]
- 14.Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151(8):3836–3846. doi: 10.1210/en.2010-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for Changes in Numbers of Synaptic Inputs onto KNDy and GnRH Neurones during the Preovulatory LH Surge in the Ewe. J Neuroendocrinol. 2015;27(7):624–35. doi: 10.1111/jne.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo RV. Kappa-opioid receptor involvement in the regulation of pulsatile luteinizing hormone release during early pregnancy in the rat. J Neuroendocrinol. 1990;2(5):685–691. doi: 10.1111/j.1365-2826.1990.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 17.Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin A concentrations in cerebrospinal fluid, and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835–1842. doi: 10.1210/en.2004-1326. [DOI] [PubMed] [Google Scholar]
- 18.Schulz R, Wilhelm a, Pirke KM, Gramsch C, Herz a. Beta-endorphin and dynorphin control serum luteinizing hormone level in immature female rats. Nature. 1981;294(5843):757–759. doi: 10.1038/294757a0. [DOI] [PubMed] [Google Scholar]
- 19.Nakahara T, Uenoyama Y, Iwase A, Oishi S, Nakamura S, Minabe S, Watanabe Y, Deura C, Noguchi T, Fujii N, Kikkawa F, Maeda KI, Tsukamura H. Chronic Peripheral Administration of Kappa-Opioid Receptor Antagonist Advances Puberty Onset Associated with Acceleration of Pulsatile Luteinizing Hormone Secretion in Female Rats. J Reprod Dev. 2013;59(5):479–484. doi: 10.1262/jrd.2013-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143(11):4366–4374. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- 21.Goodman RL, Lehman MN, Smith JT, Coolen LM, De Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 22.Foradori CD, Goodman RL, Lehman MN. Distribution of preprodynorphin mRNA and dynorphin-A immunoreactivity in the sheep preoptic area and hypothalamus. Neuroscience. 2005;130(2):409–418. doi: 10.1016/j.neuroscience.2004.08.051. [DOI] [PubMed] [Google Scholar]
- 23.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K-I, Steiner Ra, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- 25.Foradori CD, Amstalden M, Coolen LM, Singh SR, McManus CJ, Handa RJ, Goodman RL, Lehman MN. Orphanin FQ: Evidence for a role in the control of the reproductive neuroendocrine system. Endocrinology. 2007;148(10):4993–5001. doi: 10.1210/en.2007-0011. [DOI] [PubMed] [Google Scholar]
- 26.Grachev P, Li XF, Hu MH, Li SY, Millar RP, Lightman SL, O’Byrne KT. Neurokinin B signaling in the female rat: A novel link between stress and reproduction. Endocrinology. 2014;155(7):2589–2601. doi: 10.1210/en.2013-2038. [DOI] [PubMed] [Google Scholar]
- 27.Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. Kappa Opioid Receptor is Co-localized in GnRH and KNDy Cells in the Female Ovine and Rat Brain. Endocrinology. 2016;(May):en20151763. doi: 10.1210/en.2015-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: A novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whisnant CS, Goodman RL. Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biol Reprod. 1988;39(5):1032–1038. doi: 10.1095/biolreprod39.5.1032. [DOI] [PubMed] [Google Scholar]
- 30.Goodman RL, Karsch FJ. Pulsatile secretion of luteinizing hormone: Differential suppression by ovarian steroids. Endocrinology. 1980;107(5):1286–1290. doi: 10.1210/endo-107-5-1286. [DOI] [PubMed] [Google Scholar]
- 31.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300(1):E202–E210. doi: 10.1152/ajpendo.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pillon D, Caraty A, Fabre-Nys C, Bruneau G. Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J Neuroendocrinol. 2003;15(8):749–753. doi: 10.1046/j.1365-2826.2003.01054.x. [DOI] [PubMed] [Google Scholar]
- 33.Abel TW, Lou Voytko M, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84(6):2111–2118. doi: 10.1210/jcem.84.6.5689. [DOI] [PubMed] [Google Scholar]
- 34.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner Ra. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 35.Redmond JS, Baez-Sandoval GM, Spell KM, Spencer TE, Lents Ca, Williams GL, Amstalden M. Developmental Changes in Hypothalamic Kiss1 Expression during Activation of the Pulsatile Release of Luteinising Hormone in Maturing Ewe Lambs. J Neuroendocrinol. 2011;23(9):815–822. doi: 10.1111/j.1365-2826.2011.02177.x. [DOI] [PubMed] [Google Scholar]
- 36.Lehman MN, Coolen LM, Goodman RL. Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodman RL, Parfitt DB, Evans NP, Dahl GE, Karsch FJ. Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology. 1995;136(6):2412–2420. doi: 10.1210/endo.136.6.7750462. [DOI] [PubMed] [Google Scholar]
- 38.Ciofi P, Lapirot OC, Tramu G. An androgen-dependent sexual dimorphism visible at puberty in the rat hypothalamus. Neuroscience. 2007;146(2):630–642. doi: 10.1016/j.neuroscience.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 39.Mostari P, Ieda N, Deura C, Minabe S, Yamada S, Uenoyama Y, Maeda K, Tsukamura H. dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev. 2013;59(3):266–72. doi: 10.1262/jrd.2012-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grachev P, Li XF, Kinsey-Jones JS, Di Domenico AL, Millar RP, Lightman SL, O’Byrne K. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153(10):4894–4904. doi: 10.1210/en.2012-1574. [DOI] [PubMed] [Google Scholar]
- 41.Grachev P, Li XF, Lin YS, Hu MH, Elsamani L, Paterson SJ, Millar RP, Lightman SL, O’Byrne KT. GPR54-Dependent Stimulation of Luteinizing Hormone Secretion by Neurokinin B in Prepubertal Rats. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0044344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–4269. doi: 10.1210/en.2013-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schall RE, Ebling FJ, Karsch FJ, Foster DL. Postpubertal maturation of endogenous opioid regulation of luteinizing hormone secretion in the female sheep. Biol Reprod. 1991;44(5):760–768. doi: 10.1095/biolreprod44.5.760. [DOI] [PubMed] [Google Scholar]
- 44.Portoghese PS, Lipkowski AW, Takemori AE. Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists. Life Sci. 1987;40(13):1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- 45.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin b, and dynorphin by modulators of neurokinin 3 and k-opioid receptors in adult male mice. Endocrinology. 2013;154(8):2761–2771. doi: 10.1210/en.2013-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152(11):4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sannella MI, Petersen SL. Dual label in situ hybridization studies provide evidence that luteinizing hormone-releasing hormone neurons do not synthesize messenger ribonucleic acid for mu, kappa, or delta opiate receptors. Endocrinology. 1997;138(4):1667–1672. doi: 10.1210/endo.138.4.5091. [DOI] [PubMed] [Google Scholar]
- 49.Olmos G, Naftolin F, Perez J, Tranque PA, Garcia-Segura LM. Synaptic remodeling in the rat arcuate nucleus during the estrous cycle. Neuroscience. 1989;32(3):663–667. doi: 10.1016/0306-4522(89)90288-1. [DOI] [PubMed] [Google Scholar]
- 50.Naftolin F, Mor G, Horvath TL, Luquin S, Fajer AB, Kohen F, Garcia-Segura LM. Synaptic remodeling in the arcuate nucleus during the estrous cycle is induced by estrogen and precedes the preovulatory gonadotropin surge. Endocrinology. 1996;137(12):5576–5580. doi: 10.1210/endo.137.12.8940386. [DOI] [PubMed] [Google Scholar]
- 51.Burke MC, Letts PA, Krajewski SJ, Range NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]