Abstract
Purpose
Neutrophil-derived extracellular debris has been shown to accelerate bacterial biofilm formation on hydrogel and silicone hydrogel contact lens surfaces compared to lenses inoculated with bacteria alone. The purpose of this study was to evaluate the disinfection efficacy of four standard commercial contact lens cleaning regimens against neutrophil-enhanced bacterial biofilms formed on silicone hydrogel contact lenses.
Methods
Four reference strains were used: Pseudomonas aeruginosa, Serratia marcescens, Stenotrophomonas maltophilia, and Staphylococcus aureus. Human neutrophils were isolated from peripheral blood by venipuncture. Unworn Lotrafilcon B lenses were incubated overnight in each respective strain with stimulated neutrophils. Contact lenses were then cleaned using one of four contact lens care solutions according to manufacturer instructions. Bacterial viability was assessed by colony counts and confocal microscopy. Volume of residual debris on lens surfaces after cleaning was quantified using IMARIS software.
Results
All four solutions tested showed effective antimicrobial activity against each bacterial strain; however, substantial amounts of nonviable bacteria and cellular debris remained on the lens surface despite concomitant digital cleaning.
Conclusions
Necrotic cellular debris that accumulates under the posterior lens surface during wear of an inoculated contact lens is not fully removed during routine cleaning and disinfection.
Translational Relevance
The accumulation of residual cellular debris on the contact lens surface may contribute to new colonization of the lens and represents a significant risk factor for a contact lens–related adverse event. Additional studies are needed to correlate these findings with risk for corneal infiltrative and/or infectious events in a standard animal model.
Keywords: contact lens; Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Stenotrophomonas maltophilia, Serratia marcescens
Introduction
The annualized incidence of contact lens–related microbial keratitis has remained unchanged for more than three decades.1–4 Despite enhanced lens materials and care solutions, lens–related corneal infections continue to appear in otherwise healthy adults who would not normally be susceptible to infection. While the widespread implementation of silicone hydrogel contact lenses into the worldwide market has resulted in an improvement in corneal physiology, the incidence of contact lens–related adverse events has not dropped. Instead, well-controlled studies have clearly demonstrated an increased risk for corneal infiltrative events (CIEs) in response to silicone hydrogel lens wear.5–9 One of the primary risk factors identified for having a CIE during lens wear is the presence of substantial microbial bioburden on the lens surface.8
Factors relating to silicone hydrogel lens use and increased risk for CIEs include the potential for increased microbial adhesion to silicone hydrogel contact lens surfaces and issues pertaining to lens care solution biocompatibility.10–17 For a contact lens care solution to be effective and promote safe lens wear, two main criteria must be met. First, use of the care solution cannot result in any corneal surface epithelial damage. Uptake and release of biocides by the lens during wear has been shown to be associated with corneal staining.18–21 In our in vivo rabbit model, uptake and release of a biguanide-based solution was also associated with junctional disruption in surface epithelial cells with enhanced bacterial internalization into the cornea.22,23 Second, the solution must demonstrate effective disinfection activity against pathogens present on the contact lens and in the contact lens storage case.24–29
We have recently shown that Pseudomonas aeruginosa, along with other strains of bacteria, can accelerate colonization of contact lens surfaces in the presence of dying neutrophils in vitro (manuscript under review).30,31 Analysis of the microbial burden on the lens showed both an increase in viable bacteria and thickness of the newly formed biofilm. Importantly, direct targeting of neutrophil–bacterial interactions during contact lens wear in vivo has been shown to reduce bacterial internalization into the cornea.30
It has been previously reported that biofilms formed on contact lens surfaces demonstrate increased resistance to common biocides in contact lens care solutions.29 This study sought to determine the efficacy of both chemically preserved and peroxide-based contact lens care solutions in removing neutrophil-enhanced bacterial bioburden from contact lens surfaces during standard cleaning regimens. Importantly, we show that current commercial contact lens care solutions fail to fully remove cellular debris from contact lens surfaces using recommended rub and rinse cleaning practices. The residual debris may represent a new risk factor for microbial recolonization of contact lenses.
Methods
Bacterial Strains
Four reference strains were used in this study: P. aeruginosa (ATCC 9027), Staphylococcus aureus (ATCC 6538), Stenotrophomonas maltophilia (ATCC 13637), and Serratia marcescens (ATCC 13880). All strains were purchased from the American Type Culture Collection (ATCC; Manassas, VA). Bacterial stocks were stored at −80°C. For experiments, bacteria were grown on tryptic soy agar (TSA; Sigma-Aldrich Corp., St. Louis, MO) at 37°C overnight. From that plate, a single clone was isolated and grown at 37°C overnight on a TSA slant. Bacteria were then suspended in Roswell Park Memorial Institute Media (RPMI; Sigma-Aldrich Corp.) to a concentration of ∼108 CFU/mL using a spectrophotometer (absorbance was 0.3 at 650 nm) and diluted to ∼106. Inoculums were confirmed by standard colony counts.
Ethics Statement
Human subject research was performed according to the tenets of the Declaration of Helsinki. All procedures were approved by the Institutional Review Board at University of Texas Southwestern Medical Center, and each subject signed an informed consent before participation in this study.
Neutrophil Isolation
Whole blood was collected from the peripheral arm vein of healthy human volunteers as we have previously reported.30 Following collection into three 4.5-mL vacutainers containing 3.2% citrate (Becton, Dickinson and Company, Franklin Lakes, NJ), samples were combined into a single 50-mL conical tube. Peripheral blood neutrophils were then isolated using Plasma-Percoll gradient separation.32 All procedures were performed at room temperature to prevent nonspecific activation of neutrophils. Samples were then centrifuged at 300 g for 20 minutes, followed by removal of the platelet-rich plasma. The platelet-rich plasma then underwent an additional centrifugation step for 15 minutes at 2500 g. The remaining supernatant constituted the platelet-poor plasma (PPP). With the remaining sample left after the collection of the platelet-rich plasma from the initial centrifugation step, 5 mL of 6% dextran and 0.9% saline were added and then mixed by gentle inversion. After a 30-minute incubation at room temperature, the leukocyte-rich layer was removed and subject to an additional centrifugation step for 6 minutes at 275 g. The resultant pellet was then resuspended in the PPP. Platelet-poor plasma was used to make solutions containing 42% and 51% Percoll (Sigma-Aldrich Corp.). These were added, and the sample was centrifuged for 10 minutes at 275 g. Neutrophils were carefully collected from the interface between the two Percoll layers, washed with PPP, and pelleted by centrifugation at 275 g. Neutrophils were resuspended in RPMI containing 2% heat-inactivated PPP (HIPPP) and the concentration determined using a hemacytometer. To generate the 2% HIPPP, PPP was incubated for 30 minutes in a water bath at 56°C. The heated 2% HIPPP was then centrifuged to remove any particulates. Stimulated neutrophils were obtained by adding 60 ng/mL (25 nM) of phorbol 12-myristate 13-acetate (Sigma-Aldrich Corp.), a neutrophil-activating agonist, for 1 minute. Activated neutrophils were then washed in RPMI containing 2% HIPPP and resuspended to a final concentration of 16.6 × 106 cells/mL. Neutrophils were allowed to incubate for 2 hours at 37°C prior to the inoculation.
Contact Lens Incubation
Unworn soft contact lenses (Lotrafilcon B; Alcon Laboratories, Ft. Worth, TX) with a base curve of 8.6, a diameter of 14.2, and a power of −0.50 were used in this study. In a sterile hood, lenses were removed from their blister pack, and whole lenses were placed into single wells of a plastic 24-well plate. Bacteria and neutrophils were added at a 1:1 ratio in a total of 1 mL of RPMI containing 2% HIPPP. Contact lenses were then incubated overnight for approximately 18 hours at 37°C. Control lenses were incubated with neutrophils alone as an additional control to ensure no cross-contamination.
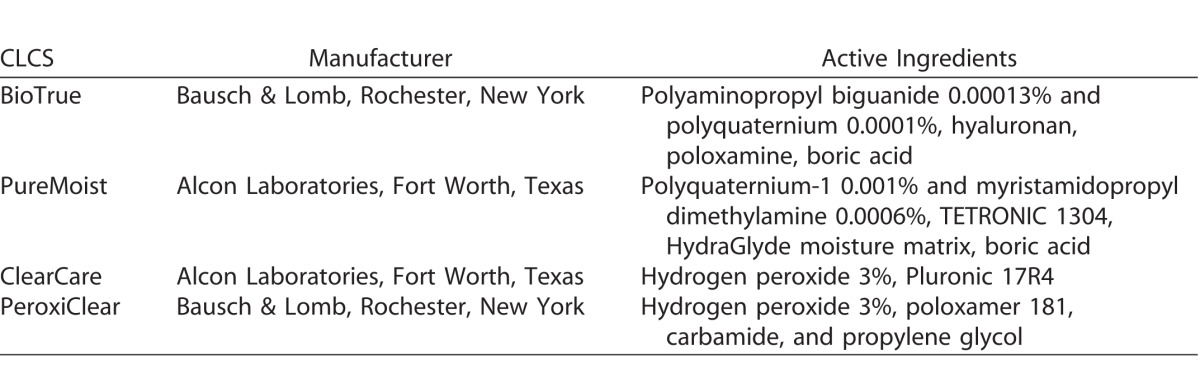
Contact Lens Care Solutions
Efficacy of four commercially available contact lens care solutions was tested. Following an overnight incubation (approximately 18 hours) at 37°C, contact lenses were rinsed in phosphate-buffered saline (PBS) to remove any nonadherent bacteria and then disinfected with either one of two chemically preserved multipurpose contact lens care solutions (MPS) or one of two hydrogen peroxide–based (HPB) lens care systems. Although both HPB solutions have 3% hydrogen peroxide as the active agent, we chose to test two different HPB care solutions due to differences in the chemical composition such as surfactants and wetting agents. The active ingredients for each care solution are detailed in the Table. In addition, PeroxiClear is reported to have a delayed-release mechanism that initially inhibits neutralization to allow for better disinfection, with complete neutralization in 4 hours. Clear Care requires 6 hours for neutralization. Cleaning, including digital rubbing, was performed according to specific manufacturer guidelines. Lenses were placed in sterile manufacturer-provided lens cases and allowed to sit at room temperature for an additional 4 or 6 hours as indicated in the manufacturer disinfection guidelines. Sterile, individually packaged nitrile surgical gloves, were used when the disinfectant procedure called for rubbing. Control lenses were rinsed with PBS and then placed into PBS for 6 hours. After respective disinfection times had elapsed, contact lenses were removed from disinfecting solutions and neutralized for 15 minutes using 1 mL of Dey-Engley broth (Sigma-Aldrich Corp.). The lenses were then stained using the Bacterial Viability Kit (Life Technologies, Grand Island, NY) or processed for colony-forming unit determination. Each strain/solution combination was tested three independent times.
Table.
Contact Lens Care Solutions (CLCS)
Laser Scanning Confocal Microscopy
After cleaning, contact lenses were stained using a Live/Dead BacLight Bacterial Viability Kit (Life Technologies). Staining was achieved using a 3-μL mixture containing 1.5 μL SYTO 9 and 1.5 μL propidium iodide (PI) at room temperature for 15 minutes. Contact lenses were then washed in PBS and transferred onto separate 35-mm-diameter glass-bottom culture dishes (MatTek Corp., Ashland, MA) and viewed using a laser scanning confocal microscope (Leica SP8; Leica Microsystems, Heidelberg, Germany). Images were sequentially scanned to minimize any spectral overlap between the emission channels. Images were processed using IMARIS software (Bitplane, South Windsor, CT). To quantify the volume of adherent debris on contact lens surfaces, the surface function in IMARIS was used.
Viable Bacterial Quantification
To determine the number of viable bacteria remaining on the contact lens after cleaning, contact lenses were placed in individual Eppendorf tubes containing PBS. Tubes were then placed in a water sonicator (Branson 2510; VWR, Radnor, PA) at 50–60 Hz and sonicated for 1 minute. Sonication was followed by immediately vortexing on high for 2 minutes to eliminate any residual bacterial clumping. Prior testing in our laboratory has shown that the water sonication/vortexing combination results in the greatest number of viable bacteria recovered from inoculated contact lenses. The resultant solution was serially diluted with PBS and plated on TSA plates in triplicate for each dilution. The plates were then incubated at 37°C overnight, and colony counts were obtained for each bacterial/solution combination. Each experiment was performed three independent times.
Statistical Analysis
Statistical analysis was performed using Sigma Plot 11.0 (Systat Software, Inc., San Jose, CA). All data are expressed as mean ± standard deviation. To assess differences in the debris volume between care solutions, a 1-way ANOVA was used. To assess differences between bacterial/solution combinations on kill rate, a 2-way ANOVA was used. Results were then tested using appropriate post hoc comparison tests. Significance was set at P < 0.05.
Results
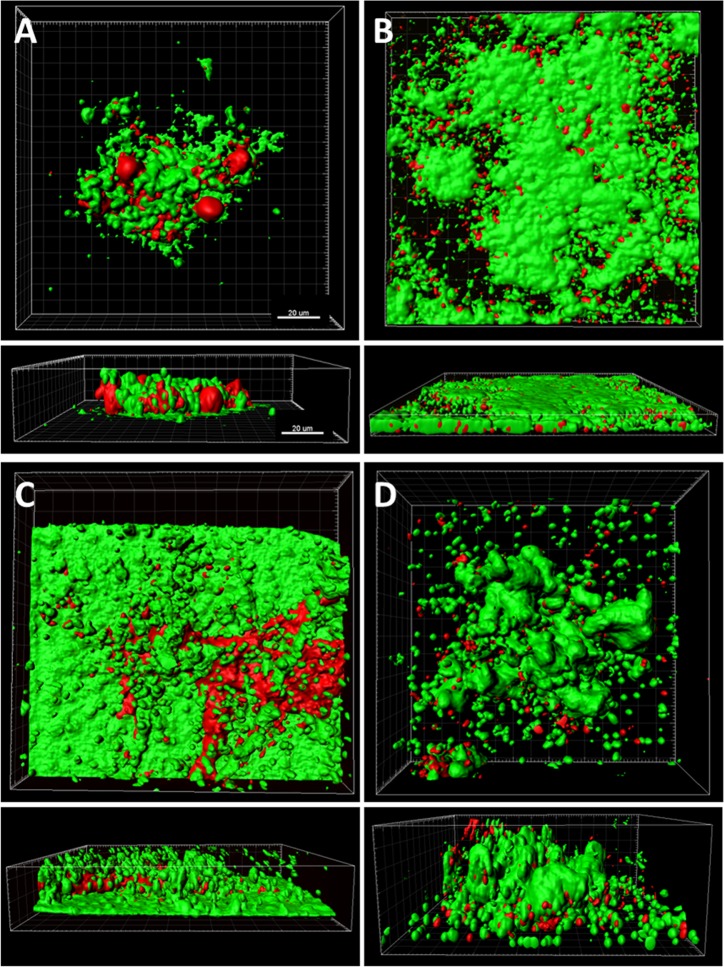
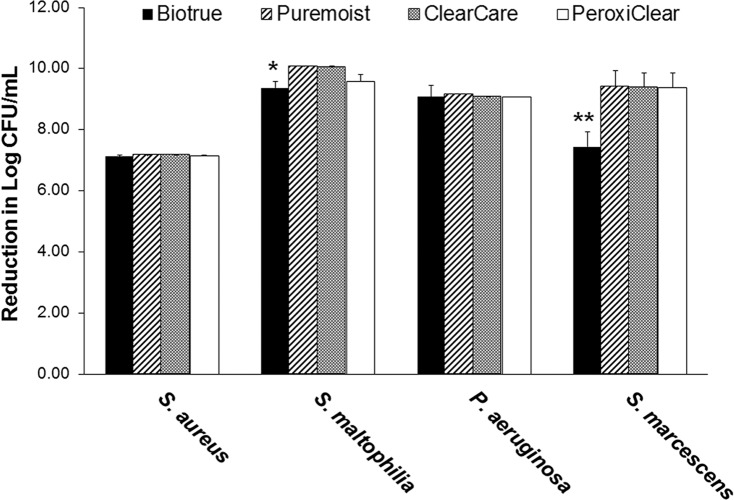
Following the initial overnight incubation with each test strain and neutrophils, lenses were treated with one of four commercially available lens care products or rinsed and incubated in PBS alone. Three-dimensional confocal analysis of colonized contact lenses following cleaning with PBS alone is shown in Figure 1. All four strains demonstrated the formation of dense biofilms on the lens surface. In addition to a large proportion of viable bacteria, both nonviable bacteria and extracellular debris were readily visible. For the PBS control groups, mean colony growth counts for each bacterial strain cultured with neutrophils were 1.5 × 107 ± 7.2 × 105 CFU/mL for S. aureus, 1.2 × 1010 ± 1.7 × 109 CFU/mL for S. maltophilia, 7.3 × 109 ± 7.5 × 108 CFU/mL for P. aeruginosa, and 2.6 × 109 ± 2.1 × 108 CFU/mL for S. marcescens. After treatment, S. aureus exhibited a 7-log reduction over PBS with all contact lens care solutions; this did not differ among the products tested (Figure 2, P = 0.609). Similarly, there was a consistent 9-log reduction for P. aeruginosa (Figure 2, P = 1.000). Stenotrophomonas maltophilia also exhibited a 9- to 10-log reduction over PBS, which was slightly lower for BioTrue (Figure 2, P = 0.040). Likewise, there was a 9-log reduction in S. marcescens for all contact lens care solutions, except BioTrue, which still had a 7-log reduction (P < 0.001) in bacteria recovered (Figure 2).
Figure 1.
Live/dead staining of PBS-treated control lenses inoculated with each test strain in the presence of dying neutrophils. Viable bacteria shown in green, nonviable bacteria and extracellular DNA shown in red. Surfaces were applied to both the green and red channels using the surface function of IMARIS. (A) Staphylococcus aureus, (B) S. maltophilia, (C) P. aeruginosa, and (D) S. marcescens. Scale: 20 μm.
Figure 2.
Bactericidal efficacy of contact lens care solutions against S. aureus, S. maltophilia, P. aeruginosa, and S. marcescens. Staphylococcus aureus consistently exhibited a 7-log reduction compared to the PBS control with all solutions tested. S. maltophilia exhibited a 9-log to 10-log reduction for all solutions, which was lowest for Biotrue (*P ≤ 0.040 compared to all other test solutions). P. aeruginosa showed a 9-log reduction, which did not differ among lens care products. Similarly, there was a 9-log reduction in bacterial load for S. marcescens, with the exception of Biotrue (**P < 0.001 compared to all other test solutions). Data expressed as mean ± standard deviation. Graph is representative of three independent experiments.
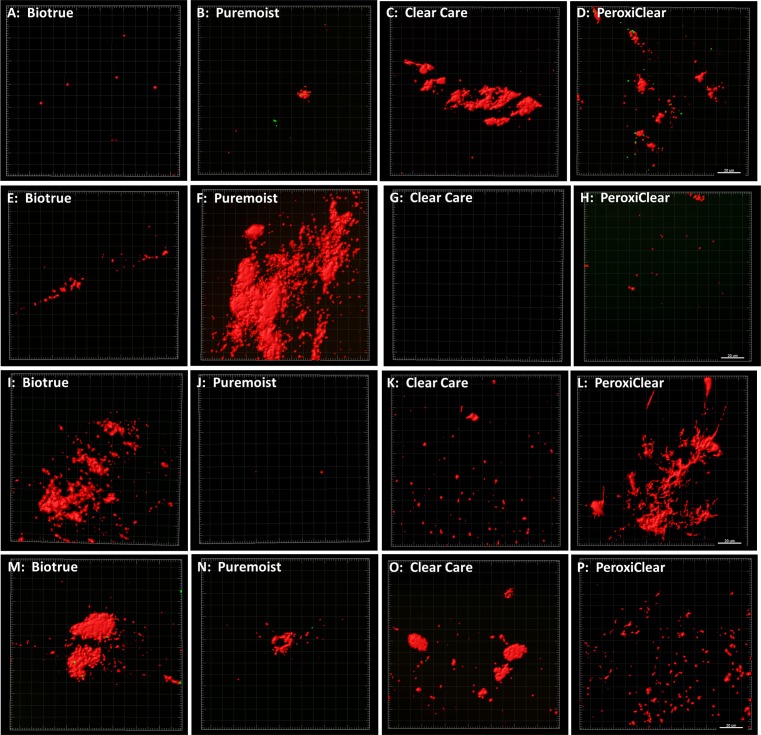
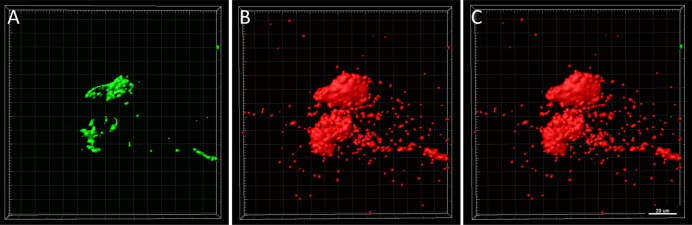
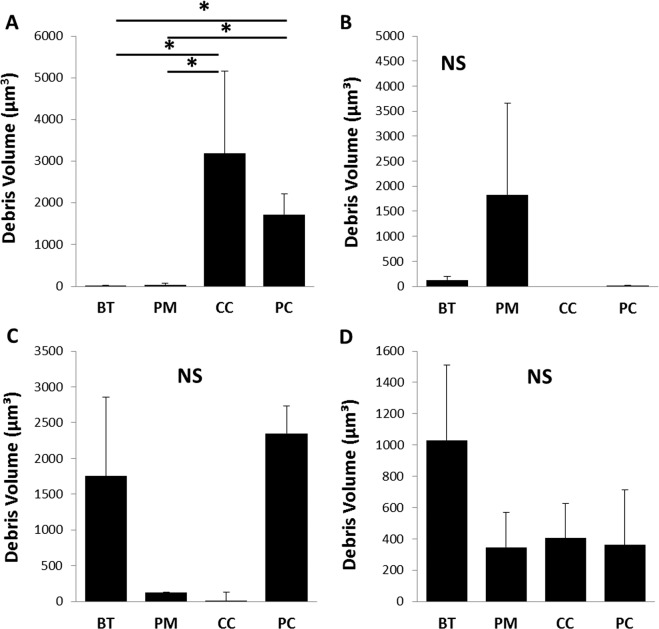
Confocal microscopy paralleled these findings, showing a substantial reduction in viable bacteria following disinfection. Interestingly, however, BacLight staining revealed the presence of substantial nonviable bacteria and extracellular debris on the lens surface after treatment for the majority of bacteria/contact lens care solution combinations tested (Fig. 3). In areas where dense clumps of nonviable bacteria and debris were present, splitting out the individual component channels showed that a small amount of viable bacteria was present buried within the debris scaffold (Fig. 4). Using the surface function in IMARIS, the volume of residual debris was quantified (Fig. 5). Due to the high variability in lens-associated debris, there were no statistical differences in debris volume between care solutions for P. aeruginosa (P = 0.052), S. marcescens (P = 0.455), or S. maltophilia (P = 0.427). A significant difference in debris volume was seen between care solutions for S. aureus (P = 0.036). Highest rates of debris were found on contact lenses cleaned with peroxide solutions.
Figure 3.
Cellular debris present on contact lens surfaces after cleaning with each bacteria/solution combination. Residual bacteria and debris were stained with BacLight. Viable bacteria (green), nonviable bacteria and extracellular DNA (red). (A–D) Lenses inoculated with S. aureus. (E–H) Lenses inoculated with S. maltophilia. (I–L) Lenses inoculated with P. aeruginosa. (MP) Lenses inoculated with S. marcescens. Scale bar: 20 μm.
Figure 4.
Viable bacteria encased within cellular debris scaffold. Representative image showing splitting of the surface function into individual channels showed viable bacteria (green) underneath large, clumpy areas of debris (red). (A) Viable bacteria, (B) nonviable bacteria and extracellular DNA, (C) overlay. Scale bar: 20 μm.
Discussion
We have previously shown that the four bacterial strains used in this study have the capacity to accelerate colonization of contact lens surfaces in the presence of necrotic cellular debris. Building on this data, in the present study we demonstrated good efficacy of commercially available peroxide-based and chemically preserved multipurpose care solutions against neutrophil-enhanced bacterial biofilms formed on silicone hydrogel contact lens surfaces. All four solutions tested met the FDA criteria of a 3-log reduction in microbial bioburden. Prior reports have shown that different contact lens care solutions have reduced efficacy against biofilms formed on contact lens surfaces and in contact lens storage cases.28,29,33 In a susceptibility study by Szczotka-Flynn and colleagues, the authors compared the ability of four chemically preserved multipurpose solutions and one hydrogen peroxide–based product against planktonic bacteria and biofilms formed on Lotrafilcon A lenses.29 While all planktonic bacteria were susceptible to killing by commonly used contact lens care solutions, bacteria present in the biofilm showed increased resistance to microbiocidal activity. The level of resistance varied between the bacterial strain and solution tested.
Several differences in study design may account for the differences in antimicrobial efficacy between studies. In our study, contact lenses were cultured overnight (approximately 18 hours) in RPMI media, whereas Szczotka-Flynn et al. used different culture media.29 A second potential contributor to the difference in study outcomes relates to the use of a digital cleaning step. All of the contact lenses in our study underwent a digital cleaning step to mechanically remove bacteria from the lens surface prior to rinsing and storing as detailed in the manufacturer guidelines. When including the digital cleaning step, all of the contact lens care solutions tested using our neutrophil-enhanced model met the FDA criterion. While some small differences were noted between products, these differences were likely too small to be of clinical relevance.
Despite good disinfection efficacy, substantial cellular debris was still visible on contact lens surfaces after cleaning. This included nonviable bacteria that remained adherent to the lens surface and extracellular DNA, illustrated by the PI staining. Extracellular DNA is a known contributor to biofilm formation and, when present with F-actin, is a key component in the formation of neutrophil-derived extracellular scaffolds.30,34–36 Further, the use of phorbol 12-myristate 13-acetate as a neutrophil-activating agonist is known to drive the release of neutrophil extracellular traps (NETs), which are composed of long strands of extracellular DNA.37–39 While this study did not address the role of NETs in mediating biofilm formation on contact lenses, we hypothesize that both NET release and the accumulation of necrotic cellular debris are likely contributors to the source of debris found on the lens surface. The clinical implication for the continual deposition of bacterial and cellular by-products on the posterior lens surface that are not fully removed during cleaning lies in the potentiation of an endogenous host inflammatory response with acceleration of bacterial recolonization of the lens. Since contact lenses act as a vector for introducing foreign pathogens to the eye and the substrate upon which the debris accumulates, the inability of current care solutions to effectively remove them would argue in favor of daily disposable lenses, which do not require any type of cleaning regimen and eliminate any residual daily buildup.
Residual bacterial and cellular debris with or without viable bacteria encased within the debris scaffold may represent an important risk factor for the development of CIEs and may explain why positive cultures are not always obtained when contact lenses are cultured following the onset of an inflammatory event. The question remains, however, whether the residual cellular debris on the lens surface potentiates the ability of organisms to recolonize the lens, particularly when stored in a contaminated lens case, and whether the ability to recolonize the lens is a risk factor for an infectious event. While further studies are needed to investigate the clinical impact of these findings, they may explain, in part, why the introduction of monthly and bimonthly disposable contact lenses failed to reduce the annualized incidence of infectious keratitis associated with lens wear. These findings also predict that, in the absence of daily disinfection, failure to discard daily disposable soft lenses as prescribed may result in rapid recolonization of the posterior lens surface, predisposing the wearer to infectious keratitis.40 Additional work is needed to correlate these findings with risk for corneal infiltrative and/or infectious events in a standard animal model.
Figure 5.
Debris volume on contact lens surfaces after cleaning. (A) Lenses inoculated with S. aureus, (B) lenses inoculated with S. maltophilia, (C) lenses incubated with P. aeruginosa, and (D) lenses inoculated with S. marsecsens. There were no significant differences in the amount of residual debris on lens surfaces between the different care solutions for contact lenses inoculated with gram-negative bacteria. There was a significant increase in residual debris for peroxide-based solutions following inoculation with S. aureus (*P = 0.036, 1-way ANOVA with Dunnet post hoc multiple comparison test). Data expressed as mean ± standard deviation. Graphs are representative of three individual lenses. BT, Biotrue; PM, Puremoist; CC, Clear Care; PC, PeroxiClear.
Acknowledgments
Supported by National Institutes of Health/National Eye Institute (Bethesda, MD, USA) Grants EY024433 (DMR), EY024546 (DMR), Core Grant for Vision Research EY020799, and by an unrestricted grant from Research to Prevent Blindness (New York, NY, USA).
Disclosure: J.A. Hinojosa, None; N.B. Patel, None; M. Zhu, None; D.M. Robertson, None
References
- 1. Poggio EC,, Glynn RJ,, Schein OD,, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989. ; 321: 779–783. [DOI] [PubMed] [Google Scholar]
- 2. Schein OD,, Glynn RJ,, Poggio EC,, Seddon JM,, Kenyon KR. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. Microbial Keratitis Study Group. N Engl J Med. 1989. ; 321: 773–778. [DOI] [PubMed] [Google Scholar]
- 3. Stapleton F,, Keay L,, Edwards K,, et al. The incidence of contact lens related microbial keratitis in Australia. Ophthalmology. 2008. ; 115: 1655–1662. [DOI] [PubMed] [Google Scholar]
- 4. Cheng KH,, Leung SL,, Hoekman HW,, et al. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999. ; 354: 181–185. [DOI] [PubMed] [Google Scholar]
- 5. Szczotka-Flynn L,, Chalmers R. Incidence and epidemiologic associations of corneal infiltrates with silicone hydrogel contact lenses. Eye Contact Lens. 2013. ; 39: 49–52. [DOI] [PubMed] [Google Scholar]
- 6. Szczotka-Flynn L,, Debanne SM,, Cheruvu VK,, et al. Predictive factors for corneal infiltrates with continuous wear of silicone hydrogel contact lenses. Arch Ophthalmol. 2007. ; 125: 488–492. [DOI] [PubMed] [Google Scholar]
- 7. Szczotka-Flynn L,, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low dk hydrogel extended contact lens wear: a meta-analysis. Optom Vis Sci. 2007. ; 84: 247–256. [DOI] [PubMed] [Google Scholar]
- 8. Szczotka-Flynn L,, Lass JH,, Sethi A,, et al. Risk factors for corneal infiltrative events during continuous wear of silicone hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2010. ; 51: 5421–5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szczotka-Flynn L,, Jiang Y,, Raghupathy S,, et al. Corneal inflammatory events with daily silicone hydrogel lens wear. Optom Vis Sci. 2014. ; 91: 3–12. [DOI] [PubMed] [Google Scholar]
- 10. Willcox MD. Microbial adhesion to silicone hydrogel lenses: a review. Eye Contact Lens. 2013. ; 39: 61–66. [DOI] [PubMed] [Google Scholar]
- 11. Borazjani RN,, Levy B,, Ahearn DG. Relative primary adhesion of Pseudomonas aeruginosa, Serratia marcescens and Staphylococcus aureus to HEMA-type contact lenses and an extended wear silicone hydrogel contact lens of high oxygen permeability. Cont Lens Anterior Eye. 2004. ; 27: 3–8. [DOI] [PubMed] [Google Scholar]
- 12. Bruinsma GM,, van der Mei HC,, Busscher HJ. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials. 2001. ; 22: 3217–3224. [DOI] [PubMed] [Google Scholar]
- 13. Dutta D,, Cole N,, Willcox MD. Factors influencing bacterial adhesion to contact lenses. Mol Vis. 2012. ; 18: 14–21. [PMC free article] [PubMed] [Google Scholar]
- 14. Henriques M,, Sousa C,, Lira M,, et al. Adhesion of Pseudomonas aeruginosa and Staphylococcus epidermidis to silicone-hydrogel contact lenses. Optom Vis Sci. 2005. ; 82: 446–450. [DOI] [PubMed] [Google Scholar]
- 15. Kodjikian L,, Casoli-Bergeron E,, Malet F,, et al. Bacterial adhesion to conventional hydrogel and new silicone-hydrogel contact lens materials. Graefes Arch Clin Exp Ophthalmol. 2008. ; 246: 267–273. [DOI] [PubMed] [Google Scholar]
- 16. Vijay AK,, Zhu H,, Ozkan J,, et al. Bacterial adhesion to unworn and worn silicone hydrogel lenses. Optom Vis Sci. 2012. ; 89: 1095–1106. [DOI] [PubMed] [Google Scholar]
- 17. Carnt NA,, Evans VE,, Naduvilath TJ,, et al. Contact lens-related adverse events and the silicone hydrogel lenses and daily wear care system used. Arch Ophthalmol. 2009. ; 127: 1616–1623. [DOI] [PubMed] [Google Scholar]
- 18. Bandamwar KL,, Garret Q,, Cheung D,, et al. Onset time course of solution induced corneal staining. Cont Lens Anterior Eye. 2010. ; 33: 199–201. [DOI] [PubMed] [Google Scholar]
- 19. Cho P,, Lui T,, Kee CS. Soft contact lens care systems and corneal staining in Hong Kong-Chinese. Cont Lens Anterior Eye. 1998. ; 21: 47–53. [DOI] [PubMed] [Google Scholar]
- 20. Garofalo RJ,, Dassanayake N,, Carey C,, Stein J,, Stone R,, David R. Corneal staining and subjective symptoms with multipurpose solutions as a function of time. Eye Contact Lens. 2005. ; 31: 166–174. [DOI] [PubMed] [Google Scholar]
- 21. Jones L,, MacDougall N,, Sorbara LG. Asymptomatic corneal staining associated with the use of balafilcon silicone-hydrogel contact lenses disinfected with a polyaminopropyl biguanide-preserved care regimen. Optom Vis Sci. 2002. ; 79: 753–761. [DOI] [PubMed] [Google Scholar]
- 22. Posch LC,, Zhu M,, Robertson DM. Multipurpose care solution-induced corneal surface disruption and Pseudomonas aeruginosa internalization in the rabbit corneal epithelium [ published online ahead of print July 2014]. Invest Ophthalmol Vis Sci. 2014. ; 55: 4229–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robertson DM. The effects of silicone hydrogel lens wear on the corneal epithelium and risk for microbial keratitis. Eye Contact Lens. 2013. ; 39: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hall BJ,, Jones L. Contact lens cases: the missing link in contact lens safety? Eye Contact Lens. 2010. ; 36: 101–105. [DOI] [PubMed] [Google Scholar]
- 25. Vermeltfoort PB,, Hooymans JM,, Busscher HJ,, van der Mei HC. Bacterial transmission from lens storage cases to contact lenses-effects of lens care solutions and silver impregnation of cases. J Biomed Mater Res B Appl Biomater. 2008. ; 87: 237–243. [DOI] [PubMed] [Google Scholar]
- 26. Willcox MDP,, Carnt NA,, Diec J,, et al. Contact lens case contamination during daily wear of silicone hydrogels. Optom Vis Sci. 2010. ; 87: 456–464. [DOI] [PubMed] [Google Scholar]
- 27. Wu YT,, Zhu H,, Harmis NY,, Iskandar SY,, Willcox M,, Stapleton F. Profile and frequency of microbial contamination of contact lens cases. Optom Vis Sci. 2010. ; 87: E152–E158. [DOI] [PubMed] [Google Scholar]
- 28. Wu YT,, Zhu H,, Willcox M,, Stapleton F. The effectiveness of various cleaning regimens and current guidelines in contact lens case biofilm removal. Invest Ophthalmol Vis Sci. 2011. ; 52: 5287–5292. [DOI] [PubMed] [Google Scholar]
- 29. Szczotka-Flynn LB,, Imamura Y,, Chandra J,, et al. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea 2009. ; 28: 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robertson DM,, Parks QM,, Young RL,, et al. Disruption of contact lens-associated Pseudomonas aeruginosa biofilms formed in the presence of neutrophils. Invest Ophthalmol Vis Sci. 2011. ; 52: 2844–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burnham GW,, Cavanagh HD,, Robertson DM. The impact of cellular debris on Pseudomonas aeruginosa adherence to silicone hydrogel contact lenses and contact lens storage cases. Eye Contact Lens. 2012. ; 38: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haslett C,, Guthrie LA,, Kopaniak MM,, Johnston RB,, Henson PM. Modulation of neutrophil function by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985. ; 119: 101–110. [PMC free article] [PubMed] [Google Scholar]
- 33. Wu YT,, Zhu H,, Willcox M,, Stapleton F. Removal of biofilm from contact lens storage cases. Invest Ophthalmol Vis Sci. 2010. ; 51: 6329–6333. [DOI] [PubMed] [Google Scholar]
- 34. Whitchurch CB,, Tolker-Nielsen T,, Ragas PC,, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002. ; 295: 1487. [DOI] [PubMed] [Google Scholar]
- 35. Walker TS,, Tomlin KL,, Worthen GS,, et al. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun. 2005. ; 73: 3693–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parks QM,, Young RL,, Poch KR,, Malcolm KC,, Vasil ML,, Nick JA. Neutrophil enhancement of Pseudomonas aeruginosa biofilm development: human F-actin and DNA as targets for therapy. J Med Microbiol. 2009. ; 58: 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brinkmann V,, Reichard U,, Goosmann C,, et al. Neutrophil extracellular traps kill bacteria. Science. 2004. ; 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 38. Gray RD,, Lucas CD,, MacKellar A,, et al. Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J Inflamm. 2013. ; 10: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yipp BG,, Kubes P. NETosis: how vital is it? Blood. 2013. ; 122: 2784–2794. [DOI] [PubMed] [Google Scholar]
- 40. Dart JK,, Radford CF,, Minassian D,, Verma S,, Stapleton F. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008. ; 115: 1647–1654. [DOI] [PubMed] [Google Scholar]