Abstract
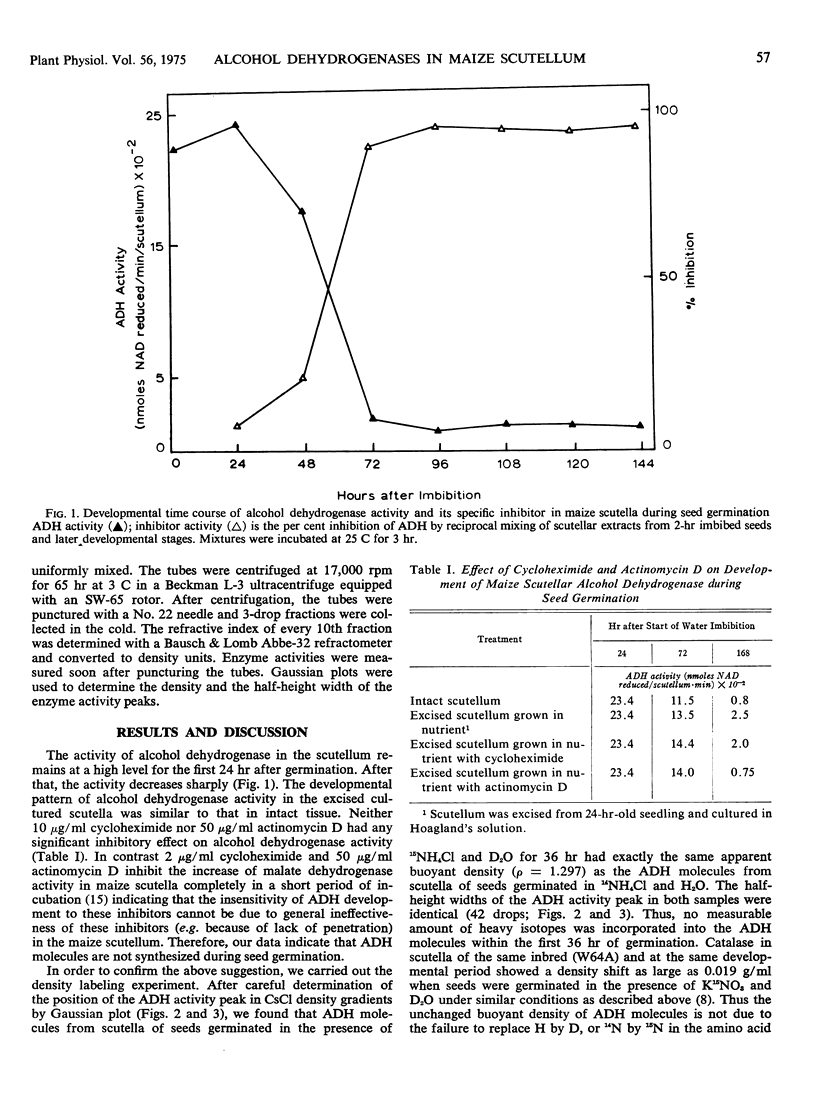
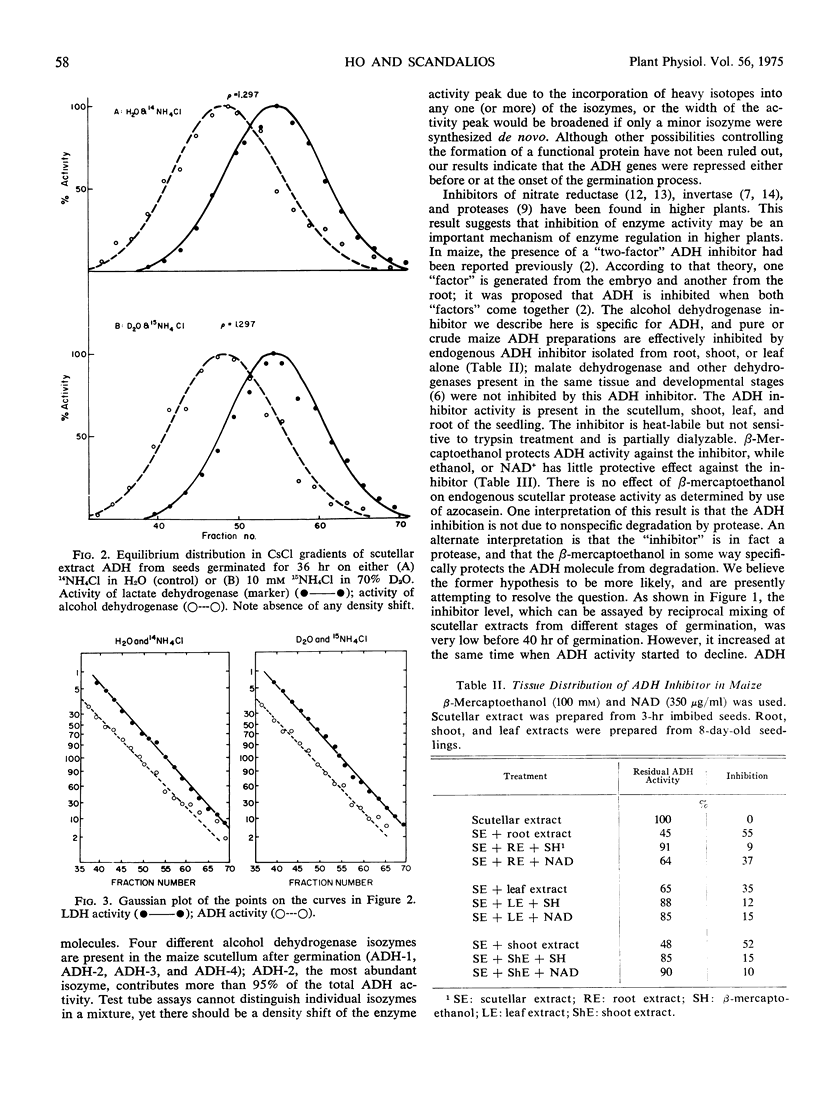
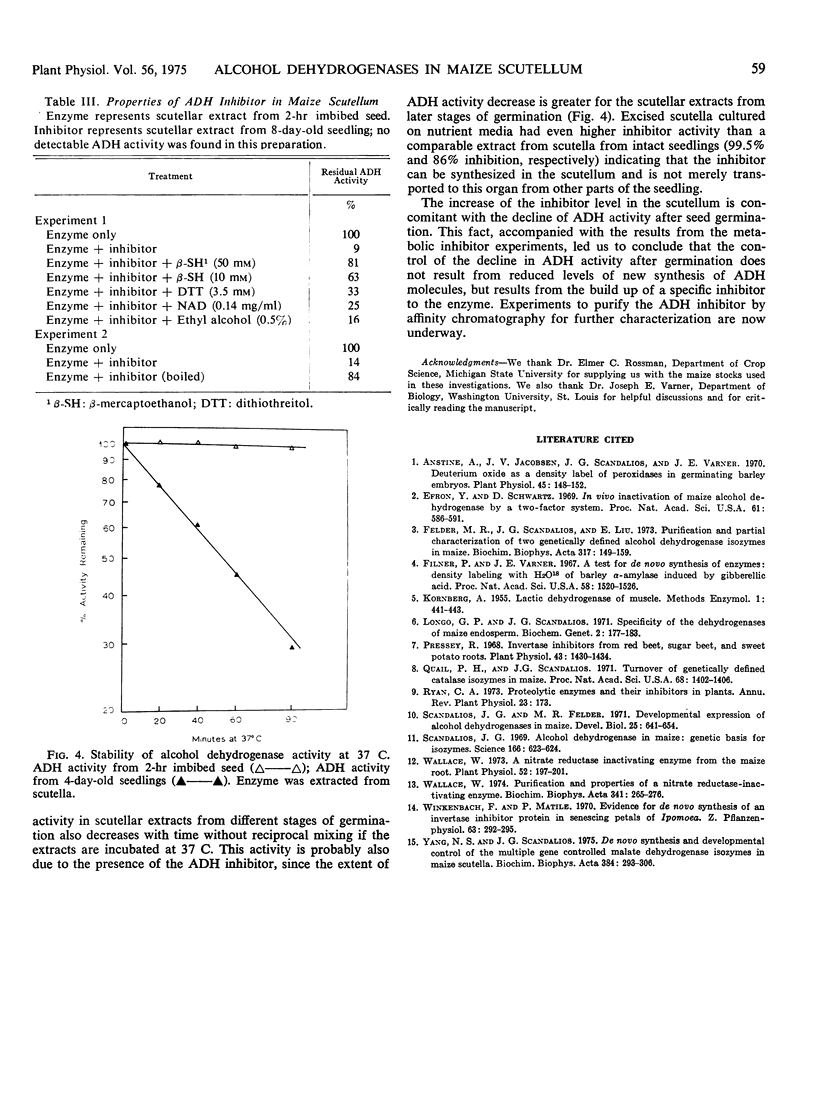
The pattern of change in the activity of alcohol dehydrogenase in maize (Zea mays L.) scutellum during seed germination is not altered by 10 μg/ml cycloheximide or 50 μg/ml actinomycin D. The enzyme does not become density labeled when maize seeds are germinated in the presence of D2O and 15NH4Cl, indicating that no new alcohol dehydrogenase molecules are synthesized after the onset of germination. However, the activity of an endogenous inhibitor for alcohol dehydrogenase is increased after germination. The increase of this inhibitor is concomitant with the decline of alcohol dehydrogenase activity, indicating that the activity of alcohol dehydrogenase during seed germination is controlled by the level of the inhibitor.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anstine W., Jacobsen J. V., Scandalios J. G., Varner J. E. Deuterium oxide as a density label of peroxidases in germinating barley embryos. Plant Physiol. 1970 Feb;45(2):148–152. doi: 10.1104/pp.45.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron Y., Schwartz D. In vivo inactivation of maize alcohol dehydrogenase by a two-factor system. Proc Natl Acad Sci U S A. 1968 Oct;61(2):586–591. doi: 10.1073/pnas.61.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder M. R., Scandalios J. G., Liu E. H. Purification and partial characterization of two genetically defined alcohol dehydrogenase isozymes in maize. Biochim Biophys Acta. 1973 Jul 12;317(1):149–159. doi: 10.1016/0005-2795(73)90207-9. [DOI] [PubMed] [Google Scholar]
- Filner P., Varner J. E. A test for de novo synthesis of enzymes: density labeling with H2O18 of barley alpha-amylase induced by gibberellic acid. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1520–1526. doi: 10.1073/pnas.58.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo G. P., Scandalios J. G. Specificity of the dehydrogenases of maize endosperm. Biochem Genet. 1968 Sep;2(2):177–183. doi: 10.1007/BF01458715. [DOI] [PubMed] [Google Scholar]
- Mürer E. H. Thrombin-induced release of calcium from blood platelets. Science. 1969 Oct 31;166(3905):623–623. doi: 10.1126/science.166.3905.623. [DOI] [PubMed] [Google Scholar]
- Pressey R. Invertase inhibitors from red beet, sugar beet, and sweet potato roots. Plant Physiol. 1968 Sep;43(9):1430–1434. doi: 10.1104/pp.43.9.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail P. H., Scandalios J. G. Turnover of genetically defined catalase isozymes in maize. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1402–1406. doi: 10.1073/pnas.68.7.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios J. G., Felder M. R. Developmental expression of alcohol dehydrogenases in maize. Dev Biol. 1971 Aug;25(4):641–654. doi: 10.1016/0012-1606(71)90009-1. [DOI] [PubMed] [Google Scholar]
- Wallace W. A nitrate reductase inactivating enzyme from the maize root. Plant Physiol. 1973 Sep;52(3):197–201. doi: 10.1104/pp.52.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. Purification and properties of a nitrate reductase-inactivating enzyme. Biochim Biophys Acta. 1974 Mar 21;341(1):265–276. doi: 10.1016/0005-2744(74)90087-4. [DOI] [PubMed] [Google Scholar]
- Yang Ning-Sun, Scandalios J. G. De novo synthesis and developmental control of the multiple gene-controlled malate dehydrogenase isozymes in maize scutella. Biochim Biophys Acta. 1975 Apr 19;384(2):293–306. doi: 10.1016/0005-2744(75)90031-5. [DOI] [PubMed] [Google Scholar]


