Abstract
Background
YM155, which inhibits the anti-apoptotic protein survivin, is known to exert anti-tumor effects in various cancers. However, there were few reports describing the inhibitory effect of YM155 on human oral squamous cell carcinoma (OSCC) cells that highly express survivin. In this study, we investigated the anti-tumor effects of YM155 on OSCC cells and then examined its molecular mechanisms.
Material/Methods
SCC9 cells of OSCC were treated with series of concentrations of YM155 (0.01, 0.1, 1, and 10 ng/ml) for 6, 12, and 24 h. The effect of YM155 on survival of SCC9 cells was detected by MTT and colony formation assay. Cell apoptosis was detected by flow cytometric analysis and the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) assays. Western blot was used to detect the protein expression of survivin, p53, and PUMA. Caspase-3 activity was measured by cleavage of the caspase-3 substrate. To test the role of PUMA and caspase-3 on YM155-induced apoptosis and growth inhibition, the SCC9 cells was transfected with PUMA siRNA or caspase-3 siRNA or control siRNA for 16 h before YM155 (1 and 10 ng/ml) treatment for 24 h. In addition, we also investigated the effect of YM155 in an in vivo xenograft model.
Results
Treatment of YM155 efficiently reduced survivin expression and increased PUMA expression and caspase-3 activation in the SCC9 cells. YM155 treatment resulted in 18–86% decrease in cell viability, 10–60% decrease in colony numbers, and 8–40% increase in cell apoptosis (p<0.05 and p<0.01). However, the induction of cell apoptosis growth inhibition was reversed by PUMA siRNA or caspase-3 transfection. In addition, animals treated with YM155 showed more than 60% tumor growth inhibition compared to the controls (p<0.05).
Conclusions
YM155 is a potent inhibitor of progression of SCC9 cells, which could be due to attenuation of survivin, and activation of the PUMA/caspase-3 cellular signaling processes. This study suggests that YM155 may be a potential molecular target with therapeutic relevance for the treatment of OSCC.
MeSH Keywords: Apoptosis, Head and Neck Neoplasms, Puma
Background
Oral cancer is among the 10 most common cancers in the world. Its high mortality rate and the disfigurement that survivors may suffer gives rise to a considerable global public health burden [1]. About 90% of malignant oral neoplasms are oral squamous cell carcinomas (OSCC), followed by adenocarcinoma and, rarely, other types of tumor [2]. Despite advances in treatment for OSCC, the 5-year survival rate remains poor [3]. As an important hallmark of OSCC, apoptosis resistance restricts the efficacy of traditional therapies [4]. Survivin (BIRC5), a member of the inhibitor of apoptosis protein (IAP) gene family, has been shown to inhibit apoptosis, enhance proliferation, and promote angiogenesis [5–7]. Survivin is selectively expressed in fetal and proliferating tissues and in various solid tumors, such as OSCC [8], and elevated survivin expression in the tissue of OSCC has been associated with poor prognosis, high recurrence rate, and resistance to chemotherapy and radiation [8]. Therefore, targeting survivin has promise for use in OSCC therapies.
Sepantronium bromide (YM155) is the first-in-class survivin inhibitor [9]. YM155 has shown potent antiproliferative effects on a variety of human cancer cell lines [10]. Numerous studies have demonstrated that YM155 alone or in combination decreases tumor growth, induces apoptosis, sensitizes resistant cells to apoptosis, and prolongs survival of tumor-bearing mice [11–13]. However, its molecular mechanism of action is still unclear.
In vitro studies revealed that YM155 triggered apoptosis of head/neck squamous cell carcinoma (HNSCC) cells in mitochondria in a death receptor-dependent manner. In addition, YM155 not only downregulated the expression of survivin, but also remarkably suppressed the activation of the mTOR signaling pathway in vitro and in vivo [14]. In human oral cancer cell lines, YM155 inhibited growth and caused caspase-dependent apoptosis in MC3 and HN22 cells; the mechanism is that YM155 causes apoptosis of human oral cancer cell lines was through downregulation of Sp1 and Mcl-1 [15]. Tang et al. showed YM155 exhibited its anti-tumor activities in oral cancer cell lines by downregulation of Mcl-1 [16]. In adenoid cystic carcinoma (ACC) cells, YM155 caused significant autophagy-dependent cell death. In addition, YM155-induced autophagy and cell death in vivo was correlated with the suppression of Erk1/2 and S6 activation, as well as increased TFEB nuclear translocation [17].
PUMA (p53 upregulated modulator of apoptosis) is a pro-apoptotic member of the BH3-only subgroup of the Bcl-2 family. It is a key mediator of p53-dependent and p53-independent apoptosis [18,19]. PUMA transduces death signals primarily to the mitochondria, where it acts indirectly on the Bcl-2 family members Bax and/or Bak by relieving the inhibition imposed by anti-apoptotic members. It directly binds and antagonizes all known anti-apoptotic Bcl-2 family members to induce mitochondrial dysfunction and caspase activation [20].
It has been shown that survivin inhibits Fas (CD95)-mediated apoptosis by supporting caspase3/p21 formation as a result of interaction with cdk4 [21]. In addition, survivin was shown to suppress the cell death induced by Bax [22]. A recent study has reported that targeting survivin resulted in increased transcription of p53 targets, such as BAX, PUMA, NOXA and p21, and increased p53-dependent breast cancer cells apoptosis [23], suggesting that PUMA signals may be regulated by survivin.
In this study, we evaluated the anticancer effects of YM155 in OSCC cell in vitro and xenografts in vivo. In addition, we investigated the effect of YM155 on PUMA signals expression to determine whether YM155 affects the PUMA pathways.
Material and Methods
Cell culture and reagents
The study was conducted in accordance with the guidelines in the Declaration of Helsinki. The human oral squamous cell carcinomas (OSCC) SCC9 cell line was purchased from the Institute of Biochemistry and Cell Biology (Shanghai, China). It was maintained in DMEM-F12 (Gibco, Shanghai, China), and supplemented with 10% fetal bovine serum and maintained at 37°C in a 5% CO2 humidified incubator. Antibodies against survivin, PUMA, activated caspase-3, and β-actin were from Santa Cruz (Shanghai, China). YM155 were purchased from Selleck Chemicals (Shanghai, China). PUMA siRNA, caspase-3 siRNA, and control siRNA were purchased from Santa (Shanghai, China).
siRNA transfection
PUMA siRNA or caspase-3 siRNA or mismatched siRNA (control siRNA) were transiently transfected into SCC9 cells using Lipofectamine 2000 reagent (Invitrogen, Inc., Carlsbad, CA) according to the manufacturer’s instructions. Briefly, SCC9 cells (2×103) were plated in each well of a 96-well plate. Experimental conditions were set in quadruplicate. After cells were attached, the culture medium was replaced with serum-free medium plus 3 μl of siRNA (20 μM) and mixed with 1 μl transfection reagent and 100 μl Lipofectamine medium supplied with the kit. Then, the siRNA transfection reagent complex was incubated with 500 μl of diluted cells (5×104 cells/well) for 24 h at 37°C and 5% CO2. The cells without siRNA transfection were used as the control. The knockdown effect was verified by Western blot analysis. The stable siRNA transfected SCC9 cells were screened by administration of 400 μg/ml G418 (Invitrogen, Carlsbad, CA) for 10–14 days.
Western blot analysis
SCC9 cells were treated with 0.01, 0.1, 1, and 10 ng/ml YM155 for 6, 12, and 24 h, respectively, or transfected with PUMA/caspase-3 siRNA or control siRNA for 16 h before YM155 (1 and 10 ng/ml) treatment for 24 h, then the cells were lysed and protein was analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The following antibodies were applied: monoclonal human anti-survivin, anti-p53 and anti-PUMA, anti-activated caspase-3, and anti-β-actin. Secondary antibodies (dilution 1: 20,000) were horseradish peroxidase-conjugated (Hangzhou, China).
Caspase-3 activity assay
Caspase-3 activity was measured by cleavage of the caspase-3 substrate. Briefly, SCC9 cells (2×104) were treated as described above. Reactions were spiked with DMSO or the Caspase-3 specific inhibitor Ac-DMQD-CHO at a final concentration of 2 mM. Measurements were done in triplicate. The mean of 3 biological replicates is shown.
Cell viability assay
Cell viability was assessed with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT) according to the manufacturer’s instructions. Briefly, SCC9 cells (1×104) were plated in each well of a 96-well plate. The following day, the cells were incubated with increasing concentrations of YM155 (ranging from 0.01 to 10 ng/ml) and incubated at 37°C for 6–24 h. To assess the role of PUMA/caspase-3 on YM155-induced growth of SCC9 cells, the SCC9 cells (1×104) were transfected PUMA siRNA or caspase-3 siRNA or control siRNA for 16 h before incubation with the concentrations of YM155 (1 and 10 ng/ml) for 24 h. We then added 50 μL of 0.15% 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma-Aldrich) to each well (after exposure to YM155 for 72 h) followed by incubation at 37°C for 4 h. The medium containing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was then removed. After the medium was removed, 200 uL of DMSO was added to each well. The optical density (OD) values were measured at 570 nm on a scanning multi-well spectrophotometer (BioRad Model 550, USA). Each assay was performed in triplicate. Absorbance values were normalized to the values for the vehicle-treated cells to determine the percent survival.
Colony formation assay
SCC9 cells, stable PUMA siRNA, or caspase-3 siRNA transfected SCC9 cells were seeded at 500 cells/well in 6-well plates and allowed to adhere for 24 h. Cells were subsequently treated with YM155 (1 and 10 ng/ml) for a period of 24 h, after which media was aspirated and cells were washed and incubated in drug-free media for approximately 2 weeks to allow colony formation. Colonies were fixed with methanol and stained with 0.1% crystal violet in 20% methanol, and then were photographed and counted. All visible colonies were quantified.
In vitro apoptosis assay
The measurement of phosphatidylserine redistribution in a plasma membrane was conducted according to the protocol outlined by the manufacturer of the Annexin V-FITC/PI apoptosis detection kit (Abcam, Hangzhou, Zhejiang, China). Briefly, SCC9 cells were treated with 0.01, 0.1, 1, and 10 ng/ml YM155 for 6, 12, and 24 h, respectively, or transfected with PUMA/caspase-3 siRNA or control siRNA for 16 h before YM155 (1 and 10 ng/ml) treatment for 24 h. The cells (1×105) were then suspended in 500 ml of Annexin V binding buffer, 5 ml of Annexin V-FITC and 5 mL of PI were added and incubated for 15 min in the dark, and 400 mL binding buffer was added to each sample. The stained cells were analyzed directly by flow cytometry using the Cell Quest program (Becton Dickinson, San Jose, CA).
TUNEL staining
For in vitro assay, SCC9 cells, PUMA siRNA, or caspase-3 siRNA-transfected SCC9 cells were treated with YM155 (1 and 10 ng/ml) for 24 h. Cell apoptosis was detected using terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) in situ cell death detection kit according to the manufacturer’s instructions. The apoptotic index was determined by dividing the number of apoptotic cells by the total number of cells in at least 20 randomly selected fields (×200).
For in vivo assay, 5-micron-thick frozen sections were cut on a cryostat, placed on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA), and stored at −70°C. TUNEL was carried out according to the manufacturer’s recommendations. TUNEL+ immunoreactivity was detected and counted under light microscopy.
In vivo experiments
Animal experiments were approved by the Ethics Committee of the Taishan Medical University and were carried out according to the Chinese guidelines for animal care and protection to minimize animal suffering. We utilized 4- to 6-week-old severe combined immunodeficient (SCID) female mice for all experiments. SCC9 (1×107) cells were injected into the flank. Tumor growth was monitored by measuring tumor volume (length×width2/2/mm3). When subcutaneous tumors reached a size of 50 mm3 (day 0), xenografted animals were randomly allocated into vehicle (saline) and YM155 (50 mg/kg) groups. YM155 was subcutaneously administered as a 3-day continuous infusion per week for 2 weeks using the Alzet Osmotic Pump® (Model 1003D). At experiment termination, mice were dissected and tumor tissue was processed for immunohistochemistry (IHC) and TUNEL assay. For statistical analysis of tumor growth, two-way ANOVA and Bonferroni post-correction analysis were applied.
Immunohistochemistry
Immunohistochemistry (IHC) was performed using standard procedures. Briefly, 4-μm sections from xenograft tumor blocks were deparaffinized and rehydrated, heated for 10 min in 10 mM citrate buffer (pH 6.0) in a pressure cooker for epitope retrieval, and then incubated for 60 min at room temperature with anti-survivin and anti-PUMA antibodies. Antibody binding was detected using the UltraVision LP detection system according to the manufacturer’s recommendations.
Statistical analysis
The significance of the results was determined using the t test. Values are expressed as mean ±SD from at least 3 separate experiments, and A P value less than 0.05 was considered to be statistically significant.
Results
YM155 inhibits growth and colony formation, and induces apoptosis of SCC9 cells
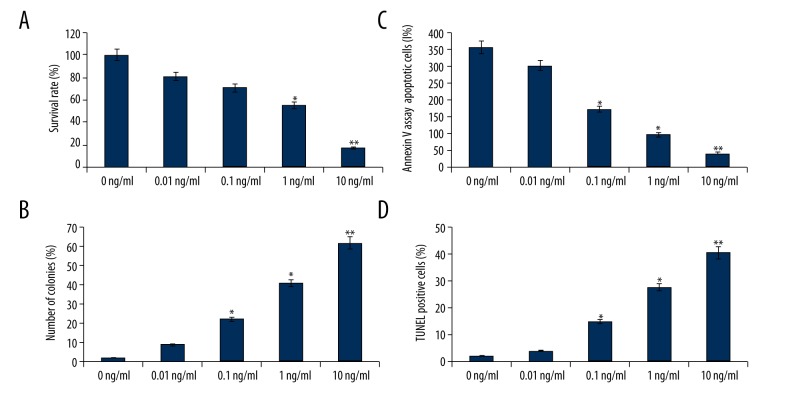
SCC9 cells were first treated with 0.01, 0.1, 1, and 10 ng/ml YM155 for 24 h. YM155 treatment resulted in an 18–86% decrease in cell viability (p<0.05 and p<0.01, Figure 1A), suggesting that YM155 treatment reduced viability of SCC9 cells in a dose-dependent manner. To confirm cell growth inhibition, we also performed cell colony formation assay, which produced results similar to those obtained using MTT assay (p<0.05 and p<0.01, Figure 1B).
Figure 1.
YM155 affects growth and colony formation, and induces apoptosis of SCC9 cells SCC9 cells were treated with 0.01, 0.1, 1, and 10 ng/ml YM155 for 24 h. (A) MTT assay; (B) Colony formation assay; (C) FACS analysis; (D) TUNEL assay. The survival rate and apoptotic rates are the means ±SD of 3 independent experiments vs. 0 ng/ml, * p<0.05, ** p<0.01
Next, we examined whether the inhibition of cell growth was also accompanied by the induction of apoptosis induced by YM155. SCC9 cells were first treated with 0.01, 0.1, 1, and 10 ng/ml YM155 for 24 h. After treatment, the degree of apoptosis was measured. The induction of apoptosis was found to be dose-dependent by flow cytometry assay (p<0.05 and p<0.01, Figure 1C) and TUNEL assay (p<0.05 and p<0.01, Figure 1D). These results provided convincing data showing that YM155 could induce apoptosis in SCC-9 cells.
We also treated the SCC9 cells with 0.01, 0.1, 1, and 10 ng/ml YM155 for 6, 12, and 24 h. The results showed that YM155 resulted in cell growth inhibition and increased apoptosis in a time-dependent manner (data not shown).
YM155 inhibits survivin and induces PUMA/caspase-3 upregulation
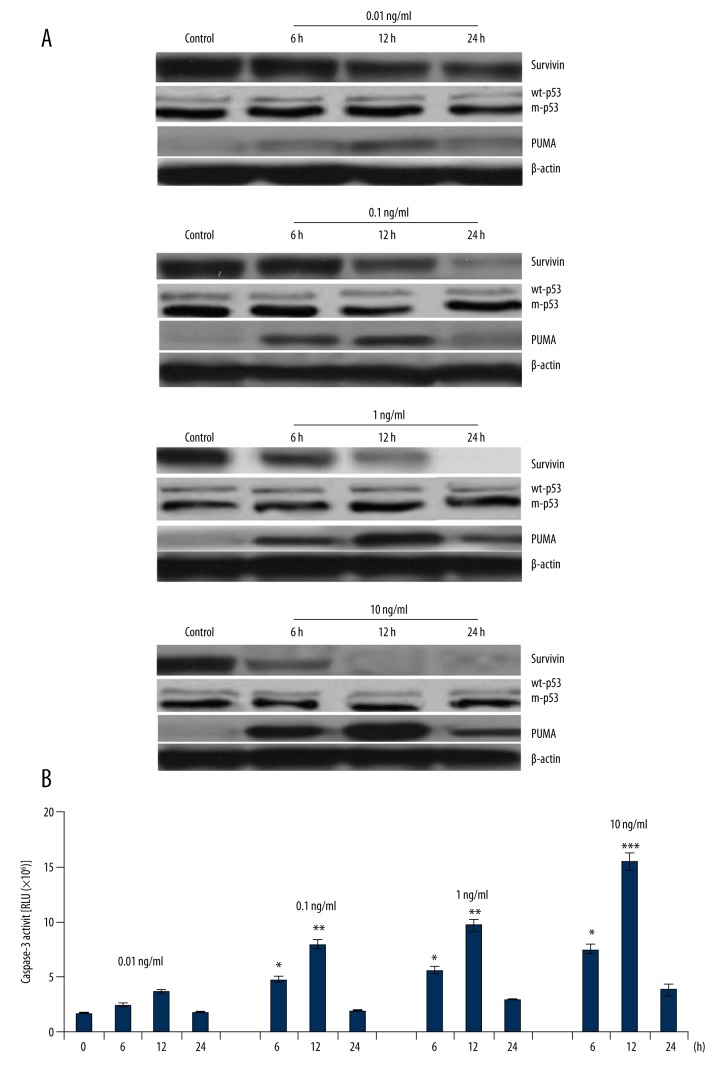
SCC9 cells were treated with 0.01, 0.1, 1, and 10 ng/ml YM155 for 6, 12, and 24 h. Survivin and PUMA expression was detected by Western blot assay. Caspase-3 activity was measured by cleavage of the caspase-3 substrate. The results showed that survivin was overexpressed in the SCC9 cells, and YM155 treatment caused survivin inhibition and PUMA upregulation in a dose- and time-dependent manner (Figure 2A). Activation of caspase-3 also occurred in a dose- and time-dependent manner (Figure 2B), indicating the specificity of the apoptotic effect of YM155 by caspase activation in SCC9 cells.
Figure 2.
Effect of YM155 on survivin, p53, PUMA expression, and caspase-3 activity. SCC9 cells were treated with 0.01, 0.1, 1, and 10 ng/ml YM155 for 6, 12, and 24 h. (A) Survivin, p53, and PUMA protein expression was detected by Western blot assay; (B) Caspase-3 activity was detected by cleavage of the caspase-3 substrate vs. 0 ng/ml, * p<0.05, ** p<0.01 and *** p<0.001.
PUMA was regulated through a p53-dependent and p53-independent pathway [18–20]. We next investigated whether p53 was regulated by YM155/survivin. As shown in Figure 2A, treatment with 0.01, 0.1, 1, and 10 ng/ml YM155 for 6, 12, and 24 h in the SCC9 cells did not affect wt-p53 expression, suggesting that PUMA was involved in p53-independent regulation by YM155 treatment.
We also found that treatment with YM155 (10 and 1 ng/ml) for 24 h resulted in complete survivin inhibition. In addition, treatment with YM155 rapidly increased the PUMA expression in the SCC9 cells at 6 h, reached the highest level at 12 h, and significantly decreased at 24 h (Figure 2A). In addition, caspase-3 activity was also highest at 12 h, and significantly decreased at 24 h (Figure 2B). It is unclear why PUMA/caspase-3 expression was downregulated after 24 h.
YM155 induces apoptosis and inhibits growth of SCC9 cells through PUMA/caspase-3 activation
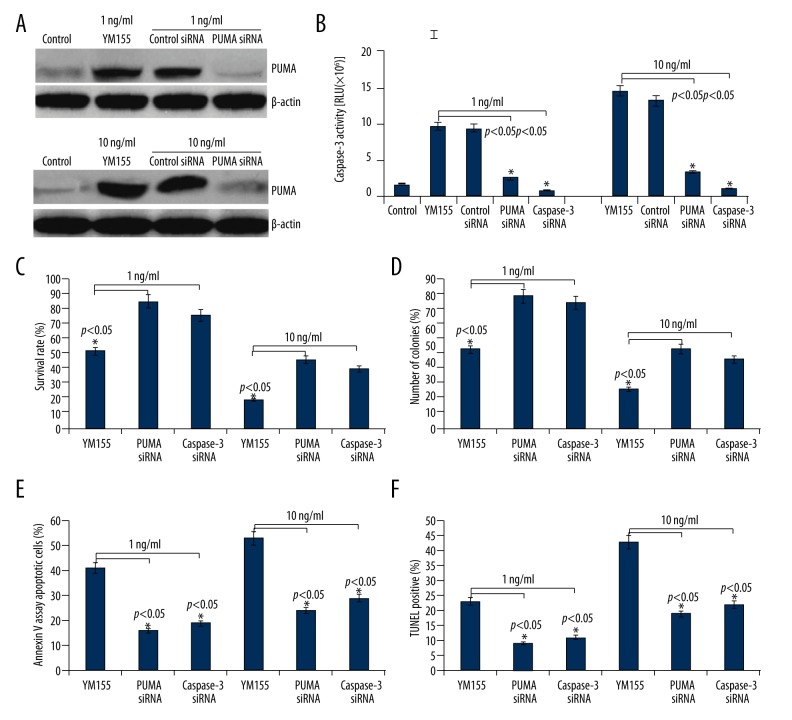
As shown in Figure 2A, treatment with 1 and 10 ng/ml of YM155 for 24 h completely inhibited survivin expression; therefore, we selected 1 and 10 ng/ml of YM155 for further study. To confirm the mechanism responsible for YM155-mediated apoptosis and growth inhibition, we transfected PUMA siRNA or caspase-3 siRNA or control siRNA into SCC9 cells, then treated the transfected cells with YM155 (1 and 10 ng/ml) for 24 h. As shown in Figure 3A, targeting PUMA by PUMA siRNA transfection inhibited YM155-induced PUMA expression and caspase-3 activation (Figure 3B). In addition, targeting caspase-3 by caspase-3 siRNA transfection also inhibited YM155-induced caspase-3 activation (Figure 3B). Transfection of SCC9 cells with PUMA siRNA or caspase-3 siRNA significantly reduced the ability of YM155 to induce cell apoptosis and inhibit growth in SCC9 cells (Figure 3C–3F). The control siRNA did not cause any significant change in cell viability or cell apoptosis (data not shown). Although PUMA siRNA or caspase-3 siRNA significantly reduced the ability of YM155 to induce cell apoptosis and growth inhibition, PUMA siRNA or caspase-3 siRNA could not completely reverse the effect of YM155, suggesting that the apoptosis-inducing effect by YM155 is partly mediated through the activation of the PUMA/caspase-3 pathway.
Figure 3.
Effect of PUMA/caspase-3 on YM155-induced apoptosis and growth inhibition of SCC9 cells. SCC9 cells were transfected with PUMA siRNA or caspase-3 siRNA for 16 h, then we treated the transfected cells with YM155 (1 and 10 ng/ml) for 24 h. (A) PUMA protein expression was detected by Western blot assay; (B) Caspase-3 activity was detected by cleavage of the caspase-3 substrate. (C) Cell survival rate was detected by MTT assay; (D) Colony formation assay; (E) Apoptosis was determined by FACS analysis; (F) Apoptosis was determined by TUNEL assay vs. control, * p<0.05.
YM155 treatment inhibited growth of SCC9 xenograft tumor in nude mice
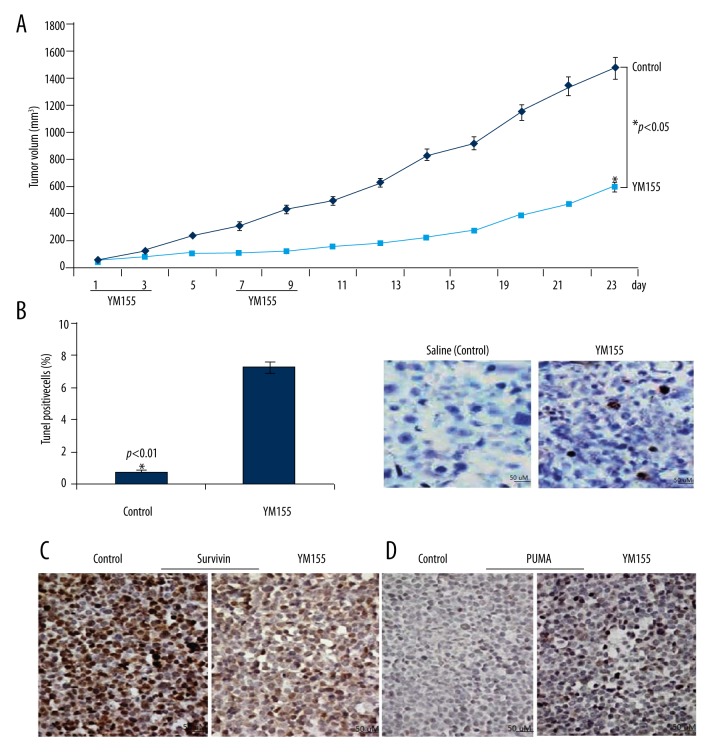
To confirm the anti-tumor effects of YM155 in vivo, we used a SCID mouse xenograft model. Animals bearing SCC9 tumors were subcutaneously administered a 3-day per week continuous infusion for 2 weeks using the Alzet Osmotic Pump® (Model 1003D). Animals treated with YM155 showed more than 60% tumor growth inhibition compared to the controls (Figure 4A). TUNEL assay showed there were more apoptotic cells in YM155-treated tumors (p<0.01, Figure 4B). In addition, survivin expression was inhibited and PUMA expression was increased with YM155 treatment, as shown by immunohistochemical assay (Figure 4C, 4D).
Figure 4.
YM155 inhibits xenograft growth of SCC9 cells. (A) Tumor xenografts were established by s.c. injection of SCC9 cells into the flanks of the mice. Animals bearing SCC9 tumors were subcutaneously administered a 3-day per week continuous infusion for 2 weeks. Tumor size was measured every 2 days. The tumor growth curve is shown. * p<0.05 compared with the control group. (B) Apoptotic cells was detected by TUNEL assay, p<0.01. (C) Survivin was detected by immunohistochemistry staining assay. (D) PUMA was detected by immunohistochemistry staining assay.
Discussion
Survivin is one of the most frequently overexpressed genes in all types of cancer. Increased survivin expression in cancer patients is an unfavorable prognostic marker correlating with decreased overall survival in several malignancies, including pancreatic cancer [24], breast carcinomas [25], non-small cell lung [26], colorectal, hepatocellular carcinoma [27], and neuroblastoma [28]. Increased survivin expression was also associated with increased risk of recurrence, lymph node invasion, and metastasis. Survivin has been shown to inhibit cell apoptosis and promote cell proliferation and angiogenesis, all of which make survivin a potentially attractive target for therapy [29]. In OSCC, survivin was found to be strikingly increased in malignant cells, and increased survivin expression was also associated with bad prognosis [30]. Therefore, targeting survivin in OSCC therapies is promising.
Several strategies have been used to suppress survivin expression [31]. One approach is to use antisense oligonucleotides or siRNA to knock down survivin expression, resulting in cell growth arrest and increased apoptosis in a broad range of tumor cell lines [32]. YM155 is a survivin suppressant identified in a high-throughput screen of compounds that selectively inhibited survivin promoter activity [9].
Our current data show that YM155 treatment induced apoptosis and decreased cell proliferation in human SCC9 cells in vitro, and that YM155 suppressed in vivo SCC9 cell growth by inducing apoptosis without apparent body weight loss (data not shown). Our results strongly suggest that survivin expression contributes to human SCC9 tumor progression, and that survivin inhibition by YM155, a novel survivin suppressant, provides an anti-tumor effect on human SCC9 cells via induction of apoptosis.
To further explore the mechanism of YM155-induced cell apoptosis and growth inhibition, possible proteins involved were detected in vitro and in vivo by Western blot analysis. Previous data suggest that survivin depletion triggers p53 activation and sensitizes cancer cells to of PARP inhibition [33]. In our study, YM155 treatment did not affect wt-p53 expression in the SCC9 cells, suggesting that there is no relation between p53 and YM155/survivin-induced apoptosis.
PUMA as a BH3-only Bcl-2 family protein that plays an essential role in p53-dependent and -independent apoptosis [18,19]. In the present study, the expression of PUMA was upregulated in SCC9 cells when treated with YM155 for 6–24 h. Therefore, PUMA upregulation was shown to be p53-independent. To assess whether YM155-induced apoptosis and growth inhibition was PUMA-dependent, SCC9 cells was transfected with PUMA siRNA to knock down PUMA expression, only to find YM155-induced apoptosis and growth inhibition was partly reversed by PUMA inhibition. We propose that the mechanism of YM155-induced apoptosis may be attributable to the downregulation of survivin and the upregulation of PUMA.
Because survivin inhibits apoptosis through both direct and indirect inhibitions of caspase-3, a downstream gene of PUMA, we next investigated whether YM155/survivin inhibition could upregulate caspase-3 activity. In the present study, increases of caspase-3 activity by YM155 treatment were observed, which is consistent with the results of PUMA, demonstrating YM155-induced apoptosis and growth inhibition may be partly due to inhibition of the survivin/PUMA/caspase-3 pathway.
Although apoptosis provoked by YM155/survivin depends on the activation of PUMA/caspase-3 signaling, how survivin regulates PUMA/caspase-3 remains unknown. Studies have reported that targeting the Ras/Raf/MEK/ERK pathway induces PUMA-dependent apoptosis in cancer cells, irrespective of p53 status [34,35], suggesting that PUMA is negatively regulated by ERK signals. Wang et al. reported that YM155-induced autophagy and cell death in vivo was correlated with the suppression of survivin/Erk1/2 and S6 activation [36]. We hypothesized in the present study that YM155/survivin might downregulate ERK1/2 signaling, resulting in upregulation of PUMA/caspase-3 expression and activation, and this hypothesis needs further investigation. We also demonstrated that although YM155 induced apoptosis and growth inhibition, targeting PUMA or caspase-3 could partly reverse the function of YM155, suggesting that some other proapoptotic signals may take part in YM155/survivin-induced apoptosis and growth inhibition of SCC9 cells.
It is interesting that in our study PUMA/caspase-3 expression reached the peak at 12 h with YM155 treatment, and was dramatically decreased at 24 h. It remains unclear why PUMA/caspase-3 expression was downregulated at 24 h. A previous study has reported that activation of ERK/Slug signaling by Cytarabine contributed to the PUMA downregulation in HL-60 cells after 48 h [37]. Further investigation is needed to determine whether some antiapoptotic signals, which inhibited PUMA upregulation, were induced by YM155 or YM155/survivin.
Conclusions
We presented experimental evidence that strongly supports the anti-tumor effects of YM155 in SCC9 cells in vitro and in vivo. Thus, we believe that YM155 could potentially be an effective therapeutic agent for the inactivation of survivin and activation of PUMA/caspase-3 signaling, resulting in the induction of cell apoptosis and inhibition of cell growth. Our study suggests that YM155 is a promising novel agent that should be developed for the treatment of OSCC.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Source of support: This study was granted from the National Natural Science Research Fund (No: 82350713) and the Nanjing Medical Technology Development Key Program (No. zkx15035)
References
- 1.Kademani D. Oral cancer. Mayo Clin Proc. 2007;82:878–87. doi: 10.4065/82.7.878. [DOI] [PubMed] [Google Scholar]
- 2.Petersen PE. Oral cancer prevention and control – the approach of the World Health Organization. Oral Oncol. 2009;45:454–60. doi: 10.1016/j.oraloncology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 3.Chen GS, Chen CH. A study on survival rates of oral squamous cell carcinoma. Kaohsiung J Med Sci. 2006;12:317–25. [PubMed] [Google Scholar]
- 4.Lim YC, Choi EC. Surgery alone for squamous cell carcinoma of the oral cavity: survival rates, recurrence patterns, and salvage treatment. Acta Oto-Laryngol. 2008;128:1132–37. doi: 10.1080/00016480801901691. [DOI] [PubMed] [Google Scholar]
- 5.Deveraux QL, Reed JC. IAP family proteins – suppressors of apoptosis. Genes Dev. 1999;13:239–52. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 6.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 7.Lo Muzio L, Pannone G, Staibano S, et al. Survivin expression in oral squamous cell carcinoma. Br J Cancer. 2003;89:2244–48. doi: 10.1038/sj.bjc.6601402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohi T, Okada K, Xia F, et al. An IAP-IAP complex inhibits apoptosis. J Biol Chem. 2004;279:34087–90. doi: 10.1074/jbc.C400236200. [DOI] [PubMed] [Google Scholar]
- 9.Nakahara T, Kita A, Yamanaka K, et al. Broad spectrum and potent antitumor activities of YM155, a novel small-molecule survivin suppressant, in a wide variety of human cancer cell lines and xenograft models. Cancer Sci. 2011;102:614–21. doi: 10.1111/j.1349-7006.2010.01834.x. [DOI] [PubMed] [Google Scholar]
- 10.Minematsu T, Iwai M, Sugimoto K, et al. Carrier-mediated uptake of YM155 monobromide, a novel small-molecule survivin suppressant, into human solid tumor and lymphoma cells. Drug Metab Dispos. 2009;37:619–28. doi: 10.1124/dmd.108.025254. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Pise-Masison CA, Shih JH, et al. Markedly additive antitumor activity with the combination of a selective survivin suppressant YM155 and alemtuzumab in adult T-cell leukemia. Blood. 2013;121:2029–37. doi: 10.1182/blood-2012-05-427773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mir R, Stanzani E, Martinez-Soler F, et al. YM155 sensitizes ovarian cancer cells to cisplatin inducing apoptosis and tumor regression. Gynecol Oncol. 2014;132:211–20. doi: 10.1016/j.ygyno.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Koike H, Nitta T, Sekine Y, et al. YM155 reverses rapamycin resistance in renal cancer by decreasing survivin. J Cancer Res Clin Oncol. 2014;140:1705–13. doi: 10.1007/s00432-014-1734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Zhang W, Wang YF, et al. Dual induction of apoptotic and autophagic cell death by targeting survivin in head neck squamous cell carcinoma. Cell Death Dis. 2015;6:e1771. doi: 10.1038/cddis.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachita K, Yu HJ, Yun JW, et al. YM155 induces apoptosis through downregulation of specificity protein 1 and myeloid cell leukemia-1 in human oral cancer cell lines. J Oral Pathol Med. 2015;44:785–91. doi: 10.1111/jop.12299. [DOI] [PubMed] [Google Scholar]
- 16.Tang H, Shao H, Yu C, Hou J. Mcl-1 downregulation by YM155 contributes to its synergistic anti-tumor activities with ABT-263. Biochem Pharmacol. 2011;82:1066–72. doi: 10.1016/j.bcp.2011.07.064. [DOI] [PubMed] [Google Scholar]
- 17.Wang YF, Zhang W, He KF, et al. Induction of autophagy-dependent cell death by the survivin suppressant YM155 in salivary adenoid cystic carcinoma. Apoptosis. 2014;19:748–58. doi: 10.1007/s10495-013-0960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeffers JR, Parganas E, Lee Y, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–28. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 19.Villunger A, Michalak EM, Coultas L, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins PUMA and NOXA. Science. 2003;302:1036–38. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 20.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27:S71–83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki A, Ito T, Kawano H, et al. Survivin initiates procaspase 3/p21 complex formation as a result of interaction with Cdk4 to resist Fas-mediated cell death. Oncogene. 2000;19:1346–53. doi: 10.1038/sj.onc.1203429. [DOI] [PubMed] [Google Scholar]
- 22.Tamm I, Wang Y, Sausville E, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–20. [PubMed] [Google Scholar]
- 23.Véquaud E, Desplanques G, Jézéquel P, et al. Survivin contributes to DNA repair by homologous recombination in breast cancer cells. Breast Cancer Res Treat. 2016;155:53–63. doi: 10.1007/s10549-015-3657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MA, Park GS, Lee HJ, et al. Survivin expression and its clinical significance in pancreatic cancer. BMC Cancer. 2005;5:127. doi: 10.1186/1471-2407-5-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nassar A, Sexton D, Cotsonis G, Cohen C. Survivin expression in breast carcinoma: correlation with apoptosis and prognosis. Appl Immunohistochem Mol Morphol. 2008;16:221–26. doi: 10.1097/PAI.0b013e3180c317bc. [DOI] [PubMed] [Google Scholar]
- 26.Dai CH, Li J, Shi SB, et al. Survivin and Smac gene expressions but not livin are predictors of prognosis in non-small cell lung cancer patients treated with adjuvant chemotherapy following surgery. Jpn J Clin Oncol. 2014;5:327–35. doi: 10.1093/jjco/hyp165. [DOI] [PubMed] [Google Scholar]
- 27.Augello C, Caruso L, Maggioni M, et al. Inhibitors of apoptosis proteins (IAPs) expression and their prognostic significance in hepatocellular carcinoma. BMC Cancer. 2009;9:125. doi: 10.1186/1471-2407-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Islam A, Kageyama H, Takada N, et al. High expression of Survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene. 2000;19:617–23. doi: 10.1038/sj.onc.1203358. [DOI] [PubMed] [Google Scholar]
- 29.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy. Expert Opin Ther Targets. 2008;12:463–76. doi: 10.1517/14728222.12.4.463. [DOI] [PubMed] [Google Scholar]
- 30.Xie S, Xu H, Shan X, et al. Clinicopathological and prognostic significance of survivin expression in patients with oralsquamous cell carcinoma: Evidence from a meta-analysis. PLoS One. 2015;10:e0116517. doi: 10.1371/journal.pone.0116517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan BM, O’Donovan N, Duffy MJ. Survivin: A new target for anti-cancer therapy. Cancer Treat Rev. 2009;35:553–62. doi: 10.1016/j.ctrv.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Lares MR, Rossi JJ, Ouellet DL. RNAi and small interfering RNAs in human disease therapeutic applications. Trends Biotechnol. 2010;28:570–79. doi: 10.1016/j.tibtech.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Véquaud E, Desplanques G, Jézéquel P, et al. Survivin contributes toDNA repair by homologous recombination in breast cancer cells. Breast Cancer res Treat. 2016;155:53–63. doi: 10.1007/s10549-015-3657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen D, Wei L, Yu J, Zhang L. Regorafenib inhibits colorectal tumor growth through PUMA-mediated apoptosis. Clin Cancer Res. 2014;20:3472–84. doi: 10.1158/1078-0432.CCR-13-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudgeon C, Peng R, Wang P, et al. Inhibiting oncogenic signaling by sorafenib activates PUMA via GSK3β and NF-κB to suppress tumor cell growth. Oncogene. 2012;31:4848–58. doi: 10.1038/onc.2011.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang YF, Zhang W, He KF, et al. Induction of autophagy-dependent cell death by the survivin suppressant YM155 in salivary adenoid cystic carcinoma. Apoptosis. 2014;19:748–58. doi: 10.1007/s10495-013-0960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu GJ, Pan GJ, Wang J, et al. Knockdown of ERK/Slug signals sensitizes HL-60 Leukemia cells to Cytarabine via upregulation of PUMA. Eur Rev Med Pharmacol Sci. 2014;18:3802–9. [PubMed] [Google Scholar]