Abstract
It has been known for decades that brown adipose tissue (BAT) plays a central role in maintaining body temperature in hibernating animals and human infants. Recently, it has become evident that there are also depots of brown fat in adult humans, and the mass of brown fat is inversely correlated with body weight. There are a variety of transcription factors implicated in the differentiation of classical Myf5+ brown preadipocytes, one of the most important of which is PRDM16. We have recently identified that in addition to PRDM16, the tyrosine kinase Tyk2 and the STAT3 transcription factor are required for the differentiation of Myf5 positive brown preadipocytes both in cell culture and in mice. Tyk2 is a member of the Jak family of tyrosine kinases, which are activated by exposure of cells to different cytokines and growth factors. In this study we report the surprising observation that a mutated form of Tyk2, which lacks tyrosine kinase activity (Tyk2KD) restores differentiation of brown preadipocytes in vitro as well as in Tyk2−/− mice. Furthermore, expression of the Tyk2KD transgene in brown fat reverses the obese phenotype of Tyk2−/− animals. Treatment of cells with Jak-selective inhibitors suggests that the mechanism by which Tyk2KD functions to restore BAT differentiation is by dimerizing with kinase active Jak1 or Jak2. These results indicate that there are redundant mechanisms by which members of the Jak family can contribute to differentiation of BAT.
Obesity is a worldwide epidemic affecting approximately 35% of the population in industrialized countries. It occurs as a consequence of accumulation of lipids in white adipose tissue (WAT). In contrast to WAT, which is involved in storage of energy in form of triglycerides, brown adipose tissue (BAT) is responsible for energy expenditure in the form of thermogenesis. Energy loss from BAT in the form of heat is mediated by uncoupling protein-1 (UCP1), which uncouples oxidative phosphorylation from ATP synthesis. The recent observation that adult humans possess active brown fat depots has generated excitement that manipulation of BAT could be used therapeutically to treat obesity (1).
The Jak/STAT pathway is activated by numerous cytokines and growth factors to stimulate tyrosine phosphorylation of the STAT transcription factors that induce the expression of early response genes. Proteins with expression regulated by the STATs control numerous biological functions including cell growth, immune function, and cell differentiation. Activation of this signaling cascade also regulates cell metabolism and the differentiation of both WAT and BAT (for reviews, see 2, 3).
There are 2 types of brown fat cells: classic brown fat cells derived from Myf5+ progenitors, which also differentiate into skeletal muscle (SKM), and Myf5– beige or brite cells, which are scattered through subcutaneous WAT and originate from a smooth muscle-like lineage (4). We have previously reported that mice that do not express the tyrosine kinase Tyk2, a member of the Jak family, become obese (5). Obesity in Tyk2−/− mice is associated with a defect in differentiation of My5+ brown preadipocytes. Expression of either the constitutively active STAT3 (CASTAT3) transcription factor or PRDM16 rectifies the defect in differentiation of Tyk2−/− classic brown preadipocytes in cell culture. Furthermore, expression of a CASTAT3 transgene in BAT in Tyk2−/− mice also restores a normal phenotype (5). In this report, we present surprising evidence that the kinase activity of Tyk2 is not required for differentiation of Myf5+ preadipocytes. Furthermore, expression of Tyk2KD in BAT of Tyk2−/− mice is sufficient to revert the obese phenotype of Tyk2−/− mice. These results highlight a novel kinase–independent mechanism by which Tyk2 controls the differentiation of brown adipocytes.
Experimental Procedures
Reagents and antibodies
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) or indicated otherwise. Antibodies were purchased from Cell Signaling Technology (Danvers, MA) (rabbit monoclonal PPAR-γ, rabbit monoclonal pStat1 Y701, rabbit polyclonal pStat3 Y705), Sigma-Aldrich (mouse monoclonal α-tubulin), Abcam (rabbit polyclonal UCP1, rabbit polyclonal PGC1-α). Rabbit Tyk2 antisera was a kind gift of Dr. Birgit Strobl (University of Veterinary Medicine, Vienna, Austria). PRDM16-specific rabbit polyclonal antibodies were kind gifts of Dr. Patrick Seale (University of Pennsylvania, School of Medicine, Philadelphia, PA). Antibodies are shown in Table 1.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog #, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| UCP1 | UCP1 | ABCAM #10983 | Rabbit polyclonal | 1000 fold | |
| Tyk2 | Tyk2 | Brigit Strobel | Rabbit polyclonal | 500 fold | |
| PPARg | PPARg | Cell Signaling #2443 | Rabbit polyclonal | 1000 fold | |
| tubulin | tubulin | Sigma-Aldrich #T8203 | Mouse monoclonal | 1000 fold | |
| Stat3 | Stat3 | Cell Signaling # 9139 | Mouse monoclonal | 1000 fold | |
| PYSTAT3 | PYSTAT3 | Cell Signaling #9145 | Rabbit polyclonal | 1000 fold | |
| PG1a | PGC1a | ABCAM #ab54481 | Rabbit polyclonal | 1000 fold | |
| PRDM16 | PRDM16 | Patrick Seale | Rabbit polyclonal | 1000 fold |
Animals
All mice were bred and maintained in the MCV/Virginia Commonwealth University animal facility according to Institutional Animal Care and Use Committee regulations. Male mice were used for these studies. Tyk2-deficient mice (C57BL/6) were kindly provided by Dr. Ana Gamero (Temple University School of Medicine, Philadelphia, PA). Mice carrying a transgene encoding the wild type (Tyk2WT) or kinase dead (Tyk2KD) form of Tyk2, including an upstream loxP-flanked stop sequence in the ubiquitously expressed Rosa26 locus were generated. These mice were crossed 5 generations to Tyk2−/− mice on a C57Bl6/J background. Mice expressing the transgenes in BAT were obtained by crossing Tyk2WT or Tyk2KD transgenic animals with mice expressing the Cre recombinase under control of the Myf5 promoter, allowing Myf5 lineage-specific expression of Cre (Tyk2WT TG-Cre+ and Tyk2KD TG-Cre+). Control animals (Tyk2+/+ TG) were obtained by crossing Tyk2WT and Tyk2KD transgenics with a wild type (Tyk2+/+) mouse (on C57Bl6/J background) for 5 generations. Animals from the same mixed background strain generation were compared. Mice were genotyped to confirm the expression of the transgene and the specific cre recombinase.
Cell culture
Brown fat preadipocytes were isolated as previously described (14). Interscapular BAT was isolated from newborn Tyk2+/+, Tyk2−/−, Tyk2−/−+WT, Tyk2−/− +KD mice, minced and subjected to collagenase A digestion (1.5 mg/mL in isolation buffer containing 123 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 5 mM glucose, 100 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 4% bovine serum albumin) for 40 minutes at 37°C. The digested tissue was filtered through a 70-μM filter. Collected cells were centrifuged at 1500 rpm at room temperature for 5 minutes and then resuspended in 1 mL of primary culture medium [Dulbecco’s modified Eagle medium (DMEM), 4500 mg/L glucose Gibco, Carlsbad, CA] containing 20% fetal bovine serum (FBS), 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 1% penicillin-streptomycin), transferred into 12 well plates, and grown in a humidified atmosphere of 5% CO2 and 95% O2 at 37°C. After 3 days of culture, cells were immortalized by infection with puromycin resistance retroviral vector pBabe encoding SV40 Large T antigen. 24 hours after infection cells were split into 10-cm dishes and maintained in primary culture media for the next 24 hours and then subjected to selection with puromycin at a concentration of 2 μg/mL in DMEM with 20% FBS for 1 week.
For differentiation, brown preadipocytes were grown to 100% confluence in the differentiation medium: DMEM containing 4500 mg/L glucose, 10% FBS, 20 nM insulin, and 1 nM triiodothyronine. Fully confluent cells were incubated for 48 hours in differentiation medium supplemented with an induction medium of 0.5 mM isobutylmethylxanthine, 0.5 μM dexamethasone and 0.125 mM indomethacin. After 48 hours of induction, cells were maintained in differentiation medium for 5 days.
For ruxolitinib treatment, cells were maintained in 1 μM ruxolitinib during the course of differentiation.
Constructs and viral transductions
293 T cells, used as packaging cells, were grown in complete DMEM medium containing 10% FBS and 1% penicillin-streptomycin. Cells were transfected in 10-cm dishes using Fugene reagent, 5 μg plasmid (vectors encoding Tyk2 or empty vector murine stem cell virus containing an internal ribosome entry site tagged to a green fluorescent protein), and 5 μg of helper vector (Roche Diagnostics, Indianapolis, IN), according to the manufacturer’s instructions. Media was changed 24 hours after transfection. The virus-containing medium was collected 48 hours after transfection, centrifuged for 10 minutes at 500 × g, and filtered through 0.45-μm filter. Viral supernatants were added to the cells for 24 hours in the presence of Polybrene (8 μg/mL polybrene, Chemicon Int., Temecula, CA), then diluted twice with the fresh medium. The next day, the viral supernatant was removed and replaced by fresh medium. Seven days following infection, GFP-positive cells were sorted by fluorescence-activated cell sorting to 100% purity and culture further for the experiments.
Glucose tolerance test
Mice were fasted overnight (16 hours), and then glucose was injected intraperitoneally (2 mg/g). Blood glucose levels were measured using a One-Touch Ultra glucometer (LifeScan, Milpitas, CA) at 0, 15, 30, 60, and 120 minutes after glucose administration.
Biochemical analysis
Mice were fasted overnight (16 hours). Blood samples were collected by heart punctures. Plasma samples were obtained by centrifuging blood in Microtainer plasma separator tubes (Becton Dickinson, Franklin Lakes, NJ). Plasma was assayed for insulin with an ultrasensitive mouse insulin ELISA kit (Crystal Chem, Downers Grove, IL).
RNA extraction and real-time qPCR
Total RNA was isolated with TRI Reagent (Molecular Research, Cincinnati, OH), according to the manufacturer’s instructions. Isolated RNA samples were treated with DNase (Promega, Madison, WI), complementary DNA was synthesized from 2 μg RNA with the Tetro complementary DNA Synthesis Kit (Bioline, Taunton, MA), and real-time quantitative polymerase chain reaction (qPCR) was performed with the SensiMix SYBR and Fluorescein Kit (Bioline, Taunton, MA) according to manufacturer’s instructions. All the samples were assayed in duplicates and analyzed with a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA).
Histology
BATs were fixed in 10% formalin and were paraffin-embedded. Multiple sections were prepared and stained with hematoxylin and eosin at the Pathology Research Service Facility (Virginia Commonwealth University Medical Center, Richmond, VA).
Immunoprecipitation
BAT was homogenized and extracts were incubated overnight at 4°C with Tyk2, PRDM16, or PGC1-α antibodies plus agarose beads or control IgG plus agarose beads in an IP buffer pH 7.4 (150 mM NaCl, 50mM Tris-HCl, 1% Triton, 1 mM EDTA with added protease and phosphatase inhibitor cocktails (Roche, Indianapolis, IN). Immunoprecipitates were washed 5 times with wash buffer (150 mM NaCl, 50mM Tris-HCl, 1% Triton, protease and phosphatase inhibitor cocktails), separated by SDS-PAGE and blotted with indicated antibodies.
Statistical analysis
Data are expressed as mean ± SEM. Comparisons among more than 2 groups were determined by 1-way analysis of variance. Data are represented from at least 3 independent experiments (as indicated in the figure legend). Significance is indicated by P ≤ 0.05 (*compared toTyk2WT, $compared with Tyk2 −/−, # compared with Tyk2−/− + Tyk2 WT, +compared with Tyk2−/− + Tyk2 KD).
Results
Kinase activity of Tyk2 is dispensable for differentiation of brown adipocytes
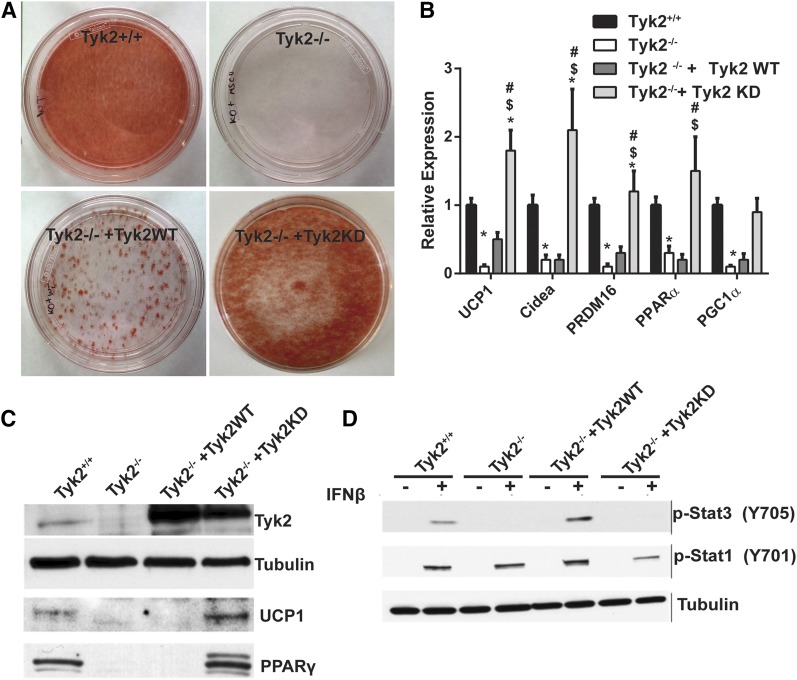
During the course of our experiments to demonstrate that differentiation of immortalized classic brown preadipocytes required the expression of Tyk2, we observed that a kinase inactive from of the enzyme also restored differentiation of Tyk2−/− preadipocytes, as monitored by Oil Red O staining of cells [Fig. 1(A)]. Levels of BAT-selective RNAs were also fully restored in Tyk2−/− preadipocytes expressing Tyk2KD [Fig. 1(B)]. In most experiments, Tyk2KD was more effective than the wild type protein in promoting differentiation. Although the levels of Tyk2WT or Tyk2KD expression in Tyk2−/− cells were approximately the same, they were elevated compared with preadipocytes from wild type mice [Fig. 1(C)]. Tyk2 levels do not change during the course of differentiation [Supplemental Fig. 3(A) (15.4MB, docx) ]. To validate that Tyk2KD does not have cytokine-induced kinase activity, we examined interferon-β (IFN-β)–induced tyrosine phosphorylation of STAT3, which is defective in Tyk2−/− cells (6). IFN-β induced tyrosine phosphorylation of STAT3 when wild type Tyk2 was introduced into Tyk2−/− cells but not in cells expressing Tyk2KD [Fig. 1(D)].
Figure 1.
Expression of kinase-inactive Tyk2 rescues differentiation of Tyk2−/− brown preadipocytes. (A) Wild type or Tyk2−/− immortalized preadipocytes were subject to differentiation (top panels). Tyk2−/− preadipocytes were reconstituted with Tyk2WT or Tyk2KD and differentiation in vitro (bottom panels). All samples were stained with Oil Red O. (B) Expression of BAT-selective RNAs in differentiated brown adipocytes quantitated by RT-PCR. (C) Representative western blot of immortalized Tyk2+/+ or Tyk2−/− preadipocytes reconstituted with Tyk2WT or Tyk2KD (n = 4 independent experiments). (D) IFN-β stimulated phosphorylation of STAT3 in wild type preadipocytes, Tyk2−/− preadipocytes and Tyk2−/− preadipocytes reconstituted with Tyk2 or Tyk2KD. Cells were incubated for 30 minutes with IFN-β prior to preparing cell extracts that were immunoblotted with a STAT3 phospho-specific antibody that recognizes phosphorylation of tyrosine 705. P < 0.05 *compared toTyk2WT, $compared with Tyk2 −/−, #compared with Tyk2−/− + Tyk2 WT, +compared with Tyk2−/− + Tyk2 KD.
Expression of kinase-inactive Tyk2 in BAT protects Tyk2−/− mice from becoming obese
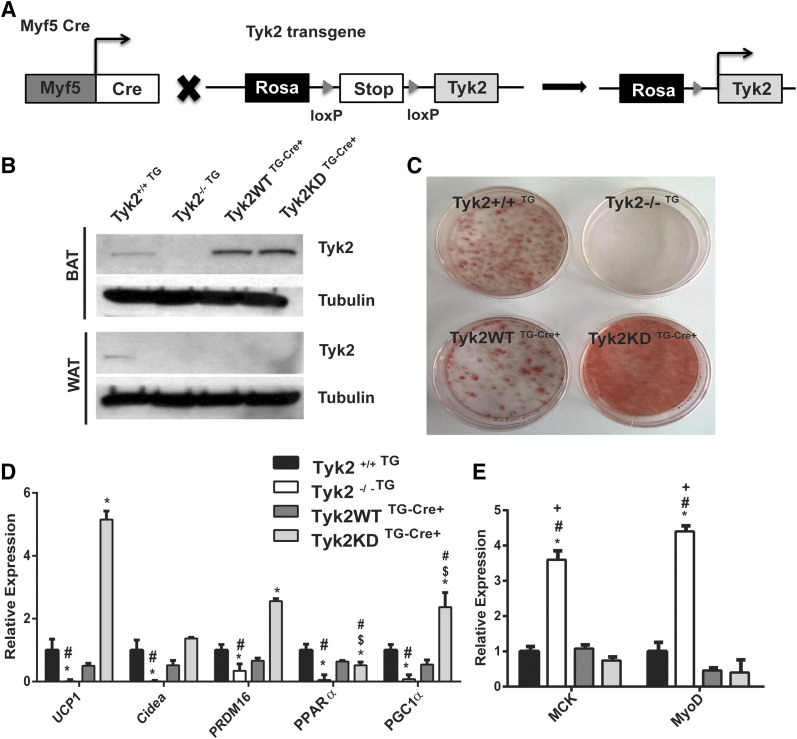
To determine whether Tyk2KD expression in Tyk2−/− mice restores a normal metabolic phenotype, we generated mice expressing either Tyk2WT or Tyk2KD transgenes in Tyk2−/− mice. These transgenes contained an upstream loxP-flanked stop sequence in the ubiquitously expressed Rosa26 locus (7). Mice were crossed with animals expressing Myf5 cre recombinase to obtain mice expressing Tyk2WT (Tyk2WT TG-Cre+) or Tyk2KD (Tyk2KD TG-Cre+) transgene in BAT [Fig. 2(A)]. The Myf5 cre restricts expression of Tyk2 to classic BAT and SKM. Tyk2 transgenes were expressed at slightly elevated levels compared with wild type mice [Fig. 2(B)].
Figure 2.
Tyk2−/− mice expressing a Tyk2KD transgene in BAT and SKM display normal differentiation of classic brown fat cells (A) Schematic representation for generation of the transgenic mouse lines expressing Tyk2WT or Tyk2KD. (B) Representative western blot from 3-month-old mice showing expression of Tyk2 and kinase-inactive Tyk2 transgene in BAT. (C) Primary preadipocytes were isolated from transgenic lines and were subjected to in vitro differentiation and stained with Oil Red O. (D) Total RNA was isolated from in vitro differentiated primary brown preadipocytes and brown fat-specific RNA levels were measured using qPCR. (E) SKM-specific RNAs in differentiated primary brown adipocytes from transgenic lines (n = 4 independent experiments). P < 0.05 *compared toTyk2+/+TG, $compared with Tyk2−/−TG, #compared with Tyk2WT TG-Cre+, +compared with Tyk2KD TG-Cre+.
We isolated classic brown fat preadipocytes and examined their ability to differentiate in vitro by staining with Oil Red O [Fig. 2(C)]. Similar to the Oil Red O staining, differentiated preadipocytes from Tyk2WT TG-Cre+or Tyk2KD TG-Cre+ displayed induced BAT-selective gene expression in differentiated cells [Fig. 2(D)]. However, levels of most but not all BAT-selective RNAs (Cidea, Ppar-α) were substantially elevated in cells expressing Tyk2 KD compared with animals expressing Tyk2WT [Fig. 2(D)]. These results are consistent with the observation that preadipocytes expressing Tyk2KD differentiated more effectively that those expressing Tyk2WT (Fig. 1). The immortalization process does not alter the gene expression profile significantly in these cells [Supplemental Fig. 1(A) (15.4MB, docx) ].
BAT and SKM are derived from common Myf5 progenitors. We have previously observed that the muscle-selective RNAs MyoD and MCK are elevated in Tyk2−/− preadipocytes (5). MyoD and MCK RNAs in differentiated brown preadipocytes from Tyk2WT and Tyk2KD transgenic mice were decreased compared with adipocytes from Tyk2−/− animals subjected to differentiation [Fig. 2(E)]. In contrast to BAT-selective RNAs, there is no significant difference in the levels of MyoD and MCK in preadipocytes expressing Tyk2WT or Tyk2KD. The reasons for this discrepancy are not readily apparent, but suggest that there are subtle differences in the targets of Tyk2, which depend upon the intrinsic kinase activity of the protein.
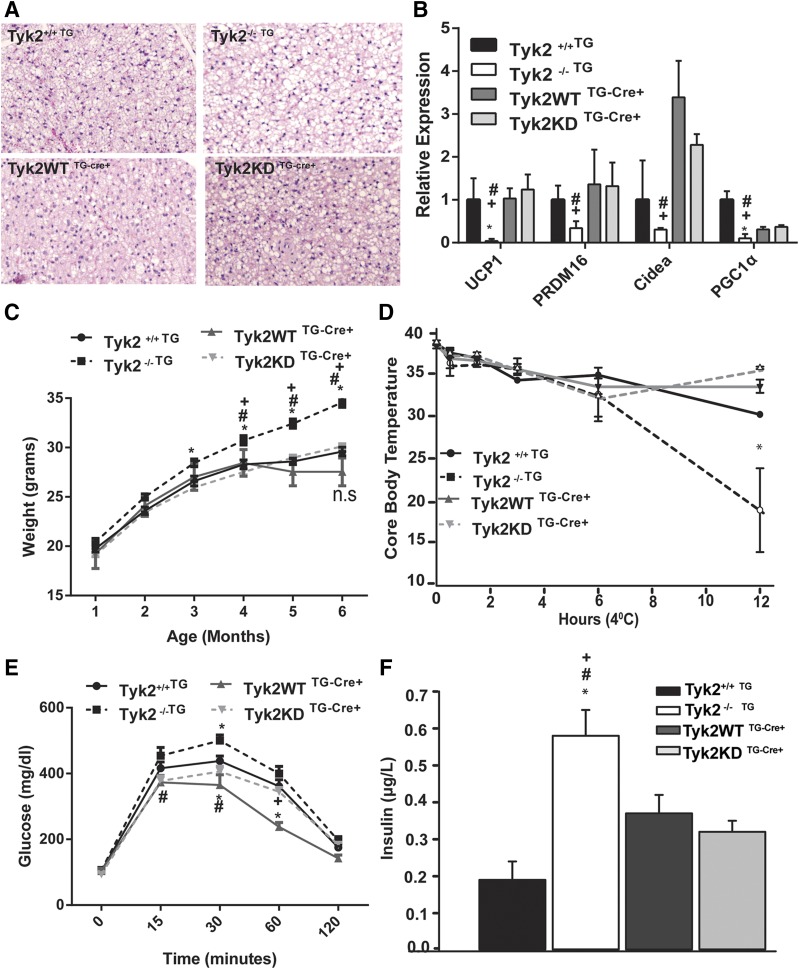
The altered BAT morphology observed in Tyk2−/− mice was restored by Tyk2WT and Tyk2KD expression and resembled the tissue in wild-type animals [Fig. 3(A)]. Expression of Tyk2 WT and Tyk2KD transgene in BAT of Tyk2−/− mice also restored the expression of brown fat-specific genes [Fig. 3(B)] and significantly lowered body weight compared with Tyk2−/− mice [Fig. 3(C)]. In addition to functional defects in BAT, Tyk2−/− mice have defects in maintaining blood glucose, are insulin resistant, are not able to maintain core body temperature, and become obese (5). Restoration of Tyk2WT or Tyk2 KD in BAT and SKM in Tyk2−/− mice is sufficient to revert the metabolic defects seen in Tyk2−/− mice [Fig. 3(D–F)]. We did not observe any larger differences in weights in mice expressing Tyk2WT or Tyk2KD from wild type mice placed on a high-fat diet [Supplemental Fig. 1(C) (15.4MB, docx) ]. Gene expression analysis shows that the rescue of obese phenotype in Tyk2−/− mice is correlated with transgene expression in BAT; however, we do see a similar induction of UCP1 in WAT of both Tyk2WT and Tyk2KD expressing transgenic mice [Supplemental Fig. 1(B) (15.4MB, docx) ]. The contribution of WAT in this phenotype and the role of Tyk2 in browning still needs further investigation.
Figure 3.
Restoring kinase-inactive Tyk2 expression in Myf5 progenitors reverses the obese phenotype of Tyk2−/− mice. (A) Hematoxylin and eosin staining of BAT. (B) Expression of brown fat selective RNAs from BAT, (n = 6 to 13 mice per group). RNA was isolated from BAT of 6-month-old mice and analyzed for RNA levels using qPCR. The values represent mean fold change ± SEM over Tyk2+/+ set as 1. (C) Body weight of transgenic mice measured over a period of 6 months. (D) Body temperatures measured with a rectal thermometer in transgenic mice placed at 4°C for various times (n = 4 to 6 mice per group). (E) Glucose tolerance test in 3-month-old transgenic mice. Mice were fasted for 16 hours and injected intraperitoneally with 2 mg glucose/g body weight. Blood was collected from the tail vein and glucose was measured at the indicated times, (n = 8 to 16 mice per group). (F) Fasted plasma insulin levels of 6-month-old transgenic mice (n = 6 to 8 mice per group). Data are expressed as mean ± SEM. P < 0.05 *compared toTyk2+/+TG, $compared with Tyk2−/−TG, #compared with Tyk2WT TG-Cre+, +compared with Tyk2KD TG-Cre+.
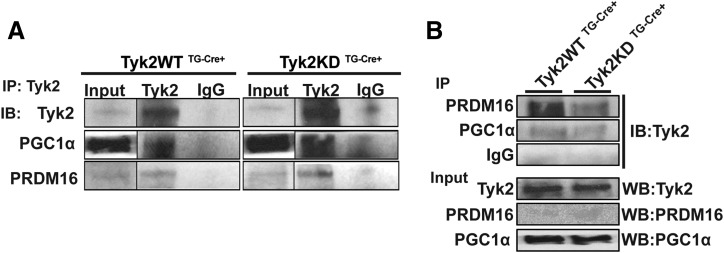
Tyk2 interacts with PGC1-α and PRDM16 in BAT
PGC1-α and PRDM16 play important roles in differentiation of brown adipocytes. It has been well defined that the N-terminal domain of Tyk2 contains a nuclear-localization sequence, and a small fraction of cellular Tyk2 has been shown to localize to the nucleus where it binds to DNA (8). We investigated whether Tyk2 associates with these transcription factors. We immunoprecipitated Tyk2 from BAT of Tyk2WT TG-Cre+or Tyk2KD TG-Cre+ mice and determined its interaction PRDM16 and PGC1-α. As seen in Fig. 4(A) and 4(B), both Tyk2WT and Tyk2KD interact with these transcription factors suggesting that Tyk2 may be a part of the transcriptional complex regulating brown fat differentiation and function. The kinase activity of Tyk2 is not required for these proteins to form a complex, which is consistent with the fact that kinase inactive Tyk2 restores differentiation of Tyk2−/− brown preadipocytes.
Figure 4.
Tyk2 interacts with PRDM16 and PGC1-α. (A) BAT extracts from Tyk2WT TG-Cre+ or Tyk2KD TG-Cre+ mice were immunoprecipitated with Tyk2 or IgG and blotted for Tyk2, PGC1-α and PRDM16. (B) BAT extracts from Tyk2WT TG-Cre+ or Tyk2KD TG-Cre+ mice were immunoprecipitated with either PRDM16, PGC1-α, or IgG. The immunoblots were probed for Tyk2, PRDM16, or PGC1-α. IB, immunoblot; IP, immunoprecipitate (n = 3 independent experiments).
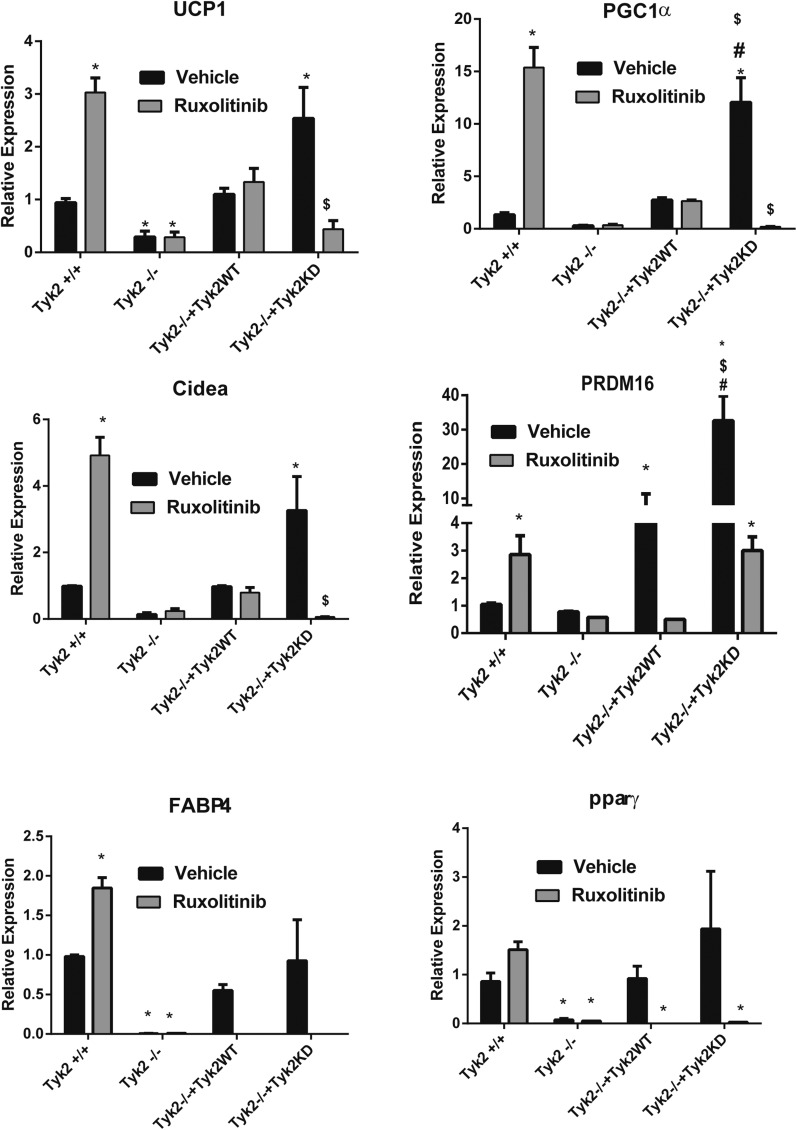
Jak inhibitor Ruxolitinib induces brown fat-specific gene expression in brown adipocytes
There are a few possible mechanisms by which Tyk2KD restores differentiation of brown preadipocytes including that Tyk2KD has a latent kinase activity mediated by the pseudo kinase domain of the protein (9). It is known that expression of Tyk2 is required for assembly of the type I interferon receptor, suggesting that Tyk2KD may function as an adaptor (10). It is also possible that either Jak1 or Jak2 can substitute for Tyk2 when its kinase activity is mutated. To test this possibility we treated Tyk2−/− preadipocytes expressing Tyk2WT or Tyk2KD with the Jak1 and Jak2 inhibitor Ruxolitinib. Tyk2 protein levels do not change in the presence of Ruxolitinib [Supplemental Fig. 3(B) (15.4MB, docx) ]. Surprisingly, the expression of BAT-selective RNAs was significantly elevated in wild type preadipocytes differentiated in the presence of Ruxolitinib (Fig. 5). In Tyk2−/− preadipocytes there was no expression of BAT-selective RNAs (Fig. 5). Tyk2−/− preadipocytes expressing Tyk2WT displayed about the same degree of expression of BAT-selective RNAs as wild type cells, but there was no increase in these RNAs in the presence of Ruxolitinib (Fig. 5). As shown in Fig. 1, Tyk2−/− cells expressing Tyk2KD displayed elevated levels of BAT-selective RNAs. Expression of these RNAs was abolished in cells treated with Ruxolitinib (Fig. 5). IFN-β induces Tyk2 phosphorylation and the kinase active Tyk2 can activate the downstream STAT transcription factors. Treatment of Tyk2+/+ brown adipocytes with IFN-β during differentiation inhibits the induction of BAT-selective RNAs, which can be overcome by treatment with Ruxolitinib [Supplemental Fig. 2(A)]. (15.4MB, docx) Similar to the Tyk2+/+ brown adipocytes, Tyk2−/− preadipocytes expressing Tyk2WT displayed a similar decrease in expression of BAT-selective RNAs with IFN-β which was overcome with Ruxolitinib treatment [Supplemental. Fig. 2(B) (15.4MB, docx) ]. However, Tyk2−/− cells expressing Tyk2KD did not show any further changes in BAT specific gene expression with IFN-β treatment [Supplemental Fig. 2(C) (15.4MB, docx) ].
Figure 5.
Pharmacologic Inhibition of JAKs induces expression of brown fat genes. Tyk2+/+, Tyk2−/−, Tyk2−/−+Tyk2, or Tyk2−/−+Tyk2KD immortalized brown preadipocytes were differentiated in the presence of Ruxolitinib or vehicle. BAT selective RNAs were measured by qPCR. P < 0.05 *compared toTyk2WT, $compared with Tyk2 −/−, #compared with Tyk2−/− + Tyk2 WT, +compared with Tyk2−/− + Tyk2 KD.
Discussion
The data presented in this report highlights a yet unreported kinase-independent role of the Jak Tyk2 in brown fat differentiation and function. Our previous study showed that global deletion of Tyk2 resulted in impaired brown fat development and energy imbalance in mice (5). Expressing kinase inactive Tyk2 in Tyk2−/− brown fat preadipocytes restored their ability to differentiate in vitro, suggesting that Tyk2 may not function as a kinase in brown fat development (Fig. 1).
Cytokine-mediated activation of the Jaks is required not only for tyrosine phosphorylation of the STATs, but also for orchestrating the responses of many other signaling cascades involved in cell growth and differentiation. The Jaks contain both a classic tyrosine kinase domain as well as a pseudo kinase domain. The tyrosine kinase activity of the classic kinase domain is well known to be required for cytokine activation of the Jaks. In contrast to the classic kinase domain, 1 of the actions of the pseudo kinase domain is to repress the activity of the classic kinase domain. Mutation of valine 617 to phenylalanine in the pseudo kinase domain of Jak2 enhances basal tyrosine kinase activity. This mutation is present in many myeloproliferative disorders (11). Mutations in the pseudo kinase domain of Jak1 have also been identified in other hematopoietic malignancies. The mechanisms by which the pseudo kinase domain regulates activity of these kinases are not evident. It has been reported that the pseudo kinase domain of Jak2 is a dual-specificity kinase, which inhibits cytokine signaling (9). Tyk2 gene contains >100 variants (12). Two of these variants have mutations in the catalytic domain, which have been associated with susceptibility to autoimmune disease. However, the variants display normal signaling in response to IFN-α/β, interleukin-10, and interleukin-6 (12). These data, suggest that 1 catalytically competent Jak is sufficient for signaling, provided that its partner functions as a kinase inactive scaffold. Our findings suggest that the numerous reported variants of Tyk2 may contribute to many other pathologies associated with cell growth, differentiation and alterations in innate and adaptive immunity. The substrates for the pseudo kinase domain remain to be identified.
Both Jak2 and Tyk2 have been observed to be in the nucleus, and Jak2 has been reported to phosphorylate histone H3 (8, 13). Our results suggest that Tyk2 may have a nuclear role in brown fat differentiation. Tyk2 interacts with the 2 important transcription factors required for brown fat differentiation, PRDM16 and PGC1-α, in a kinase-independent fashion (Fig. 4). This suggests that kinase activity of Tyk2 is dispensable for its nuclear localization and function in BAT differentiation. Our previous study reported hypermethylation of UCP1 and cidea promoters in Tyk2−/− preadipocytes, suggesting an alternative role of nuclear Tyk2 in chromatin remodeling (5). It is also possible that the pseudo kinase domain of Tyk2 phosphorylates or regulates the phosphorylation of H3 or other substrates in the nucleus.
The results of our studies indicate that the classic tyrosine kinase activity of Tyk2, which is activated by IFN-α/β and other cytokines, is not required for differentiation of brown preadipocytes in vitro (Fig. 5 and Supplemental Fig. 2 (15.4MB, docx) ) and in mice expressing Tyk2KD in BAT and SKM. Not only do mice expressing a Tyk2KD transgene restore classic brown fat cell differentiation, they also restore glucose tolerance, insulin sensitivity, and core body temperature, and they maintain a normal weight (Fig. 3). These results highlight the importance of Tyk2 expression in brown fat to maintain normal metabolism. It should be noted that the Myf5 Cre transgene drives expression of Tyk2 in SKM. It is therefore possible that some of the phenotypic changes observed in our transgenic mice are the result of Tyk2 expression in SKM as well as BAT. Our previous findings indicate that expression of a constitutively active STAT3 transgene in BAT alone is sufficient to restore a normal phenotype in Tyk2−/− mice (5). Because STAT3 is activated byTyk2, these results suggest, but do not prove, that the expression of Tyk2 in SKM is not required to restore a normal metabolic phenotype.
The mechanism by which Tyk2KD restores differentiation of brown fat preadipocytes still remains unclear. Two possibilities are that (a) the pseudo kinase domain of Tyk2, but not the classic activity of the kinase is responsible for differentiation of brown preadipocytes, and (b) Tyk2 heterodimerizes with either Jak1 or Jak2, whose kinase activity can then substitute for wild type Tyk2 to induce differentiation. The later possibility is more attractive because Ruxolitinib has no effect on the expression of BAT-selective RNAs in Tyk2−/− cells expressing wild type Tyk2, but completely inhibited induction of these RNAs in cells expressing Tyk2KD (Fig. 5). In cells expressing Tyk2KD, the activity of Jak1 and Jak2 are blocked by Ruxolitinib so the cell has no classic kinase activity provided by the Jak members. Understanding the complexities of how a kinase-inactive Tyk2 restores function will require defining domains in Tyk2KD required for its function both in differentiation and control of the kinase activity of Jak1 and Jak2.
Acknowledgments
This work was supported by R01 DK099732 (to A.C.L.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- brown adipose tissue
- DMEM
- Dulbecco's modified Eagle medium
- FBS
- fetal bovine serum
- IFN-β
- interferon-β
- qPCR
- quantitative polymerase chain reaction
- SKM
- skeletal muscle
- Tyk2−/− TG
- Tyk2−/− mice carrying inactive transgene
- Tyk2−/−+Tyk2 KD
- Tyk2−/− cells reconstituted with Tyk2 KD construct
- Tyk2−/−+Tyk2 WT
- Tyk2−/− cells reconstituted with Tyk2 WT construct
- Tyk2+/+ TG
- Tyk2-+/+ mice carrying inactive transgene
- Tyk2KD
- Tyk2 that has its classic kinase inactivated by mutating K to R
- Tyk2KDTG-Cre+
- Tyk2−/− mice expressing Tyk2KD under Myf5 cre
- Tyk2WT
- wild type Tyk2
- Tyk2WTTG-Cre+
- Tyk2−/− mice expressing Tyk2 WT under Myf5 cre
- UCP1
- uncoupling protein-1
- WAT
- white adipose tissue
References
- 1.Lee P, Greenfield JR. Non-pharmacological and pharmacological strategies of brown adipose tissue recruitment in humans. Mol Cell Endocrinol. 2015;418(Pt 2):184–190. [DOI] [PubMed] [Google Scholar]
- 2.Gurzov EN, Stanley WJ, Pappas EG, Thomas HE, Gough DJ. The JAK/STAT pathway in obesity and diabetes. FEBS J. 2016;283(16):3002–3015. [DOI] [PubMed] [Google Scholar]
- 3.Richard AJ, Stephens JM The role of JAK-STAT signaling in adipose tissue function. Biochim Biophys Acta. 2014;1842(3):431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, Castellot JJ Jr, Rosen ED, Spiegelman BM. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19(5):810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derecka M, Gornicka A, Koralov SB, Szczepanek K, Morgan M, Raje V, Sisler J, Zhang Q, Otero D, Cichy J, Rajewsky K, Shimoda K, Poli V, Strobl B, Pellegrini S, Harris TE, Seale P, Russell AP, McAinch AJ, O’Brien PE, Keller SR, Croniger CM, Kordula T, Larner AC. Tyk2 and Stat3 regulate brown adipose tissue differentiation and obesity. Cell Metab. 2012;16(6):814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karaghiosoff M, Neubauer H, Lassnig C, Kovarik P, Schindler H, Pircher H, McCoy B, Bogdan C, Decker T, Brem G, Pfeffer K, Müller M. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13(4):549–560. [DOI] [PubMed] [Google Scholar]
- 7.Mesaros A, Koralov SB, Rother E, Wunderlich FT, Ernst MB, Barsh GS, Rajewsky K, Brüning JC. Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell Metab. 2008;7(3):236–248. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed CM, Noon-Song EN, Kemppainen K, Pascalli MP, Johnson HM. Type I IFN receptor controls activated TYK2 in the nucleus: implications for EAE therapy. J Neuroimmunol. 2013;254(1–2):101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ungureanu D, Wu J, Pekkala T, Niranjan Y, Young C, Jensen ON, Xu CF, Neubert TA, Skoda RC, Hubbard SR, Silvennoinen O. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat Struct Mol Biol. 2011;18(9):971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragimbeau J, Dondi E, Alcover A, Eid P, Uzé G, Pellegrini S. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J. 2003;22(3):537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen E, Staudt LM, Green AR. Janus kinase deregulation in leukemia and lymphoma. Immunity. 2012;36(4):529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Gakovic M, Ragimbeau J, Eloranta ML, Rönnblom L, Michel F, Pellegrini S. Two rare disease-associated Tyk2 variants are catalytically impaired but signaling competent. J Immunol. 2013;190(5):2335–2344. [DOI] [PubMed] [Google Scholar]
- 13.Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1a from chromatin. Nature. 2010;461(7265):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR. beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J Biol Chem. 1999;274(49):34795–34802. [DOI] [PubMed] [Google Scholar]