Abstract
The steroid hormone progesterone acting via the nuclear progesterone receptor (PR) isoforms, progesterone receptor A (PR-A) and progesterone receptor B (PR-B), is essential for the maintenance of uterine quiescence during pregnancy. Inhibition of PR signaling augments uterine contractility and induces labor. Human parturition is thought to be triggered by modulation of PR signaling in myometrial cells to induce a functional progesterone withdrawal. One mechanism for functional progesterone withdrawal is increased abundance of PR-A, which decreases progesterone responsiveness by inhibiting the transcriptional activity of PR-B. Human parturition also involves tissue-level inflammation within the myometrium. This study examined the control of PR-A abundance and transrepressive activity in myometrial cells and the role of the inflammatory stimuli in the form of interleukin-1β (IL-1β) and lipopolysaccharide (LPS) in these processes. We found that abundance of PR-A was markedly increased by progesterone and by exposure to IL-1β and LPS via posttranslational mechanisms involving increased PR-A protein stability. In contrast, progesterone decreased abundance of PR-B by increasing its rate of degradation. Together, progesterone and proinflammatory stimuli induced a PR-A–dominant state in myometrial cells similar to that observed in term laboring myometrium. IL-1β and LPS also increased the capacity for PR-A to inhibit the transcriptional activity of PR-B. Taken together, our data suggest that proinflammatory stimuli increase the steady-state levels of PR-A and its transrepressive activity in myometrial cells and support the hypothesis that tissue-level inflammation triggers parturition by inducing PR-A–mediated functional progesterone withdrawal.
Progesterone acting via the nuclear progesterone receptor (PR) isoforms, progesterone receptor A (PR-A) and progesterone receptor B (PR-B), is essential for the establishment and maintenance of pregnancy, and withdrawal of PR-mediated progestational actions is a principal trigger for parturition (1, 2). In all viviparous species examined to date, disruption of PR signaling by administration of PR antagonists such as mifepristone (RU486) or onapristone (ZK98299) increases myometrial contractility and induces labor and delivery at all stages of pregnancy (3). Because parturition in women occurs without a systemic decrease in maternal progesterone levels (4), it is hypothesized that the progesterone withdrawal trigger for human parturition is mediated by altered PR activity in uterine target cells, especially myometrial cells, to cause a functional progesterone withdrawal. Possible mechanisms for functional progesterone/PR withdrawal in myometrial cells include changes in the relative levels and function of PR isoforms (5–8), changes in the activity and level of PR coregulators (9), and metabolism of progesterone to an inactive form that cannot bind the PR (10). The studies described here explore the control of PR isoform abundance and function in human myometrial cells.
Human PR is encoded by a single gene (PGR) controlled by 2 promoters leading to the production of 2 principal isoforms: the full-length PR-B and the truncated (by 164 N-terminal amino acids) PR-A (11). Both receptors bind progesterone with equal affinity and function as ligand-activated transcription factors to affect the expression of distinct cohorts of genes (12–15). Responsiveness to progesterone, therefore, represents the combined transcriptional activity of PR-A and PR-B (16). At some gene promoters, PR-A represses the transcriptional activity of PR-B, an effect referred to as transrepression that is directly related to the abundance of PR-A relative to PR-B (i.e., the PR-A:PR-B ratio) (17). The PR-A:PR-B ratio in human myometrium increases in association with advancing gestation and the onset of labor at term as a result of the increased abundance of PR-A (5–7). In human myometrial cell cultures, PR-A inhibits the transcriptional activity of PR-B (7, 18) suggesting that the labor-associated increase in the PR-A:PR-B ratio decreases myometrial progesterone responsiveness. Those data led to the hypothesis that the functional progesterone withdrawal trigger for human parturition involves increased abundance and transrepressive activity of PR-A in myometrial cells. In this study, we examined factors controlling the abundance and transrepressive activity of PR-A in human myometrial cells.
Multiple studies show that parturition is an inflammatory process and that labor is associated with sterile inflammation within the uterine tissue compartments (myometrium, cervix, and decidua) (19–22). We tested the hypothesis that a functional interaction exists between tissue-level inflammation and the abundance and transrepressive activity of PR-A in myometrial cells such that inflammatory signals induce parturition by affecting myometrial cell PR-A abundance and function. To this end, we used a human myometrial cell line and explant cultures of term myometrium to determine the effect of inflammatory stimuli on PR isoform abundance and activity. We found that proinflammatory stimuli increased PR-A abundance and the PR-A:PR-B ratio by increasing PR-A protein stability. Importantly, proinflammatory stimuli also increased the transrepressive effects of PR-A on PR-B independently of the PR-A:PR-B ratio. Taken together, our findings provide evidence for a functional association between inflammation and PR-A mediated functional progesterone withdrawal. We propose that tissue-level inflammation in response to multiple prolabor/stress signals (e.g., intrauterine infection, fetal stress, uterine wall distention) triggers parturition by augmenting the stability and transrepressive activity of PR-A to induce functional progesterone withdrawal.
Material and Methods
Cell culture and treatments
Generation and characterization of the human telomerase reverse transcriptase-immortalized-human myometrial (hTERT-HM)A/B cell line have been described (23). The cell line was derived from the hTERT-HM cell line, which was produced from a primary culture of myometrial cells derived from a term uterus (24). hTERT-HM and hTERT-HMA/B cells are morphologically identical to primary cultures of myometrial cells derived from term myometrium and express genes typical of a myometrial cell phenotype (e.g., caldesmon, smooth muscle actin, connexin-43, oxytocin receptor) and respond to oxytocin with increased intracellular free Ca2+ and retraction (24, 25). As with primary myometrial cell cultures prepared from term myometrium (26), PGR expression in the cells is low, with only a weak signal for PR-A and none for PR-B that is detectable by immunoblotting. Consequently, the cells are minimally responsive to progesterone under basal conditions (7), which is a recognized limitation of this cell line. The hTERT-HMA/B cell line was therefore produced to overcome the low level of PGR expression and as a model in which the function of PR-A and PR-B could be examined in a myometrial cell context. To do this, hTERT-HM cells (provided by Dr William Rainey, Medical College of Georgia at Augusta University, Augusta, GA) (24) were genetically modified to express PR-A and PR-B from a stably incorporated transgene controlled by independent promoters. In hTERT-HMA/B cells, abundance of PR-A is controlled by the Tet-On Advanced Inducible Expression System (Takara Bio USA, Mountain View, CA), in which expression of a PR-A transgene is induced by doxycycline (DOX; Takara Bio USA), and abundance of PR-B is controlled by the RheoSwitch system (New England Biolabs, Ipswich, MA) in which expression of a PR-B transgene is induced by diacylhydrazine (DAH; Alinda Chemical, Riga, Latvia). The PR-A and PR-B transgenes are silent in the absence of the DOX and DAH inducers and activated within 12 to 24 hours in a DOX and DAH dose-dependent manner. Thus, the PR-A:PR-B ratio can be experimentally controlled. hTERT-HMA/B cells were cultured in Dulbecco’s modified Eagle medium/Ham’s F12 (1:1) supplemented with 5% fetal bovine serum, 1% penicillin-streptomycin, 0.1 mg/mL geneticin, and 2 mM l-glutamine (Life Technologies, Grand Island, NY) at 37°C in a 5% CO2 humidified incubator.
Transfection
For DNA transfection, hTERT-HMA/B cells at 80% confluence were harvested by trypsinization, pooled into an electroporation cuvette at a concentration of 1 × 106 cells/100 µL of mammalian smooth muscle cell transfection solution (Lonza, Walkersville, MD) containing the DNA to be transfected, and subjected to nucleofection using program A-033 in an Amaxa Nucleofector apparatus (Lonza). The cells were then replated and allowed to stabilize for 16 hours before exposure to experimental conditions.
Myometrial tissue and explant culture
Full-thickness uterus was obtained with informed consent (Institutional Review Board approval no. 11-06-04) from women undergoing a scheduled term (≥ 39 weeks of gestation) cesarean section delivery performed at MacDonald Women’s Hospital, University Hospitals Case Medical Center, Cleveland, OH. Patients lacked signs of infection or known pregnancy complications and were clinically defined as not in labor on the basis of intact membranes, a closed cervix, and a quiescent uterus. After delivery of the placenta, myometrium (1–2 cm3) was excised from the upper incisional margin of the lower uterine segment and immediately washed in ice-cold phosphate-buffered saline (PBS). Myometrium was carefully isolated from connective tissue, perimetrium, decidua, and blood clots by microscope-aided dissection, and 1–2 mm3 fragments were used for explant tissue culture. Immediately after dissection, myometrium fragments were incubated in phenol red–free Dulbecco's modified Eagle medium at 37°C in a 5% CO2 incubator for up to 24 hours in the presence or absence of test substances.
RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was isolated from hTERT-HMA/B cells using the Total RNA Isolation/NucleoSpin RNA II kit (Macherey-Nagel, Bethlehem, PA), treated with DNase (DNA-free; Life Technologies), and quantified by absorbance at 260 nM. RNA (300 ng) was then reverse transcribed with random hexamer priming using Superscript III Random Prime Synthesis kit for real-time polymerase chain reaction (RT-PCR) (Thermo Fisher Scientific, Grand Island, NY). Aliquots of complementary DNA were then used as a template for quantitative RT-PCR (qRT-PCR) with primer sets for FK506 binding protein-5 (FKBP5; forward: 5′ ATGCCATTTACTGTGCAAACCAG 3′ and reverse: 5′ AAGAGAGTTGCATTCGAGGGAA 3′) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA (5′ TTGCCATCAATGACCCCTTCA 3′ and 5′ CGCCCCACTTGATTTTGGA 3′). Assays were optimized and validated by confirming that single amplicons of correct size and sequence were generated and that priming and amplification efficiencies of all primer pairs were identical. PCR was performed on an ABI PRISM 7500 sequence detector (Thermo Fisher Scientific, Waltham, MA) with SYBR Green I (Thermo Fisher Scientific) as the fluorescent indicator. Abundance of FKBP5 messenger RNA (mRNA) relative to GAPDH mRNA was calculated using the ΔCT method [relative mRNA abundance = 2 − (CT gene of interest − CT GAPDH)].
Immunoblot analysis
Myometrium tissue explants were homogenized using a bead mill (Bullet Blender STORM; Next Advance, Averill Park, NY) in radioimmunoprecipitation assay buffer (Sigma, St. Louis, MO) supplemented with protease (Roche, Indianapolis, IN) and phosphatase inhibitors (Sigma), and then centrifuged at 16,000 × g for 10 minutes at 4°C. hTERT-HMA/B cells were washed in PBS, collected by scraping and centrifugation, and then lysed in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors, and centrifuged at 16,000 × g for 10 minutes at 4°C. Supernatants were assayed for protein content using the bicinchoninic acid protein assay (Thermo Fisher Scientific). Lysates containing equal amounts of protein were diluted in gel loading buffer [375 mM Tris-HCl, 6% sodium dodecyl sulfate (SDS), 48% glycerol, 9% β-mercaptoethanol, and 0.03% bromophenol blue, pH 6.8], heated for 5 minutes at 100°C, and subjected to denaturing SDS polyacrylamide gel electrophoresis on precast 4% to 20% tris-glycine polyacrylamide gels with the Novex electrophoresis system (Life Technologies). Proteins were then transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). For immunodetection, membranes were first incubated in blocking buffer [5% nonfat milk in tris-buffered saline containing 0.1% Tween-20 (Fisher Scientific, Pittsburgh, PA) (TBST)] at room temperature for 1 hour and then with primary antibodies overnight at 4°C. Antibodies included Dako catalog no. M3568 (Agilent Technologies, Santa Clara, CA) at a 1:750 dilution, which detects PR-A and PR-B with equal affinity; Santa Cruz Biotechnology catalog no. sc-32233 (Santa Cruz, CA) at a 1:50,000 dilution, which detects GAPDH; and Cell Signaling Technology catalog no. 2146 (Danvers, MA) at a 1:10,000 dilution, which detects β-tubulin (Table 1). The following day, membranes were washed 3 times with TBST and incubated at room temperature for 1 hour with horseradish peroxidase–conjugated anti-mouse immunoglobulin G or anti-rabbit immunoglobulin G antibodies. After 3 washes with TBST, immunoreactive proteins were visualized using the HyGlo Chemiluminescent HRP Antibody Detection Reagent (Denville Scientific, South Plainfield, NJ). Chemiluminescence was detected and quantified with the FluorChem E processor (ProteinSimple, San Jose, CA).
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| PR-A and PR-B | 1294 | Dako, M3568 | Monoclonal mouse | 1:750 | AB_2252608 | |
| GAPDH | GAPDH | Santa Cruz Biotechnology, sc-32233 | Monoclonal mouse | 1:50,000 | AB_627679 | |
| β-tubulin | β-tubulin | Cell Signaling Technology, 2146 | Polyclonal rabbit | 1:1000 | AB_2210545 |
Abbreviation: RRID, Research Resource Identifier.
Luciferase assay
Lysate was prepared from hTERT-HMA/B cells transiently transfected with a firefly luciferase reporter DNA controlled by tandem progesterone response elements (PREs) [PRE2 luciferase (PRE2-LUC) provided by Dr Zafar Nawaz, University of Miami Sylvester, Braman Family Breast Cancer Institute, Miami, FL] and a renilla luciferase (REN-LUC) DNA (Promega, Madison, WI) at a 10:1 molar ratio. Luciferase activity was measured with the Dual-Luciferase Reporter Assay (Promega) with luminosity assayed using a GloMax 20/20 Luminometer (Promega). Data were normalized to REN-LUC activity.
Statistical analysis
Data shown are representative of 3 or more time-separated experiments and are expressed as mean ± standard error of the mean. Data were subjected to a normalcy test and groups were compared by the Wilcoxon rank-sum test. Some normally distributed data were compared by analysis of variance and Student t test. Differences were considered statistically significant when P < 0.05.
Results
Effect of progesterone on PR isoform abundance
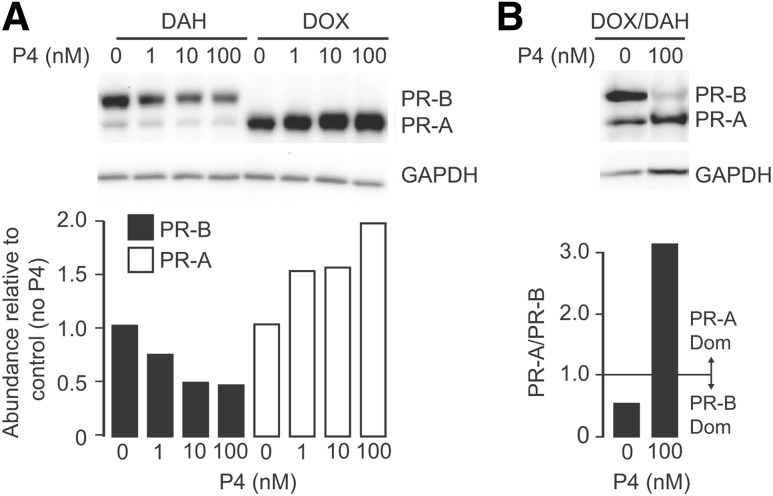
In hTERT-HMA/B cells exposed to DOX or DAH to induce expression of the PR-A and PR-B respectively, progesterone (Sigma; 100 nM) increased the abundance of PR-A and decreased the abundance of PR-B [Fig. 1(A)]. In cells exposed to DOX and DAH to produce a PR-B-dominant state (i.e., PR-A:PR-B < 1), the presence of progesterone for 24 hours increased PR-A and decreased PR-B and converted the PR-A:PR-B ratio from PR-B-dominance to PR-A-dominance [i.e., PR-A:PR-B > 1; Fig. 1(B)]. PR mRNA abundance, assessed by qRT-PCR, was not affected by progesterone (Supplemental Fig. 1 (258.9KB, docx) ). These outcomes suggest that progesterone affected PR-A and PR-B protein abundance via posttranslational mechanisms.
Figure 1.
Effect of progesterone PR-A and PR-B abundance in hTERT-HMA/B cells. (A) Representative immunoblot analysis of PR-A, PR-B, and GAPDH in whole cell lysate from hTERT-HMA/B cells treated with DOX (30 ng/mL) or DAH (400 nM) for 48 hours to induce PR-A or PR-B, respectively, and then exposed to vehicle or progesterone (P4; 1 nM, 10 nM, or 100 nM) for the final 16 hours. Histogram shows relative levels of PR-A and PR-B in each condition. (B) Immunoblot analysis of PR-A, PR-B, and GAPDH in whole cell lysate from hTERT-HMA/B cells treated with DOX (80 ng/mL) or DAH (200 nM) for 48 hours to induce PR-A and PR-B, respectively, and exposed to vehicle or progesterone (P4) for the final 24 hours. Histograms show relative levels of PR-A and PR-B in each condition. Note that progesterone treatment induced transition from PR-B dominance to PR-A dominance.
To determine whether progesterone affects the turnover of PR-A and PR-B in hTERT-HMA/B cells, immunoblot assays were performed to determine the effect of progesterone on the rate of PR-A and PR-B decay after washing cells thoroughly to remove the DOX and DAH inducers (Fig. 2). After removal of DOX and DAH, and in the absence of progesterone, PR-A and PR-B abundance decreased such that by 48 hours, only 10% to 20% of the initial PR-A and PR-B remained. In PR-A–expressing cells, progesterone increased PR-A (by 10% to 20%,relative to levels immediately after DOX removal) during the first 12 hours, leading to an upward shift in the rate of PR-A decay and to an increase in the PR-A half-life (t1/2) from ∼16 hours in the absence of progesterone to ∼26 hours in the presence of progesterone. After the initial increase in PR-A abundance induced by progesterone, the decay of PR-A in progesterone- and vehicle-treated cells was similar, but the absolute level of PR-A remained higher in progesterone-treated cells compared with vehicle-treated cells for the remainder of the time course. In PR-B–expressing cells, progesterone increased the rate of PR-B decay (t1/2 ∼11 hours) compared with vehicle-treated cells (t1/2 ∼17 hours), leading to a net decrease in PR-B abundance.
Figure 2.
Effect of progesterone on PR-A and PR-B turnover. (A) Representative immunoblot analyses of PRs and GAPDH in hTERT-HMA/B cells induced to express PR-A (left) by exposure with DOX (100 ng/mL) or PR-B (right) by exposure to DAH (300 nM) for 48 hours and then, after removal of the inducers, exposed to progesterone or vehicle for the indicated times. Histograms show mean (± standard error of the mean; n = 3) PR/GAPDH levels between vehicle- and progesterone-treated cells at various times. Decay curves show the half-life (T1/2) of the PRs.
To determine whether PR isoform decay was mediated by the proteasome, PR-A and PR-B decay was monitored in the presence and absence of the proteasome inhibitor, MG132 (Sigma). Inhibition of proteasome activity augmented the stability (i.e., delayed the turnover) of PR-A and PR-B, indicating that PR turnover in myometrial cells is caused by proteasome-mediated degradation (Fig. 3).
Figure 3.
Role of the 26S proteosome in PR-A and PR-B degradation. Immunoblot analysis of PRs and GAPDH in hTERT-HMA/B cells induced to express PR-A and PR-B with DOX (100 ng/mL) and DAH (300 nM), respectively, and then washed and exposed to progesterone (100 nM) ± the 26S proteasome inhibitor MG132 (10 µM) for 16 hours and 24 hours.
Effect of proinflammatory stimuli on PR isoform stability
Abundance of PR-A in the human pregnancy myometrium increases with advancing gestation, leading to a labor-associated increase in the PR-A:PR-B ratio (7, 8). Because tissue-level inflammation is an integral component of the parturition cascade (20, 22), we hypothesized that proinflammatory stimuli associated with tissue-level inflammation increase PR-A abundance in myometrial cells by augmenting the stability of PR-A. To test this hypothesis, PR decay studies were performed in hTERT-HMA/B cells induced to express equivalent levels of both PRs by exposure to DOX and DAH for 48 hours. The cells were then washed in PBS 3 times to ensure removal of the inducers and exposed for various times to either progesterone (100 nM) alone or to progesterone and IL-1β (IL-1β; Cell Signaling Technology; 1 ng/mL) or LPS (Escherichia coli 055:B5; Sigma; 1 µg/mL). As with decay studies of cells expressing only PR-A (Fig. 2), progesterone increased PR-A abundance during the first 4 to 8 hours, and this was not affected by IL-1β or LPS. However, compared with cells exposed to progesterone alone, IL-1β and LPS decreased PR-A decay after 4 to 8 hours, leading to a significant increase in PR-A abundance at 24 and 48 hours relative to cells exposed to progesterone alone [Fig. 4(A)]. PR-B decay was slightly increased by IL-1β and LPS at 24 hours and 48 hours, but overall, the effect of IL-1β and LPS on PR-B t1/2 was small. The abundance of mRNAs encoding the PRs was not affected by IL-1β or LPS with or without progesterone and decreased to preinduction levels within 6 to 12 hours (Supplemental Fig. 1 (258.9KB, docx) ).
Figure 4.
Effect of IL-1β and LPS PR-A and PR-B abundance in hTERT-HMA/B cells. (A) Representative immunoblot analysis of lysate from hTERT-HMA/B cells treated with DOX (100 ng/mL) and DAH (300 nM) for 48 hours to induce expression of PR-A and PR-B and then washed and exposed to progesterone (P4; 100 nM) and IL-1β (1 ng/mL) or LPS (1 μg/mL) for various times. Histogram shows mean (± SEM; n = 3) relative abundance of PR-A and PR-B at various times after inducer withdrawal (*P < 0.05). (B) Immunoblot analysis of lysate from hTERT-HMA/B cells treated with DOX and DAH to express PR-A and PR-B in the presence of vehicle or 10 nM R5020 with increasing concentrations of IL-1β for 24 hours. Histogram shows effect of R5020 and IL-1β on the relative abundance of PR-A and PR-B and the PR-A:PR-B ratio. Immunoblotting for tubulin was used to monitor protein loading.
To further assess the effects of inflammatory stimuli on PR-A and PR-B protein stability, hTERT-HMA/B cells were induced to express PR-A and PR-B by exposure to DOX and DAH in the presence and absence of the progesterone agonist R5020 [promegestone: 17,21-dimethylpregna-4,9(10)-diene-3,20-dione; PerkinElmer, Waltham, MA] and increasing amounts of IL-1β for 24 hours. As expected, without inflammatory stimuli, R5020 increased the abundance of PR-A and decreased the abundance of PR-B, converting the PR-A:PR-B ratio from PR-B dominance to PR-A dominance [Fig. 4(B)]. R5020 also decreased the mobility of PR-A and PR-B and resulted in a doublet PR-B, which was indicative of ligand-induced phosphorylation. IL-1β further increased the abundance of PR-A in a dose-dependent manner but did not affect the abundance of PR-B. Thus, IL-1β increased the PR-A:PR-B ratio in myometrial cells in a milieu of a high progestin, analogous to human pregnancy.
Studies were also conducted in explant cultures of myometrium obtained from the lower uterine segment of women undergoing elective cesarean section delivery. In this model system, abundance of PR isoforms and PR mRNA rapidly decrease to almost undetectable levels within 3 to 12 hours (data not shown), and we previously determined that tissue viability is maintained for up to 72 hours (27). Decay of PR isoforms was monitored after treatments of explants with progesterone and IL-1β, or LPS, for 30 minutes, 6 hours, and 24 hours (Fig. 5). At 30 minutes, IL-1β stabilized both PR isoforms, whereas LPS appeared to slightly stabilize PR-A. At 6 and 24 hours, only PR-A remained when progesterone and either proinflammatory stimulus was present. Unlike its effect in hTERT-HMA/B cells, progesterone alone did not affect PR-A decay in myometrium explant cultures.
Figure 5.
Effect of IL-1β and LPS on PR-A abundance in myometrium explants. Immunoblot analysis of PR-A and PR-B levels in response to P4 (100 nM) and IL-1β (1 ng/mL) or LPS (1 µg/mL) in myometrial explants after 30 minutes, 6 hours, or 24 hours of treatment. Each time represents myometrium from different women. Histogram shows relative levels of PR-A at the 24-hour time point.
Effect of inflammation on PR-A transrepression of PR-B
The PR isoform switching (or PR-A/PR-B) hypothesis for functional progesterone withdrawal posits that increased PR-A abundance in myometrial cells causes functional progesterone withdrawal via PR-A–mediated transrepression of PR-B. Given that proinflammatory stimuli increased the PR-A:PR-B ratio in hTERT-HMA/B cells to favor PR-A-dominance, we aimed to determine whether this affected the capacity for PR-A to transrepress PR-B. hTERT-HMA/B cells induced to express equivalent levels of PR-A and PR-B were transiently transfected with PRE2 luciferase and REN-LUC and then exposed for 24 hours to progesterone (100 nM) or vehicle with or without increasing concentrations of IL-1β (25 to 100,000 pg/mL) or LPS (1 to 1000 ng/mL). As expected, progesterone increased luciferase activity in cells expressing only PR-B, and this effect was inhibited by coexpression of PR-A, demonstrating the transrepressive activity of PR-A. Exposure of PR-A/B–expressing cells to progesterone and IL-1β and LPS further decreased luciferase activity, suggesting that the transrepressive activity of PR-A was increased by the inflammatory stimuli [Fig. 6(A)]. There was no significant difference in luciferase activity in cells expressing only PR-B upon treatment with progesterone and IL-1β or LPS, which indicated that IL-1β and LPS augmented the transrepressive activity of PR-A rather than directly inhibiting of PR-B.
Figure 6.
Effect of IL-1β and LPS on PR-A transrepression of PR-B. (A) Relative luciferase activity [mean ± standard error of the mean (SEM); n = 3] in hTERT-HMA/B cells transiently transfected with PRE2-LUC and REN-LUC, and then treated with DAH ± DOX to induce PR-B alone and PR-B and PR-A expression, respectively, for 48 hours. Progesterone (P4; 100 nM) ± increasing levels of IL-1β (25 pg/mL, 100 pg/mL, 1 ng/mL, or 100 ng/mL) or LPS (1 ng/mL, 10 ng/mL, 100 ng/mL, or 1 µg/mL) were added for the final 24 hours. (B) Relative luciferase activity (mean ± SEM; n = 3) in hTERT-HMA/B cells transiently transfected with PRE2-LUC and REN-LUC, and then treated with DAH (200 nM) and increasing levels of DOX (1× = 10 ng/mL; 2× = 50 ng/mL; or 20× = 200 ng/mL) to induce a fixed amount of PR-B with increasing amounts of PR-A, respectively, for 48 hours. Progesterone (P4; 100 nM) was added for the final 24 hours, and vehicle, IL-1β (1 ng/mL), or LPS (1 µg/mL) for the final 30 minutes (*P < 0.05). Immunoblot shows PR-A and PR-B levels achieved at the end of the experiment. (C) Effect of progesterone (100 nM for 24 hours) and IL-1β (1 ng/mL for the final 4 hours of incubation) on the abundance of FKBP5 mRNA in hTERT-HMA/B cells conditioned to be PR-B dominant (left), PR-A dominant (middle), or PR-A/B equivalent (right). Upper panel shows qRT-PCR analysis of FKBP5 mRNA relative abundance. Lower panel show immunoblot analysis of PR isoforms and GAPDH in each experimental condition (n = 3; *P < 0.05).
We next determined whether increased transrepressive activity of PR-A in myometrial cells induced by IL-1β and LPS was due to increased PR-A abundance and/or increased PR-A transrepressive activity independent of the PR-A:PR-B ratio. For these studies, hTERT-HMA/B cells were transfected with the PRE2-LUC and REN-LUC reporters and induced to express a fixed amount of PR-B and increasing amounts of PR-A by increasing the DOX concentration 1×, 5×, or 20×, resulting in an average PR-A:PR-B of 7:1, 26:1, and 61:1, respectively. Cells were then exposed to progesterone or vehicle for 24 hours and IL-1β or LPS was added for the final 30 minutes before luciferase activity was measured. At the lowest PR-A:PR-B ratio (7:1), exposure to IL-1β and LPS for 30 minutes significantly enhanced the transrepressive activity of PR-A [Fig. 6(B)]. LPS, but not IL-1β, also increased the transrepressive activity of PR-A activity when the level of PR-A:PR-B was increased to 26:1. At a large excess of PR-A (PR-A:PR-B of 61:1), PR-A transrepression was maximal and was not further increased by IL-1β and LPS. PR isoform levels were not significantly changed by the 30 minutes of treatment with the proinflammatory stimuli.
Effects of IL-1β on PR-A transrepression of PR-B were also determined at an endogenous progesterone/PR-B target gene, FKBP5, which we previously showed is a progesterone/PR-B–responsive gene in hTERT-HMA/B cells (23). In hTERT-HMA/B cells that were induced to express predominantly PR-B, progesterone (100 nM for 24 hours) increased the abundance of mRNA encoding FKBP5, and this was not affected by IL-1β (1 ng/mL; for the final 4 hours). In cells expressing only PR-A, progesterone with or without IL-1β had no effect on FKBP5 mRNA abundance. In cells expressing PR-A and PR-B, the induction of FKBP5 expression by progesterone via PR-B was not affected by PR-A. However, exposure to IL-1β for the final 4 hours significantly (P < 0.05) decreased progesterone/PR-B–induced FKBP5 mRNA abundance [Fig. 6(C)]. Thus, as shown with our PRE2-LUC studies, IL-1β increased the capacity for PR-A to inhibit progesterone/PR-B transcriptional activity at an endogenous target gene.
Discussion
Appropriate progesterone/PR signaling is essential for the establishment and maintenance of pregnancy, and withdrawal of progesterone or disruption of PR signaling induces parturition. Because labor and delivery in women occur without a systemic decrease in maternal progesterone levels, it is hypothesized that human parturition is triggered by a physiologically controlled modulation of PR signaling that causes functional progesterone withdrawal. One mechanism for functional progesterone withdrawal is by PR-A–mediated transrepression of PR-B due, in part, to increased PR-A abundance (5–8). In this context, understanding the factors controlling the abundance and transrepressive activity of PR-A in human myometrial cells is critical for unraveling the hormonal control of human parturition.
Steady-state levels of PR-A and PR-B are the net effect of PR synthesis (PGR expression controlled by the PR-A and PR-B promoters and PR mRNA processing/translation) and the rate of PR protein degradation. Therefore, the labor-associated increase in PR-A protein in the human myometrium at term could be a result of increased transcriptional activity of PGR from the PR-A promoter, increased stability of PR mRNA by progestins (28), and/or decreased degradation of the PR-A protein. Previous studies have suggested that PR-A mRNA levels (estimated by qRT-PCR) in term myometrium increase several-fold with the onset of term labor, which suggests that PR-A promoter activity in myometrial cells is increased as part of the parturition process (6, 8). PR-A transcription levels in term myometrium are increased by prostaglandins (PGs) (29) and by activating epigenetic modifications of histones such as acetylation and methylation at the PR-A promoter (8, 30, 31). However, the PCR-based assay used to measure PR-A transcripts is problematic because it cannot discriminate between transcripts encoding PR-A and PR-B (the PR-A sequence is included in the longer PR-B transcript). We observed that in hTERT-HMA/B cells that are conditioned to express only PR-A (therefore avoiding the problem of PR-A mRNA ambiguity in the qRT-PCR assay), progesterone and proinflammatory stimuli (IL-1β and LPS) increased PR-A protein levels without a concordant increase in the abundance of PR-A mRNA (Supplemental Fig.1 (258.9KB, docx) ). Those findings led us to hypothesize that PR-A abundance in myometrial cells can also be affected by posttranslational processes such as changes in PR-A stability/turnover. We therefore conducted the current study to examine whether progesterone and inflammatory stimuli affect the steady-state level of PR-A in human myometrial cells via changes in PR-A protein stability/turnover. PR isoform stability is typically measured by pulse chase experiments, in which the PR isoforms are radiolabeled, followed by a chase of media containing ligands for defined time periods, and immunoprecipitated protein is monitored for decay by immunoblotting (32). Alternatively, cell lines with stably expressed PRs (using exogenous promoters) are monitored for protein decay after ligand treatment with immunoblotting (33). Differential PR isoform stability has not been examined in myometrial cells. We used our myometrial cell line, hTERT-HMA/B, to study the properties of each isoform independently of transcriptional contributions from the endogenous PR promoters.
Studies of PR protein turnover, performed mainly in breast cancer cell lines, have shown that degradation of the PRs through the 26S proteasome is affected by posttranslational modifications, especially site-specific serine phosphorylation and ubiquitination/sumoylation, in a cell-type and context-dependent manner (34). PR-A stability is mediated by phosphorylation of serine 390 by glycogen synthase kinase-3β in the Brca-1–deficient mammary gland (32), whereas p38-mitogen–activated protein kinase activation stabilizes PR-A in MDA-MB-231 breast cancer cells and endometrial (Ishikawa) cells (33). In T47D breast cancer cells, ligand binding induces phosphorylation of PR-B on serine 294, which augments its transcriptional activity and promotes its ubiquitination and degradation by the 26S proteasome (35). This causes a paradoxical decrease in PR-B abundance in response to progesterone, even though transcriptional activity is increased. In contrast, phosphorylation of PR-A at serine 294 causes its sumoylation, which increases its stability by preventing its degradation by the proteasome (36). In our studies, inhibition of the 26S proteasome almost completely inhibited the decay of PR-A and PR-B in hTERT-HMA/B cells, which suggests that proteasomal processing is the principal pathway for PR protein degradation (Fig. 3). We also found that progesterone decreased the rate of PR-A and PR-B migration in SDS–polyacrylamide gel electrophoresis [e.g., Figs. 1 and 4(B)], which is indicative of ligand-induced phosphorylation. Consistent with breast cancer cell studies, progesterone decreased the abundance of PR-B and increased the abundance of PR-A in hTERT-HMA/B myometrial cells, which suggests that the ligand-induced processes that affect PR isoform turnover are conserved across multiple cell types. Thus, we propose that in response to progesterone, PR-A and PR-B are differentially phosphorylated in myometrial cells such that proteasomal degradation of PR-B is increased, whereas proteasomal degradation of PR-A is decreased.
To investigate PR processing mechanisms in greater detail, decay studies were performed that took advantage of the inducible PR transgenes in hTERT-HMA/B cells (Fig. 2). In hTERT-HMA/B cells, PR-B resides mainly in the nucleus regardless of its liganded state, whereas PR-A is predominantly cytoplasmic in the absence of progesterone and translocates to the nucleus in response to progesterone (data not shown). As expected, progesterone increased the rate of PR-B decay (t1/2 of ∼17 hours for vehicle-treated cells vs t1/2 of ∼11 hours for progesterone-treated cells). This is consistent with ligand-induced phosphorylation of PR-B, which increases the efficiency with which it is degraded by the proteasome. In contrast, we found that progesterone increased the t1/2 of PR-A by approximately 10 hours (t1/2 of ∼26 hours in progesterone-treated cells vs ∼16 hours in vehicle-treated cells). Interestingly, progesterone increased the abundance of PR-A to 10% to 20% above levels that were measured immediately after DOX removal [i.e., time-0 (t0)] during the first 8 to 12 hours of progesterone exposure, but thereafter, the rate of PR-A decay was similar in both progesterone- and vehicle-treated cell conditions. One possible explanation for this dynamic is that PR-A stability was increased during its transitions from the unliganded to the liganded state, which involves a conformational change and dissociation from cytoplasmic chaperones, dimerization, and translocation to the nucleus. Once in the nucleus, processing of PR-A may be identical to that of the cytoplasmic form, causing the liganded and unliganded decay curves to be parallel. The initial transient increase in PR-A abundance would cause a right-shift in the PR-A decay curve. It should be noted, however, that in vivo and especially during pregnancy, when progesterone levels are high [100 to 200 ng/mL (318 to 636 nM; Kd of the PR, ∼1nM) (4)], the cytoplasmic pool of PR-A is constantly exposed to ligand and replenished by PGR expression, and as such, effects of any cytoplasm-to-nuclear transition on PR-A stability would be continuous and would not explain the labor-associated increase in PR-A. This also would explain why progesterone alone had no effect on PR-A abundance in term myometrium explants (Fig. 5), given that the tissues were derived from the high progesterone environment.
We therefore hypothesized that other factors, especially proinflammatory stimuli, promote the parturition-associated increase in PR-A in myometrial cells. To test this, we measured the effects of IL-1β and LPS on PR-A and PR-B stability/turnover and found that both factors increased PR-A stability in progesterone-treated hTERT-HMA/B cells and in explant cultures of term myometrium but had minimal effects on PR-B. These data suggest that inflammatory stimuli promote the labor-associated increase in PR-A abundance in term myometrium. We next examined whether IL-1β and LPS also affected the function of PR-A and especially its transrepression of PR-B.
Multiple studies in a variety of cell types show that PR-A transrepression of PR-B is directly related to its levels relative to PR-B (i.e., the PR-A:PR-B ratio). The increase in PR-A induced by proinflammatory stimuli would therefore be expected to decrease PR-B transcriptional activity in myometrial cells. To address this issue, we examined the effect of IL-1β and LPS on the capacity for PR-A to transrepress PR-B and found that IL-1β and LPS increased PR-A transrepressive activity and that this effect was independent of the PR-A:PR-B ratio (Fig. 6). In fact, exposure to IL-1β and LPS for only 30 minutes increased the transrepressive activity of PR-A without changing the PR-A:PR-B ratio [Fig. 6(B)]. Importantly, IL-1β also increased the transrepressive activity of PR-A at the promoter of a progesterone/PR-B–responsive gene, FKBP5, suggesting that the PRE2-LUC readout is applicable to at least a subset of PR-B–responsive genes. This was also confirmed with another PR-B–responsive gene, AC069155.1, for which transrepressive activity of PR-A was also increased by IL-1β (data not shown). These effects occurred only in cells expressing PR-A; there was no effect of IL-1β or LPS on PRE2-LUC activity or FKBP5 mRNA levels in cells expressing PR-B alone, suggesting that IL-1β or LPS increased PR-A transrepression of PR-B, rather than inhibiting PR-B transcriptional activity directly. Taken together, the data support the hypothesis that the transrepressive activity of PR-A in myometrial cells is stimulated by proinflammatory stimuli.
Human parturition is associated with sterile tissue-level inflammation within the uterine tissues (myometrium, cervix, and decidua) and increased expression of proinflammatory cytokines (including IL-1β) and chemokines by myometrial cells (20, 22, 37–39). Preterm birth is strongly associated with intrauterine infection/inflammation (21) and is induced in animal models by treatment with IL-1β or LPS (40–42). The current consensus, therefore, is that proinflammatory stimuli contribute causally to the human parturition process. In this context, our finding that IL-1β and LPS increased the abundance of PR-A (and the PR-A:PR-B ratio) and the capacity for PR-A to inhibit the transcriptional activity of PR-B in myometrial cells suggests that tissue-level inflammation contributes to the induction of human parturition by promoting PR-A–mediated functional progesterone withdrawal. However, progesterone is considered to be an anti-inflammatory agent, and this effect has been proposed as a mechanism by which it promotes uterine quiescence during pregnancy (43, 44). Indeed, we (23) and others (43) have found that ligand activation of PR-B in human myometrial cells inhibits responsiveness to IL-1β and LPS. Those data support the hypothesis proposed by Sitteri et al. (44) in the 1970s that progesterone maintains pregnancy by functioning as an anti-inflammatory hormone in the uterine tissues. We propose that for most of pregnancy, progesterone via PR-B promotes uterine quiescence, at least in part, by suppressing responsiveness to proinflammatory/prolabor signals. Importantly, we found that PR-A, likely via its transrepressive activity, blocked the anti-inflammatory actions of PR-B in myometrial cells (23). Thus, net PR-A/PR-B activity may be a critical determinant of the inflammatory state of the pregnancy myometrium. In this context, our proposal that tissue-level inflammation induces PR-A–mediated functional progesterone withdrawal presents a “chicken-egg” conundrum: does inflammation increase PR-A, or does increased PR-A augment inflammation via suppression of PR-B? We propose that both events occur. Our working model (Fig. 7) is that for most of pregnancy, the PR-B–mediated anti-inflammatory action of progesterone dominates to desensitize the myometrium to prolabor/proinflammatory stimuli, which we defined as the net inflammatory load from multiple prolabor influences. However, as we propose, that inflammatory load increases during pregnancy because of the accumulating effects of factors such as uterine distention, fetal stress, and placental senescence, or in pathophysiologic states such as intrauterine infection, and an inflammatory load threshold exists which, when surpassed, triggers signaling cascades in myometrial cells, leading to increases in PR-A stability and transrepressive activity, which may cause the functional withdrawal of PR-B–mediated anti-inflammatory activity. Thus, PR-B dominates when the inflammatory load is low, and PR-A is activated when the inflammatory load exceeds a threshold level. A consequence of PR-A transrepression of PR-B is unrestrained tissue-level inflammation within uterine tissues. This may increase the local concentration of multiple inflammatory cytokines, including PGs (mainly PGF2α and PGE2) that transform the myometrium to the laboring phenotype and promote the softening of the cervix that is required for uterine emptying. According to this model, targeting the mechanism by which inflammation augments the transrepressive activity of PR-A could be an effective therapeutic strategy to prevent and/or delay preterm birth.
Figure 7.
Working model. During most of pregnancy, progesterone via PR-B promotes myometrial cell quiescence in part by repressing responsiveness to proinflammatory stimuli (left). With advancing gestation, prolabor signals increase the inflammatory load on the uterus until a threshold is reached. The threshold is the point at which inflammatory stimuli augment PR-A stability and transrepressive activity to trigger PR-A–mediated (via transrepression of PR-B) functional progesterone withdrawal (right), which causes a proinflammatory tissue-level state that leads to the local production of PGs that increase myometrial contraction and promote cervical softening to facilitate labor.
Acknowledgments
We thank Junye Wang for technical assistance.
Acknowledgments
This work was supported by National Institutes of Health Grant RO1HD069819, Global Alliance to Prevent Prematurity and Stillbirth Preventing Preterm Birth Initiative 12004, and the March of Dimes Prematurity Research Center Ohio Collaborative.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DAH
- diacylhydrazine
- DOX
- doxycycline
- FKBP5
- endogenous progesterone/progesterone receptor B target gene
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- hTERT-HM
- human telomerase reverse transcriptase-immortalized-human myometrial
- IL-1β
- interleukin-1β
- LPS
- lipopolysaccharide
- mRNA
- messenger RNA
- PBS
- phosphate-buffered saline
- PG
- prostaglandin
- PR
- progesterone receptor
- PR-A
- progesterone receptor A
- PR-B
- progesterone receptor B
- PRE
- progesterone response element
- PVDF
- polyvinylidene difluoride
- qRT-PCR
- quantitative real-time polymerase chain reaction
- REN-LUC
- renilla luciferase
- RT-PCR
- real-time polymerase chain reaction
- SDS
- sodium dodecyl sulfate
- TBST
- tris-buffered saline containing 0.1% Tween-20
References
- 1.Liggins GC. Endocrinology of parturition. In: Novy MJ, Resko JA, eds. Fetal Endocrinology. New York, NY: Academic Press, Inc.; 1981:211–237. [Google Scholar]
- 2.Young IR, Renfree MB, Mesiano S, Shaw G, Jenkin G, Smith R. The comparative physiology of parturition in mammals: Hormones and parturition in mammals. In: Norris D, Lopez K, eds. Hormones and Reproduction in Vertebrates, volume 5 London: Academic Press; 2010:95–116. [Google Scholar]
- 3.Avrech OM, Golan A, Weinraub Z, Bukovsky I, Caspi E. Mifepristone (RU486) alone or in combination with a prostaglandin analogue for termination of early pregnancy: a review. Fertil Steril. 1991;56(3):385–393. [DOI] [PubMed] [Google Scholar]
- 4.Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112(8):1095–1100. [DOI] [PubMed] [Google Scholar]
- 5.Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod. 2001;7(9):875–879. [DOI] [PubMed] [Google Scholar]
- 6.Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87(6):2924–2930. [DOI] [PubMed] [Google Scholar]
- 7.Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92(5):1927–1933. [DOI] [PubMed] [Google Scholar]
- 8.Ke W, Chen C, Luo H, Tang J, Zhang Y, Gao W, Yang X, Tian Z, Chang Q, Liang Z. Histone deacetylase 1 regulates the expression of progesterone receptor A during human parturition by occupying the progesterone receptor A promoter. Reprod Sci. 2016;23(7):955–964. [DOI] [PubMed] [Google Scholar]
- 9.Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA. 2003;100(16):9518–9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadeem L, Shynlova O, Matysiak-Zablocki E, Mesiano S, Dong X, Lye S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat Commun. 2016;7:11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9(5):1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68(10-13):771–778. [DOI] [PubMed] [Google Scholar]
- 13.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289(5485):1751–1754. [DOI] [PubMed] [Google Scholar]
- 14.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100(17):9744–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen DX, Xu YF, Mais DE, Goldman ME, McDonnell DP. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol. 1994;14(12):8356–8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–5218. [DOI] [PubMed] [Google Scholar]
- 17.Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20(9):3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20(4):764–775. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166(5):1515–1528. [DOI] [PubMed] [Google Scholar]
- 20.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9(1):41–45. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31(3):553–584. [DOI] [PubMed] [Google Scholar]
- 22.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–236. [PubMed] [Google Scholar]
- 23.Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97(5):E719–E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW, Rainey WE. Telomerase immortalization of human myometrial cells. Biol Reprod. 2002;67(2):506–514. [DOI] [PubMed] [Google Scholar]
- 25.Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci USA. 2010;107(48):20828–20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgiou EX, Lei K, Lai PF, Yulia A, Herbert BR, Castellanos M, May ST, Sooranna SR, Johnson MR. The study of progesterone action in human myometrial explants. Mol Hum Reprod. 2016;22(8):877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh T, Johnson M, Yi L, Tan H, Rahman R, Merlino A, Zakar T, Mesiano S. Estrogen receptor (ER) expression and function in the pregnant human myometrium: estradiol via ERα activates ERK1/2 signaling in term myometrium. J Endocrinol. 2012;212(2):227–238. [DOI] [PubMed] [Google Scholar]
- 28.Tseng L, Zhu HH. Regulation of progesterone receptor messenger ribonucleic acid by progestin in human endometrial stromal cells. Biol Reprod. 1997;57(6):1360–1366. [DOI] [PubMed] [Google Scholar]
- 29.Madsen G, Zakar T, Ku CY, Sanborn BM, Smith R, Mesiano S. Prostaglandins differentially modulate progesterone receptor-A and -B expression in human myometrial cells: evidence for prostaglandin-induced functional progesterone withdrawal. J Clin Endocrinol Metab. 2004;89(2):1010–1013. [DOI] [PubMed] [Google Scholar]
- 30.Chai SY, Smith R, Zakar T, Mitchell C, Madsen G. Term myometrium is characterized by increased activating epigenetic modifications at the progesterone receptor-A promoter. Mol Hum Reprod. 2012;18(8):401–409. [DOI] [PubMed] [Google Scholar]
- 31.Chai SY, Smith R, Fitter JT, Mitchell C, Pan X, Ilicic M, Maiti K, Zakar T, Madsen G. Increased progesterone receptor A expression in labouring human myometrium is associated with decreased promoter occupancy by the histone demethylase JARID1A. Mol Hum Reprod. 2014;20(5):442–453. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Li Y, Hsu PH, Lee SY, Kim Y, Lee EY. Progesterone receptor A stability is mediated by glycogen synthase kinase-3β in the Brca1-deficient mammary gland. J Biol Chem. 2013;288(36):26265–26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan JA, Amazit L, Bellance C, Guiochon-Mantel A, Lombès M, Loosfelt H. p38 and p42/44 MAPKs differentially regulate progesterone receptor A and B isoform stabilization. Mol Endocrinol. 2011;25(10):1710–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdel-Hafiz HA, Horwitz KB. Post-translational modifications of the progesterone receptors. J Steroid Biochem Mol Biol. 2014;140:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA. 2000;97(3):1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniel AR, Faivre EJ, Lange CA. Phosphorylation-dependent antagonism of sumoylation derepresses progesterone receptor action in breast cancer cells. Mol Endocrinol. 2007;21(12):2890–2906. [DOI] [PubMed] [Google Scholar]
- 37.Osman I, Young A, Jordan F, Greer IA, Norman JE. Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J Soc Gynecol Investig. 2006;13(2):97–103. [DOI] [PubMed] [Google Scholar]
- 38.Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, Dong Z, Nhan-Chang CL, Chaiworapongsa T, Lye S, Kusanovic JP, Lipovich L, Mazaki-Tovi S, Hassan SS, Mesiano S, Kim CJ. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med. 2010;38(6):617–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan YW, van den Berg HA, Moore JD, Quenby S, Blanks AM. Assessment of myometrial transcriptome changes associated with spontaneous human labour by high-throughput RNA-seq. Exp Physiol. 2014;99(3):510–524. [DOI] [PubMed] [Google Scholar]
- 40.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171(6):1660–1667. [DOI] [PubMed] [Google Scholar]
- 41.Baggia S, Gravett MG, Witkin SS, Haluska GJ, Novy MJ. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996;3(3):121–126. [DOI] [PubMed] [Google Scholar]
- 42.Hirsch E, Muhle R. Intrauterine bacterial inoculation induces labor in the mouse by mechanisms other than progesterone withdrawal. Biol Reprod. 2002;67(4):1337–1341. [DOI] [PubMed] [Google Scholar]
- 43.Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20(11):2724–2733. [DOI] [PubMed] [Google Scholar]
- 44.Siiteri PK, Febres F, Clemens LE, Chang RJ, Gondos B, Stites D. Progesterone and maintenance of pregnancy: is progesterone nature’s immunosuppressant? Ann N Y Acad Sci. 1977;286:384–397. [DOI] [PubMed] [Google Scholar]