Abstract
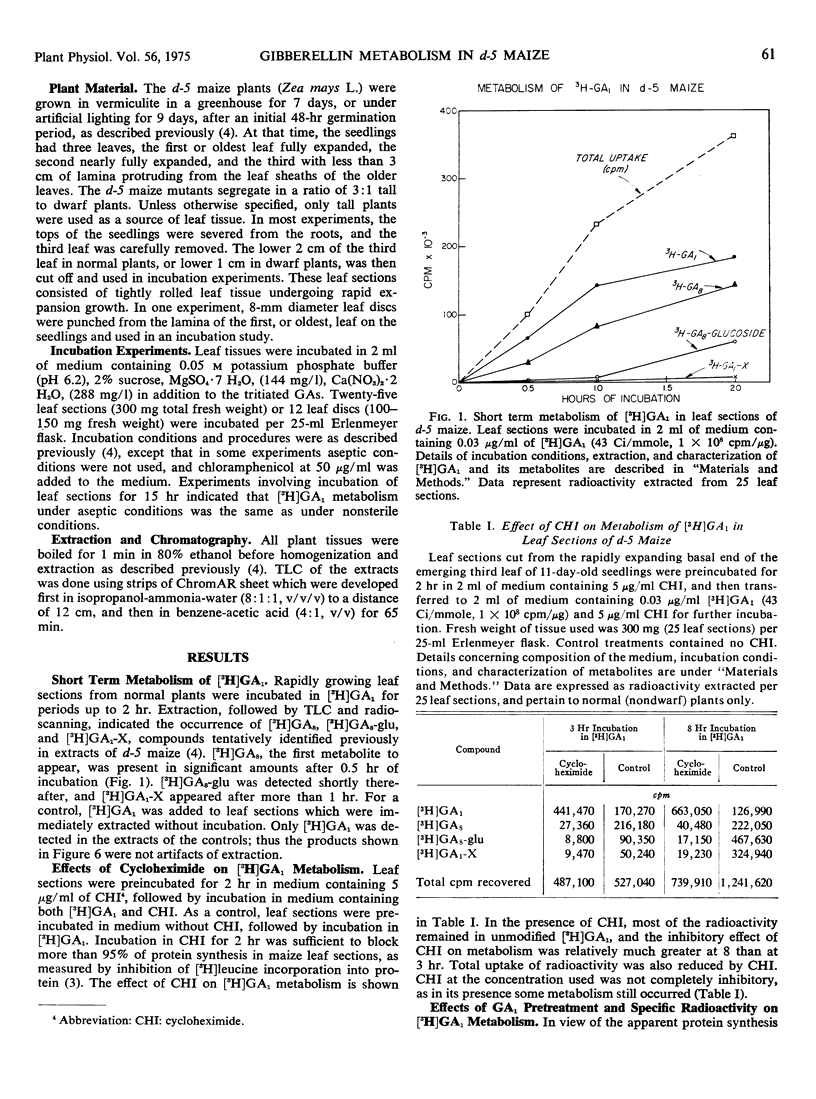
After 30 minutes of incubation of young leaf sections of d-5 maize (Zea mays L.) in [3H]gibberellin A1 ([3H]GA1), the metabolite [3H]GA8 was present in significant amounts, with a second metabolite, [3H]GA8-glucose ([3H]GA8-glu), appearing soon after. A third [3H]GA1 metabolite, the polar uncharacterized conjugate [3H]GA1-X, took more than 1 hour to appear. The protein synthesis inhibitor cycloheximide inhibited the production of all [3H]GA1 metabolites, indicating a possible protein synthesis requirement for [3H]GA1 metabolism.
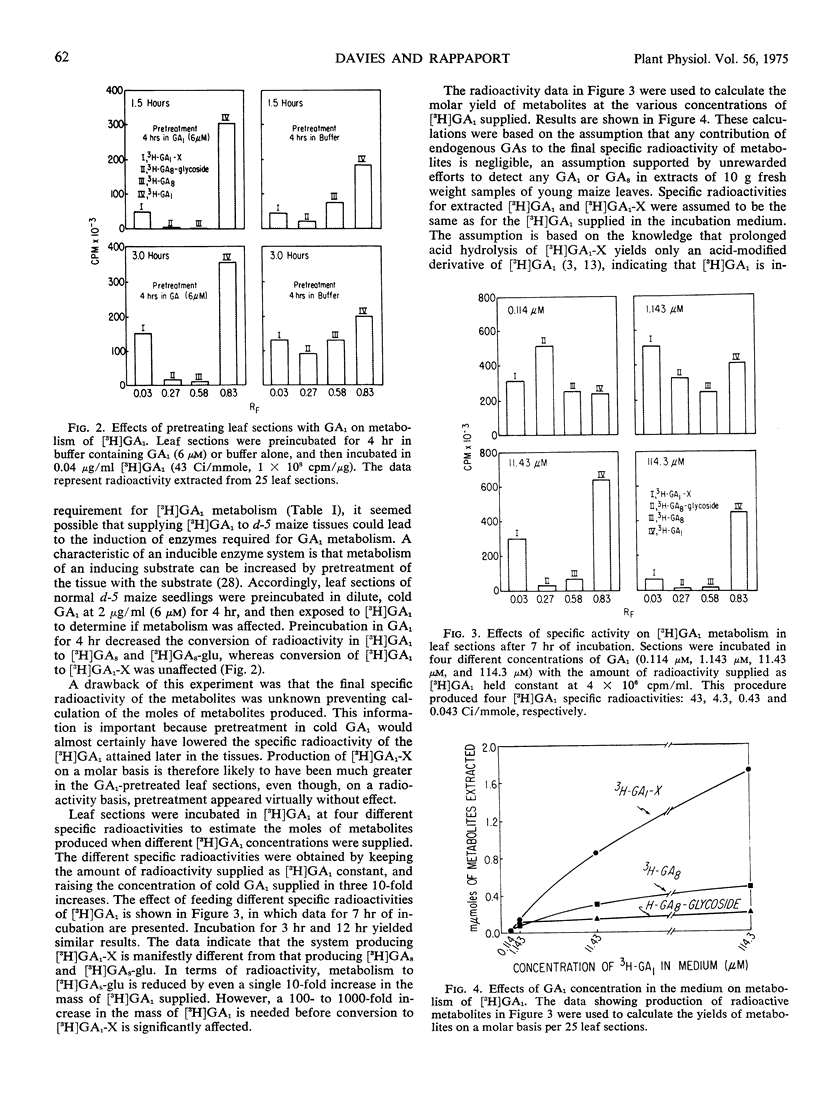
By preincubating leaf sections in unlabeled GA1 before exposure to [3H]GA1 or by reducing the specific radioactivity of the [3H]GA1 supplied, it was possible to reduce greatly the conversion of radioactive GA1 to [3H]GA8-glu, without affecting conversion to [3H]GA1-X. Increasing the molar concentration of the [3H]GA1 fed greatly increased the molar yield of [3H]GA1-X, whereas the molar yields of [3H]GA8 and [3H]GA8-glu were much less affected.
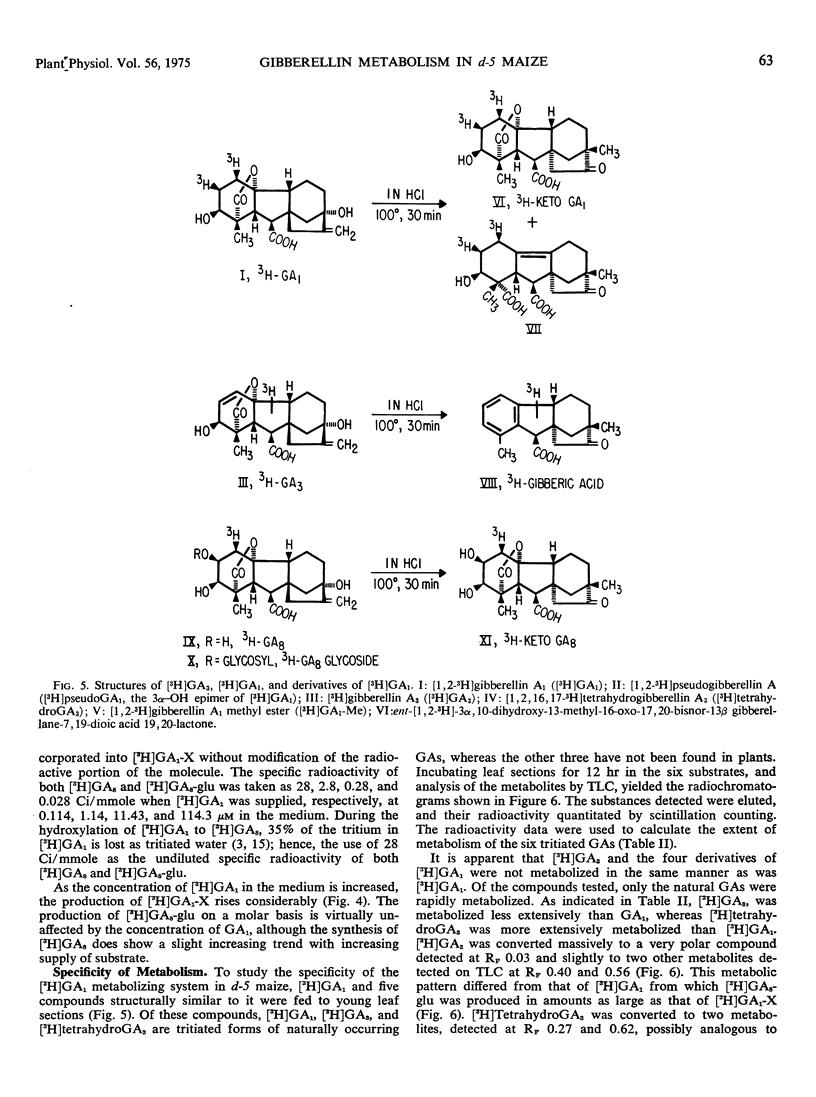
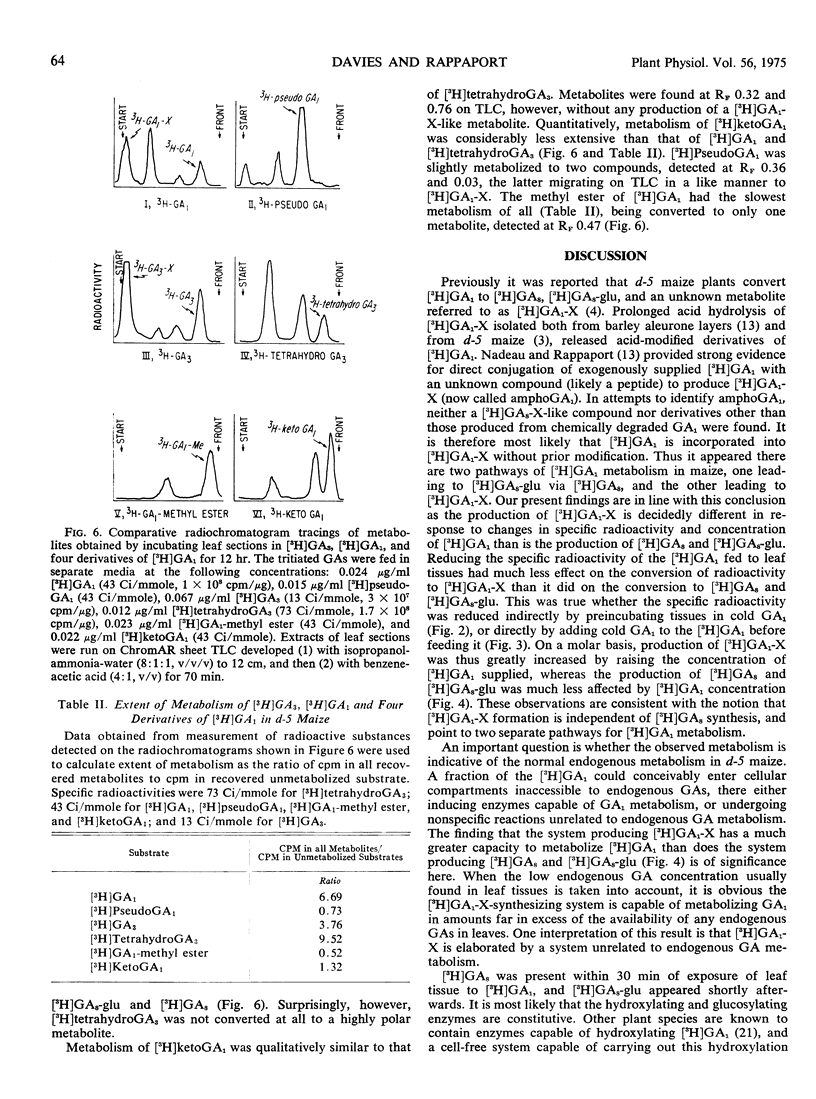
The principal metabolite of [3H]GA3 was a very polar compound having chromatographic properties similar to those of the conjugate [3H]GA1-X produced from [3H]GA1. The naturally occurring GAs [3H]GA1, [3H]GA3, and [3H]tetrahydroGA3 were metabolized to a much greater extent than were the artifical derivatives [3H]ketoGA1, [3H]GA1-methyl ester, and [3H]pseudoGA1. Only [3H]GA1 and [3H]GA3, with their identical D ring structures, were converted to [3H]GA1-X type compounds; [3H]-ketoGA1 and [3H]tetrahydroGA3, with modified D rings, were not converted to this type of conjugate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies L. J., Rappaport L. Metabolism of Tritiated Gibberellins in d-5 Dwarf Maize: I. In Excised Tissues and Intact Dwarf and Normal Plants. Plant Physiol. 1975 Apr;55(4):620–625. doi: 10.1104/pp.55.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., Macdonald I. R. Specificity of cycloheximide in higher plant systems. Plant Physiol. 1970 Aug;46(2):227–232. doi: 10.1104/pp.46.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J. T. Failure to detect effects of cycloheximide on energy metabolism in Euglena gracilis. Nature. 1970 Apr 11;226(5241):182–182. doi: 10.1038/226182a0. [DOI] [PubMed] [Google Scholar]
- Nadeau R., Rappaport L. An amphoteric conjugate of [h]gibberellin a(1) from barley aleurone layers. Plant Physiol. 1974 Dec;54(6):809–812. doi: 10.1104/pp.54.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Railton I. D., Durley R. C., Pharis R. P. Metabolism of Tritiated Gibberellin A(9) by Shoots of Dark-grown Dwarf Pea, cv. Meteor. Plant Physiol. 1974 Jul;54(1):6–12. doi: 10.1104/pp.54.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart J., Breidenbach W., Nadeau R., Rappaport L. Selective binding of (3H)gibberellin A1 by protein fractions from dwarf pea epicotyls. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3255–3259. doi: 10.1073/pnas.71.8.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp C. F., Nadeau R., Rappaport L. Effect of Abscisic Acid on Uptake and Metabolism of [H]Gibberellin A(1) and [H]Pseudogibberellin A(1) by Barley Half-seeds. Plant Physiol. 1973 Dec;52(6):546–548. doi: 10.1104/pp.52.6.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venis M. A. Auxin-induced Conjugation Systems in Peas. Plant Physiol. 1972 Jan;49(1):24–27. doi: 10.1104/pp.49.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]


