Abstract
The peroxisome-proliferator activated receptor γ (PPARγ) is expressed in the hypothalamus in areas involved in energy homeostasis and glucose metabolism. In this study, we created a deletion of PPARγ brain-knockout (BKO) in mature neurons in female mice to investigate its involvement in metabolism and reproduction. We observed that there was no difference in age at puberty onset between female BKOs and littermate controls, but the BKOs gave smaller litters when mated and fewer oocytes when ovulated. The female BKO mice had regular cycles but showed an increase in the number of cycles with prolonged estrus. The mice also had increased luteinizing hormone (LH) levels during the LH surge and histological examination showed hemorrhagic corpora lutea. The mice were challenged with a 60% high-fat diet (HFD). Metabolically, the female BKO mice showed normal body weight, glucose and insulin tolerance, and leptin levels but were protected from obesity-induced leptin resistance. The neuronal knockout also prevented the reduction in estrous cycles due to the HFD. Examination of ovarian histology showed a decrease in the number of primary and secondary follicles in both genotypes due to the HFD, but the BKO ovaries showed an increase in the number of hemorrhagic follicles. In summary, our results show that neuronal PPARγ is required for optimal female fertility but is also involved in the adverse effects of diet-induced obesity by creating leptin resistance potentially through induction of the repressor Socs3.
Thiazolidinediones (TZDs) are a class of drugs that activate the nuclear receptor peroxisome-proliferator activated receptor γ (PPARγ) and improve blood glucose control and insulin sensitivity in patients with type 2 diabetes mellitus (1). TZDs also induce weight gain in humans and rodent models not only by promoting adipogenesis and fluid retention, but also by increasing food intake (2, 3). Consistent with this, PPARγ is expressed in key brain areas involved in energy homeostasis and glucose metabolism (4). We have previously shown that neuronal PPARγ increases weight gain in male mice when placed on a high-fat diet (HFD), mediates the HFD-induced hypothalamic leptin resistance, and is required for the improvement of liver insulin sensitivity upon rosiglitazone treatment (5). Food intake and energy homeostasis are controlled by a hypothalamic melanocortin circuit involving neuropeptide Y (NPY), proopiomelanocortin (POMC), agouti-related peptide (AgRP), and cocaine and amphetamine-regulated transcript (CART) expressing neurons in the arcuate nucleus (ARC) (6). Insulin and leptin inhibit NPY/AGRP neurons and activate POMC/CART neurons to suppress food intake and increase energy expenditure integrating central and peripheral metabolism (7). Obesity causes both hypothalamic insulin (8) and leptin resistance (9) and also causes inflammation peripherally and in the hypothalamus (10, 11).
Gonadotropin-releasing hormone (GnRH) is the central regulator of the hypothalamic-pituitary-gonadal (HPG) axis that controls mammalian reproductive function (12). It is released from the median eminence of the hypothalamus in a pulsatile manner and acts on anterior pituitary gonadotropes to stimulate the synthesis and release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (13). GnRH neuronal activity is controlled by kisspeptin release from Kiss1 neurons in the ARC and anteroventral periventricular nuclei (14), and kisspeptin signaling is an essential regulator of fertility and puberty in numerous mammalian species, including humans (15, 16). Interestingly, Kiss1 neurons arise from POMC progenitor cells (17), and activation of Kiss1 neurons triggers glutamatergic signaling to POMC and AGRP neurons, leading to depolarization or hyperpolarization, respectively (18). NPY/AGRP neurons inhibit (19) but POMC/CART neurons stimulate the HPG axis (20), and melanocortin fibers make synaptic contact with Gnrh1 and Kiss1 neurons. Leptin is essential for pubertal development, and mice lacking either leptin (ob/ob) or its receptor (db/db) are infertile in addition to being obese (21, 22). The effects of leptin on fertility seem to be mediated by AgRP, as ablation of AgRP neurons restores fertility in the db/db mice, and AgRP overexpression impairs fertility (23). Although the effects of leptin on puberty are well documented, the lack of pubertal development in knockout mice has hindered studies of leptin’s effects on adult fertility (21, 22).
The role of PPARγ in the regulation of reproduction is less clear. Previously, PPARγ was reported to influence reproduction, acting in either the pituitary or the ovary (24, 25). Genetic studies have linked polymorphisms in PPARγ to polycystic ovary syndrome (PCOS), suggesting a connection between PPARγ activation and HPG axis function (26, 27). PCOS is the major cause of anovulation and infertility and affects 5% to 10% of women of reproductive age (28). One of the characteristics of PCOS is increased circulating LH and normal or decreased FSH levels (29, 30). Alterations in LH pulses have also been observed, suggesting a hypothalamic or a pituitary defect. TZD therapy in PCOS decreases serum LH and androgen levels and increases ovulation rate (31). Although these drugs are known to have insulin-sensitizing effects, the mechanism underlying the action on the HPG axis is not understood. Clinical studies by Mehta et al. (32) showed that pioglitazone therapy reduces the amplitude of serum LH response but not the GnRH dose response, indicating that pituitary response is altered in vivo. This could be a direct effect of TZDs on the pituitary, as we have previously published that PPARγ impairs GnRH signaling in immortalized pituitary gonadotropes, and loss of PPARγ causes elevated LH (33) or an indirect effect resulting from changes in circulating androgens. Furthermore, PPARγ action in the ovary is also important for ovulation (25). In this study, we deleted PPARγ in neurons to investigate the role of neuronal PPARγ in metabolism and reproduction in female mice.
Methods
Animals
Male Ppargflox/flox mice (34, 35) were bred with female Syn1-Cre mice (36) to generate female Ppargflox/+:Cre mice, which were then bred to Ppargflox/flox male mice to obtain Ppargflox/flox:Cre mice [referred to as brain-knockout (BKO)] and Ppargflox/flox littermate controls (referred to as Flox). The Syn-Cre allele was always maintained on the female side, as Syn-Cre expresses in the testis (37). Syn-cre mice express the cre transgene as early as e12.5 in differentiated neurons throughout the brain (36). Recombination has been observed in neurons in the cornu ammonis 3 and dentate gyrus of the hippocampus, layers IV/V of the cortex, cerebellum, thalamus, and brain stem and in multiple hypothalamic nuclei, including the arcuate, dorsal medial, ventral medial, paraventricular, and suprachiasmatic nucleus (38–40). However, the transgene has been reported to have only limited expression in NPY and POMC neurons in the ARC and in cornu ammonis 1 neurons in the hippocampus (39, 40). Mice were housed in a 12-hour light, 12-hour dark cycle. Males and females had access to standard chow and water ad libitum. At 15 weeks of age, cohorts of female and male mice were fed either a 60% HFD (Research Diets D12492) or a 10% low-fat diet (LFD; Research Diets 12450B). Major lipid components of the HFD are derived from lard and include 20% palmitic acid, 11% stearic acid, 34% oleic acid, and 29% linoleic acid, resulting in 32% saturated fat and 36% monounsaturated and 32% polyunsaturated fat. Food intake and body weight were measured weekly. Mouse procedures conformed to the Guide for Care and Use of Laboratory Animals of the US National Institutes of Health and were approved by the Institutional Animal Use and Care Committee of the University of California, San Diego.
Puberty onset and fertility assessment
Male and female pups were weaned at 21 days of age. Weights were recorded daily from 21 to 37 days of age for both sexes. Female pups were checked for vaginal opening as a sign of onset of puberty. Six- to 8-week-old BKO and Flox mice were paired in different combinations and bred over a period of 6 months. Average days before the first litter and average number of pups born to the indicated breeding pairs were recorded and analyzed. Estrous cycles were monitored in female mice by vaginal cytology for 3 weeks starting at 12 weeks of age, or after 6, 12, or 20 weeks on diets. Ovulation was assessed by superovulating mice using a pregnant mare serum gonadotropins/human chorionic gonadotropin (hCG) protocol (5 IU of pregnant mare serum gonadotropins subcutaneous followed 48 hours later by 5 IU of hCG subcutaneous). Mice were euthanized 16 hours post hCG, and the oocytes were harvested from the oviducts and counted manually under a light microscope (41).
Tissue collection and histology
Ovaries, brain, pituitaries, and other tissues were harvested at euthanasia for both histology and RNA extraction. Paraffin-embedded sections (5 μm) were cut, dewaxed, and stained with hematoxylin and eosin. Follicle number and stage and corpora lutea number were counted on 3 to 5 sections from ovaries from 3 to 4 mice per group and are presented as mean number per ovary (42). Follicle stages were defined as follows: 1° as having a single layer of cuboidal granulosa cells (GCs), 2° as having 2 or more layers of cuboidal GCs but no antrum, early antral as having small patches of clear space between GCs, antral as having clearly defined antrum, and atretic as having irregular oocyte morphology. Ovarian sections examined were separated by 25 µm. Images were scanned using Aperio ImageScope and analyzed using the Imagescope software (Leica, Buffalo Grove, IL).
Gene expression
Total RNA was extracted from the tissues using RNAbee (Tel-Test Inc., Friendswood, TX) and RNA purification kits from QIAGEN (Germantown, MD) or Macherey-Nagel (Bethlehem, PA) following the manufacturers’ instructions. First-strand complementary DNA was synthesized using a High-Capacity cDNA Synthesis Kit (Applied Biosystems, Waltham, MA). Quantitative polymerase chain reaction (qPCR) assays were run in 20-μL triplicate reactions on a MJ Research Chromo4 instrument (Bio-Rad, Hercules, CA) or in 7-nL reactions on a BioMark HD System (Fluidigm, South San Francisco, CA). Gene expression levels were calculated after normalization to the housekeeping genes m36B4, Gapdh, or RpII, using the 2–∆∆Ct method and expressed as relative messenger RNA (mRNA) levels compared with the control.
Gonadotropin measurements
Blood (20 µL) was collected from the tail vein of males and from females at diestrus and proestrus, and plasma was prepared. Plasma LH and FSH levels were measured by custom duplex Luminex assay based on the rat pituitary panel (catalog number RPT86K; Millipore, Temecula, CA) in singlets. Sensitivity of the assay was as follows: LH, 4.9 pg/mL, and FSH, 47.7 pg/mL with an intra-assay coefficient of variation of 15%. After 6, 12, and 20 weeks on the diets, female mice were bled in the morning of diestrus and at the time of the proestrus surge (6 pm). Plasma LH and FSH levels were measured by Luminex assay (catalog number MPTMAG-49K). Sensitivity of the assays was as follows: LH, 4.9 pg/mL, and FSH, 24.4 pg/mL. For the GnRH stimulation test, tail vein blood was collected before and 10 minutes after intraperitoneal (IP) injection of 1 μg/kg GnRH, and gonadotropins were measured.
IP glucose tolerance and insulin tolerance tests
Female mice were subjected to IP glucose tolerance tests after 17 weeks on diets and insulin tolerance tests after 20 weeks. Mice were fasted for 6 hours starting at 6 am and then injected intraperitoneally with glucose (1 g/kg body weight) or insulin (0.4 for LFD or 0.75 U/kg body weight for HFD). Tail vein blood glucose was measured at 0, 15, 30, 45, 60, 90, and 120 minutes after injection using a glucometer (OneTouch Ultra; Bayer, Pittsburgh, PA).
Insulin, leptin, and steroid measurements
Animals were fasted for 6 hours. Fasting blood glucose concentration was measured on tail vein blood using a glucometer (OneTouch Ultra; LifeScan Inc, Milpitas, CA). Blood (50 µL) was drawn from the tail vein in EDTA-coated capillary tubes, and plasma was obtained by centrifugation at 4°C. Fasting insulin and leptin were measured on plasma using the Mouse Metabolic Kit (Meso Scale Discovery, K15124C-2, Rockville, MD). Sensitivity of the assay was: leptin, 43 pg/mL; insulin, 15 pg/mL; and coefficient of variation, 6% and 12%, respectively. Estrogen, progesterone, and testosterone were measured using a Custom Steroid Hormone Panel Kit (Meso Scale Discovery). Sensitivity of the assay was: estradiol, 5 pg/mL; progesterone, 70 pg/mL; testosterone 20 pg/mL; and coefficient of variation, 7%, 15%, and 22%, respectively.
In vivo leptin sensitivity test
We measured food intake in individually housed mice injected with leptin (0.5 or 1 mg/kg intraperitoneally; National Hormone and Peptide Program, Torrance, CA) at 12-hour intervals for a total of 4 consecutive doses. Food intake and body weight were measured throughout the 48-hour period and compared with a 48-hour period in which animals received twice daily IP injections of vehicle (phosphate-buffered saline).
Statistical analysis
Data were analyzed by 1-way or 2-way analysis of variance followed by Tukey multiple comparison posttest or Student t test as appropriate using Prism (Graph Pad, La Jolla, CA). Normality was assessed by the D’Agostino-Pearson omnibus normality test. Results were expressed as mean ± SE and considered significant with P < 0.05.
Results
Neuronal deletion of PPARγ
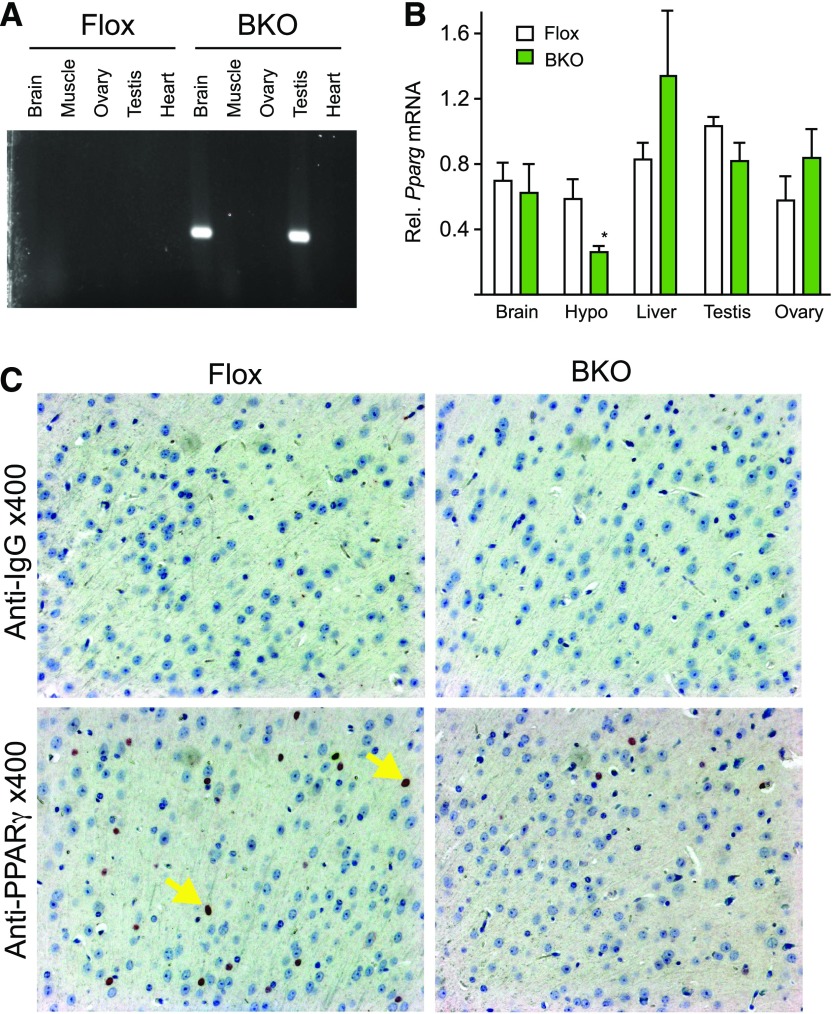
PPARγ was deleted from mature neurons by crossing Pparg floxed mice with mice expressing cre recombinase under control of the synapsin promoter (syn-cre) as published previously (5). Littermates that lacked the syn-cre allele were used as controls (Flox). Neuronal deletion was confirmed by polymerase chain reaction amplification of the recombined allele [Fig. 1(A)]. Recombination was observed in brain and also in testis, so the cre allele was always bred from the maternal side to prevent germline recombination. To verify brain-specific deletion of PPARγ in BKO mice, we isolated RNA from brain regions and from peripheral tissues for measurement of PPARγ mRNA abundance. Pparg expression was quantified by qPCR. There was a significant reduction in Pparg gene expression in the hypothalamus but not in other tissues [Fig. 1(B)]. Surprisingly, the expression was not reduced in whole brain, but Pparg is also expressed in astrocytes that may mask changes in expression in neurons. Immuno-histochemical staining for PPARγ protein showed reduced staining in cortical slices in the BKO brain compared with the Flox controls [Fig. 1(C)].
Figure 1.
PPARγ gene targeting. (A) DNA was extracted from the indicated tissues and polymerase chain reaction analysis was carried out for recombination. (B) Total RNA was isolated from the indicated tissues of WT (white) and BKO (green) mice. PPARγ mRNA levels were measured by qPCR analysis. Data shown are the fold induction of gene expression normalized with housekeeping gene and expressed as mean ± standard error of the mean (SEM). Asterisk indicates statistical significance (*P < 0.05). (C) Immunohistochemical analysis of coronal sections of brain from WT and BKO mice. Arrows indicate nuclear (blue) and PPARγ (black) staining. WT, wild type.
Deletion of PPARγ does not alter timing of puberty in female mice but impairs fertility
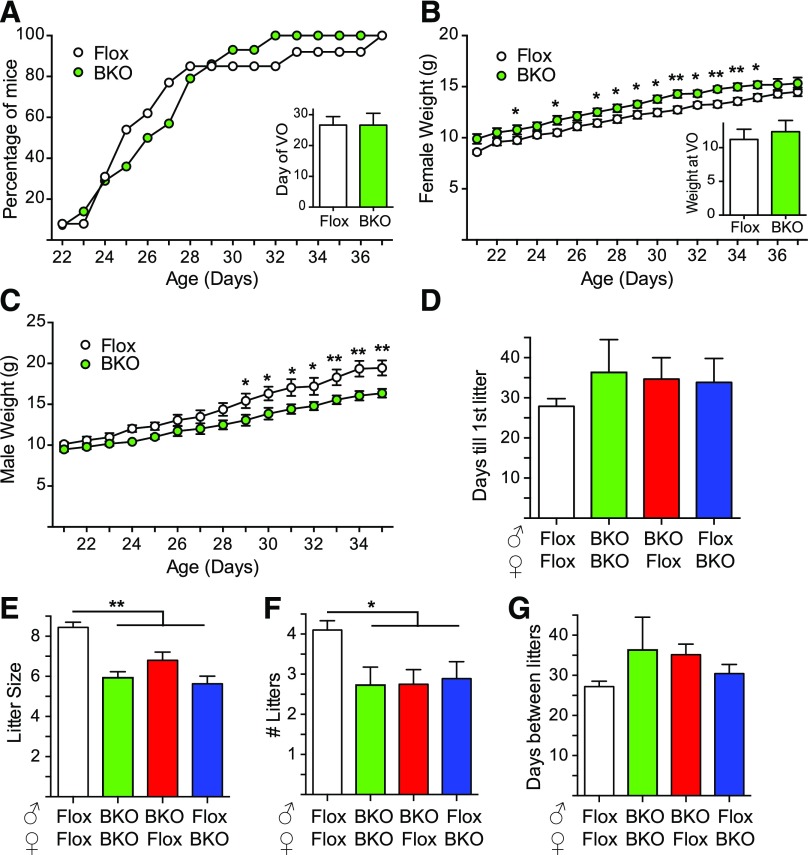
Female BKO underwent vaginal opening at the same time as Flox controls [Fig. 2(A)]. Female BKO were slightly heavier [Fig. 2(B)] and male BKO lighter [Fig. 2(C)] than their Flox littermates during the peripubertal period (21 to 35 days of age), but the weight at vaginal opening was unaltered in females [Fig. 2(B)]. Fertility was assessed by mating pairs of mice over a period of 6 months. None of the breeding pairs showed a difference in the days until first litter [Fig. 2(D)]. Litter size was significantly smaller for the BKO pairs irrespective of whether the BKO was paternal or maternal, suggesting that the deletion impairs both female and male fertility [Fig. 2(E)]. The BKO breeding pairs also had fewer litters than Flox/Flox breeding pairs over the course of the study [Fig. 2(F)]. Like the time until first litter, the time between litters was no different for BKO pairs [Fig. 2(G)].
Figure 2.
Puberty and fertility assessment in BKO mice. (A) Percentage of mice undergoing vaginal opening over time. Inset shows day of vaginal opening [mean ± standard error of the mean (SEM)]. Flox mice are shown in white, and BKO mice are shown in green. (B) Female weights during puberty. Inset shows weight at vaginal opening (mean ± SEM). Asterisks indicate statistical significance for BKO versus Flox mice (*P < 0.05, **P < 0.01 by 2-way ANOVA). (C) Male body weights after weaning (mean ± SEM). (D) Six-week-old BKO and Flox mice were paired in different combinations and bred over a period of 6 months. Average days before the first litter was born to the breeding pair (mean ± SEM). Flox/Flox breeding is shown in white, BKO/BKO breeding is shown in green, and the mixed Flox/BKO breeding is shown in red and blue. (E) Average number of pups born to the indicated breeding pairs (mean ± SEM). Asterisk indicates statistical significance versus Flox:Flox (**P < 0.01 by ANOVA). (F) Number of litters over 6 months (mean ± SEM). Asterisk indicates statistical significance versus Flox:Flox (*P < 0.05 by ANOVA). (G) Days between litters over 6 months (mean ± SEM). VO, vaginal opening.
Neuronal deletion of PPARγ causes hemorrhagic corpora lutea in female mice
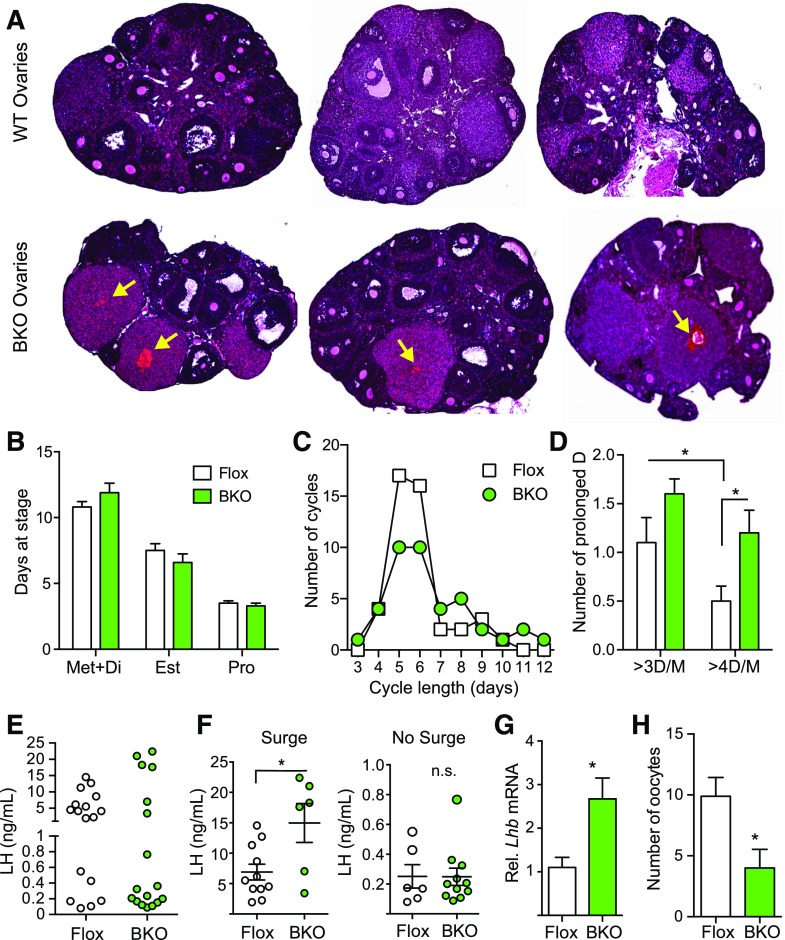
Ovarian histology was assessed from female BKO and Flox mice. The most striking difference was the presence of hemorrhagic corpora lutea in the BKO [Fig. 3(A), arrows] that were not seen in the ovaries from the Flox littermates. Estrous cycles were monitored for 21 days, and there was no difference between BKO and Flox mice in the number of cycles or mean cycle length [Supplemental Fig. 1(A) and 1(B) (1.3MB, eps) ] or in the total days spent at each stage of the cycle [Fig. 3(B)]. Both genotypes showed a median cycle length of 5 to 6 days, but the BKO mice tended to have more long cycles [Fig. 3(C)] and the number of instances of prolonged diestrus/metestrus was increased [Fig. 3(D)]. Serum gonadotropins during diestrus were unchanged [Supplemental Fig. 1C (1.3MB, eps) ] as were levels during afternoon (6 pm) of proestrus [Supplemental Fig. 1(D) (1.3MB, eps) ]. Plotting individual LH values clearly indicated that there were 2 populations of mice, those with an LH surge and those without [Fig. 3(E)]. The mean proestrus LH surge value was significantly higher in the BKO mice but the nonsurge value was unaltered [Fig. 3(F)]. The BKO mice had fewer surges than the Flox mice (6/17 for BKO versus 11/17 for Flox). Hypothalamic Gnrh mRNA was unaltered [Supplemental Fig. 1(E) (1.3MB, eps) ] but pituitary Lhb mRNA was higher in the BKO females [Fig. 3(G)] and Fshb was unchanged [Supplemental Fig. 1(E) (1.3MB, eps) ]. Diestrus estradiol and progesterone levels were unchanged [Supplemental Fig. 1(F) (1.3MB, eps) ]. We assessed the ovulatory capacity of the mice by superovulating and collecting oocytes. The BKO showed a significant decrease in the number of oocytes released [Fig. 3(H)], confirming an ovulation defect.
Figure 3.
Histological analysis of ovaries, estrous cycles, and hormone levels from adult Flox and BKO mice. (A) Hematoxylin and eosin stained sections of ovaries from 13-week-old mice. Arrows indicate hemorrhagic corpora lutea. (B) Percentage of days in metestrus/diestrus, estrus, or proestrus [mean ± standard error of the mean (SEM)]. Flox mice are shown in white, and BKO mice are shown in green. (C) Distribution of estrous cycle length in Flox and BKO mice. Cycle length was defined as the end of estrus to the end of the next estrus. (D) Number of cycles with prolonged diestrus. A prolonged diestrus stage was defined as greater than 3 or 4 days of metestrus or diestrus (mean ± SEM). BKO mice showed a greater number of prolonged cycles (genotype effect, P = 0.005 by 2-way ANOVA). (E) Individual plasma LH levels measured at 6 pm on the day of proestrus. (F) Plasma LH values separated into mice that showed an LH surge and those that did not. Individual values are shown along with mean ± SEM. (G) Pituitary Lhb mRNA expression by qPCR. (H) Number of oocytes released following induction of ovulation. Asterisks indicate statistical significance as indicated by Student t test or post hoc testing following 2-way ANOVA (*P < 0.05). Est, estrus; Met+Di, mestestrus/diestrus; Pro, proestrus; WT, wild type.
Diet-induced obesity impairs metabolism in female BKO mice
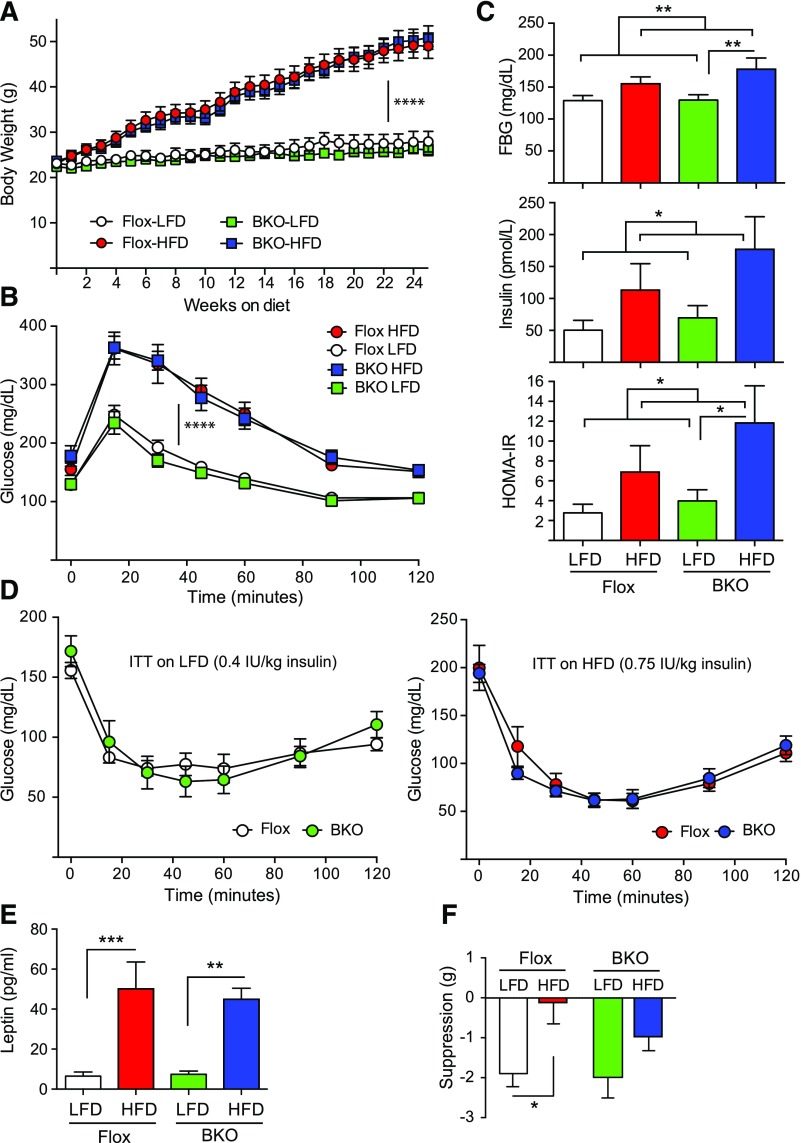
We had previously published that high-fat feeding in wild-type mice reduces serum FSH levels in males and disrupts the LH proestrus surge in females (43). Therefore we placed female BKO mice and Flox littermates on a 60% HFD for 24 weeks to test the effect of diet-induced obesity on female BKO mice. A defined 10% LFD was used as a control instead of normal chow to avoid spurious results due to undefined nutrients in the normal chow. We did not observe any difference in weight gain over the 24 weeks of high-fat feeding for the 2 genotypes [Fig. 4(A)]. Female BKO mice gained weight at the same rate as female Flox littermates, unlike their male counterparts (5). Glucose tolerance tests indicated the BKO and Flox mice become equally glucose intolerant on HFD [Fig. 4(B)]. Fasting blood glucoses were significantly higher on HFD as were fasting insulin levels [Fig. 4(C)], and as a result, homeostatic model assessment of insulin resistance (HOMA-IR) was also significantly higher on HFD. Insulin tolerance tests indicated that the BKO and Flox mice had the same insulin sensitivity as Flox mice whether on LFD or HFD [Fig. 4(D)]. Leptin levels were equivalently elevated in both BKO and Flox mice after 24 weeks of HFD [Fig. 4(E)] but leptin sensitivity was altered. Flox mice on LFD showed the expected suppression of food intake after leptin injection, but Flox mice on HFD were completely resistant to leptin’s anorexigenic effect [Fig. 4(F)]. BKO mice showed the same leptin sensitivity on LFD that was not significantly different on HFD [Fig. 4(F)], as we had observed for male BKO mice (5). Thus like the male BKO mice, the female BKO mice are protected from obesity-induced leptin resistance despite having equivalent body weights.
Figure 4.
Body weights and metabolic characterization of adult female BKO mice on HFD. Mice were placed on a 60% HFD or a matched 10% LFD for 24 weeks. (A) Body weight over time on diets [mean ± standard error of the mean (SEM)]. The Flox mice on LFD group is shown in white, the Flox mice on HFD group is shown in red, the BKO mice on LFD group is shown in green, and the BKO mice on HFD group is shown in blue. Flox mice are shown by circles, and BKO mice are shown by squares. Body weights on LFD or HFD show a time effect (P < 0.0001) but no genotype effect or interaction by repeated measures 2-way ANOVA. (B) IP glucose tolerance tests (1 g/kg body weight) performed on mice after 17 weeks on diets. Tail vein glucose was measured over 120 minutes (mean ± SEM; ****P < 0.0001) for diet effect. (C) Fasting blood glucose (FBG), fasting insulin, and HOMA-IR measurements for Flox and BKO mice (mean ± SEM). Fasting blood glucose, fasting insulin, and HOMA-IR showed significant diet effects (P = 0.005, 0.028, and 0.025, respectively) by 2-way ANOVA. (D) Insulin tolerance tests performed on mice after 20 weeks on diets. Mice on LFD received 0.4 IU/kg insulin, whereas those on HFD received 0.75 IU/kg due to their insulin resistance. Tail vein glucose was measured over 120 minutes (mean ± SEM). (E) Fasted leptin levels in Flox and BKO mice after 20 weeks on LFD or HFD (mean ± SEM). Leptin showed a very significant diet effect (P < 0.0001). (F) Leptin suppression of food intake. Mice received daily injections of leptin for 2 days and then injections of saline for 2 days, and food intake was measured (mean ± SEM). Suppression of food intake showed a significant diet effect (P = 0.0041) primarily in the Flox mice. Asterisks indicate statistical significance as indicated (*P < 0.05, ** P < 0.01, ***P < 0.001, and ****P < 0.0001). ITT, insulin tolerance test.
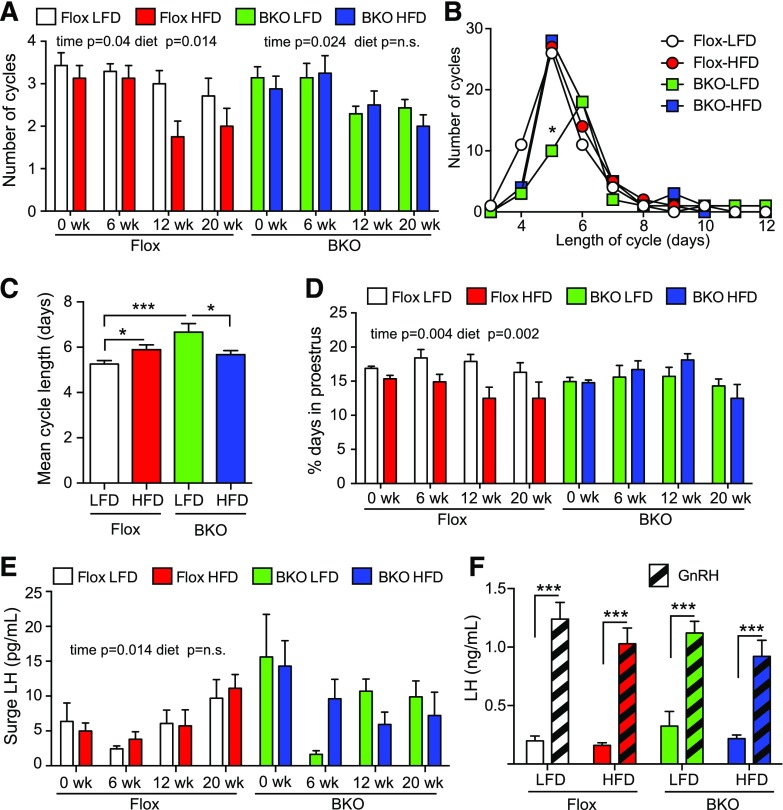
Female BKO mice are protected from obesity-induced estrous cycle impairment
Female BKO mice were assessed for estrous cycles by vaginal cytology for 21 days at weeks 0, 6, 12, and 20. Representative cyclegrams for 3 mice from each group are shown in Supplemental Fig. 2 (1,001.2KB, eps) . The Flox mice showed a time- and diet-dependent decrease in the number of cycles [P = 0.04 and 0.014, respectively; Fig. 5(A)]. In contrast, the BKO mice only showed a time-dependent decrease (P = 0.024), and there was no further effect of HFD [Fig. 5(A)]. The length of the estrous cycles also varied. Cycle data were combined for the 6-, 12-, and 20-week time points to increase statistical power. The Flox mice showed a median cycle length of 5 days irrespective of diet, but the BKO mice showed a median cycle length of 6 days on the LFD, with a significant decrease in the number of 5-day cycles [Fig. 5(B)]; however, the HFD group showed a median 5-day cycle like the Flox mice. The mean cycle length was also significantly longer for the BKO mice compared with the Flox mice on the LFD [Fig. 5(C)]. When the time spent in each stage was assessed, the Flox mice, but not the BKO mice, showed a time- and diet-dependent decrease in the days at proestrus [P = 0.004 and 0.002, respectively; Fig. 5(D)]. No significant differences were found in the time in metestrus/diestrus or estrus [Supplemental Fig. 3(A) and 3(B) (1.5MB, eps) ]. Diestrus LH and FSH levels were not different in either BKO or Flox animals [Supplemental Fig. 3(C) and 3(E) (1.5MB, eps) ]. No difference was observed in FSH levels or LH levels in mice that did not surge at proestrus [Supplemental Fig. 3(D) and 3(F) (1.5MB, eps) ], but the surge LH values showed a time-dependent increase in Flox mice (P = 0.014) that was not seen on BKO mice [Fig. 5(E)]. We also analyzed pituitary LH release in response to GnRH and observed that pituitaries responded similarly regardless of the genotype and diet [Fig. 5(F)].
Figure 5.
Estrous cycles over time on HFD diet. Estrous cycles were monitored by vaginal cytology for 21 days at 6, 12, and 20 weeks on diets. (A) Number of cycles over time on diets. A cycle was defined as a day of diestrus, followed by a day of proestrus and then a day of estrus. The Flox mice on LFD group is shown in white, the Flox mice on HFD group is shown in red, the BKO mice on LFD group is shown in green, and the BKO mice on HFD group is shown in blue. Data are shown as mean ± standard error of the mean (SEM). Flox mice showed a significant time and diet effect by 2-way ANOVA, but BKO did not show a difference with diet. (B) Distribution of cycle length for Flox and BKO on LFD and HFD. Cycle length was defined as the end of estrus to the end of the next estrus. Data from 6, 12, and 20 weeks was combined. (C) Mean cycle length (mean ± SEM). Cycle length showed a genotype effect (P = 0.01) and a significant interaction of genotype and diet (P = 0.0004) by 2-way ANOVA. (D) Percentage of days in proestrus. Data are shown as mean ± SEM. The Flox mice showed significant time and diet effects (P = 0.004 and 0.002, respectively). (E) Surge LH values during the afternoon (6 pm) of proestrus. The Flox mice showed significant time effect (P = 0.014). (F) GnRH stimulated LH release. Mice received GnRH (1 µg/kg) in the morning after 18 weeks on diets. Tail vein blood was taken before and 10 minutes after GnRH injection (GnRH, striped bars). Data are shown as mean ± SEM. LH values showed a very significant GnRH effect (P < 0.0001) but no differences between groups. Asterisks indicate statistical significance by testing following 2-way ANOVA (* = P < 0.05, ***P < 0.001). n.s., not significant.
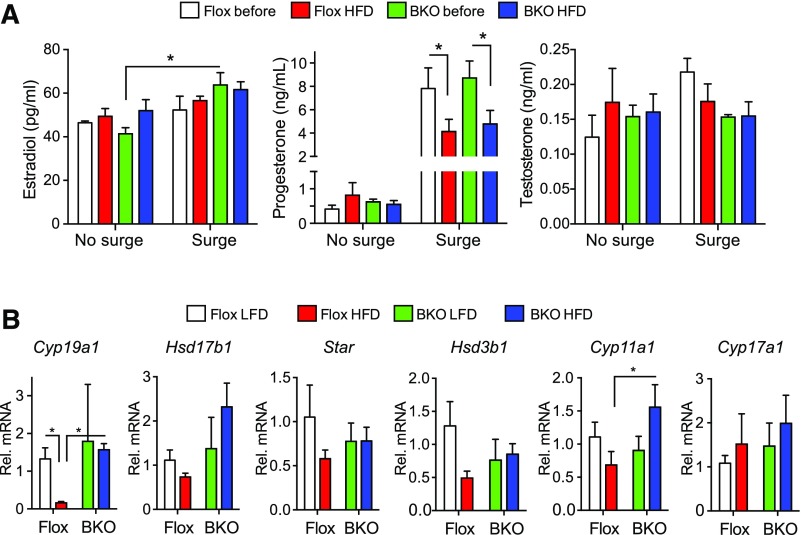
Steroid levels were measured in serum samples taken from mice before and after HFD. Estradiol and testosterone levels were not changed by diet or genotype irrespective of the LH surge [Fig. 6(A)], but progesterone was markedly increased (P = 0.0001) in samples from mice with LH surges [Fig. 6(A)], confirming that a surge had occurred, and putting the mice on the HFD decreased the surge progesterone in both genotypes [Fig. 6(A)]. We assessed ovarian gene expression after euthanasia. Aromatase expression (Cyp19a1) was strongly suppressed by the HFD in the Flox mice but was unchanged in the BKO mice [Fig. 6(B)]. Cyp11a1 increased in the BKO mice on HFD but Star, Hsd17b1, Hsd3b1, and Cyp17a1 did not change significantly.
Figure 6.
Steroid levels and ovarian gene expression. (A) Plasma steroid levels during proestrus for mice with or without LH surges, before or after HFD. Data are shown as mean ± standard error of the mean (SEM). Estradiol and progesterone showed significant surge increases (P = 0.0027 and < 0.0001, respectively) by 2-way ANOVA, and the surge progesterone values showed a HFD-dependent decrease (P = 0.015). (B) Ovarian expression of steroid biosynthetic genes Cyp19a1, Hsd17b1, Star, Hsd3b1, Cyp11a1, and Cyp17a1 by qPCR. The Flox mice on LFD group is shown in white, the Flox mice on HFD group is shown in red, the BKO mice on LFD group is shown in green, and the BKO mice on HFD group is shown in blue. Cyp19a1 expression showed a significant interaction of diet and genotype (P = 0.0089) by 2-way ANOVA. Asterisks indicate statistical significance by testing after 2-way ANOVA as indicated (*P < 0.05).
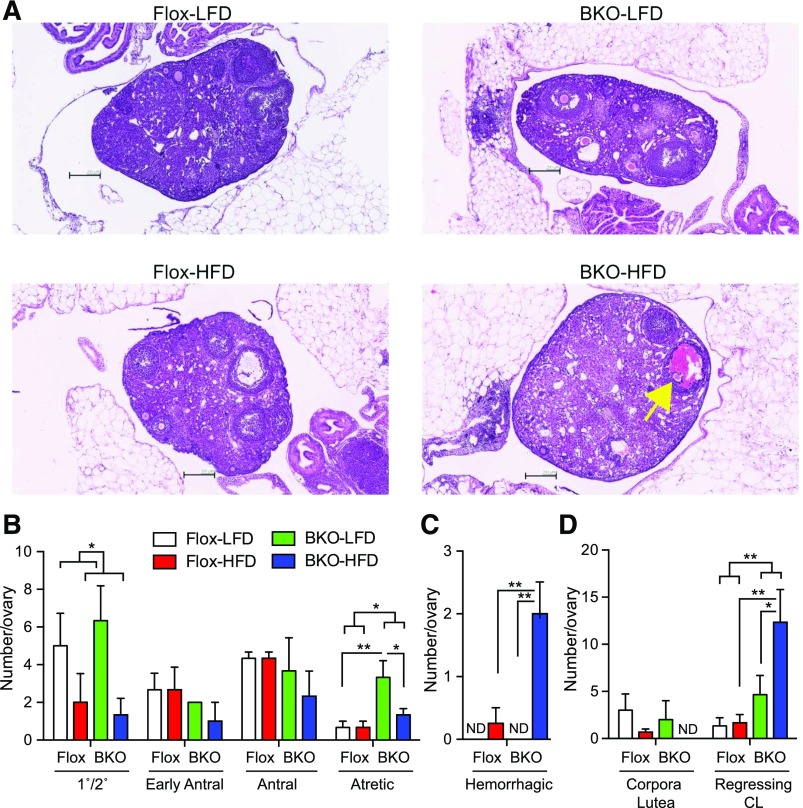
Examination of ovarian histology showed significant differences between groups [Fig. 7(A)]. The HFD decreased the number of primary/secondary follicles in both the Flox and BKO mice [Fig. 7(B); P = 0.03]. The number of early antral and antral follicles was no different in BKO mice or Flox mice on either diet, but the number of atretic follicles was selectively increased in the BKO mice (P = 0.019), especially on the LFD. The number of hemorrhagic follicles [Fig. 7(A)] was significantly higher in the BKO on HFD [Fig. 7(C)]. The number of new corpora lutea (with a defined boundary) was unchanged, but the number of regressing corpora lutea (no well-defined boundary) was increased in the BKO (P = 0.01), particularly on the HFD [Fig. 7(D)], suggesting impaired luteolysis in the BKO mice.
Figure 7.
Ovarian histology for mice on HFD. Mice were euthanized after 24 weeks on diets. (A) Representative Hematoxylin and eosin stained sections of ovaries from Flox and BKO mice on LFD and HFD. Yellow arrow indicates hemorrhagic follicle. (B) Number of staged follicles per 3 ovary sections [mean ± standard error of the mean (SEM)]. Primary/secondary follicle number showed a diet effect (P = 0.03), and atretic follicle number showed a genotype effect (P = 0.013) by 2-way ANOVA. (C) Number of hemorrhagic follicles per 3 ovary sections (mean ± SEM). Diet effect (P = 0.005), genotype effect (P = 0.02), and interaction (P = 0.02) by 2-way ANOVA. (D) Number of corpora lutea per 3 ovary sections (mean ± SEM). Genotype effect (P = 0.01) by 2-way ANOVA. The Flox mice on LFD group is shown in white, the Flox mice on HFD group is shown in red, the BKO mice on LFD group is shown in green, and the BKO mice on HFD group is shown in blue. Asterisks indicate statistical significance by post hoc testing after 2-way ANOVA as indicated (*P < 0.05, **P < 0.01, and ***P < 0.001). CL, corpora lutea; ND, none detected.
To gain insight into potential explanations for the reproductive phenotype, we measured the expression of selected inflammation and neuroendocrine genes by qPCR in hypothalamic RNA from a second cohort of male BKO mice on HFD. As expected, Pparg showed a genotype-dependent decrease in expression in the hypothalamus due to the knockout [Supplemental Fig. 4(A) (1.4MB, eps) ]. Gfap expression was unchanged, indicating that loss of PPARγ did not cause astrocyte activation, but the janus kinase/signal transducer and activator of transcription (STAT) negative feedback regulator suppressor of cytokine signaling 3 (Socs3) showed an HFD-dependent increase in the Flox mice but not the BKO [Supplemental Fig. 4(A) (1.4MB, eps) ]. Gnrh1 did not change with genotype or diet [Supplemental Fig. 4(B) (1.4MB, eps) ], whereas Kiss1 and Cart showed an HFD-dependent increase in expression in both genotypes, and Agrp showed an HFD-dependent increase only in the Flox mice [Supplemental Fig. 4(B) (1.4MB, eps) ]. Other neuropeptide genes implicated in reproduction, including Npy, Npvf (GnIH), Tac2 (NKB), and Hcrt (orexinA), did not vary with diet or genotype.
Discussion
PPARγ regulates a number of biological processes, including adipocyte differentiation, lipid and glucose homeostasis, and control of inflammatory responses. The effects in reproduction are less known, but PPARγ actions have been demonstrated in the pituitary and ovary (24, 25). Loss of PPARγ in neurons in male mice reduces their food intake and body weight gain when fed a HFD and abolishes leptin resistance (5). We show here that loss of neuronal PPARγ in female mice also prevented HFD-induced leptin resistance, but in contrast to male mice, food intake and weight gain were normal, suggesting a sexually dimorphic feeding response. If the phenotype of the male BKO mice is due to the absence of leptin resistance, then the normal feeding behavior in female BKO mice might have a number of causes. It could indicate that HFD does not induce leptin expression in the BKO mice, that feeding is less sensitive to leptin feedback, or that different neuronal circuits control body weight or compensate for the loss of PPARγ. The first explanation can be discounted, as leptin levels were increased equivalently in both genotypes. The second explanation is unlikely, as we observed that experimental suppression of food intake by leptin injection is no different between male and female mice. The notion that different neuronal circuits control feeding is supported by the observation that male and female mice have different food anticipatory responses (44, 45), different relaxin 3–dependent alcohol consumption responses (46), and different reward-related behaviors (47). At the molecular level, SOCS3 mediates hypothalamic leptin resistance due to high-fat feeding (48, 49), and we observed that Socs3 was increased in Flox mice fed a HFD, but not in the BKO mice, consistent with their leptin-sensitive phenotype. Other authors have shown that PPARγ knockdown prevented Socs3 transactivation induced by docosahexaenoic acid (50) and that PPARγ agonists were shown to induce Socs3 in glial cells and the pancreas (51, 52). Leptin signaling via the leptin receptor (LepR) and STAT activation is also required for induction of Socs3 in the hypothalamus (53), but how PPARγ and STAT signaling combine to increase Socs3 expression is not known. Knockout of Socs3 in neurons using Syn-cre, the same transgene used in this report, or in whole brain using Nestin-cre, increases sensitivity for reduction of food intake or body weight following acute leptin treatment (49). Interestingly, the whole brain knockout of Socs3 decreases body weight gain on HFD in both males and females and improves glucose and insulin tolerance in male mice (49). This is consistent with our previous study in male Pparg knockout mice but inconsistent with the data presented here in females. Unfortunately, the authors do not present any data on body weight gain and glucose tolerance in the Syn-cre Socs3 knockout, and interpretation of the whole brain knockout is confounded by the loss of Socs3 in astrocytes.
At the reproductive level, female BKO mice went through puberty normally but were subfertile when mated over 6 months, with smaller litter size and frequency while on normal chow. The fertility defect was due to an ovulation defect, as BKO mice released fewer oocytes when superovulated. The Cre transgene is not expressed in the ovary, so we believe that the defect is central. Female BKO had a reduced number of LH surges but the surge LH concentrations were higher. The elevated LH surge values may be due to the elevated pituitary Lhb mRNA expression, but the reduced frequency of surges could be related to altered hypothalamic input. We also observed that the BKO mice presented with hemorrhagic follicles and corpora lutea and increased numbers of atretic follicles. The hemorrhage could have been due to the elevated surge LH, as ovarian hyperstimulation causes vascular leakage (54). There also appeared to be a defect in luteolysis in the BKO with the persistence of regressing corpora lutea. When placed on an HFD, female Flox mice had a reduced number of estrous cycles and a reduction in the time spent in proestrus, but female BKO mice did not show this impairment, suggesting that the effect was centrally mediated and required functional PPARγ in neurons. Obesity can also alter ovarian function (55). Indeed, the dramatic reduction of aromatase (Cyp19a1) expression in obese mice is consistent with the elevated leptin, as leptin induces Cart expression in granulosa cells to lower Cyp19a1 expression (56). Interestingly, aromatase down-regulation was not seen in the BKO, but we do not know whether this reflected a difference in leptin signaling in the ovary or whether the effect was centrally mediated. At the pituitary, we did not see a change in sensitivity to a bolus of GnRH, although it has been reported that obesity and/or hyperinsulinemia increase pituitary gonadotrope sensitivity to GnRH (43, 57).
At the hypothalamic level, the LepR is not expressed in Gnrh1 neurons but is coexpressed in a subset of Kiss1 neurons that become leptin responsive after puberty (58). Loss of LepR in mature neurons prevents puberty and causes infertility, but loss in Kiss1 neurons alone does not (59). Leptin stimulates Kiss1 expression, so the effect of HFD on Kiss1 expression in our study may be mediated by the increased leptin levels in the obese animals. The observation that Kiss1 was elevated in both Flox and BKO mice also suggests that the Kiss1 neuron itself is not leptin resistant. Leptin normally suppresses AgRP neuronal activity and gene expression (19), but diet-induced obesity causes leptin resistance and persistent activation of these neurons (60), which is consistent with the observed increase in Agrp expression in obesity. Furthermore, ablation of AgRP neurons in LepR-deficient mice restores fertility, indicating a central role for AgRP in reproductive suppression due to leptin deficiency (23). Interestingly, the only hypothalamic gene to show differential expression in the BKO mice was Agrp. The lack of induction of Agrp by HFD in the BKO mice suggests that PPARγ mediates leptin resistance in AgRP neurons. A conditional knockout of the LepR in AgRP neurons causes increased adiposity, but neither a detailed analysis of reproductive function nor a study of the effect of HFD was performed (61, 62). In support of our model, intraperitoneal administration of the PPARγ agonist rosiglitazone increases Agrp mRNA in the ARC in hamsters and mice (63). Furthermore, Agrp neurons are more responsive to low levels of leptin and are more prone to SOCS3 induction and leptin resistance (64). GnRH neuron activity, rather than gene expression, may be restrained by PPARγ through AgRP induction, as AgRP fibers form synapses on GnRH neurons in mice (65) and infusion of AgRP suppresses pulsatile LH release in ovariectomized monkeys (66).
In conclusion, the results presented here show that neuronal PPARγ was necessary for normal LH surges and female fertility but was also involved in the adverse effects of diet-induced obesity on estrous cycles by creating leptin resistance in Agrp neurons potentially through induction of the repressor gene Socs3. Our data are consistent with clinical observations, as TZD therapy in women with PCOS decreases serum LH and increases ovulation rate (31, 32). Further detailed studies using genetic deletions in specific neuronal populations will be necessary to dissect the individual contributions of PPARγ, SOCS3, and AgRP to leptin resistance and reproductive function in cases of lipid excess and obesity.
Acknowledgments
We acknowledge the assistance of the Histology Core at the Moores’ Cancer Center and the Genomics Core at the Sanford Consortium for Regenerative Medicine.
Current address: M.O.F. is with the Laboratory of Neuroendocrinology, Instituto de Biología y Medicina Experimental, CONICET. Vuelta de Obligado 2490, C1428ADN, Buenos Aires, Argentina.
Acknowledgments
This work is funded in part by National Institute for Health grants HD012303, CA155435, CA023100, and CA196853 (to N.J.G.W.) and DK033651, DK074868, DK063491, and DK09062 (to J.M.O.) and VA Merit Review award I01BX000130 (to N.J.G.W.).
Footnotes
- AgRP
- agouti-related peptide
- ARC
- arcuate nucleus
- BKO
- brain-knockout
- CART
- cocaine and amphetamine-regulated transcript
- FSH
- follicle-stimulating hormone
- GnRH
- gonadotropin-releasing hormone
- HFD
- high-fat diet
- HOMA-IR
- homeostatic model assessment of insulin resistance
- HPG
- hypothalamic-pituitary-gonadal
- IP
- intraperitoneal
- LepR
- leptin receptor
- LFD
- low-fat diet
- LH
- luteinizing hormone
- mRNA
- messenger RNA
- NPY
- neuropeptide Y
- PCOS
- polycystic ovary syndrome
- POMC
- proopiomelanocortin
- PPARγ
- peroxisome-proliferator activated receptor γ
- qPCR
- quantitative polymerase chain reaction
- STAT
- signal transducer and activator of transcription
- TZD
- thiazolidinedione
References
- 1.Semple RK, Chatterjee VK, O’Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116(3):581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123(6):993–999. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu H, Tsuchiya T, Sato N, Shimomura Y, Kobayashi I, Mori M. Troglitazone reduces plasma leptin concentration but increases hunger in NIDDM patients. Diabetes Care. 1998;21(9):1470–1474. [DOI] [PubMed] [Google Scholar]
- 4.Sarruf DA, Yu F, Nguyen HT, Williams DL, Printz RL, Niswender KD, Schwartz MW. Expression of peroxisome proliferator-activated receptor-gamma in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology. 2009;150(2):707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G, Nalbandian S, Fan W, Gayen JR, Mahata SK, Webster NJ, Schwartz MW, Olefsky JM. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med. 2011;17(5):618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keen-Rhinehart E, Ondek K, Schneider JE. Neuroendocrine regulation of appetitive ingestive behavior. Front Neurosci. 2013;7:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobrino Crespo C, Perianes Cachero A, Puebla Jiménez L, Barrios V, Arilla Ferreiro E. Peptides and food intake. Front Endocrinol (Lausanne). 2014;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro AV, Kolka CM, Kim SP, Bergman RN. Obesity, insulin resistance and comorbidities? Mechanisms of association. Arq Bras Endocrinol Metabol. 2014;58(6):600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crujeiras AB, Carreira MC, Cabia B, Andrade S, Amil M, Casanueva FF. Leptin resistance in obesity: an epigenetic landscape. Life Sci. 2015;140:57–63. [DOI] [PubMed] [Google Scholar]
- 10.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdearcos M, Xu AW, Koliwad SK. Hypothalamic inflammation in the control of metabolic function. Annu Rev Physiol. 2015;77:131–160. [DOI] [PubMed] [Google Scholar]
- 12.Herbison AE. Physiology of the GnRH neuronal network. In: Neill JD, ed. Knobil and Neill’s Physiology of Reproduction. 3rd ed. San Diego, CA: Elsevier, Academic Press; 2006:1415–1482. [Google Scholar]
- 13.Pawson AJ, McNeilly AS. The pituitary effects of GnRH. Anim Reprod Sci. 2005;88(1-2):75–94. [DOI] [PubMed] [Google Scholar]
- 14.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauffman AS. Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol. 2010;324(1-2):51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semaan SJ, Tolson KP, Kauffman AS. The development of kisspeptin circuits in the mammalian brain. Adv Exp Med Biol. 2013;784:221–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanz E, Quintana A, Deem JD, Steiner RA, Palmiter RD, McKnight GS. Fertility-regulating Kiss1 neurons arise from hypothalamic POMC-expressing progenitors. J Neurosci. 2015;35(14):5549–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nestor CC, Qiu J, Padilla SL, Zhang C, Bosch MA, Fan W, Aicher SA, Palmiter RD, Rønnekleiv OK, Kelly MJ. Optogenetic stimulation of arcuate nucleus Kiss1 neurons reveals a steroid-dependent glutamatergic input to POMC and AgRP neurons in male mice. Mol Endocrinol. 2016;30(6):630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klenke U, Constantin S, Wray S. Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors. Endocrinology. 2010;151(6):2736–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.True C, Verma S, Grove KL, Smith MS. Cocaine- and amphetamine-regulated transcript is a potent stimulator of GnRH and kisspeptin cells and may contribute to negative energy balance-induced reproductive inhibition in females. Endocrinology. 2013;154(8):2821–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratra DV, Elias CF. Chemical identity of hypothalamic neurons engaged by leptin in reproductive control. J Chem Neuroanat. 2014;61-62:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellefontaine N, Elias CF. Minireview: metabolic control of the reproductive physiology: insights from genetic mouse models. Horm Behav. 2014;66(1):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheffer-Babila S, Sun Y, Israel DD, Liu SM, Neal-Perry G, Chua SC Jr. Agouti-related peptide plays a critical role in leptin’s effects on female puberty and reproduction. Am J Physiol Endocrinol Metab. 2013;305(12):E1512–E1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma S, Sharma PM, Mistry DS, Chang RJ, Olefsky JM, Mellon PL, Webster NJ. PPARG regulates gonadotropin-releasing hormone signaling in LbetaT2 cells in vitro and pituitary gonadotroph function in vivo in mice. Biol Reprod. 2011;84(3):466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Sato M, Li Q, Lydon JP, Demayo FJ, Bagchi IC, Bagchi MK. Peroxisome proliferator-activated receptor gamma is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol. 2008;28(5):1770–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korhonen S, Heinonen S, Hiltunen M, Helisalmi S, Hippeläinen M, Koivunen R, Tapanainen JS, Laakso M. Polymorphism in the peroxisome proliferator-activated receptor-gamma gene in women with polycystic ovary syndrome. Hum Reprod. 2003;18(3):540–543. [DOI] [PubMed] [Google Scholar]
- 27.Orio F Jr, Matarese G, Di Biase S, Palomba S, Labella D, Sanna V, Savastano S, Zullo F, Colao A, Lombardi G. Exon 6 and 2 peroxisome proliferator-activated receptor-gamma polymorphisms in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(12):5887–5892. [DOI] [PubMed] [Google Scholar]
- 28.Lujan ME, Chizen DR, Pierson RA. Diagnostic criteria for polycystic ovary syndrome: pitfalls and controversies. J Obstet Gynaecol Can. 2008;30(8):671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dereli D, Dereli T, Bayraktar F, Ozgen AG, Yilmaz C. Endocrine and metabolic effects of rosiglitazone in non-obese women with polycystic ovary disease. Endocr J. 2005;52(3):299–308. [DOI] [PubMed] [Google Scholar]
- 30.Khan KA, Stas S, Kurukulasuriya LR. Polycystic ovarian syndrome. J Cardiometab Syndr. 2006;1(2):125–132. [DOI] [PubMed] [Google Scholar]
- 31.Azziz R, Ehrmann D, Legro RS, Whitcomb RW, Hanley R, Fereshetian AG, O’Keefe M, Ghazzi MN; PCOS/Troglitazone Study Group . Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2001;86(4):1626–1632. [DOI] [PubMed] [Google Scholar]
- 32.Mehta RV, Patel KS, Coffler MS, Dahan MH, Yoo RY, Archer JS, Malcom PJ, Chang RJ. Luteinizing hormone secretion is not influenced by insulin infusion in women with polycystic ovary syndrome despite improved insulin sensitivity during pioglitazone treatment. J Clin Endocrinol Metab. 2005;90(4):2136–2141. [DOI] [PubMed] [Google Scholar]
- 33.Sharma S, Sharma PM, Mistry DS, Chang RJ, Olefsky JM, Mellon PL, Webster N. PPARG regulates gonadotropin-releasing hormone signaling in LbetaT2 cells in vitro and pituitary gonadotroph function in vivo in mice. Biol Reprod. 2010;84(3):466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA. 2003;100(26):15712–15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat Med. 2003;9(12):1491–1497. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15(7):859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rempe D, Vangeison G, Hamilton J, Li Y, Jepson M, Federoff HJ. Synapsin I Cre transgene expression in male mice produces germline recombination in progeny. Genesis. 2006;44(1):44–49. [DOI] [PubMed] [Google Scholar]
- 38.Yamanaka T, Tosaki A, Kurosawa M, Akimoto K, Hirose T, Ohno S, Hattori N, Nukina N. Loss of aPKCλ in differentiated neurons disrupts the polarity complex but does not induce obvious neuronal loss or disorientation in mouse brains. PLoS One. 2013;8(12):e84036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren H, Plum-Morschel L, Gutierrez-Juarez R, Lu TY, Kim-Muller JY, Heinrich G, Wardlaw SL, Silver R, Accili D. Blunted refeeding response and increased locomotor activity in mice lacking FoxO1 in synapsin-Cre-expressing neurons. Diabetes. 2013;62(10):3373–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43(1):31–42. [DOI] [PubMed] [Google Scholar]
- 41.Pelusi C, Ikeda Y, Zubair M, Parker KL. Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biol Reprod. 2008;79(6):1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127(5):569–580. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S, Morinaga H, Hwang V, Fan W, Fernandez MO, Varki N, Olefsky JM, Webster NJ. Free fatty acids induce Lhb mRNA but suppress Fshb mRNA in pituitary LβT2 gonadotropes and diet-induced obesity reduces FSH levels in male mice and disrupts the proestrous LH/FSH surge in female mice. Endocrinology. 2013;154(6):2188–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michalik M, Steele AD, Mistlberger RE. A sex difference in circadian food-anticipatory rhythms in mice: interaction with dopamine D1 receptor knockout. Behav Neurosci. 2015;129(3):351–360. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, Wang Y, Sun KK, Wang K, Sun ZS, Zhao M, Wang J. Sex-related difference in food-anticipatory activity of mice. Horm Behav. 2015;70:38–46. [DOI] [PubMed] [Google Scholar]
- 46.Shirahase T, Aoki M, Watanabe R, Watanabe Y, Tanaka M. Increased alcohol consumption in relaxin-3 deficient male mice. Neurosci Lett. 2016;612:155–160. [DOI] [PubMed] [Google Scholar]
- 47.Seu E, Groman SM, Arnold AP, Jentsch JD. Sex chromosome complement influences operant responding for a palatable food in mice. Genes Brain Behav. 2014;13(6):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu ZJ, Bian J, Zhao YL, Zhang X, Zou N, Li D. Lentiviral vector-mediated knockdown of SOCS3 in the hypothalamus protects against the development of diet-induced obesity in rats. Diabetes Obes Metab. 2011;13(10):885–892. [DOI] [PubMed] [Google Scholar]
- 49.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10(7):739–743. [DOI] [PubMed] [Google Scholar]
- 50.Berger H, Végran F, Chikh M, Gilardi F, Ladoire S, Bugaut H, Mignot G, Chalmin F, Bruchard M, Derangère V, Chevriaux A, Rébé C, Ryffel B, Pot C, Hichami A, Desvergne B, Ghiringhelli F, Apetoh L. SOCS3 transactivation by PPARγ prevents IL-17-driven cancer growth. Cancer Res. 2013;73(12):3578–3590. [DOI] [PubMed] [Google Scholar]
- 51.Park EJ, Park SY, Joe EH, Jou I. 15d-PGJ2 and rosiglitazone suppress Janus kinase-STAT inflammatory signaling through induction of suppressor of cytokine signaling 1 (SOCS1) and SOCS3 in glia. J Biol Chem. 2003;278(17):14747–14752. [DOI] [PubMed] [Google Scholar]
- 52.Yu JH, Kim KH, Kim H. SOCS 3 and PPAR-gamma ligands inhibit the expression of IL-6 and TGF-beta1 by regulating JAK2/STAT3 signaling in pancreas. Int J Biochem Cell Biol. 2008;40(4):677–688. [DOI] [PubMed] [Google Scholar]
- 53.Pedroso JA, Buonfiglio DC, Cardinali LI, Furigo IC, Ramos-Lobo AM, Tirapegui J, Elias CF, Donato J Jr. Inactivation of SOCS3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Mol Metab. 2014;3(6):608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delbaere A, Smits G, De Leener A, Costagliola S, Vassart G. Understanding ovarian hyperstimulation syndrome. Endocrine. 2005;26(3):285–290. [DOI] [PubMed] [Google Scholar]
- 55.Huang-Doran I, Franks S. Genetic rodent models of obesity-associated ovarian dysfunction and subfertility: insights into polycystic ovary syndrome. Front Endocrinol (Lausanne). 2016;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma X, Hayes E, Prizant H, Srivastava RK, Hammes SR, Sen A. Leptin-induced CART (cocaine- and amphetamine-regulated transcript) is a novel intraovarian mediator of obesity-related infertility in females. Endocrinology. 2016;157(3):1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brothers KJ, Wu S, DiVall SA, Messmer MR, Kahn CR, Miller RS, Radovick S, Wondisford FE, Wolfe A. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010;12(3):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cravo RM, Frazao R, Perello M, Osborne-Lawrence S, Williams KW, Zigman JM, Vianna C, Elias CF. Leptin signaling in Kiss1 neurons arises after pubertal development. PLoS One. 2013;8(3):e58698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donato J Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121(1):355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baver SB, Hope K, Guyot S, Bjørbaek C, Kaczorowski C, O’Connell KM. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J Neurosci. 2014;34(16):5486–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG Jr, Schwartz GJ, Chua SC Jr. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149(4):1773–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo N, Marcelin G, Liu SM, Schwartz G, Chua S Jr. Neuropeptide Y and agouti-related peptide mediate complementary functions of hyperphagia and reduced energy expenditure in leptin receptor deficiency. Endocrinology. 2011;152(3):883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garretson JT, Teubner BJ, Grove KL, Vazdarjanova A, Ryu V, Bartness TJ. Peroxisome proliferator-activated receptor γ controls ingestive behavior, agouti-related protein, and neuropeptide Y mRNA in the arcuate hypothalamus. J Neurosci. 2015;35(11):4571–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olofsson LE, Unger EK, Cheung CC, Xu AW. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc Natl Acad Sci USA. 2013;110(8):E697–E706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turi GF, Liposits Z, Moenter SM, Fekete C, Hrabovszky E. Origin of neuropeptide Y-containing afferents to gonadotropin-releasing hormone neurons in male mice. Endocrinology. 2003;144(11):4967–4974. [DOI] [PubMed] [Google Scholar]
- 66.Vulliémoz NR, Xiao E, Xia-Zhang L, Wardlaw SL, Ferin M. Central infusion of agouti-related peptide suppresses pulsatile luteinizing hormone release in the ovariectomized rhesus monkey. Endocrinology. 2005;146(2):784–789. [DOI] [PubMed] [Google Scholar]