Abstract
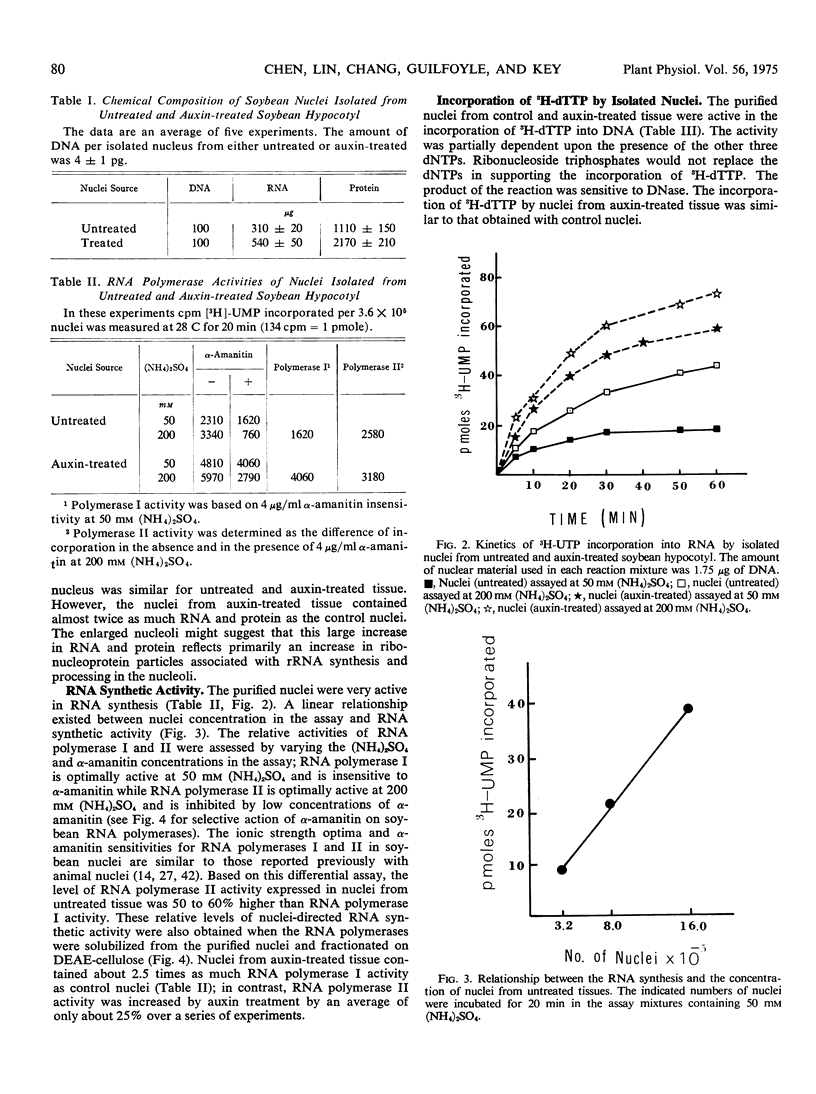
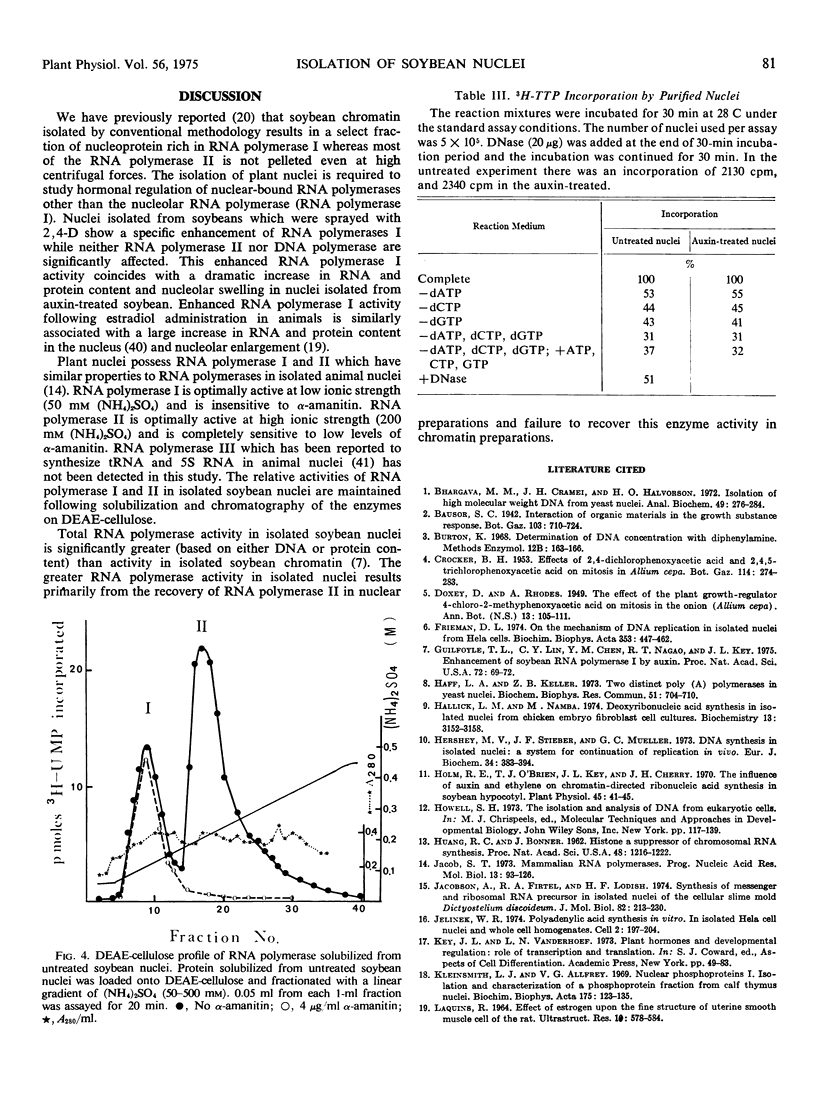
A quick procedure for the isolation of nuclei with good yield from soybean hypocotyl (Glycine max var. Wayne) was developed. The isolated nuclei appeared to retain their structural integrity. They were typically ellipsoidal with minima and maxima diameter of about 6 and 8 to 10 micrometers. While the nuclei were similar in size, the nucleoli were significantly larger in nuclei from auxin-treated tissue. The DNA content per nucleus was 4 ± 1 picograms for both untreated and auxin-treated tissues. The DNA: RNA: protein ratio of isolated nuclei in untreated and auxin-treated tissues was 1: 3.1: 11 and 1: 5.4: 21.7, respectively. The purified nuclei were active in RNA synthesis; the level of RNA polymerase II activity expressed in the nuclei from untreated tissue was 50 to 60% higher than RNA polymerase. I. The nuclei from auxin-treated tissues contained about 2.5 times as much RNA polymerase I activity as nuclei from untreated tissue. The purified nuclei from both untreated and auxin-treated tissues were also active in the incorporation of 3H-TTP into DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhargava M. M., Cramer J. H., Halvorson H. O. Isolation of high molecular weight DNA from yeast nuclei. Anal Biochem. 1972 Sep;49(1):276–284. doi: 10.1016/0003-2697(72)90266-7. [DOI] [PubMed] [Google Scholar]
- Friedman D. L. On the mechanism of DNA replication in isolated nuclei from HeLa cells. Biochim Biophys Acta. 1974 Jul 24;353(4):447–462. doi: 10.1016/0005-2787(74)90051-3. [DOI] [PubMed] [Google Scholar]
- Guilfoyle T. J., Lin C. Y., Chen Y. M., Nagao R. T., Key J. L. Enhancement of soybean RNA polymerase I by auxin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):69–72. doi: 10.1073/pnas.72.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG R. C., BONNER J. Histone, a suppressor of chromosomal RNA synthesis. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1216–1222. doi: 10.1073/pnas.48.7.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haff L. A., Keller E. B. Two distinct poly(A) polymerases in yeast nuclei. Biochem Biophys Res Commun. 1973 Apr 2;51(3):704–710. doi: 10.1016/0006-291x(73)91372-7. [DOI] [PubMed] [Google Scholar]
- Hallick L. M., Namba M. Deoxyribonucleic acid synthesis in isolated nuclei from chicken embryo fibroblast cell cultures. Biochemistry. 1974 Jul 16;13(15):3152–3158. doi: 10.1021/bi00712a023. [DOI] [PubMed] [Google Scholar]
- Hershey H. V., Stieber J. F., Mueller G. C. Dna synthesis in isolated HeLa nuclei. A system for continuation of replication in vivo. Eur J Biochem. 1973 Apr;34(2):383–394. doi: 10.1111/j.1432-1033.1973.tb02770.x. [DOI] [PubMed] [Google Scholar]
- Holm R. E., O'brien T. J., Key J. L., Cherry J. H. The Influence of Auxin and Ethylene on Chromatin-directed Ribonucleic Acid Synthesis in Soybean Hypocotyl. Plant Physiol. 1970 Jan;45(1):41–45. doi: 10.1104/pp.45.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S. T. Mammalian RNA polymerases. Prog Nucleic Acid Res Mol Biol. 1973;13:93–126. doi: 10.1016/s0079-6603(08)60101-4. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Firtel R. A., Lodish H. F. Synthesis of messenger and ribosomal RNA precursors in isolated nuclei of the cellular slime mold Dictyostelium discoideum. J Mol Biol. 1974 Jan 15;82(2):213–230. doi: 10.1016/0022-2836(74)90342-8. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R. Poly adenylic acid synthesis in vitro in isolated HeLa cell nuclei and whole cell homogenates,. Cell. 1974 Jul;2(3):197–204. doi: 10.1016/0092-8674(74)90094-4. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G. Nuclear phosphoproteins. I. Isolation and characterization of a phosphoprotein fraction from calf thymus nuclei. Biochim Biophys Acta. 1969 Feb 4;175(1):123–135. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin C. Y., Guilfoyle T. J., Chen Y. M., Nagao R. T., Key J. L. The separation of RNA polymerases I and II achieved by fractionation of plant chromatin. Biochem Biophys Res Commun. 1974 Sep 23;60(2):498–506. doi: 10.1016/0006-291x(74)90268-x. [DOI] [PubMed] [Google Scholar]
- Lynch W. E., Brown R. F., Umeda T., Langreth S. G., Lieberman I. Synthesis of deoxyribonucleic acid by isolated liver nuclei. J Biol Chem. 1970 Aug 10;245(15):3911–3916. [PubMed] [Google Scholar]
- Marzluff W. F., Jr, Murphy E. C., Jr, Huang R. C. Transcription of the genes for 5S ribosomal RNA and transfer RNA in isolated mouse myeloma cell nuclei. Biochemistry. 1974 Aug 27;13(18):3689–3696. doi: 10.1021/bi00715a011. [DOI] [PubMed] [Google Scholar]
- O'Brien T. J., Jarvis B. C., Cherry J. H., Hanson J. B. Enhancement by 2,4-dichlorophenoxyacetic acid of chromatin RNA polymerase in soybean hypocotyl tissue. Biochim Biophys Acta. 1968 Nov 20;169(1):35–43. doi: 10.1016/0005-2787(68)90006-3. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. A distinct RNA polymerase activity, synthesizing 5-5 s, 5 s and 4 s RNA in nuclei from adenovirus 2-infected HeLa cells. J Mol Biol. 1972 Oct 14;70(3):435–450. doi: 10.1016/0022-2836(72)90551-7. [DOI] [PubMed] [Google Scholar]
- Raskas H. J. Release of adenovirus messenger RNA from isolated nuclei. Nat New Biol. 1971 Sep 29;233(39):134–136. doi: 10.1038/newbio233134a0. [DOI] [PubMed] [Google Scholar]
- Raynaud-Jammet C., Biéri F., Baulieu E. E. Effects of oestradiol, -amanitin and ionic strength on the in vitro synthesis of RNA by uterus nuclei. Biochim Biophys Acta. 1971 Oct 14;247(2):355–360. doi: 10.1016/0005-2787(71)90683-6. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Roeder R. G. Ribosomal RNA synthesis in isolated nuclei. J Mol Biol. 1972 Jun 28;67(3):433–441. doi: 10.1016/0022-2836(72)90461-5. [DOI] [PubMed] [Google Scholar]
- Rickwood D., Riches P. G., Maggillivray A. J. Studies of the in vitro phosphorylation of chromatin non-histone proteins in isolated nuclei. Biochim Biophys Acta. 1973 Feb 23;299(1):162–171. doi: 10.1016/0005-2787(73)90408-5. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Schumm D. E., Webb T. E. Modified messenger ribonucleic acid release from isolated hepatic nuclei after inhibition of polyadenylate formation. Biochem J. 1974 Apr;139(1):191–196. doi: 10.1042/bj1390191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumm D. E., Webb T. E. Transport of informosomes from isolated nuclei of regenerating rat liver. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1259–1265. doi: 10.1016/0006-291x(72)90847-9. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Sussman M. Transcription in isolated nuclei of the sline mold Dictyostelium discoideum. Biochim Biophys Acta. 1973 Sep 7;319(3):312–322. doi: 10.1016/0005-2787(73)90171-8. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Spelsberg T. C., Kleinsmith L. J. Nonhistone chromosomal proteins and gene regulation. Science. 1974 Mar 1;183(4127):817–824. doi: 10.1126/science.183.4127.817. [DOI] [PubMed] [Google Scholar]
- Tautvydas K. J. Mass isolation of pea nuclei. Plant Physiol. 1971 Apr;47(4):499–503. doi: 10.1104/pp.47.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T., Allfrey V. G. Studies of nuclear acidic proteins. Evidence for their phosphorylation, tissue specificity, selective binding to deoxyribonucleic acid, and stimulation effects on transcription. J Biol Chem. 1971 Jun 10;246(11):3597–3609. [PubMed] [Google Scholar]
- WIDNELL C. C., TATA J. R. EVIDENCE FOR TWO DNA-DEPENDENT RNA POLYMERASE ACTIVITIES IN ISOLATED RAT-LIVER NUCLEI. Biochim Biophys Acta. 1964 Jul 22;87:531–533. doi: 10.1016/0926-6550(64)90133-1. [DOI] [PubMed] [Google Scholar]
- Weinmann R., Roeder R. G. Role of DNA-dependent RNA polymerase 3 in the transcription of the tRNA and 5S RNA genes. Proc Natl Acad Sci U S A. 1974 May;71(5):1790–1794. doi: 10.1073/pnas.71.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg J. A., Kooistra T., Geert A. B., Gruber M. Effect of estradiol on the RNA content and the activity of nucleolar RNA polymerase from rooster liver. Biochem Biophys Res Commun. 1974 Nov 6;61(1):367–374. doi: 10.1016/0006-291x(74)90576-2. [DOI] [PubMed] [Google Scholar]