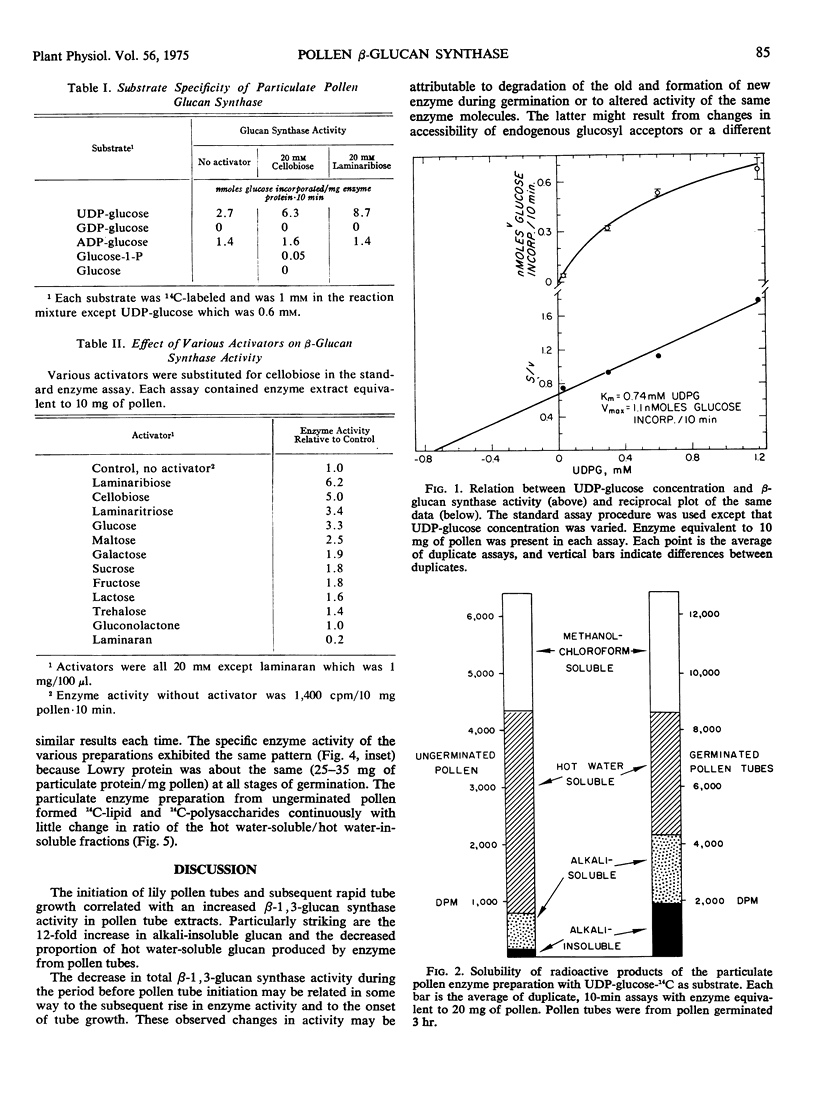
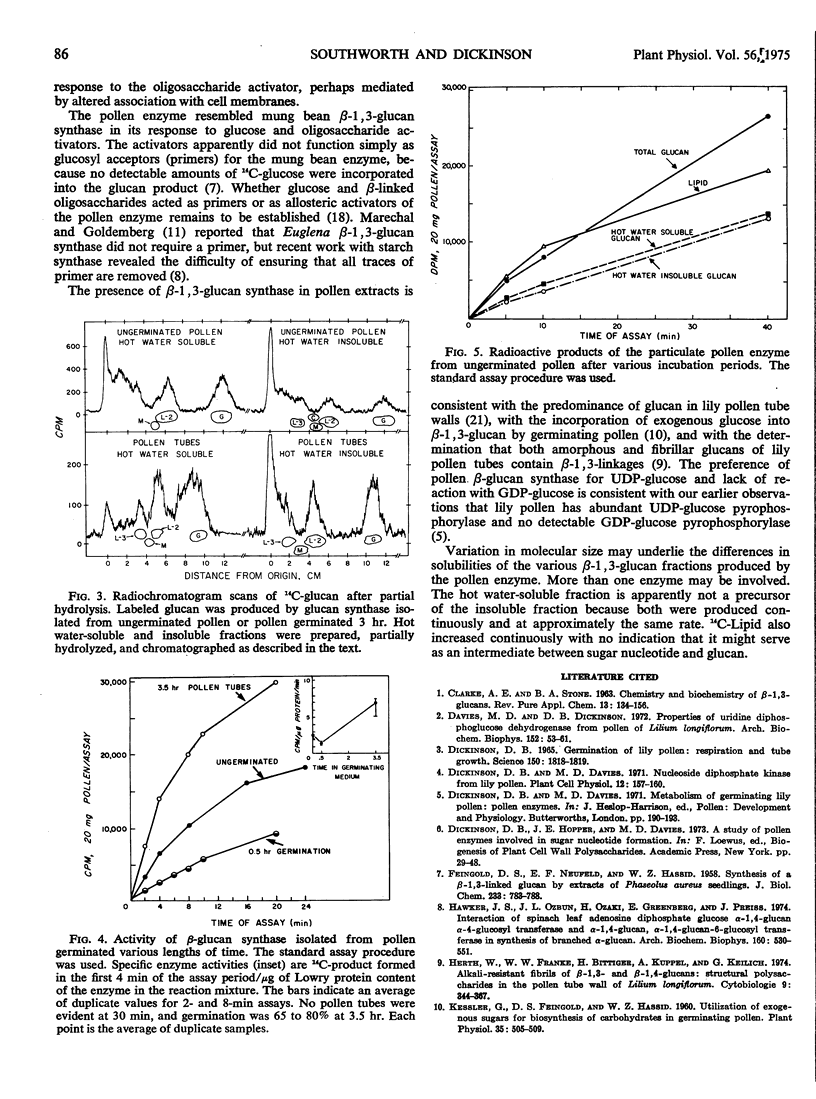
Abstract
A particulate fraction from pollen tubes and ungerminated pollen of Lilium longiflorum incorporated 14C-glucose from UDP-glucose-14C into a lipid fraction and into β-1, 3-glucan. Partial hydrolysis of the glucan yielded laminaribiose as the only radioactive disaccharide. The preferred substrate was UDP-glucose, and enzyme activity was stimulated by glucose and by β-linked di- and trisaccharides. Enzyme from growing pollen tubes synthesized β-1, 3-glucan more rapidly and produced a higher proportion of alkali-insoluble glucan than did enzyme from ungerminated pollen. The onset of pollen tube growth may be dependent on altered activity of β-1, 3-glucan synthase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies M. D., Dickinson D. B. Properties of uridine diphosphoglucose dehydrogenase from pollen of Lilium longiflorum. Arch Biochem Biophys. 1972 Sep;152(1):53–61. doi: 10.1016/0003-9861(72)90192-0. [DOI] [PubMed] [Google Scholar]
- Dickinson D. B. Germination of Lily Pollen: Respiration and Tube Growth. Science. 1965 Dec 31;150(3705):1818–1819. doi: 10.1126/science.150.3705.1818. [DOI] [PubMed] [Google Scholar]
- FEINGOLD D. S., NEUFELD E. F., HASSID W. Z. Synthesis of a beta-1, 3-linked glucan by extracts of Phaseolus aureus seedlings. J Biol Chem. 1958 Oct;233(4):783–788. [PubMed] [Google Scholar]
- Hawker J. S., Ozbun J. L., Ozaki H., Greenberg E., Preiss J. Interaction of spinach leaf adenosine diphosphate glucose alpha-1,4-glucan alpha-4-glucosyl transferase and alpha-1,4-glucan, alpha-1,4-glucan-6-glycosyl transferase in synthesis of branched alpha-glucan. Arch Biochem Biophys. 1974 Feb;160(2):530–551. doi: 10.1016/0003-9861(74)90430-5. [DOI] [PubMed] [Google Scholar]
- Kessler G., Feingold D. S., Hassid W. Z. Utilization of Exogenous Sugars for Biosynthesis of Carbohydrates in Germinating Pollen. Plant Physiol. 1960 Jul;35(4):505–509. doi: 10.1104/pp.35.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARECHAL L. R., GOLDEMBERG S. H. URIDINE DIPHOSPHATE GLUCOSE-BETA-1,3-GLUCAN BETA-3-GLUCOSYLTRANSFERASE FROM EUGLENA GRACILIS. J Biol Chem. 1964 Oct;239:3163–3167. [PubMed] [Google Scholar]
- Pharr D. M., Dickinson D. B. Partial Characterization of C(x) Cellulase and Cellobiase from Ripening Tomato Fruits. Plant Physiol. 1973 Mar;51(3):577–583. doi: 10.1104/pp.51.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore G., Maclachlan G. A. Indoleacetic acid stimulates cellulose deposition and selectively enhances certain beta-glucan synthetase activities. Biochim Biophys Acta. 1973 Dec 5;329(2):271–282. doi: 10.1016/0304-4165(73)90292-4. [DOI] [PubMed] [Google Scholar]
- Spencer F. S., Ziola B., Maclachlan G. A. Particulate glucan synthetase activity: dependence on acceptor, activator, and plant growth hormone. Can J Biochem. 1971 Dec;49(12):1326–1332. doi: 10.1139/o71-192. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Hassid W. Z. Substrate Activation of beta-(1 --> 3) Glucan Synthetase and Its Effect on the Structure of beta-Glucan Obtained from UDP-d-glucose and Particulate Enzyme of Oat Coleoptiles. Plant Physiol. 1973 Jun;51(6):998–1001. doi: 10.1104/pp.51.6.998. [DOI] [PMC free article] [PubMed] [Google Scholar]



