Abstract
CD40, a tumor necrosis factor receptor, is a major immune-modulating susceptibility gene for Graves disease (GD) as well as for a variety of other autoimmune diseases. Its broad association with autoimmunity underscores its paramount role in the development of a normal adaptive immune response, primarily in coordinating effective antigen presentation. The molecular pathways by which CD40 activation in the thyroid induces GD are unknown. In this study, we investigated whether NF-κB, a ubiquitious family of transcription factors, mediates the downstream effects of thyroid-specific CD40 activation. Cultured primary human thyrocytes, from patients with and without GD, underwent CD40 stimulation. Once stimulated, cytokines and transcription factors specific for either the canonical nuclear factor κB (NF-κB)1 pathway [interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α], which primarily recruits cells for innate immunity, or the noncanonical NF-κB2 pathway [B cell–activating factor of the TNF family, CC chemokine ligand (CCL)21], which directs B cell viability, were analyzed. Significant upregulation in the messenger RNA and protein levels of both canonical and noncanonical pathway cytokines was observed. Western blot analyses of the specific transcription factors for the NF-κB1 and NF-κB2 pathways (p65 and p100/p52, respectively) demonstrated that p65 is constitutively expressed. In contrast, CD40 stimulation robustly increased the expression of the NF-κB2 p52 transcription factor, and the upregulation was significantly more profound in the GD tissue than in the normal thyroid tissue. Our data show that CD40 activity in thyrocytes is prominently mediated via NF-κB and furthermore suggest that the NF-κB1 and NF-κB2 pathways both contribute to the triggering and the progression of GD.
Graves disease (GD) is characterized by thyrotoxicosis due to stimulating thyrotropin receptor antibodies. It is a leading cause for hyperthyroidism and represents one of the most common autoimmune diseases with a prevalence of approximately 1% in the United States (1–5). Genetic susceptibility has been consistently shown to play a major role in the etiology of GD, with some epidemiological studies estimating that genetic factors contribute 80% of the liability to develop GD (6–9).
CD40 has emerged as a key genetic player in the development of GD and autoimmunity in general (1, 10–22). CD40 is a tumor necrosis factor (TNF) receptor found on antigen-presenting cells (APCs) and thyrocytes and is required for an effective adaptive immune response (20, 23). The interaction with its ligand, CD154 (expressed on T cells), provides the needed costimulatory signals to stimulate B cell proliferation, immunoglobulin class switching, and germinal center formation. CD40 guides immune-tolerant immature dendritic cells to develop into potent immunostimulatory cells and enhances T cell priming (10, 24–29). CD40’s central role in coordinating T cell– and B cell–mediated immunity is underscored by the profound immune system aberrations that develop with both CD40 deficiency (X-linked hyper-IgM syndrome) (25) and CD40 overactivity, including GD (20, 21, 23, 30). Indeed, transgenic mouse models constitutively overexpressing thyroidal CD40 develop more severe experimental autoimmune GD and thyrotoxicosis, whereas blockade of CD40 stimulation in experimental animal models suppresses progression to overt thyroiditis (21, 29, 31).
In B cells, CD40 activates various transcription factors, including NF-κB. NF-κB activates the promoter regions of many cytokines (32) and is independently implicated in the development of impaired self-tolerance (33). In fact, corticosteroid-mediated immune suppression is believed to be mainly through NF-κB pathway interference (34–36). The association between CD40 overactivation and GD has been firmly established, but the definitive molecular steps that link thyroid-specific CD40 activity to GD remain unknown. Whether local thyroidal CD40 acts through the NF-κB signaling cascade has not been previously studied. The aim of this study was to examine the downstream molecular effects of CD40 in thyroid follicular cells and to test the hypothesis that thyroid-specific NF-κB is the central effector pathway by which CD40 triggers GD.
Materials and Methods
Primary thyrocyte cell cultures
Studies were approved by the Icahn School of Medicine institutional review board. Human primary thyroid specimens were obtained from the Department of Pathology. A total of 6 specimens was obtained (4 from non-autoimmune thyroid disease patients and 2 from GD patients) and was digested in type II collagenase, at a concentration of 1.25 mg collagenase/100 mg thyroid tissue for 45 minutes at 37°C. The layer containing cells was strained through a 40-µm filter and neutralized with primary medium (HyClone medium 199/Earle's Balanced Salt Solution with Earle’s balanced salts and l-glutamine) at 1:1 volume. Cells were centrifuged at 1000 rpm, 5 minutes, 4°C; the resulting pellet was resuspended in 2 mL medium and set aside. The process was repeated two more times with the remaining partially collagenase-treated thyroid tissue. The resulting pellets were suspended in 2 mL medium and pooled together for a total of 6 mL resuspended cells. These cells were then plated directly and distributed equally into a 6-well plate, reaching >80% confluence. Cells were maintained at 37°C in 5% CO2.
CD40 stimulation of primary thyrocytes
A B cell hybridoma (American Type Culture Collection, Manassas, VA; HB-9110) was used to produce a 64.64 mg stock of G28.5 (QED Bioscience, San Diego, CA), a CD40-stimulating monoclonal antibody. Plated primary thyrocytes with ≥80% confluence were stimulated, in duplicates, with 20 or 40 µg G28.5/mL. After 12 hours of incubation at 37°C, supernatants and cells were collected. Cells were also stimulated using MegaCD40L, a recombinant CD154 (Enzo Life Sciences, Farmingdale, NY; ALX-522-015-C010), at 250 ng/mL.
Reverse transcription polymerase chain reaction
RNA was extracted from thyroid cells using TRIzol reagent (Life Technologies, Waltham, MA; 15596026) and a polyacryl carrier (MRC, Cincinnati, OH; PC 152) as per manufacturer’s protocol. Complementary DNA was synthesized by reverse transcription using the Superscript III First-Strand Synthesis kit (Life Technologies, Waltham, MA; 18080-051). Quantitative messenger RNA (mRNA) expression levels of interleukin (IL)-6, IL-8, TNF-α, and B cell–activating factor of the TNF family (BAFF) were assessed using SYBR Green PCR Master Mix (Affymetrix, Santa Clara, CA; 75600) and 10 µM primers in a 20 µL reaction. The reaction was set to run at 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute, utilizing the Applied Biosystems 7300 Real-Time PCR System. IL-6 primers: forward, 5′- CGGTACATCCTCGACGGCAT-3′ and reverse, 5′-CTGCCAGTGCCTCTTTGCTG-3′. IL-8: forward, 5′-GTGGCTCTCTTGGCAGCCTT-3′ and reverse, 5′-AAATTTGGGGTGGAAAGGTT-3′. TNF-α: forward, 5′-GCCCAGGCAGTCAGATCATC-3′ and reverse, 5′-GGTTTGCTACAACATGGGCTACA-3′. BAFF: forward, 5′-AGAACAGCAGAAATAAGCGT-3′ and reverse, 5′-TGCAATCAGTTGCAAGCAGT-3′. CD40: forward, 5′-GCTGTCCATCCAGAACCACC-3′ and reverse, 5′-CAAAGCCGGGCGAGCATGAG-3′. Results were normalized to glyceraldehyde-3-phosphate dehydrogenase, a housekeeping gene. Experiments were performed in triplicates.
Cytokine analysis
Supernatants collected from primary thyrocyte cultures were assayed using an EMD Millipore (Billerica, MA; HCYTOMAG-60K) multiplex bead-based assay, according to the manufacturer’s protocol. Cytokine levels of IL-6, IL-8, TNF-α, IL-12(p40), IL-1b, interferon-γ, and IL-17 were measured on a Luminex xMAP 200 platform. Standards and quality controls were run in parallel to determine cytokine concentrations. Samples were performed in duplicates.
Western blot
After appropriate incubation times, thyroid cells were rinsed twice with phosphate-buffered saline, collected on ice with a cell scraper, and pooled. Cells were centrifuged at 14,000 G, 4°C for 10 minutes and subsequently lysed with 1× radioimmunoprecipitation assay (radioimmunoprecipitation assay 10×, 9806), 1:100 phenylmethanesulfonyl fluoride solution (Sigma-Aldrich, St. Louis, MO; 93482), and 1:100 NaF. Samples were then vortexed every 5 minutes for a total of 40 minutes and then centrifuged for 45 minutes, 14,000g, 4°C to remove insoluble materials. The protein content was quantitated using the Bradford protein assay. Cell lysates, matched for protein content, were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Membranes were blotted with anti-phospho–NF-κB p65 (Cell Signaling, Danvers, MA; 3033) (37), anti–NF-κB p100/p52 (Abcam, Cambridge, MA; ab71108) (38), and anti–proliferating cell nuclear antigen primary antibodies (Cell Signaling, Danvers, MA; 2586) (39) (Table 1). The anti-p65 and anti-p100/p52 antibodies were validated in the BSM (human B lymphoblastoid) cell line as a positive control. The primary antibodies were detected using anti-mouse and anti-rabbit secondary Abs (Licor, Lincoln, NE; 926-32210, 926-68071). Protein bands were visualized using the Licor Odyssey Fc imaging system.
Table 1.
Primary Antibody Table
| Peptide/Protein Target | Antigen Sequence (If Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID No. |
|---|---|---|---|---|---|---|
| p65 | Anti-phospho–NF-κB p65 (Ser536) | Cell Signaling, 3033 | Rabbit | 1000 | AB_331284 | |
| p100/p52 | Anti–NF-κB p100/p52 | Abcam, ab71108 | Mouse | 1000 | AB_1281035 | |
| PCNA | Anti-PCNA (PC10) | Cell Signaling, 2586 | Mouse | 2000 | AB_2160343 |
RRID, Research Resource Identifier.
Statistical analyses
Data are presented as mean ± standard error of the mean. Statistical analyses were determined by a two-tailed t test, using the GraphPad Prism 5 software (San Diego, CA). Differences were considered statistically significant when the p values were <0.05.
Results
Thyroidal CD40 activation specifically upregulates NF-κB
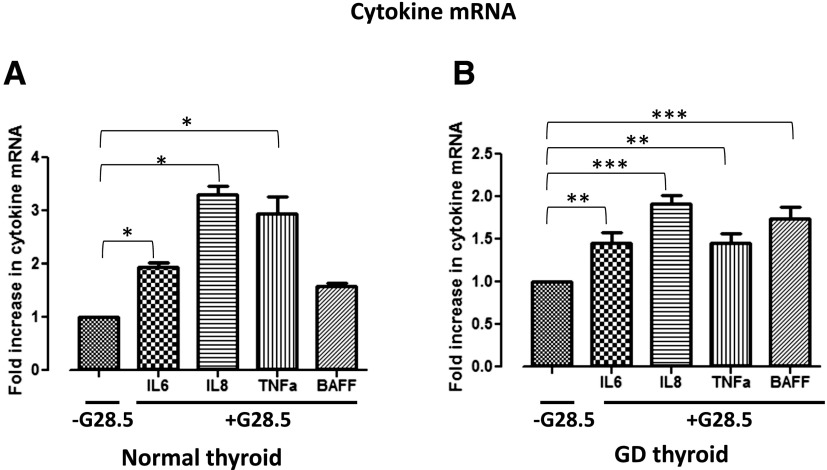
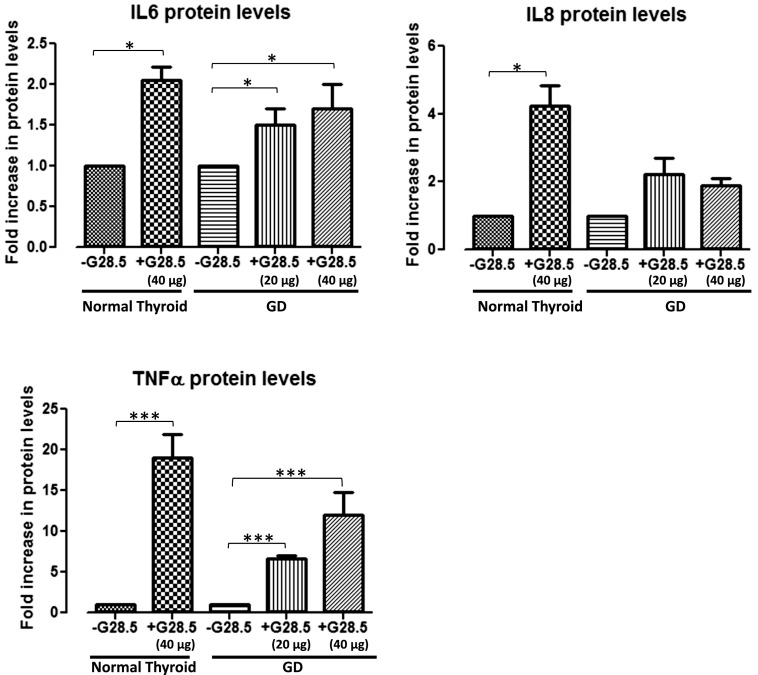
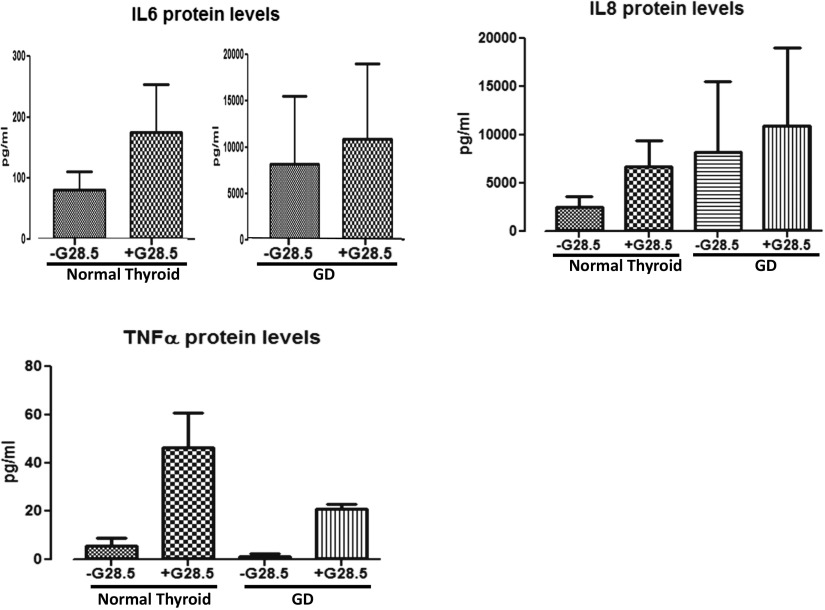
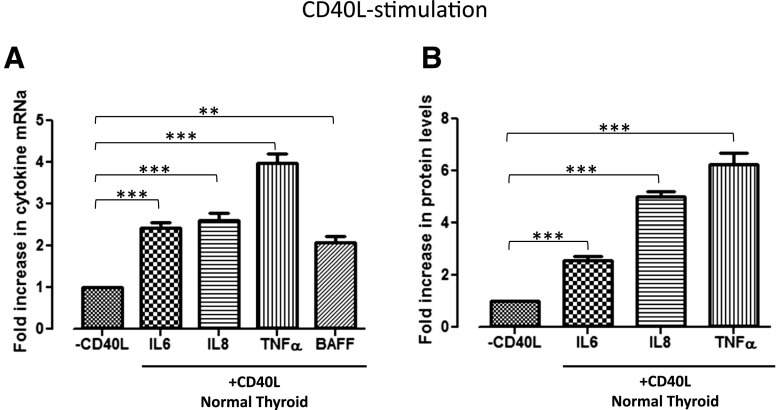
To investigate the specific downstream effectors of thyroidal CD40, thyrocytes isolated from both normal and GD thyroid specimens were exposed to 0, 20, or 40 μg/mL G28.5. Normal thyrocytes exposed to G28.5 showed significant upregulation of IL-6, IL-8, and TNF-α mRNA [Fig. 1(A)], whereas each, including BAFF, was significantly upregulated in the GD thyrocytes [Fig. 1(B); Supplemental Fig. 1 (97.6KB, pptx) ]. The CD40-mediated effect was likewise observed at the protein level, demonstrating significant increases in the protein expression in the normal and the GD thyroid cells. Normal thyrocytes demonstrated an upregulation only when stimulated with the higher G28.5 concentration of 40 μg/mL, whereas GD thyrocytes were responsive to G28.5 at both the 20 μg/mL and 40 μg/mL concentrations (Fig. 2; BAFF had very high background due to technical challenges with commercial BAFF assays; data not shown). Although the normal thyrocytes showed a greater relative increase in several of the cytokines, the absolute measured levels (pg/mL) of the NF-κB–specific cytokines, IL-6 and IL-8, were consistently and significantly higher in the GD specimens (Fig. 3). Indeed, when we measured CD40 expression on thyrocytes in response to G28.5 stimulation, the levels increased significantly more in GD thyrocytes than in normal thyrocytes (Supplemental Fig. 2 (51.3KB, pptx) ). Other cytokines (IL-1β, IL-12p40, IL-17α, and interferon-γ) were undetectable with CD40 activation, suggesting that CD40’s downstream effects are not broad spectrum, but NF-κB specific. These experiments were replicated by stimulating thyroid cells with CD40 ligand (MegaCD40L) and demonstrated similar increases in cytokine mRNA and protein upon stimulation (Fig. 4).
Figure 1.
Upregulation of the mRNA of NF-κB cytokines after G28.5 stimulation of CD40 in (A) normal and (B) GD primary human thyroid cells. RNA was extracted from thyrocytes after they were cultured with and without G28.5 for 12 hours. Samples were analyzed by real-time reverse transcription polymerase chain reaction for NF-κB1– and NF-κB2–specific target cytokines; values are normalized to glyceraldehyde-3-phosphate dehydrogenase, the internal standard. The mRNA levels are relative to cells not exposed to G28.5. *P < 0.05; **P < 0.01; ***P < 0.001. Experiments were performed in triplicates.
Figure 2.
Fold increase in NF-κB–specific cytokine levels with G28.5 stimulation versus without G28.5 exposure. Primary normal and GD thyroid specimens were either treated or untreated with G28.5 (20 or 40 μg/mL) for 12 hours. Supernatants were collected and analyzed by a multiplex bead-based assay, for NF-κB–specific cytokines (IL-6, IL-8, TNF-α), among others (IL-17a, IL-1b, interferon-γ, and IL-12p40). Normal thyroids showed significant upregulation of the NF-κB–specific cytokines only with 40 μg/mL G28.5, but no change in cytokine levels with 20 μg/mL G28.5 (20 μg/mL; data not shown). GD thyroids showed significant upregulation in IL-6 and TNF-α levels with both the lower 20 μg/mL and 40 μg/mL G28.5. IL-17a, IL-1b, interferon-γ, and IL-12p40 were undetectable regardless of G28.5 exposure (results also not depicted). *P < 0.05; ***P < 0.001. Experiments were performed in triplicates.
Figure 3.
Absolute values in pg/mL NF-κB–specific cytokines with and without G28.5 stimulation. Primary normal and GD thyroid specimens were treated with G28.5 for 12 hours. Supernatants were collected and analyzed by Luminex for IL-6, IL-8, and TNF-α. Non–NF-κB cytokines (IL-17a, IL-1b, interferon-γ, and IL-12p40) were also measured and were undetectable regardless of G28.5 exposure (results are not depicted).
Figure 4.
Fold increase in the NF-κB–specific cytokine levels with CD40L stimulation versus without CD40L exposure in normal human thyroid cells. Primary normal thyroid specimens were treated with 0 or 250 ng/mL CD40L for 12 hours. Extracted mRNA samples were analyzed by real-time reverse transcription polymerase chain reaction, and supernatants were analyzed by a Luminex bead-based assay. Upregulation of both the (A) mRNA and (B) protein levels of the NF-κB cytokines with CD40L is comparable to the upregulation with G28.5 stimulation. Non–NF-κB–specific cytokines, including IL-17a, IL-1b, interferon-γ, and IL-12p40, were measured and undetectable with or without CD40L (results are not depicted). **P < 0.01; ***P < 0.001.
Thyroidal NF-κB2 (noncanonical) activity is more pronounced in GD than in normal thyroids
To further clarify whether the 2 NF-κB pathways, the canonical (NF-κB1) and the noncanonical (NF-κB2), are differentially activated by CD40, transcription factors representative of each cascade were measured. In unstimulated cells, the NF-κB family of dimeric transcription factors remains dormant in the cytoplasm. p65(RelA)/p50, the key heterodimeric transcription factor of the canonical (NF-κB1) pathway, is bound and rendered inactive in the cytoplasm by inhibitory NF-κB (IκB), until the IκB kinase complex is activated, leading to IκB’s rapid degradation. Once freed from IκB, the p65/p50 transcription factor quickly translocates into the nucleus and activates target gene transcription. Similarly, the noncanonical (NF-κB2) transcription factors are inactive at baseline, in the form of a precursor protein p100. When stimulated, the NF-κB–inducing kinase activates IκB kinase α, which phosphorylates p100, generating p52. p52 subsequently forms a heterodimer with Rel B and translocates into the nucleus to activate gene transcription.
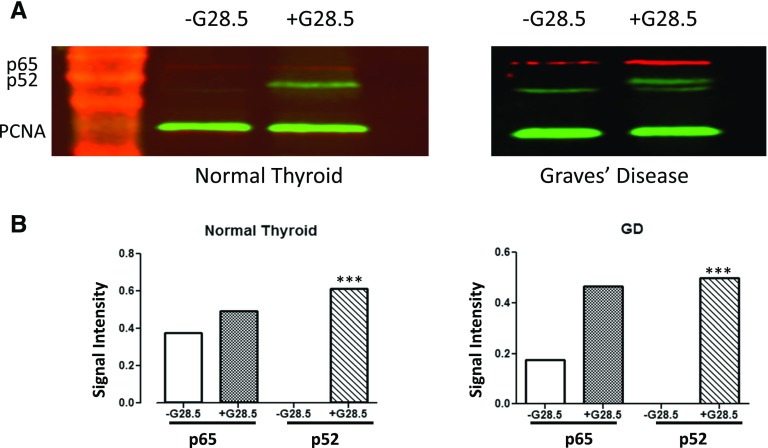
We tested whole protein lysates by Western blot for protein levels of p65 and p52. G28.5 stimulation essentially revealed no change in p65 constitutive expression in the normal thyrocytes, whereas there was a slight increase in the GD thyrocytes, indicating that the NF-κB1 (canonical) pathway is active in thyrocytes (Fig. 5). In contrast, p52 (noncanonical NF-κB2 pathway) was undetectable at baseline and was robustly expressed only after CD40 activation. Interestingly, the upregulation of p52 was more pronounced in the GD thyrocytes, suggesting involvement of the noncanonical pathway in triggering GD.
Figure 5.
CD40 activation upregulates p65 and p52 transcription factors. Primary normal and GD thyroid specimens were cultured; these were either exposed to or not exposed to 40 μg G28.5, a stimulating CD40 mAb, for 12 hours. Whole-cell protein lysates (40 μg GD sample and 60 μg normal thyroid sample) were analyzed by immunoblotting. Filters were probed with primary antibodies against p65 (of the NF-κB1 pathway) and p52 (of the NF-κB2 pathway); proliferating cell nuclear antigen was used as the housekeeping protein. (A) Band intensities show faint background, yet constitutive expression of p65 (red) with visible upregulation in the GD thyrocytes. p52 (green) is undetectable at baseline and is robustly measurable after CD40 stimulation. (B) Band intensities, normalized to proliferating cell nuclear antigen, were quantified by densitometry, using ImageJ software. ***P < 0.001.
Discussion
CD40, a critical receptor in B cell development, T cell priming, antigen presentation, and overall proper adaptive immunity, is expressed on thyroid follicular cells (10, 23–29). CD40 is also a major susceptibility gene for GD (1, 2), as shown by the numerous clinical associations between its single-nucleotide polymorphism (SNP) variants and GD. The C/C genotype of the CD40’s Kozak sequence SNP predisposes to GD, giving an odds ratio of 1.2–1.9, and has also been associated with more frequent relapses (22, 40–42). The T/T genotype, in contrast, has been shown to be protective and even linked to later-onset GD (43, 44). Mechanistically, the C/C variant increases the translational efficiency of CD40, most likely resulting in enhanced CD40 activity (45). Furthermore, CD40 polymorphisms have been significantly associated with an array of other autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, Crohn disease, and Behcet disease (12, 13, 19, 24, 46). This broad range of autoimmunity underscores the critical role of CD40 in antigen presentation. The observation that CD40 overactivity leads to tissue-specific disease rather than multiorgan autoimmunity as in GD suggests that thyroid-specific CD40 expression plays a prominent part in the development of GD. Indeed, our laboratory has previously demonstrated that thyroidal CD40 overexpression leads to increased thyrotropin receptor antibodies and more severe thyrotoxicosis in a GD mouse model (21). However, the thyroidal signaling pathways through which CD40 may trigger GD have been unknown until now.
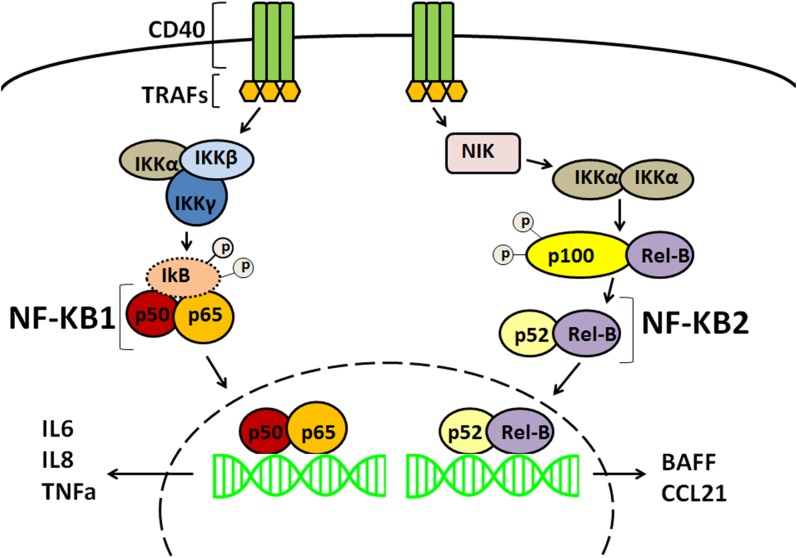
This study investigated the downstream effects of CD40 in thyroid cells. We focused on the NF-κB pathway because it is known to be activated by CD40 in professional immune cells (32, 47–51). In B cells, CD40 trimerizes after CD40–CD154 binding, and TNF receptor–associated factors aggregate to activate NF-κB (52). Subsequently, two different NF-κB pathways with distinct immune regulatory functions are activated (53). The canonical NF-κB1 pathway classically involves the immediate activation of the heterodimeric transcription factors p65(RelA)/p50 and p50/c-Rel. It primarily encodes for cytokines, chemokines, and adhesion molecules responsible for the recruitment of inflammatory and phagocytic cells of the innate immune system (Fig. 6). The noncanonical NF-κB2 pathway mainly activates the p52/RelB transcription factor, upregulating a different panel of proteins that are needed for B cell viability and function, proper antigen presentation, and adaptive immunity. IL-6, IL-8, and TNF-α are main end products of the canonical NF-κB1 arm, whereas BAFF is a key target of the noncanonical NF-κB2 arm. It is therefore expected that aberrations in the NF-κB pathway would lead to immune dysregulation (54, 55). Increased levels of IL-6, IL-8, TNF-α, BAFF, and CCL21 have been associated with various autoimmune diseases, including GD (56, 57), Graves ophthalmopathy (58), and systemic lupus erythematosus (59–61), among others.
Figure 6.
The canonical (NF-κB1) pathway requires the NEMO (NF-κB essential modulator) complex (IκB kinase α, IκB kinase β, IκB kinase γ) to phosphorylate IκB, leading to its degradation. The p65/p50 transcription factor is released and translocates into the nucleus. The noncanonical (NF-κB2) pathway is NEMO (NF-κB essential modulator) independent and requires phosphorylation of the p100 precursor. p100 is processed into p52, allowing the p52/Rel-B transcription factor to translocate into the nucleus. Once in the nucleus, the dimeric transcription factors can activate specific gene expression.
CD40 and NF-κB individually play significant roles in general immune homeostasis. In this study, we have shown that these two important players also act locally at the level of the thyrocyte and that their pathways are linked: thyroid-specific CD40 directly recruits NF-κB signaling. We showed that thyroidal CD40 activates the transcription and translation of NF-κB cytokines and that their upregulation is more pronounced in GD. p65 (NF-κB1) is constitutively expressed, whereas p52 (NF-κB2) is expressed only with CD40 stimulation. These findings suggest that GD thyrocytes are inherently more responsive to CD40 and that both NF-κB1 and NF-κB2 pathways are pivotal to GD progression. A possible explanation behind why both NF-κB pathways’ cytokines were equally upregulated by CD40, despite the more prominent p52 response, may be due to the slower kinetics of the NF-κB2 noncanonical pathway (47). The rapid-onset NF-κB1 pathway, in contrast, may allow for repeated activation and production of its cytokine end products, resulting in the measurement of a cumulative NF-κB1 response. The NF-κB1 and NF-κB2 pathways most likely each contribute functionally distinct roles in the progression of GD: NF-κB1 (p65) activity exacerbates the local, nonspecific inflammatory changes within the thyroid, whereas NF-κB2 activity (p52) coordinates the efficient development and influx of thyroid-targeting APCs and B cells (53). This is especially intriguing and pertinent as GD is predominantly a Th2-mediated, antibody-driven disease.
Our study provides a framework on the role of thyroidal CD40 in the progression to GD. Our proposed two-hit mechanism is that, in individuals who carry the GD-associated CD40 Kozak SNP genotype (first hit), secondary environmental exposures (second hit; e.g., iodine, viral infection) cause an upregulation of thyroidal CD40, resulting in NF-κB signal transduction with augmentation of both the canonical NF-κB1 and noncanonical NF-κB2 pathways. This leads to IL-6, IL-8, and TNF-α production (NF-κB1 cytokines), as well as expression of NF-κB2–driven pro-B cell responses like BAFF, B lymphocyte chemoattractant, and CCL21 (53). Antigen presentation by resident APCs and B cells would be facilitated in this proinflammatory background, leading to aberrant and efficient production of thyroid-specific, GD-inducing antibodies.
In conclusion, our data demonstrated that the NF-κB pathways may be the mechanistic link between thyroidal CD40 and thyroid-targeting immune cells and autoantibodies in GD. Our proposed mechanism of tissue-specific CD40–NF-κB pathway activation may be applicable to other autoimmune diseases. In fact, treatments for systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, and psoriasis use monoclonal antibodies that block IL-6, TNF-α, and BAFF (62–64). Our results highlight the translational potential of the thyroid-specific CD40–NF-κB pathway and may provide future therapeutic targets for the treatment of GD.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK061659 and DK073681.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- APC
- antigen-presenting cell
- BAFF
- B cell–activating factor of the TNF family
- CCL
- CC chemokine ligand
- GD
- Graves disease
- IκB
- inhibitory NF-κB
- IL
- interleukin
- mRNA
- messenger RNA
- NF-κB
- nuclear factor κB
- SNP
- single-nucleotide polymorphism
References
- 1.Jacobson EM, Tomer Y. The genetic basis of thyroid autoimmunity. Thyroid. 2007;17:949–961. [DOI] [PubMed] [Google Scholar]
- 2.Marinò M, Latrofa F, Menconi F, Chiovato L, Vitti P. Role of genetic and non-genetic factors in the etiology of Graves’ disease. J Endocrinol Invest. 2015;38(3):283–294. [DOI] [PubMed] [Google Scholar]
- 3.Tomer Y, Huber A. The etiology of autoimmune thyroid disease: a story of genes and environment. J Autoimmun. 2009;32(3-4):231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carle A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, Laurberg P. Epidemiology of subtypes of hyperthyroidism in Denmark: a population-based study. Eur J Endocrinol. 2011;164:801–809. [DOI] [PubMed] [Google Scholar]
- 5.Abraham-Nordling M, Bystrom K, Torring O, Lantz M, Berg G, Calissendorff J, Nystrom HF, Jansson S, Jorneskog G, Karlsson FA, Nystrom E, Ohrling H, Orn T, Hallengren B, Wallin G. Incidence of hyperthyroidism in Sweden. Eur J Endocrinol. 2011;165:899–905. [DOI] [PubMed] [Google Scholar]
- 6.Brix TH, Kyvik KO, Christensen K, Hegedüs L. Evidence for a major role of heredity in Graves’ disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001;86(2):930–934. [DOI] [PubMed] [Google Scholar]
- 7.Tomer Y. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu Rev Pathol. 2014;9:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomer Y, Hasham A, Davies TF, Stefan M, Concepcion E, Keddache M, Greenberg DA. Fine mapping of loci linked to autoimmune thyroid disease identifies novel susceptibility genes. J Clin Endocrinol Metab. 2013;98(1):E144–E152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomer Y, Ban Y, Concepcion E, Barbesino G, Villanueva R, Greenberg DA, Davies TF. Common and unique susceptibility loci in Graves and Hashimoto diseases: results of whole-genome screening in a data set of 102 multiplex families. Am J Hum Genet. 2003;73(4):736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munroe ME. Functional roles for T cell CD40 in infection and autoimmune disease: the role of CD40 in lymphocyte homeostasis. Semin Immunol. 2009;21(5):283–288. [DOI] [PubMed] [Google Scholar]
- 11.Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol. 2009;21(5):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danese S, Katz JA, Saibeni S, Papa A, Gasbarrini A, Vecchi M, Fiocchi C. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut. 2003;52(10):1435–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filion LG, Matusevicius D, Graziani-Bowering GM, Kumar A, Freedman MS. Monocyte-derived IL12, CD86 (B7-2) and CD40L expression in relapsing and progressive multiple sclerosis. Clin Immunol. 2003;106(2):127–138. [DOI] [PubMed] [Google Scholar]
- 14.Joo YB, Park BL, Shin HD, Park SY, Kim I, Bae SC. Association of genetic polymorphisms in CD40 with susceptibility to SLE in the Korean population. Rheumatology. 2013;52(4):623–630. [DOI] [PubMed] [Google Scholar]
- 15.Blanco-Kelly F, Matesanz F, Alcina A, Teruel M, Díaz-Gallo LM, Gómez-García M, López-Nevot MA, Rodrigo L, Nieto A, Cardeña C, Alcain G, Díaz-Rubio M, de la Concha EG, Fernandez O, Arroyo R, Martín J, Urcelay E. CD40: novel association with Crohn’s disease and replication in multiple sclerosis susceptibility [published correction appears in PLoS One 2010:5(8)]. PLoS One. 2010;5(7):e11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu CJ, Guo J, Luo HC, Wei CD, Wang CF, Lan Y, Wei YS. Association of CD40 polymorphisms and haplotype with risk of systemic lupus erythematosus. Rheumatol Int. 2016;36(1):45–52. [DOI] [PubMed] [Google Scholar]
- 17.Wagner M, Sobczynski M, Bilinska M, Pokryszko-Dragan A, Cyrul M, Kusnierczyk P, Jasek M.. MS risk allele rs1883832T is associated with decreased mRNA expression of CD40. J Mol Neurosci. 2015;56(3):540–545. [DOI] [PubMed] [Google Scholar]
- 18.Raychaudhuri S, Remmers EF, Lee AT, Hackett R, Guiducci C, Burtt NP, Gianniny L, Korman BD, Padyukov L, Kurreeman FA, Chang M, Catanese JJ, Ding B, Wong S, van der Helm-van Mil AH, Neale BM, Coblyn J, Cui J, Tak PP, Wolbink GJ, Crusius JB, van der Horst-Bruinsma IE, Criswell LA, Amos CI, Seldin MF, Kastner DL, Ardlie KG, Alfredsson L, Costenbader KH, Altshuler D, Huizinga TW, Shadick NA, Weinblatt ME, de Vries N, Worthington J, Seielstad M, Toes RE, Karlson EW, Begovich AB, Klareskog L, Gregersen PK, Daly MJ, Plenge RM. Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat Genet. 2008;40(10):1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.İnal EE, Rüstemoğlu A, İnanır A, Ekinci D, Gül Ü, Yiğit S, Ateş Ö. Associations of rs4810485 and rs1883832 polymorphisms of CD40 gene with susceptibility and clinical findings of Behçet’s disease. Rheumatol Int. 2015;35(5):837–843. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson EM, Huber AK, Akeno N, Sivak M, Li CW, Concepcion E, Ho K, Tomer Y. A CD40 Kozak sequence polymorphism and susceptibility to antibody-mediated autoimmune conditions: the role of CD40 tissue-specific expression. Genes Immun. 2007;8(3):205–214. [DOI] [PubMed] [Google Scholar]
- 21.Huber AK, Finkelman FD, Li CW, Concepcion E, Smith E, Jacobson E, Latif R, Keddache M, Zhang W, Tomer Y. Genetically driven target tissue overexpression of CD40: a novel mechanism in autoimmune disease. J Immunol. 2012;189(6):3043–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomer Y, Concepcion E, Greenberg DA. A C/T single-nucleotide polymorphism in the region of the CD40 gene is associated with Graves' disease. Thyroid. 2002;12(12):1129–1135. [DOI] [PubMed] [Google Scholar]
- 23.Faure GC, Bensoussan-Lejzerowicz D, Bene MC, Aubert V, Leclere J. Coexpression of CD40 and class II antigen HLA-DR in Graves’ disease thyroid epithelial cells. Clin Immunol Immunopathol. 1997;84(2):212–215. [DOI] [PubMed] [Google Scholar]
- 24.Toubi E, Shoenfeld Y. The role of CD40-CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity. 2004;37(6-7):457–464. [DOI] [PubMed] [Google Scholar]
- 25.Kehry MR. CD40-mediated signaling in B cells: balancing cell survival, growth, and death. J Immunol. 1996;156(7):2345–2348. [PubMed] [Google Scholar]
- 26.Stout RD, Suttles J. The many roles of CD40 in cell-mediated inflammatory responses. Immunol Today. 1996;17(10):487–492. [DOI] [PubMed] [Google Scholar]
- 27.Bishop GA. The many faces of CD40: multiple roles in normal immunity and disease. Semin Immunol. 2009;21(5):255–256. [DOI] [PubMed] [Google Scholar]
- 28.Bishop GA, Hostager BS. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 2003;14(3-4):297–309. [DOI] [PubMed] [Google Scholar]
- 29.Carayanniotis G, Masters SR, Noelle RJ. Suppression of murine thyroiditis via blockade of the CD40-CD40L interaction. Immunology. 1997;90(3):421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resetkova E, Kawai K, Enomoto T, Arreaza G, Togun R, Foy TM, Noelle RJ, Volpe R. Antibody to gp39, the ligand for CD40 significantly inhibits the humoral response from Graves' thyroid tissues xenografted into severe combined immunodeficient (SCID) mice. Thyroid. 1996;6(4):267–273. [DOI] [PubMed] [Google Scholar]
- 31.Chen CR, Aliesky HA, Guo J, Rapoport B, McLachlan SM. Blockade of costimulation between T cells and antigen-presenting cells: an approach to suppress murine Graves' disease induced using thyrotropin receptor-expressing adenovirus. Thyroid. 2006;16(5):427–434. [DOI] [PubMed] [Google Scholar]
- 32.Berberich I, Shu GL, Clark EA. Cross-linking CD40 on B cells rapidly activates nuclear factor-kappa B. J Immunol. 1994;153(10):4357–4366. [PubMed] [Google Scholar]
- 33.Kuryłowicz A, Nauman J. The role of nuclear factor-kappaB in the development of autoimmune diseases: a link between genes and environment. Acta Biochim Pol. 2008;55(4):629–647. [PubMed] [Google Scholar]
- 34.Beck IM, Vanden Berghe W, Vermeulen L, Yamamoto KR, Haegeman G, De Bosscher K. Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr Rev. 2009;30(7):830–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garside H, Stevens A, Farrow S, Normand C, Houle B, Berry A, Maschera B, Ray D. Glucocorticoid ligands specify different interactions with NF-kappaB by allosteric effects on the glucocorticoid receptor DNA binding domain. J Biol Chem. 2004;279(48):50050–50059. [DOI] [PubMed] [Google Scholar]
- 36.Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS Jr. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol. 1995;15(2):943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang C, Zhang DM, Song ZB, Hou YQ, Bao YL, Sun LG, Yu CL, Li YX. Protumoral TSP50 regulates macrophage activities and polarization via production of TNF-alpha and IL-1beta, and activation of the NF-kappaB signaling pathway. PLoS One. 2015;10:e0145095.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed SU, Milner J. Basal cancer cell survival involves JNK2 suppression of a novel JNK1/c-jun/Bcl-3 apoptotic network. PLoS ONE. 2009;4:e7305.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L, Zhou X, Fan LX, Yao Y, Swenson-Fields KI, Gadjeva M, Wallace DP, Peters DJ, Yu A, Grantham JJ, Li X. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J Clin Invest. 2015;125:2399–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim TY, Park YJ, Hwang JK, Song JY, Park KS, Cho BY, Park DJ. A C/T polymorphism in the 5′-untranslated region of the CD40 gene is associated with Graves' disease in Koreans. Thyroid. 2003;13(10):919–925. [DOI] [PubMed] [Google Scholar]
- 41.Ban Y, Tozaki T, Taniyama M, Tomita M, Ban Y.. Association of a C/T single-nucleotide polymorphism in the 5′ untranslated region of the CD40 gene with Graves' disease in Japanese. Thyroid. 2006;16(5):443–446. [DOI] [PubMed] [Google Scholar]
- 42.Kurylowicz A, Kula D, Ploski R, Skorka A, Jurecka-Lubieniecka B, Zebracka J, Steinhof-Radwanska K, Hasse-Lazar K, Hiromatsu Y, Jarzab B, Bednarczuk T.. Association of CD40 gene polymorphism (C-1T) with susceptibility and phenotype of Graves' disease. Thyroid. 2005;15(10):1119–1124. [DOI] [PubMed] [Google Scholar]
- 43.Mukai T, Hiromatsu Y, Fukutani T, Ichimura M, Kaku H, Miyake I, Yamada K. A C/T polymorphism in the 5′ untranslated region of the CD40 gene is associated with later onset of Graves’ disease in Japanese. Endocr J. 2005;52(4):471–477. [DOI] [PubMed] [Google Scholar]
- 44.Hsiao JY, Hsieh MC, Hsiao CT, Weng HH, Ke DS. Association of CD40 and thyroglobulin genes with later-onset Graves' disease in Taiwanese patients. Eur J Endocrinol. 2008;159(5):617–621. [DOI] [PubMed] [Google Scholar]
- 45.Jacobson EM, Concepcion E, Oashi T, Tomer Y. A Graves’ disease-associated Kozak sequence single-nucleotide polymorphism enhances the efficiency of CD40 gene translation: a case for translational pathophysiology. Endocrinology. 2005;146(6):2684–2691. [DOI] [PubMed] [Google Scholar]
- 46.Koshy M, Berger D, Crow MK. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J Clin Invest. 1996;98(3):826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shih VF, Tsui R, Caldwell A, Hoffmann A. A single NFκB system for both canonical and non-canonical signaling. Cell Res. 2011;21(1):86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. [DOI] [PubMed] [Google Scholar]
- 49.Hostager BS, Bishop GA. CD40-mediated zctivation of the NF-κB2 pathway. Front Immunol. 2013;4:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanagawa Y, Onoé K. Distinct regulation of CD40-mediated interleukin-6 and interleukin-12 productions via mitogen-activated protein kinase and nuclear factor kappaB-inducing kinase in mature dendritic cells. Immunology. 2006;117(4):526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsing Y, Bishop GA. Requirement for nuclear factor-kappaB activation by a distinct subset of CD40-mediated effector functions in B lymphocytes. J Immunol. 1999;162(5):2804–2811. [PubMed] [Google Scholar]
- 52.Hsing Y, Hostager BS, Bishop GA. Characterization of CD40 signaling determinants regulating nuclear factor-kappa B activation in B lymphocytes. J Immunol. 1997;159(10):4898–4906. [PubMed] [Google Scholar]
- 53.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25(6):280–288. [DOI] [PubMed] [Google Scholar]
- 54.Seetharaman R, Mora AL, Nabozny G, Boothby M, Chen J. Essential role of T cell NF-kappa B activation in collagen-induced arthritis. J Immunol. 1999;163(3):1577–1583. [PubMed] [Google Scholar]
- 55.Li J, Li J, Chen R, Cai G. Targeting NF-κΒ and TNF-α activation by electroacupuncture to suppress collagen-induced rheumatoid arthritis in model rats. Altern Ther Health Med. 2015;21(4):26–34. [PubMed] [Google Scholar]
- 56.Qi Y, Li X, Zhang Q, Huang F, Lin D, Zhou Y, Hong J, Cui B, Wang W, Ning G, Wang S. Increased chemokine (C-C motif) ligand 21 expression and its correlation with osteopontin in Graves’ disease. Endocrine. 2015;50(1):123–129. [DOI] [PubMed] [Google Scholar]
- 57.Campi I, Tosi D, Rossi S, Vannucchi G, Covelli D, Colombo F, Trombetta E, Porretti L, Vicentini L, Cantoni G, Curro N, Beck-Peccoz P, Bulfamante G, Salvi M.. B cell activating factor (BAFF) and BAFF receptor expression in autoimmune and nonautoimmune thyroid diseases. Thyroid. 2015;25(9):1043–1049. [DOI] [PubMed] [Google Scholar]
- 58.Gillespie EF, Raychaudhuri N, Papageorgiou KI, Atkins SJ, Lu Y, Charara LK, Mester T, Smith TJ, Douglas RS. Interleukin-6 production in CD40-engaged fibrocytes in thyroid-associated ophthalmopathy: involvement of Akt and NF-κB. Invest Ophthalmol Vis Sci. 2012;53(12):7746–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF. Association of serum B cell activating factor from the tumour necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) with central nervous system and renal disease in systemic lupus erythematosus. Lupus. 2013;22(9):873–884. [DOI] [PubMed] [Google Scholar]
- 60.Mackay F, Sierro F, Grey ST, Gordon TP. The BAFF/APRIL system: an important player in systemic rheumatic diseases. Curr Dir Autoimmun. 2005;8:243–265. [DOI] [PubMed] [Google Scholar]
- 61.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway J, Mauri D, Cavill D, Gordon TP, Mackay CR, Mackay F. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s syndrome. J Clin Invest. 2002;109(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dougados M, Kissel K, Sheeran T, Tak PP, Conaghan PG, Mola EM, Schett G, Amital H, Navarro-Sarabia F, Hou A, Bernasconi C, Huizinga TW. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis. 2013;72(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Genovese MC, Fleischmann R, Furst D, Janssen N, Carter J, Dasgupta B, Bryson J, Duncan B, Zhu W, Pitzalis C, Durez P, Kretsos K. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb study. Ann Rheum Dis. 2014;73(9):1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jordan N, D’Cruz DP. Belimumab for the treatment of systemic lupus erythematosus. Expert Rev Clin Immunol. 2015;11(2):195–204. [DOI] [PubMed] [Google Scholar]