Abstract
During the female reproductive cycle, estradiol exerts negative and positive feedback at both the central level to alter gonadotropin-releasing hormone (GnRH) release and at the pituitary to affect response to GnRH. Many studies of the neurobiologic mechanisms underlying estradiol feedback have been done on ovariectomized, estradiol-replaced (OVX+E) mice. In this model, GnRH neuron activity depends on estradiol and time of day, increasing in estradiol-treated mice in the late afternoon, coincident with a daily luteinizing hormone (LH) surge. Amplitude of this surge appears lower than in proestrous mice, perhaps because other ovarian factors are not replaced. We hypothesized GnRH neuron activity is greater during the proestrous-preovulatory surge than the estradiol-induced surge. GnRH neuron activity was monitored by extracellular recordings from fluorescently tagged GnRH neurons in brain slices in the late afternoon from diestrous, proestrous, and OVX+E mice. Mean GnRH neuron firing rate was low on diestrus; firing rate was similarly increased in proestrous and OVX+E mice. Bursts of action potentials have been associated with hormone release in neuroendocrine systems. Examination of the patterning of action potentials revealed a shift toward longer burst duration in proestrous mice, whereas intervals between spikes were shorter in OVX+E mice. LH response to an early afternoon injection of GnRH was greater in proestrous than diestrous or OVX+E mice. These observations suggest the lower LH surge amplitude observed in the OVX+E model is likely not attributable to altered mean GnRH neuron activity, but because of reduced pituitary sensitivity, subtle shifts in action potential pattern, and/or excitation-secretion coupling in GnRH neurons.
Gonadotropin-releasing hormone (GnRH) neurons of the medial preoptic area and hypothalamus comprise the final common pathway for the central regulation of fertility. GnRH controls the synthesis (1, 2) and release of the pituitary gonadotropins, luteinizing hormone (LH), and follicle-stimulating hormone, which activate gonadal steroidogenesis and gametogenesis. For most of the female reproductive cycle, estradiol exerts negative feedback on GnRH release, resulting in inhibition of LH release (3, 4). At the end of the follicular phase (proestrus in rodents), estradiol switches from negative to positive feedback action. This induces a surge of GnRH release and increases pituitary responsiveness to GnRH (5, 6), ultimately leading to the LH surge and ovulation (7–12).
To better understand the neurobiologic mechanisms underlying estradiol feedback, different experimental models of positive feedback induced by ovariectomy (OVX) followed by replacement with high physiologic/supraphysiologic levels of estrogen have been used. These replacement regimens include daily subcutaneous injection of 17β-estradiol benzoate for 5 to 6 days after OVX (13), insertion of Silastic implants containing 17β-estradiol designed to produce a slightly supraphysiologic circulating estradiol level at the time of OVX followed by injection of 17β-estradiol benzoate 6 days later (14), and implantation of Silastic capsules that produce constant high physiologic levels of 17β-estradiol. The latter avoids injections, which produce varying levels of estrogen throughout the day; nocturnal rodents treated in this manner exhibit daily LH surges peaking at lights out (15–17). These daily transitions from estradiol negative to positive feedback occur despite a constant estradiol level; changes in circulating levels of this steroid thus do not contribute to the change in feedback status. In mice prepared in this daily surge model [ovariectomized, estradiol-replaced (OVX+E)], both GnRH neuron firing rate and LH are suppressed in the morning during negative feedback and both are elevated at night during positive feedback compared with OVX controls, which do not exhibit time of day–dependent shifts (15).
The steady estradiol levels in the daily LH surge model facilitate understanding of how estradiol and time of day contribute to changes in GnRH neuron activity and release. It is important to point out, however, that although estradiol is critical for inducing the LH surge (10), it is not the only ovarian steroid that changes in an estrous cycle-dependent manner (18). Progesterone levels are also dynamic, and there is evidence for a role for progesterone in mediating estrogen positive feedback (19, 20). Of interest in this regard, the amplitude of the daily estradiol-induced LH surge appears to be lower than that previously reported for the proestrous surge in mice (8). Numerous variables, including strain, LH assay reference standards and methods, housing conditions, and diet, differ among these studies, precluding a direct comparison with the exception of one recent study in which OVX+E implant followed by estradiol injection produced lower amplitude surges than observed on proestrus (21). No studies that we are aware of have directly compared models at both the pituitary and hypothalamic levels. Because many of the electrophysiologic studies of GnRH neurons have been performed in the daily OVX+E GnRH/LH surge model using constant in vivo physiologic estradiol treatment, it is important to determine if the neurobiologic changes induced were similar to those that occur on proestrus. We thus investigated the LH surge, GnRH neuron firing rate and pattern, and pituitary response to GnRH generated by this daily surge OVX+E model vs that in diestrous and proestrous mice to test the hypotheses that the hormonal changes of proestrus induce greater positive feedback responses at both the pituitary and hypothalamus.
Materials and Methods
Animals
Female GnRH-green fluorescent protein (GFP) mice (22) on a C57Bl6/J or B6CBAF1 background and aged 59 to 137 days were used. All mice were provided with water and Harlan 2916 chow (Harlan Laboratories, Indianapolis, IN) ad libitum and were held on a 14-hour light, 10-hour dark cycle, with lights on at 4:00 am Eastern Standard Time (EST). For studies during the estrous cycle, vaginal cytology was monitored for at least a week before experiments to determine estrous cycle stage; mice were studied on diestrus or proestrus. For studies with controlled estradiol feedback (OVX+E), mice were ovariectomized and received a subcutaneous Silastic (Dow Corning, Midland, MI) implant that contained 0.625 µg 17β-estradiol in sesame oil in the scapular region; surgery was done under isoflurane general anesthesia with bupivacaine as a local analgesic. Studies were performed 2 to 3 days after surgery. The Institutional Animal Care and Use Committee of the University of Michigan approved all procedures. Independent groups of mice were used for the 3 experiments.
Experiment 1: LH surge in proestrous and OVX+E mice
Vaginal cytology of ovary-intact mice was determined for ≥10 days prior to sampling to confirm normal estrous cyclicity. Trunk blood was collected within 30 minutes of lights out from proestrous mice (2 trials, n = 3 and n = 6; n = 9 total) or OVX+E mice on day 2 postsurgery (3 trials, n = 8, n = 4, and n = 3; n = 15 total). For all samples, serum was separated by centrifugation and stored at −20°C until assay.
Experiment 2: electrophysiology and pattern analysis
Slice preparation and cell identification
Chemicals were purchased from Sigma Chemical Company (St. Louis, MO) unless noted. All solutions were bubbled with 95% O2/5% CO2 throughout the experiments and for at least 30 minutes before exposure to tissue. At 4:00 to 4:30 pm EST, near the expected onset of the LH surge, the brain was rapidly removed and placed in ice-cold sucrose saline solution containing 250 mM sucrose, 3.5 mM KCl, 26 mM NaHCO3, 10 mM d-glucose, 1.25 mM NaHPO4, 1.2 mM MgSO4, and 3.8 mM MgCl2. Coronal (300-µm) slices were cut with a Leica VT1200S (Leica Biosystems). Slices were incubated in a 1:1 mixture of sucrose saline and artificial cerebrospinal fluid (ACSF) containing 135 mM NaCl, 3.5 mM KCl, 26 mM NaHCO3, 10 mM d-glucose, 1.25 mM Na2HPO4, 1.2 mM MgSO4, and 2.5 mM CaCl2 (pH 7.4) for 30 minutes at room temperature (21°C to 23°C) and then transferred to 100% ACSF for an additional 30 to 180 minutes at room temperature before recording. For recording, slices were placed into a chamber continuously perfused with ACSF at a rate of 3 mL/min with oxygenated ACSF heated to 31°C ± 1°C with an inline-heating unit (Warner Instruments, Hamden, CT). GFP-positive GnRH neurons were identified by brief illumination at 488 nm on an Olympus BX51WI microscope (Olympus, Center Valley, PA). Recorded cells were mapped to an atlas (23) to determine if any trends based on anatomic location emerged; no such trends were apparent in these data sets. Recordings were performed 1 to 4 hours after brain slice preparation; no difference in firing patterns was evident based on time after brain slice preparation. No more than 2 cells per animal were included for analysis, and at least 6 animals were tested per parameter.
Extracellular recording
Recording micropipettes were pulled from borosilicate capillary glass (type 7052, 1.65-mm outer diameter and 1.12-mm inner diameter; World Precision Instruments, Inc., Sarasota, FL) using a Flaming/Brown P-97 puller (Sutter Instruments, Novato, CA) to obtain pipettes with a resistance of 2 to 3 MΩ when filled with the appropriate pipette solution. Recordings were made with an EPC-8 with ITC-18 interface or 1 channel of an EPC-10 dual-patch clamp amplifier (HEKA Elektronik, Holliston, MA). Data acquisition was controlled via Patchmaster software (HEKA Elektronik).
Targeted extracellular recordings were used to record long-term (1-hour) firing activity from diestrous (n = 14), proestrous (n = 17), and OVX+E (n = 11) mice. This method maintains the internal milieu and has minimal impact on the firing rate of neurons (24–26). Recording pipettes were filled with HEPES-buffered solution containing 150 mM NaCl, 10 mM HEPES, 10 mM glucose, 2.5 mM CaCl2, 1.3 mM MgCl2, and 3.5 mM KCl, and low-resistance (diestrus: 12 ± 4 MΩ; proestrus: 13 ± 3 MΩ; OVX+E: 11 ± 4 MΩ) seals were formed between the pipette and neuron after first exposing the pipette to the slice tissue in the absence of positive pressure. Recordings were made in voltage-clamp mode with a 0-mV pipette holding potential. Signals were acquired and filtered at 10 kHz. Resistance of the loose seal was checked frequently during the first 5 minutes of recordings to ensure a stable baseline, and also between each 10-minute recording period; data were not used if seal resistance changed >30% or was >25 MΩ.
Analysis of extracellular recordings
Action currents (events), the membrane currents associated with action potential firing, were detected off-line using custom programs in Igor Pro 6.31 (Wavemetrics, Lake Oswego, OR). Mean firing rate (hertz) was calculated by dividing the total number of events by the duration of recording. Data were binned at 60-second intervals and were transferred to Excel (Microsoft, Redmond, WA) for evaluation of percent quiescence (1-minute bins containing <1 event). In addition to overall activity, the burst pattern of action potentials can potentially affect neurosecretion (27, 28). Action potential grouping (bursts) was detected using software that systematically adjusted the maximum time between events (burst window) for inclusion in a burst from 0.01 to 1.5 seconds, in 10-ms intervals. Burst windows of 0.01, 0.15, 0.21, 0.5, 1.0, and 1.5 seconds were chosen for comparison among groups. The shortest window (0.01 seconds) encompasses intraburst intervals in typically bursting neurons of the cortex and hippocampus (29). The 0.21-second burst window was defined based on whole-cell current-clamp recordings of GnRH neurons; this was the longest time between action potentials in which there was a continuous depolarization toward threshold for the next action potential (30). Because the whole-cell configuration alters intracellular milieu and thus may affect action potential generation, additional burst windows were included that span the typical duration of action potential–induced increases in intracellular calcium levels in cell types for which this is well characterized (31, 32), and the afterdepolarization (ADP) of GnRH neurons, during which increased firing can occur [1.0 to 1.5 seconds (33)]. For each burst window analyzed, the percentage of spikes in bursts was calculated, and the distribution of burst durations was compared using the Kolmogorov-Smirnov test; because this test is valid only for 2 distributions, and our primary interest was comparisons between OVX+E and proestrous mice, these groups were chosen for comparison. Interspike interval was also examined by comparing the mean and distribution of the log10 of this parameter over entire recordings (34, 35).
Experiment 3: pituitary responsiveness to exogenous GnRH
Pituitary responsiveness to exogenous GnRH in the diestrous, proestrous, and OVX+E mice was evaluated in the afternoon, before onset of positive feedback in either proestrous or OVX+E mice (GnRH injections 1:00 to 2:00 pm EST). A baseline blood sample (∼ 14 µL) was obtained from the tail tip. Mice were then injected with GnRH (Bachem, Torrence, CA, H4005, 150 ng/kg in 0.9% saline; diestrus n = 6, proestrus n = 10, OVX+E on day 2 postsurgery n = 8 (36). Trunk blood was collected 15 minutes after GnRH injection. Serum from baseline and trunk blood samples was separated and stored at –20°C until assay for LH. Uterine mass was determined to confirm proestrous (uterine mass >100 mg) and diestrous (uterine mass <80 mg) stages. Pituitaries were snap frozen in liquid nitrogen and maintained at –80°C until lysis. Pituitaries were lysed in 350 µL of buffer containing 20 mM HEPES, 150 mM KCl, 10 mM MgCl2, 2.5 mM dithiothreitol plus 1× EDTA-free protease inhibitor (Roche), and 0.5% vol/vol RNase inhibitor (Protector RNase Inhibitor; Roche, Indanapolis, IN).
LH assays
Serum LH was measured by the University of Virginia Center for Research and Reproduction Ligand Assay and Analysis Core. For trunk blood (Fig. 1, experiment 1), LH was measured in singlicate by a sensitive 2-site sandwich immunoassay (37, 38) using monoclonal antibodies against bovine LH (no. 581B7; Medix Kauniainen, Espoo, Finland) and against the human LH-beta subunit (no. 5303; Medix Kauniainen) as described previously (38). Pituitary content (experiment 3) was measured in duplicate in pituitary lysate diluted 1:20 prior to assay using the 2-site sandwich assay, and was normalized to protein content (subsequently discussed). The tracer antibody (no. 518B7) was kindly provided by Dr. J. Roser (Department of Animal Science, University of California, Davis, Davis, California) (39) and was iodinated by the chloramine T method and purified on Sephadex G-50 columns. The capture antibody (no. 5303) was biotinylated and immobilized on avidin-coated polystyrene beads (7 mm; Epitope Diagnostics, Inc., San Diego, CA). Mouse LH reference prep (AFP5306A; provided by Dr. A.F. Parlow and the National Hormone and Peptide program, http://www.humc.edu/hormones/) was used as standard. The assay had a sensitivity of 0.04 ng/mL, the intra-assay had a coefficient of variation (CV) of 4.5%, and the interassay had a CV of 8.3%.
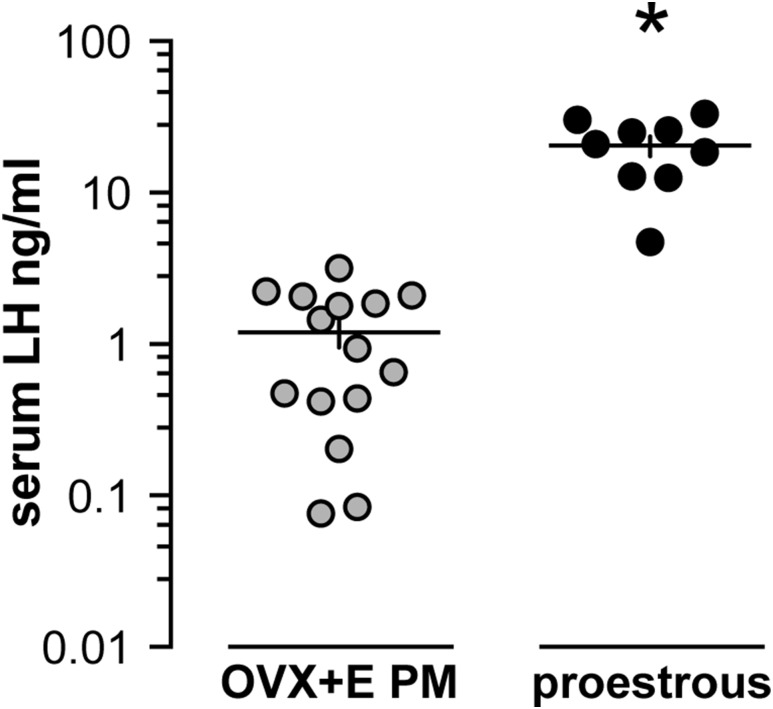
Figure 1.
The estradiol-induced LH surge is a lower amplitude than the preovulatory proestrous surge. Serum LH measured within 30 minutes of lights out in OVX+E mice (gray circles, n = 15) and proestrous mice (black circles, n = 9). Note the log scale; each symbol represents 1 mouse; means ± standard error of means are shown by the horizontal and vertical lines, respectively. *P < 0.001 calculated by Student t test with Welch correction.
For pituitary response [experiment 3], serum was diluted 1:10 in assay buffer (0.2% bovine serum albumin and 0.05% Tween-20 in phosphate-buffered saline), and LH was determined using ultra-sensitive enzyme-linked immunosorbent assay based on Steyn et al. (40). The capture monoclonal antibody (anti-bovine LH beta subunit, 518B7) was provided by J. Roser, University of California. The detection polyclonal antibody (rabbit LH antiserum, AFP240580Rb) was provided by the National Hormone and Peptide Program. Horseradish peroxidase-conjugated polyclonal antibody (goat anti-rabbit) was purchased from DakoCytomation (D048701-2; DakoCytomation, Glostrup, Denmark). Mouse LH reference prep (AFP5306A) was used as the assay standard. The limit of quantitation (functional sensitivity) was defined as the lowest concentration that demonstrates accuracy within 20% of expected values and intra-assay percent CV <20%, and was determined by serial dilutions of a defined sample pool. Intra-assay and interassay percent CVs were 6.5% and 8.6%, respectively; functional sensitivity was 0.16 ng/mL.
Pituitary RNA extraction and real-time polymerase chain reaction
Total RNA was isolated with on-column DNasing (RNeasy; Qiagen, Germantown, MD) from 100 µL pituitary lysate. Total protein was measured in 2 µL of lysate (BCA assay; ThermoFisher, Waltham, MA). Pituitary RNA (100 ng) and a standard curve of pooled mouse pituitary RNA 400ng-98pg (1:4 dilution) was reverse transcribed as described (36). Pituitary cDNA (1 ng/µL final concentration for samples; 4 ng/µL to 1 pg/µL for standards) was assayed in duplicate for: Cga, Lhb, Fshb, Gnrhr, Egr1, Actin, and Ppia messenger RNAs (mRNAs) by hydrolysis probe-based quantitative PCR chemistry (TaqMan, ThermoFisher). All primers and probes were purchased from Integrated DNA Technologies (Coralville, IA); Table 1 shows primer and probe sequences and PrimeTime assay number (Integrated DNA Technologies). PCR was conducted using Applied Biosystems Gene Expression Mastermix (ThermoFisher). Normalized relative expression of transcripts was determined by the ΔΔCt method (41), the average of Actin and Ppia expression were used for normalization.
Table 1.
qPCR Primers and Probes for Pituitary Gene Expression
| Transcript (Accession No.) | Primers | IDT Prime Time qPCR Assay No. |
|---|---|---|
| Actb (NM_007393) | FWD 5′-GAT TAC TGC TCT GGC TCC TAG-3′ | Mm.PT.39a.22214843.g |
| REV 5′-GAC TCA TCG TAC TCC TGC TTG-3′ | ||
| Probe 5′-CTG GCC TCA CTG TCC ACC TTC C-3′ | ||
| Ppia (NM_008907) | FWD 5′-CAA ACA CAA ACG GTT CCC AG-3′ | Mm.PT.39a.2.gs |
| REV 5′-TTC ACC TTC CCA AAG ACC AC-3′ | ||
| Probe 5′-TGC TTG CCA TCC AGC CAT TCA G-3′ | ||
| Gnrhr (NM_010323) | FWD 5′-TCA GCA TTG TCT TTG CAG GA-3′ | Mm.PT.45.16240237 |
| REV 5′-TCA CAC ATT GCG AGA AGA CTG-3′ | ||
| Probe 5′-TGA TCT ACC TAG CAG ACG GCT CTG G-3′ | ||
| Lhb (NM_008497) | FWD 5′-CCA GTC TGC ATC ACC TTC AC-3′ | Mm.PT.45.5612498 |
| REV 5′-GAG GCA CAG GAG GCA AAG-3′ | ||
| Probe 5′-AGT ACT CGG ACC ATG CTA GGA CAG T-3′ | ||
| Fshb (NM_008045) | FWD 5′-TTC AGC TTT CCC CAG AAG AG-3′ | Mm.PT.45.17694677 |
| REV 5′-TCC AGC ACC AGA ATA AGA TGC-3′ | ||
| Probe 5′-AGC TAC GTC CTG TGC AGT CAG C-3′ | ||
| Cga (NM_009889) | FWD 5′-AGC ATG ACC AGA ATG ACA GC-3′ | Mm.PT.58.31855537 |
| REV 5′-CCT CAG ATC GAC AAT CAC CTG-3′ | ||
| Probe 5′-CCC TCA AAA AGT CCA GAG CTT GCA GA-3′ | ||
| Egr1 (NM_007913) | FWD 5′-AGC GCC TTC AAT CCT CAA G-3′ | Mm.PT.45.13313108 |
| REV 5′-GAT AAC TCG TCT CCA CCA TCG-3′ | ||
| Probe 5′-ATG AGC ACC TGA CCA CAG AGT CC-3′ |
Abbreviations: FWD, forward primer; IDT, Integrated DNA Technologies; qPCR, quantitative polymerase chain reaction; REV, reverse primer.
Statistics
Statistical analyses were performed using Prism 7 (GraphPad Software, La Jolla, CA). The number of cells or mice per group is indicated by n. Data are reported as individual values with mean ± standard error of the mean. Data distribution was tested using Shapiro-Wilk normality test. Data distribution and experimental design were used to select appropriate statistical comparisons, which are specified for each data set in the results. For nonparametric tests, the median, 25th and 75th percentile, and interquartile range are reported in Table 2. The null hypothesis was rejected if P < 0.05.
Table 2.
Median, 25th and 75th Percentile, and Interquartile Range for Nonparametric Analyses
| Parameter (Figure) | Median | 25th Percentile | 75th Percentile | Interquartile Range |
|---|---|---|---|---|
| Mean firing rate, Hz [Fig. 2(B)] | ||||
| Diestrus | 0.02 | 0 | 0.12 | 0.12 |
| Proestrus | 0.18 | 0.01 | 0.64 | 0.63 |
| OVX+E | 0.32 | 0.19 | 0.48 | 0.29 |
| Quiescence, % [Fig. 2(C)] | ||||
| Diestrus | 82 | 55 | 100 | 45 |
| Proestrus | 50 | 2 | 63 | 61 |
| OVX+E | 18 | 3 | 46 | 43 |
| Spikes in bursts, % [Fig. 3(A)] | ||||
| BW 0.15 s | ||||
| Diestrus | 0 | 0 | 3 | 3 |
| Proestrus | 2 | 1 | 6 | 5 |
| OVX+E | 16 | 2 | 28 | 26 |
| BW 0.21 s | ||||
| Diestrus | 1 | 0 | 16 | 16 |
| Proestrus | 13 | 6 | 22 | 16 |
| OVX+E | 35 | 7 | 40 | 33 |
| BW 0.5 s | ||||
| Diestrus | 67 | 0 | 80 | 80 |
| Proestrus | 73 | 61 | 89 | 28 |
| OVX+E | 80 | 64 | 87 | 23 |
| BW 1.0 s | ||||
| Diestrus | 90 | 0 | 97 | 97 |
| Proestrus | 94 | 78 | 99 | 21 |
| OVX+E | 92 | 77 | 98 | 21 |
| BW 1.5 s | ||||
| Diestrus | 94 | 0 | 99 | 99 |
| Proestrus | 97 | 85 | 100 | 15 |
| OVX+E | 96 | 85 | 99 | 14 |
| Lhb (Fig. 5) | ||||
| Diestrus | 1.2 | 1.1 | 1.5 | 0.4 |
| Proestrus | 0.8 | 0.6 | 1.0 | 0.4 |
| OVX+E | 0.8 | 0.8 | 1.0 | 0.2 |
| Cga (Fig. 5) | ||||
| Diestrus | 1.9 | 1.4 | 2.3 | 0.9 |
| Proestrus | 0.9 | 0.6 | 1.1 | 0.5 |
| OVX+E | 1.2 | 0.7 | 1.4 | 0.7 |
| Fshb (Fig. 5) | ||||
| Diestrus | 0.5 | 0.1 | 0.8 | 0.7 |
| Proestrus | 0.4 | 0.3 | 0.6 | 0.3 |
| OVX+E | 6.6 | 5.9 | 6.7 | 0.8 |
Abbreviation: BW, burst window.
Results
Experiment 1: LH surge amplitude is lower in OVX+E mice than proestrus mice
The OVX+E daily surge model presents a constant physiologic level of estradiol in the circulation that induces a daily LH surge for several days and facilitates studies of generation and timing of GnRH surges (15). During the estrous cycle of rodents, estradiol positive feedback occurs on proestrus (42). Comparison of the LH surge amplitude between the estradiol-induced daily surge model (n = 15) and proestrous mice (n = 9) under the same husbandry and assay conditions reveals the amplitude of the estradiol-induced LH surge is lower than the proestrous surge (P < 0.001, Student t test with Welch correction) (Fig. 1).
Experiment 2: overall firing rate is similar in GnRH neurons from OVX+E and proestrous mice
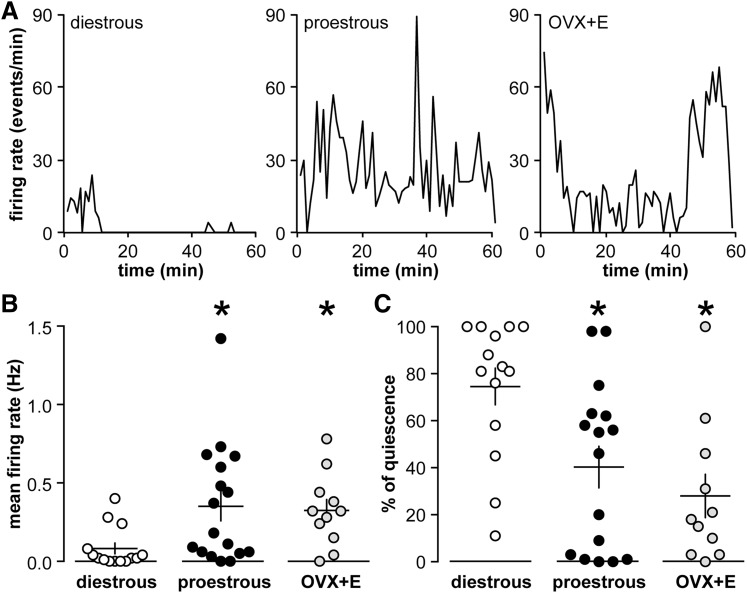
To determine if changes in GnRH neuron activity are associated with the difference in LH surge amplitude between proestrous and OVX+E mice, firing rate was monitored using targeted single-unit extracellular recordings made in the late afternoon from GFP-identified GnRH neurons in brain slices from females in diestrus (negative feedback, n = 14), proestrus (positive feedback, n = 17), or OVX+E (estradiol-induced positive feedback, n = 11). Fig. 2(A) shows representative firing patterns during 1-hour recordings. There was no difference in mean firing rate of GnRH neurons between OVX+E and proestrous mice, but both of these groups had a higher firing rate than cells from diestrous mice (Kruskal-Wallis/Dunn, both P < 0.05) [Fig. 2(B)]. Similarly, the percent of quiescent 1-minute bins (defined as ≤1 event per minute) was lower (P < 0.05) in both OVX+E (38%) and proestrous (37%) mice than diestrous mice (74%, Kruskal-Wallis/Dunn) [Fig. 2(C)]. We further looked for age-related changes in firing rate and did not find substantial correlation (r2 for firing rate as a function of age: diestrous mice, 0.003; proestrous mice, 0.004; and OVX+E, 0.132). Thus, the overall pattern of GnRH activity does not account for the difference in LH surge amplitude.
Figure 2.
GnRH neuron activity is elevated and similar during the estradiol-induced daily surge and preovulatory proestrous surge. (A) Representative firing patterns for GnRH neurons recorded from diestrous (left), proestrous (middle), and OVX+E mice (right). (B) Individual values for mean firing rate and percentage of quiescence in GnRH neurons from diestrous (open circles), proestrous (black circles), and OVX+E (gray circles) mice. Each symbol represents 1 mouse; means ± standard error of means are shown by the horizontal and vertical lines, respectively. *P < 0.05 calculated by Kruskal-Wallis/Dunn test.
Action potential spike patterning in GnRH neurons from OVX+E and proestrous mice
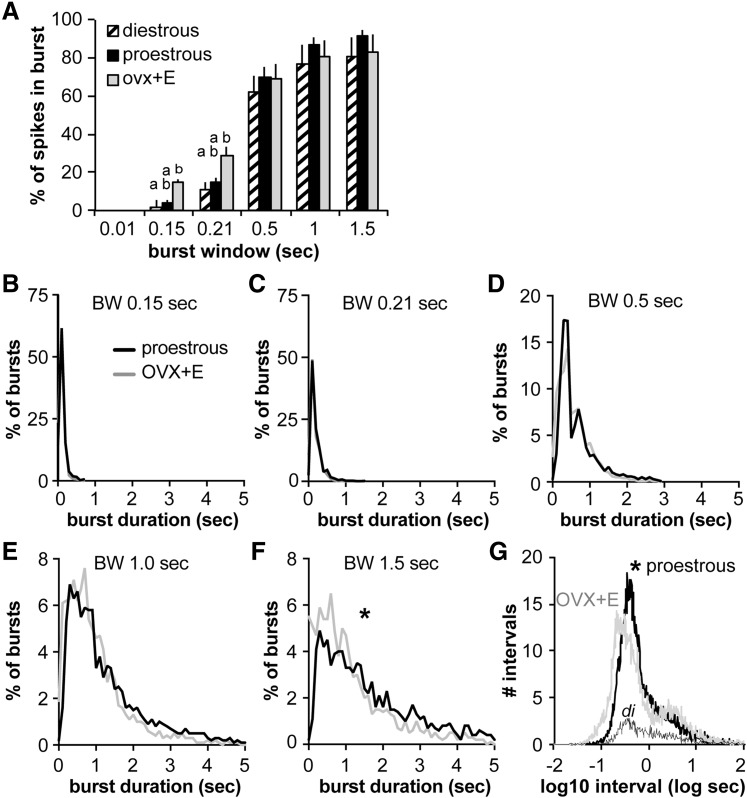
Overall firing activity provides a simple measure of neuronal activity, but the activity likely associated with hormone release occurs over much shorter time windows, referred to as burst firing. To better understand the pattern of action currents in GnRH neurons, the percentage of total spikes contained within bursts for selected burst windows was compared. No difference was observed between proestrous and OVX+E mice in the percent of spikes contained within bursts at any burst window studied (P < 0.05, Kruskal-Wallis/Dunn) [Fig. 3(A)]. In contrast, at burst windows of 0.15 and 0.21 seconds, the percent of spikes in bursts is lower in cells from diestrous mice. Burst duration was then analyzed for selected burst windows [Fig. 3(B–F)]. At a burst window of 0.01 seconds, typical of intraburst spike frequency in other neurons (29), no bursts were observed in GnRH neurons from any groups, consistent with previous reports in GnRH neurons (30, 43–45). Burst duration was not different between GnRH neurons from OVX+E and proestrous mice, except at the longest window examined, 1.5 seconds, in which burst duration was longer in proestrous mice (P < 0.05, Kolmogorov-Smirnov test). Interspike interval was shifted to shorter durations in OVX+E mice than proestrous mice (P < 0.05, Kolmogorov-Smirnov test) [Fig. 3(G)].
Figure 3.
GnRH neuron burst duration is increased on proestrus compared with OVX+E mice. (A) Mean ± standard error of mean percentage of spikes in bursts at different burst windows (BWs) in GnRH neurons from diestrous (hatched), proestrous (black), and OVX+E (gray) mice. Different lower case letters indicate P < 0.05 (Kruskal-Wallis/Dunn). (B–F) Histograms of the percentage of bursts of different duration in cells from proestrous (black line) and OVX+E (gray line) mice for burst windows indicated. (G) Log10 interval histogram in proestrous (black), OVX+E (gray), and diestrus (di, thin blackline) mice. *P < 0.05 proestrous vs OVX+E mice, Kolmogorov-Smirnov test.
Experiment 3: pituitary responsiveness to exogenous GnRH is greater in proestrous mice
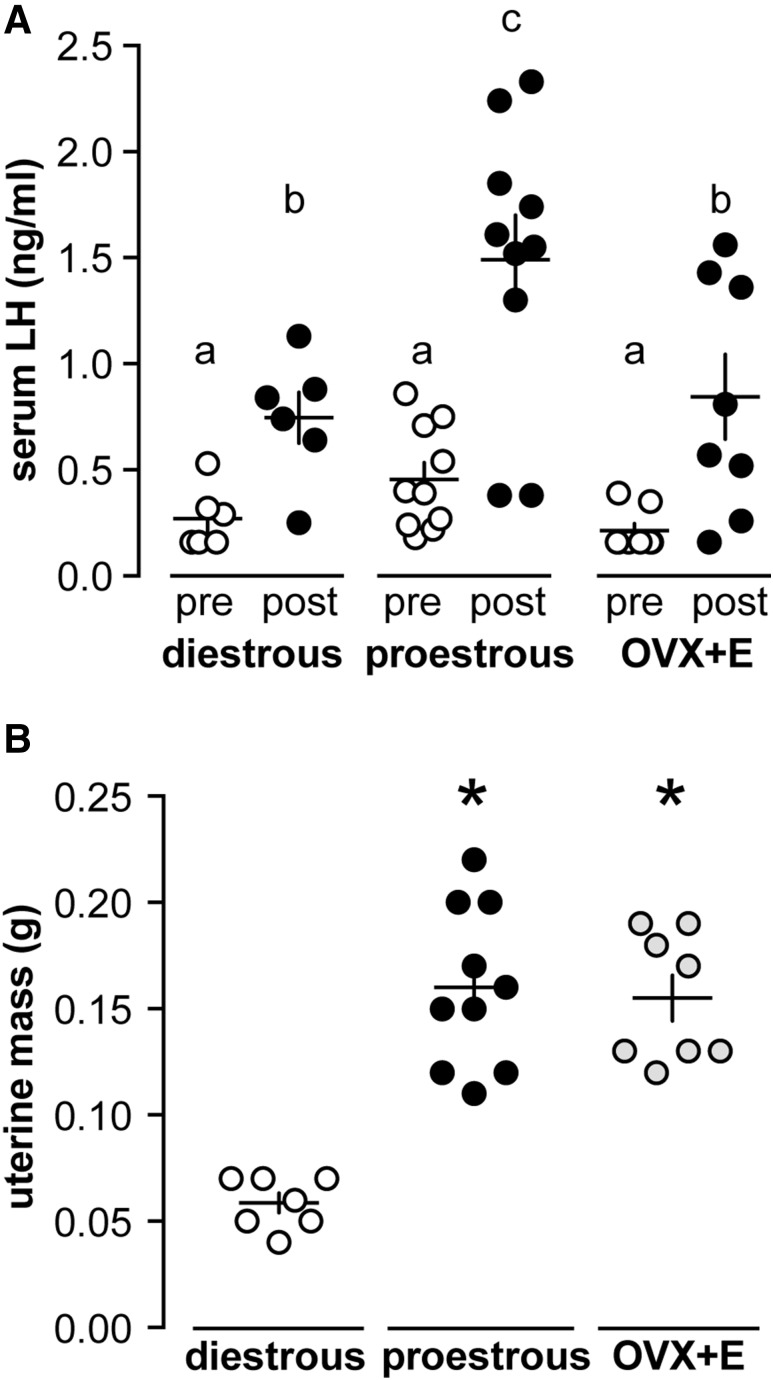
The observed reduction in LH surge amplitude (Fig. 1) could be because of reduced pituitary response in the daily surge model. To test this hypothesis, we evaluated pituitary responsiveness to exogenous GnRH in diestrous (n = 6), proestrous (n = 10), and OVX+E (n = 8) mice during the early afternoon before surge onset in the positive feedback models. Serum LH was monitored before and 15 minutes after intraperitoneal injection of 150 ng/kg GnRH (Fig. 4). No difference was observed in LH levels before GnRH injection. All 3 groups responded with an increase in serum LH, but LH values in proestrous mice were greater than the other 2 groups during the postinjection sample [2-way repeated-measures analysis of variance (ANOVA)/Holm-Sidak, P < 0.05]. Uterine mass was not different between proestrous and OVX+E mice but was greater than diestrous mice (1-way ANOVA/Tukey, P < 0.05) [Fig. 4(B)]. This suggests estradiol levels were elevated but similar in both surge models compared with diestrous mice (46).
Figure 4.
Pituitary response is lower in the daily estradiol-induced surge model. (A) Serum LH before (pre, open circles) and 15 minutes after (post, black circles) intraperitoneal injection 150 ng/kg GnRH in diestrous, proestrous, and OVX+E mice. Each symbol represents 1 mouse; means ± standard error of means are shown by the horizontal and vertical lines, respectively. Different lower case letters indicate P < 0.05 calculated by 2-way repeated-measures ANOVA/Holm-Sidak. (B) Uterine mass of diestrous (open circles), proestrous (black circles), and OVX+E (gray circles) mice. *P < 0.05 calculated by 1-way ANOVA/Tukey.
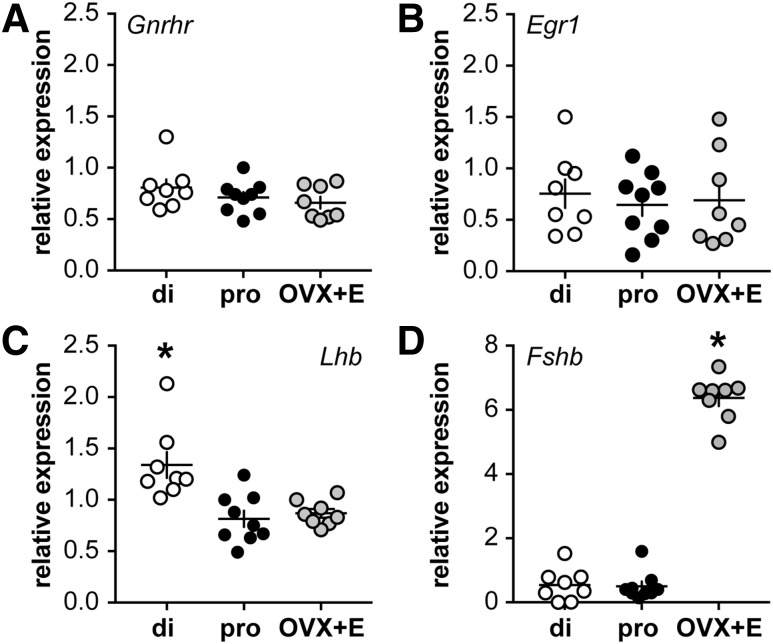
The greater LH response in proestrous mice could be attributable to differences in pituitary gene expression or LH content. To begin to test this, quantitative real-time PCR was used to evaluate the steady-state mRNA levels of specific genes. No difference in steady-state mRNA for Gnrhr or Egr1, an immediate early gene downstream of GnRH receptor signaling and required for Lhb expression (47), was observed among groups (1-way ANOVA/Tukey, P > 0.05) [Fig. 5(A) and 5(B)]. Lhb and Cga mRNA were greater (P < 0.05) in diestrous compared with proestrous and OVX+E mice, which were not different from one another (Lhb, Cga not shown, diestrous, 1.9 ± 0.2; proestrous, 0.9 ± 0.1; OVX+E, 1.1 ± 0.1 relative to mean of Actin and Ppia, Kruskal-Wallis/Dunn) [Fig. 5(C)]. For Cga, 1 point was removed from both the diestrous and proestrous data sets based on positive Grubb outlier tests. Despite reduced mRNA in proestrous and OVX+E mice, pituitary LH content was not different among groups (diestrous, 33 ± 7; proestrous, 52 ± 39; OVX+E, 41 ± 35 pg LH/µg total protein, 1-way ANOVA/Tukey, P > 0.05). Expression of Fshb was greater in OVX+E mice than both diestrous and proestrous mice (Kruskal-Wallis/Dunn, P < 0.05) [Fig. 5(D)].
Figure 5.
Relative steady-state mRNA levels of selected pituitary genes, normalized to mean expression of Actin and Ppia. (A) Gnrhr, (B) Egr1, (C) Lhb, and (D) Fshb in pituitaries from diestrous (open circles), proestrous (black circles), and OVX+E (gray circles) mice. Each symbol represents 1 mouse; the horizontal and vertical lines show mean ± standard error of mean, respectively. Note change in scale of y-axis in panel D. *P < 0.05 calculated by 1-way ANOVA/Tukey or Kruskal-Wallis/Dunn. di, diestrous; pro, proestrous.
Discussion
Preovulatory GnRH and LH surges are essential for successful reproduction in most species. The GnRH surge is initiated by high physiologic estradiol levels caused by a switch in feedback action of this steroid from negative to positive feedback on both the GnRH network and pituitary. The importance of estradiol in the control of ovulation led to the development of models in which estradiol levels were modified in ovariectomized animals to induce the positive feedback response while reducing other ovarian variables (13–17). These models have facilitated studies on the dependence and contributions of estradiol to central neuronal and pituitary changes underlying surge generation. Here we showed that the amplitude of the LH surge in an OVX+E mouse model (15) is lower than that during proestrus. Our findings suggest this may be because of a reduced response in the pituitary in combination with subtle shifts in action potential patterning, rather than differences in the mean firing rate of GnRH neurons.
This study revealed that despite a higher amplitude LH surge, the increase in mean GnRH firing rate over 1 hour was not different between proestrous and estradiol-induced surges. This suggests replacement of estradiol alone is able to recapitulate many of the positive feedback mechanisms induced by peripheral sex steroids to increase GnRH neuron activity. The present data support and extend previous studies of the firing rate of GnRH neurons during the natural cycle, specifically a higher firing rate on proestrus compared with metestrus (48). In contrast, a recent study showed a greater firing rate of GnRH neurons on diestrus than proestrus (49). This latter observation may be attributable to a short duration of recordings (5 minutes vs 1 hour in the current study), or differences in the timing of slice preparation, which was 4 to 5 hours before expected onset of the LH surge in the former study and just before expected onset of the LH surge in the current study.
Bursts of action potentials are related to neuroendocrine secretion (27, 28). Burst firing in GnRH neurons tends to be lower frequency (30, 43–45) than other cells in which burst firing has been studied (29, 50, 51). Of interest in this regard, detailed analysis of the firing pattern of GnRH neurons revealed burst duration increased in proestrous compared with OVX+E mice when longer burst windows were examined. Longer bursts of action potentials, as observed on proestrus, could thus lead to increased GnRH release, possibly by maintaining increased cytoplasmic calcium levels needed for vesicle fusion. Few measurements of intracellular calcium have been done in GnRH neurons, and limitations of the sampling rate or physical properties of the calcium indicator preclude exact conclusion of the duration of calcium elevation, but these calcium signals appear to be prolonged in GnRH neurons (43, 52). Of interest, the long burst window that revealed a difference in burst duration is similar in duration to the ADP potential of GnRH neurons (33, 53). The ADP is estradiol-sensitive in these cells, and the firing rate can be increased throughout the ADP. In addition to action potential bursts, interspike interval has also been used to classify firing cells (34, 35). Interspike intervals were shifted to shorter durations in OVX+E mice vs proestrous mice. This may indicate a shorter action potential refractory period in the former, but this did not generate longer burst durations. Thus, although both groups present similar overall firing frequency, the short-term patterning observed on proestrus might lead to greater GnRH release, which could contribute to the increased LH surge amplitude observed in this group.
Estradiol positive feedback acts not only centrally to alter GnRH release, but at the pituitary to alter responsiveness to GnRH (5, 6). During the early afternoon, before onset of the LH surge, pituitary responsiveness to exogenous GnRH was increased in proestrous mice compared with OVX+E and diestrous mice, which were similar to one another. These observations suggest that the increased amplitude of the LH surge in proestrous mice may be in part because of a difference in pituitary rather than central mechanisms (2, 5, 54). There was no difference in steady-state levels of Gnrhr mRNA. Differential translation of this mRNA pool, subcellular receptor localization, and signaling are alternative mechanisms that could account for lower amplitude LH surges in OVX+E mice. With regard to the latter, there was also no difference in expression of Egr1, an immediate early gene induced by GnRH receptor signaling (47). Other signaling pathways could be different among the groups, for example the gonadotropin-inhibitory hormone (GnIH) pathway, which reduces LH release in response to GnRH (55–57). There was also no difference in pituitary LH content among groups, despite expected suppression of Lhb and Cga mRNA by higher estradiol levels (58, 59) in proestrous and OVX+E mice, indicated by increased uterine mass. The high levels of Fshb levels in OVX+E mice compared with diestrous and proestrous mice are likely attributable to reduced levels of circulating inhibin after OVX (60). None of these changes in steady-state mRNA seem poised to contribute to a difference in LH surge amplitude between the two models.
Although the similarity of overall GnRH neuron activity between OVX+E and proestrous mice is consistent with a primary role for estradiol in surge induction, it is important to consider other steroid hormone changes during the cycle (18). Centrally produced progesterone has been reported to enhance estradiol-induced LH surges (19, 20). A role for ovarian progesterone is perhaps more likely to affect continuation of, rather than initiation of, positive feedback because serum levels of this hormone increase after onset of the LH surge (18). Expression of progesterone receptor is required for estradiol positive feedback; however, these actions may be ligand-independent (61, 62). Of interest regarding action of peripheral progesterone, activity of kisspeptin neurons of the anteroventral periventricular area, a region hypothesized to be critical for induction of positive feedback (63), is not different between OVX+E mice and OVX+E mice also treated with progesterone (50), again suggesting primary neuronal activity changes are mainly an estradiol effect.
In summary, despite a marked difference in LH surge amplitude between proestrous and OVX+E mice, overall GnRH neuron firing was fairly similar between these models, with subtle shifts toward patterns that may induce increased hormone release on proestrus. Pituitary response was also enhanced on proestrus, and the combination of these changes may contribute to increased LH release. Although it is not possible to rule out differences in parameters that were not examined (e.g., specific biophysical properties of GnRH neurons, other factors such as GnIH), these data suggest the OVX+E model recapitulates many aspects of the proestrous surge. A recent study demonstrated more consistent induction of LH surges with this type of constant estradiol implant model (64). This reliability, in combination with similarity to proestrous surges, make these models useful for studying the mechanisms of estradiol negative and positive feedback. Moreover, steroid replacement is the only choice for investigating feedback in genetic models that do not exhibit reproductive cycles, such as kisspeptin knockouts and vasopressin knockouts (65, 66).
Acknowledgments
We thank the University of Virginia Center for Research and Reproduction Ligand Assay and Analysis Core (NIH P50HD28934) for hormone assays, and Siena DeFazio for help developing the burst detection algorithm.
This study was supported by the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01 HD41469. M.S. was supported in part by the Brazilian Federal Agency for Support and Evaluation of Graduate Education and the Ministry of Education (CAPES).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- ACSF
artificial cerebrospinal fluid
- ADP
afterdepolarization potential
- ANOVA
analysis of variance
- CV
coefficient of variation
- EST
Eastern Standard Time
- GFP
green fluorescent protein
- GnIH
gonadotropin-inhibitory protein
- GnRH
gonadotropin-releasing hormone
- LH
luteinizing hormone
- mRNA
messenger RNA
- OVX
ovariectomy
- OVX+E
ovariectomized, estradiol-replaced
- PCR
polymerase chain reaction
References
- 1. Haisenleder DJ, Burger LL, Walsh HE, Stevens J, Aylor KW, Shupnik MA, Marshall JC. Pulsatile gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription in rat pituitaries: evidence for the involvement of Jun N-terminal kinase but not p38. Endocrinology. 2008;149(1):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376–385. [DOI] [PubMed] [Google Scholar]
- 3. Karsch FJ, Cummins JT, Thomas GB, Clarke IJ. Steroid feedback inhibition of pulsatile secretion of gonadotropin-releasing hormone in the ewe. Biol Reprod. 1987;36(5):1207–1218. [DOI] [PubMed] [Google Scholar]
- 4. Caraty A, Locatelli A, Martin GB. Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J Endocrinol. 1989;123(3):375–382. [DOI] [PubMed] [Google Scholar]
- 5. Clarke IJ, Cummins JT. Direct pituitary effects of estrogen and progesterone on gonadotropin secretion in the ovariectomized ewe. Neuroendocrinology. 1984;39(3):267–274. [DOI] [PubMed] [Google Scholar]
- 6. Turgeon JL, Barraclough CA. Regulatory role of estradiol in pituitary responsiveness to luteinizing hormone-releasing hormone on proestrus in the rat. Endocrinology. 1977;101(2):548–554. [DOI] [PubMed] [Google Scholar]
- 7. Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology. 1990;127(3):1375–1384. [DOI] [PubMed] [Google Scholar]
- 8. Bronson FH, Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology. 1979;104(5):1247–1255. [DOI] [PubMed] [Google Scholar]
- 9. Sarkar DK, Chiappa SA, Fink G, Sherwood NM. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature. 1976;264(5585):461–463. [DOI] [PubMed] [Google Scholar]
- 10. Döcke F, Dörner G. The mechanism of the induction of ovulation by oestrogens. J Endocrinol. 1965;33(3):491–499. [DOI] [PubMed] [Google Scholar]
- 11. Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology. 1991;129(3):1175–1182. [DOI] [PubMed] [Google Scholar]
- 12. Clarke IJ, Thomas GB, Yao B, Cummins JT. GnRH secretion throughout the ovine estrous cycle. Neuroendocrinology. 1987;46(1):82–88. [DOI] [PubMed] [Google Scholar]
- 13. Zhang C, Tonsfeldt KJ, Qiu J, Bosch MA, Kobayashi K, Steiner RA, Kelly MJ, Rønnekleiv OK. Molecular mechanisms that drive estradiol-dependent burst firing of Kiss1 neurons in the rostral periventricular preoptic area. Am J Physiol Endocrinol Metab. 2013;305(11):E1384–E1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52(2):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA. 2005;102(43):15682–15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96(1):57–62. [DOI] [PubMed] [Google Scholar]
- 17. Norman RL. Estrogen and progesterone effects on the neural control of the preovulatory LH release in the golden hamster. Biol Reprod. 1975;13(2):218–222. [DOI] [PubMed] [Google Scholar]
- 18. Walmer DK, Wrona MA, Hughes CL, Nelson KG. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology. 1992;131(3):1458–1466. [DOI] [PubMed] [Google Scholar]
- 19. Micevych P, Sinchak K. The neurosteroid progesterone underlies estrogen positive feedback of the LH surge. Front Endocrinol (Lausanne). 2011;2:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78(1):29–35. [DOI] [PubMed] [Google Scholar]
- 21. Czieselsky K, Prescott M, Porteous R, Campos P, Clarkson J, Steyn FJ, Campbell RE, Herbison AE. Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology. 2016;157(12):4794–4802. [DOI] [PubMed] [Google Scholar]
- 22. Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141(1):412–419. [DOI] [PubMed] [Google Scholar]
- 23. Paxinos G, Franklin K.. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Elsevier Academic; 2001. [Google Scholar]
- 24. Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online. 2003;5:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alcami P, Franconville R, Llano I, Marty A. Measuring the firing rate of high-resistance neurons with cell-attached recording. J Neurosci. 2012;32(9):3118–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology. 2002;143(6):2284–2292. [DOI] [PubMed] [Google Scholar]
- 27. Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979;290(2):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985;369:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen L, Deng Y, Luo W, Wang Z, Zeng S. Detection of bursts in neuronal spike trains by the mean inter-spike interval method. Progress in Natural Science. 2009;19:229–235. [Google Scholar]
- 30. Chu Z, Takagi H, Moenter SM. Hyperpolarization-activated currents in gonadotropin-releasing hormone (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. J Neurosci. 2010;30(40):13373–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kerr JN, Greenberg D, Helmchen F. Imaging input and output of neocortical networks in vivo. Proc Natl Acad Sci USA. 2005;102(39):14063–14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Komiyama T, Sato TR, O’Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464(7292):1182–1186. [DOI] [PubMed] [Google Scholar]
- 33. Chu Z, Moenter SM. Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci. 2006;26(46):11961–11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nowak LG, Azouz R, Sanchez-Vives MV, Gray CM, McCormick DA. Electrophysiological classes of cat primary visual cortical neurons in vivo as revealed by quantitative analyses. J Neurophysiol. 2003;89(3):1541–1566. [DOI] [PubMed] [Google Scholar]
- 35. Romanò N, Yip SH, Hodson DJ, Guillou A, Parnaudeau S, Kirk S, Tronche F, Bonnefont X, Le Tissier P, Bunn SJ, Grattan DR, Mollard P, Martin AO. Plasticity of hypothalamic dopamine neurons during lactation results in dissociation of electrical activity and release. J Neurosci. 2013;33(10):4424–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci. 2014;34(45):15060–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fallest PC, Trader GL, Darrow JM, Shupnik MA. Regulation of rat luteinizing hormone beta gene expression in transgenic mice by steroids and a gonadotropin-releasing hormone antagonist. Biol Reprod. 1995;53(1):103–109. [DOI] [PubMed] [Google Scholar]
- 38. Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132(4):1687–1691. [DOI] [PubMed] [Google Scholar]
- 39. Matteri RL, Roser JF, Baldwin DM, Lipovetsky V, Papkoff H. Characterization of a monoclonal antibody which detects luteinizing hormone from diverse mammalian species. Domest Anim Endocrinol. 1987;4(3):157–165. [DOI] [PubMed] [Google Scholar]
- 40. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29(1):23–39. [DOI] [PubMed] [Google Scholar]
- 42. Blake CA. A detailed characterization of the proestrous luteinizing hormone surge. Endocrinology. 1976;98(2):445–450. [DOI] [PubMed] [Google Scholar]
- 43. Lee K, Duan W, Sneyd J, Herbison AE. Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30(18):6214–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gaskins GT, Moenter SM. Orexin a suppresses gonadotropin-releasing hormone (GnRH) neuron activity in the mouse. Endocrinology. 2012;153(8):3850–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chu Z, Tomaiuolo M, Bertram R, Moenter SM. Two types of burst firing in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2012;24(7):1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shim WS, Conaway M, Masamura S, Yue W, Wang JP, Kmar R, Santen RJ. Estradiol hypersensitivity and mitogen-activated protein kinase expression in long-term estrogen deprived human breast cancer cells in vivo. Endocrinology. 2000;141(1):396–405. [DOI] [PubMed] [Google Scholar]
- 47. Yuen T, Wurmbach E, Ebersole BJ, Ruf F, Pfeffer RL, Sealfon SC. Coupling of GnRH concentration and the GnRH receptor-activated gene program. Mol Endocrinol. 2002;16(6):1145–1153. [DOI] [PubMed] [Google Scholar]
- 48. Farkas I, Vastagh C, Sárvári M, Liposits Z. Ghrelin decreases firing activity of gonadotropin-releasing hormone (GnRH) neurons in an estrous cycle and endocannabinoid signaling dependent manner. PLoS One. 2013;8(10):e78178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Piet R, Dunckley H, Lee K, Herbison AE. Vasoactive intestinal peptide excites gnrh neurons in male and female mice. Endocrinology. 2016;157(9):3621–3630. [DOI] [PubMed] [Google Scholar]
- 50. Wang L, DeFazio RA, Moenter SM. Excitability and burst generation of AVPV kisspeptin neurons are regulated by the estrous cycle via multiple conductances modulated by estradiol action. eNeuro. 2016;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Llinás R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297(5865):406–408. [DOI] [PubMed] [Google Scholar]
- 52. Terasawa E, Schanhofer WK, Keen KL, Luchansky L. Intracellular Ca(2+) oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci. 1999;19(14):5898–5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Y, Garro M, Dantzler HA, Taylor JA, Kline DD, Kuehl-Kovarik MC. Age affects spontaneous activity and depolarizing afterpotentials in isolated gonadotropin-releasing hormone neurons. Endocrinology. 2008;149(10):4938–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shupnik MA. Gonadal hormone feedback on pituitary gonadotropin genes. Trends Endocrinol Metab. 1996;7(8):272–276. [DOI] [PubMed] [Google Scholar]
- 55. Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275(2):661–667. [DOI] [PubMed] [Google Scholar]
- 56. Ciccone NA, Dunn IC, Boswell T, Tsutsui K, Ubuka T, Ukena K, Sharp PJ. Gonadotrophin inhibitory hormone depresses gonadotrophin alpha and follicle-stimulating hormone beta subunit expression in the pituitary of the domestic chicken. J Neuroendocrinol. 2004;16(12):999–1006. [DOI] [PubMed] [Google Scholar]
- 57. Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA. 2006;103(7):2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nilson JH, Nejedlik MT, Virgin JB, Crowder ME, Nett TM. Expression of alpha subunit and luteinizing hormone beta genes in the ovine anterior pituitary. Estradiol suppresses accumulation of mRNAS for both alpha subunit and luteinizing hormone beta. J Biol Chem. 1983;258(20):12087–12090. [PubMed] [Google Scholar]
- 59. Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol. 2004;33(3):559–584. [DOI] [PubMed] [Google Scholar]
- 60. Dalkin AC, Knight CD, Shupnik MA, Haisenleder DJ, Aloi J, Kirk SE, Yasin M, Marshall JC. Ovariectomy and inhibin immunoneutralization acutely increase follicle-stimulating hormone-beta messenger ribonucleic acid concentrations: evidence for a nontranscriptional mechanism. Endocrinology. 1993;132(3):1297–1304. [DOI] [PubMed] [Google Scholar]
- 61. Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141(4):1477–1485. [DOI] [PubMed] [Google Scholar]
- 62. Chappell PE, Schneider JS, Kim P, Xu M, Lydon JP, O’Malley BW, Levine JE. Absence of gonadotropin surges and gonadotropin-releasing hormone self-priming in ovariectomized (OVX), estrogen (E2)-treated, progesterone receptor knockout (PRKO) mice. Endocrinology. 1999;140(8):3653–3658. [DOI] [PubMed] [Google Scholar]
- 63. Simerly RB, Swanson LW. The distribution of neurotransmitter-specific cells and fibers in the anteroventral periventricular nucleus: implications for the control of gonadotropin secretion in the rat. Brain Res. 1987;400(1):11–34. [DOI] [PubMed] [Google Scholar]
- 64. Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88(6):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and clock mutant mice. Biol Reprod. 2006;75(5):778–784. [DOI] [PubMed] [Google Scholar]
- 66. d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104(25):10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]