Abstract
Prior work has identified alterations in activity of the hypothalamic-pituitary-adrenal axis as a potential mechanism underlying stress-induced emotional health problems, which disproportionately impact girls beginning in mid-adolescence. How adolescent girls differ from one another in dispositional coping tendencies and shift specific coping strategies in response to varying stressors have been theorized as important predictors of their adaptation, health, and well-being during this dynamic period of development. The goal of this study was to examine whether individual and day-to-day (within-person) differences in adolescent girls’ coping responses are associated with daily patterns of hypothalamic-pituitary-adrenal axis activity, indexed by cortisol. Participants were 122 early adolescent girls (Mage = 12.39) who provided three saliva samples per day for 3 days and completed daily coping reports, as well as a standard coping survey. Participants and primary caregivers also completed objective life stress interviews. On average, girls who were more likely to respond to interpersonal stress with voluntary engagement (active) coping exhibited generally adaptive daily physiological regulation—steeper diurnal cortisol slopes, lower total diurnal cortisol output, and lower cortisol awakening responses. Chronic interpersonal stress level significantly moderated these associations in different ways for two distinct components of the diurnal pattern—the slope and cortisol awakening responses. Regarding within-person differences, using active coping more than usual was associated with higher waking cortisol the following morning, which may help to prepare adolescent girls for perceived daily demands. These findings highlight the interactive influence of stress and coping in the prediction of daily hypothalamic-pituitary-adrenal axis activity and support the stress-buffering role of active coping for adolescent girls.
Keywords: Coping, Interpersonal stress, Diurnal cortisol, Daily diaries, Adolescent girls
Introduction
Conflict with friends, social rejection, and other forms of interpersonal stress grow in salience during adolescence, particularly for young girls (Rudolph and Hammen 1999). These interpersonal stressors disproportionately impact girls’ health and well-being compared to boys (Conley and Rudolph 2009; Hampel and Petermann 2006). For example, interpersonal stress contributes to the development of girls’ depression (Ge et al. 2001), which begins to increase in prevalence during mid-adolescence (Rohde et al. 2009). There are also potentially long-lasting physiological consequences of interpersonal stress during adolescence, such as alterations to hypothalamic-pituitary-adrenal (HPA) axis activity (Peters et al. 2011), which are theorized to play a role in the development of depression (Nolen-Hoeksema 2001; Slavich et al. 2010). Yet adolescent girls differ from one another in their characteristic styles of responding to interpersonal stress; some coping tendencies are thought to be more adaptive than others, such as using active rather than avoidant strategies (Compas et al. 2001; Zahn-Waxler et al. 2000). On average, girls tend to score higher on putatively maladaptive coping responses relative to boys, such as emotional venting and rumination (Hampel and Petermann 2006; Malooly et al. in press). Thus, it is particularly critical to consider differences in coping responses to interpersonal stress among adolescent girls, who are at elevated risk for depression and other internalizing psychopathology by virtue of their gender and developmental status (Gore et al. 1993; Nolen-Hoeksema 2001).
Most available research has considered biological and psychological components of stress responses separately, but examining biopsychosocial connections is critical to better understand mechanisms underlying stress-induced emotional and physical health problems (Miller et al. 2011; Slavich et al. 2010). The present study focused on how individual differences in early adolescent girls’ psychological responses to interpersonal stress (i.e., coping tendencies) were associated with daily patterns of cortisol, a hormonal product of the HPA axis. Taking into account the dynamic role of context in coping processes (DeLongis and Holtzman 2005; Lazarus and Folkman 1984), this study also examined day-to-day (within-person) differences in coping assessed via daily diaries in relation to diurnal cortisol.
Diurnal Cortisol Activity
The HPA axis is responsible for activating cortisol production in response to environmental threat (de Kloet 2004). Socially evaluative stressors (e.g., public speaking) consistently produce the cortisol response in laboratory settings (Dickerson and Kemeny 2004). This effect is more pronounced among adolescents compared to younger children (Stroud et al. 2009) and for women compared to men (Stroud et al. 2002). Cortisol also follows a typical daily pattern: relatively high levels at waking, an increase 30 min post-waking (the cortisol awakening response), and then a general decrease across the day (Adam and Kumari 2009). Alterations in the diurnal cortisol rhythm, such as less rapid rates of daily decline (i.e., flatter slopes), are considered to reflect HPA dysregulation that can contribute to health problems over time (Miller et al. 2007). Specifically, elevated flatter diurnal cortisol slopes have been linked with severity of mental health symptoms among middle school students (Shirtcliff and Essex 2008), internalizing problems among adolescent girls (Klimes-Dougan et al. 2001), and diagnoses of major depression among late adolescents (Doane et al. 2013).
Evidence suggests that the cortisol awakening response (CAR) is distinct from variations in cortisol later in the day (Clow et al. 2010). A greater average cortisol awakening response has prospectively predicted major depression in late adolescence (Vrshek-Schallhorn et al. 2013), but a smaller-than-average cortisol awakening response can also reflect burnout and other health problems (Saxbe 2008; see Chida and Steptoe 2009, for review of other mixed findings). Given that over 75 % of the variance in the cortisol awakening response is attributable to day-to-day fluctuations rather than differences between individuals (Ross et al. 2014), researchers have recently focused greater attention on daily demands, specifically anticipation of and preparation for the upcoming day, that may contribute to within-person (i.e., day-to-day) alterations in the size of the cortisol awakening response (Adam et al. 2006; Fries et al. 2009).
Various forms of interpersonal stress have been associated with diurnal cortisol patterns during adolescence. For example, cortisol levels were higher when adolescents reported being alone in their daily lives (Adam 2006), and loneliness and social exclusion have been associated with flatter diurnal slopes (Doane and Adam 2010; Peters et al. 2011). Psychological coping responses theoretically influence adolescents’ regulatory capacity when facing stressful social demands (Compas et al. 2001), but most empirical studies have focused solely on the impact of stress or adversity on HPA functioning (Obradović 2012). To address this limitation, the present study took advantage of a traditional survey measure and daily diaries to consider both coping style (i.e., individual differences in dispositional coping tendencies) and day-to-day variation in coping (i.e., within-person differences) as predictors of multiple indices of diurnal HPA activity.
Coping with Interpersonal Stress
Coping is an ongoing dynamic process including efforts to manage external or internal demands appraised as exceeding a person’s resources (Lazarus and Folkman 1984). Adolescents’ voluntary coping responses that involve deliberate attempts to manage stress have most consistently been linked with positive adjustment, whereas involuntary responses to stress that are not deliberate have generally been linked with regulatory dysfunction and other adverse outcomes (Compas et al. 2001; Connor-Smith et al. 2000). Voluntary engagement coping includes efforts directed toward a stressor (e.g., problem-solving) or one’s reactions to the stressor (e.g., acceptance). Involuntary responses to stress may take the form of engagement (e.g., intrusive thoughts) or disengagement (e.g., inaction; Connor-Smith et al. 2000). Moreover, adolescents’ maladaptive involuntary responses to interpersonal stress have helped to explain associations between several risk factors (e.g., deficits in emotional clarity) and depressive symptoms (Flynn and Rudolph 2007, 2010).
Covariation between individuals’ coping style and diurnal cortisol activity can inform how, for whom, and under what circumstances interpersonal stress leads to regulatory problems. For example, involuntary engagement responses (e.g., intrusive thoughts) were associated with greater cortisol reactivity to a lab-based social stressor among adolescent girls (Sontag et al. 2008). Similarly, putatively maladaptive coping strategies, including fighting or breaking things, were associated with flatter diurnal cortisol slopes among children of parents with HIV/AIDS (Slatcher et al. 2015). In another study, girls with mothers diagnosed with depression who scored higher on involuntary responses to stressors associated with maternal depression exhibited greater overall diurnal cortisol production, compared to girls who more frequently used voluntary coping (Foland-Ross et al. 2014). As of yet, fewer studies have focused on adaptive coping and its theorized role in promoting physiological regulation among early adolescent girls.
This next step is important because an active coping style is generally thought to be one characteristic of adolescents that can moderate the impact of stress on emotional adjustment and health, potentially via physiological processes (Grant et al. 2003). Indeed, numerous studies have demonstrated that active coping buffers adolescents from the adverse outcomes typically associated with stressful circumstances (Dumont and Provost 1999; Herman-Stahl and Petersen 1996; Marceau et al. 2014). However, whether certain coping responses can be utilized effectively also depends on the type and severity of stress exposure (Compas et al. 2001; Graber and Sontag 2009). When mismatched with the burden of chronic stress, a generally active coping style may not be linked with the same degree of physiological regulatory benefit. Thus, the present study also explored whether associations between girls’ coping style and diurnal cortisol patterns varied with levels of past year chronic interpersonal stress.
Coping in the Context of Daily Life
Lazarus and Folkman (1984) emphasized the role of context in determining individuals’ ability to cope effectively. Accordingly, many have argued for a daily process approach to capture within-person, dynamic changes in coping (e.g., Tennen et al. 2000). Followers of this approach argue that measuring the ongoing dynamic process of coping via daily diary reports gathers different information than more traditional self-report coping measures, which charge participants with the difficult task of remembering how they typically respond to stressors irrespective of context (Almeida 2005; DeLongis and Holtzman 2005). Supporting this, research indicates that trait-like measures of coping style are poor predictors of coping responses captured by daily reports, with studies using both methods reporting between 0 and 37 % shared variance between the two (Ptacek et al. 1994; Schwartz et al. 1999; Smith et al. 1999). For example, one study indicated that a trait-like self-report measure assessing typical responses to hypothetically stressful events and an average measure from diary reports only shared 0–12 % of their variance across 16 types of coping responses (Schwartz et al. 1999).
Thus, trait-like measures of coping style and daily reports are both important tools in the study of stress and coping; the former are better suited to examine differences between individuals, and the latter are better suited to examine differences across days within a single individual. Applying diary reports of coping to cortisol research has been limited. In one study, cortisol levels were higher during late adolescents’ particularly stressful real-life situations only when they also reported more active coping (Sladek et al. 2016), highlighting the adaptive nature of the cortisol response in context. Here, we built upon this prior work by collecting multiple diary reports from early adolescent girls in coordination with saliva sampling outside of the laboratory to consider daily coping responses to temporally proximal, naturally occurring stressors.
Present Study and Hypotheses
We tested two primary questions to better understand how early adolescent girls’ coping responses to interpersonal stress are associated with daily stress physiology. First, we examined individual differences in girls’ coping style in response to interpersonal stress (assessed using a standard survey measure) in relation to several diurnal cortisol indices: cortisol levels at waking, the cortisol awakening response, diurnal cortisol slope, and total diurnal cortisol output. We expected that girls who were more likely to use voluntary engagement (active) coping strategies relative to other responses to interpersonal stress would exhibit average diurnal cortisol patterns reflecting generally adaptive physiological regulation, specifically steeper slopes and lower total output (Hypothesis 1a). Due to generally mixed literature regarding the cortisol awakening response (Chida and Steptoe 2009) and its distinction from the rest of the diurnal pattern (Clow et al. 2010), we did not make a specific hypothesis for the between-person association of active coping style with the cortisol awakening response. We also expected that an active coping style would be more strongly associated with physiological regulation (steeper slopes, lower output) for girls experiencing lower to average levels of chronic interpersonal stress in the last year. Under more chronically stressful conditions, active coping would not provide the same degree of regulatory benefit (Hypothesis 1b). Next, we considered whether day-to-day (within-person) changes in voluntary engagement coping were associated with daily differences in the same diurnal cortisol indices. Although prior work is limited, we expected that using active coping to a greater extent than usual would be associated with greater cortisol production the following morning (waking cortisol or the cortisol awakening response) as a daily boost to prepare for upcoming demands (Adam et al. 2006; Hypothesis 2).
Methods
Participants
Early adolescent girls (N = 122, Mage = 12.39 years, SD = 0.76; 87 % White) completed a daily saliva collection protocol as part of a larger study examining biopsychosocial predictors of emotional disorders (N = 132 daughters and primary caregivers, herein called mothers, in the larger study). Three siblings of participants and one father participated in the saliva collection protocol. All results remained the same when these individuals were excluded from analyses. Girls who completed the saliva collection protocol did not differ from those who did not on age, parents’ income, pubertal status, or coping style (ps > .10). Participants were recruited from two rural New England counties through advertisements/flyers, referrals, and local schools. Most families were middle to upper class (17.6 % < $40k/year, 19.4 % $41–60k, 24.1 % $61–100k, 38.9 % > $100k).
Procedure
The college Institutional Review Board approved all procedures. During a laboratory visit, adolescents and their mothers completed assent and consent forms, respectively, and were invited to ask questions to ensure adequate understanding of study procedures. Subsequently, girls and their mothers each completed separate objective stress and diagnostic interviews, along with a packet of questionnaires (including a measure of pubertal status). Girls also completed online questionnaires at home, including a measure of coping style. Of the 122 girls who completed the laboratory visit and saliva collection, 108 completed the online questionnaires. Girls who completed the questionnaires did not differ from those who did not on race/ethnicity, parent income, pubertal status, chronic interpersonal stress, or cortisol variables (ps > .35). At the laboratory visit, participants were provided instructions and materials for saliva collection. Participants were scheduled to complete the collection on three consecutive weekdays within approximately 1 week of the visit (M = 7.48 days; SD = 8.86), avoiding atypical days such as vacations or birthdays. Participants were asked to provide three saliva samples each day at waking, 30 min after waking, and bedtime. Participants recorded the time and completed a diary assessment for each saliva sample. Saliva was collected by passive drool. Families were contacted the night before and on the second day of collection to ensure adherence to the protocol. On average, participants provided 8.66 saliva samples (SD = 0.82).
To obtain an objective sampling time, straws were stored in a container with a MEMS 6™ (Aardex) track cap that electronically recorded time of openings. Waking samples were considered compliant if track cap detected-time was within 15 min of self-reported wake time. Samples scheduled for 30 min after waking were considered compliant if provided between 23 and 37 min after the waking sample according to track cap data, and if the track cap was used (Doane and Zeiders 2014). Given the importance of fidelity to the sampling protocol in order to accurately characterize the cortisol awakening response (Kudielka et al. 2003), this outcome measure was treated as missing for participants who failed to use the track cap (n = 31). For other diurnal cortisol outcomes, dummy variables for individuals who did not use the track cap and specific non-compliant days were created and tested as covariates (see below). Participants who did not use the track cap (M = 2.25, SD = 0.46) had significantly higher past year chronic interpersonal stress compared to those who did use the track cap (M = 2.02, SD = 0.48), t(120) = −2.29, p = .02. These groups did not significantly differ on average diurnal cortisol activity, coping style, internalizing symptoms, or demographic factors (ps > .12).
Measures
Active Coping Style
Adolescents completed the 57-item Responses to Stress Questionnaire—social stress version (RSQ; Connor-Smith et al. 2000). Participants indicated on a 4-point scale how much they respond in different ways when they have “problems with other kids” (1 = not at all to 4 = a lot). We computed a mean score for voluntary engagement coping, including primary control (“I do something to try to fix the problem or take action to change things”) and secondary control (“I think about the things I’m learning from the situation, or something good that will come from it”). Consistent with original factor analyses (Connor-Smith et al. 2000), primary and secondary control were positively correlated, r = .43, p < .001, and thus collapsed to collectively assess voluntary engagement (18 items; α = .81). We also computed a mean score for involuntary responses, including involuntary engagement (“When I have problems with other kids I can’t stop thinking about what I did or said”) and involuntary disengagement (“My mind just goes blank when I have problems with other kids, I can’t think at all”). Similar to original factor analyses, these two dimensions were positively correlated, r = .83, p < .001, and thus collapsed to assess involuntary responses (27 items; α = .92). To account for individual differences in overall rates of item endorsement, we formed proportion scores by dividing voluntary engagement coping scores by involuntary responses scores (see Foland-Ross et al. 2014). Thus, higher scores (>1.00) reflected a greater tendency to use active voluntary coping strategies to handle interpersonal stress rather than involuntary responses. The RSQ also includes items that assess voluntary disengagement coping (e.g., denial, avoidance) but these were not included given the focus of the present analyses.
Chronic Interpersonal Stress
A modified version of the UCLA Life Stress Interview (LSI; adapted from Hammen 1991; Rudolph et al. 2000) was used to assess adolescents’ past year chronic stress. Mothers and daughters completed separate semi-structured interviews with the same interviewer, and interviewers were blind to other study data. Probes were used to elicit behavioral descriptions of adolescents’ ongoing objective stress in five interpersonal domains: parent-child relationship, close friendships, peer social life, romantic relationships/dating, parents’ marital (or cohabiting) romantic relationship (if applicable). Using the behavioral descriptions, interviewers assessed and rated each domain of life stress on a 9-point scale from 1 (excellent/optimal circumstances) to 5 (very bad circumstances) in half-point increments. Inter-rater reliability was good (ICCs: M = .80 [.72–.89] for daughter; M = .83 [.70–.91] for mother). We computed a chronic interpersonal stress composite from the mean of ratings of the interpersonal domains. The composites based on mother and daughter report were highly correlated, r = .74, p < .001; thus, we used the mean of mother and daughter ratings (Rudolph et al. 2000; see Stroud et al. 2016).
Daily Engagement Coping
At bedtime the night before the first day of saliva sampling and after each sampling day (4 diary reports per person), participants were asked to report how much they used active coping strategies throughout the course of the day (1 = not at all to 4 = a lot) without a specific type of stress specified. Nine items were modified from the RSQ to specifically assess daily voluntary engagement coping (e.g., “Overall today, how much did you try to fix your problems or take action to change things?”; αs = .71 to .74, across days).
Diurnal Cortisol
Samples were returned to the lab by mail and then stored at −20 °C until sent by courier on dry ice over 3 days to the Biochemisches Labor at the University of Trier, Germany, for assay. Samples were assayed for cortisol in duplicate, using a solid phase time-resolved fluorescence immunoassay with fluorometric endpoint detection (DELFIA; Dressendörfer et al. 1992). The intra-assay coefficients of variation ranged from 4.0 to 6.7 % and the inter-assay coefficients of variation ranged from 7.1 to 9.0 %. Cortisol values were natural log transformed and four samples were windsorized at 1.81 μg/dl (Nicolson 2008). In addition to cortisol levels at waking, we calculated three daily indices: (1) cortisol awakening response, using the formula for area under the curve with respect to increase for the two morning samples (AUCi; Pruessner et al. 2003), (2) hourly rate of change from waking to bedtime (diurnal slope), found by computing the difference between log transformed waking and bedtime cortisol values and dividing by total time awake, and (3) total diurnal cortisol output, using the formula for area under the curve with respect to ground for all samples (AUCg; Pruessner et al. 2003).
Potential Covariates
We also considered between-person and day-to-day factors that have been associated with diurnal cortisol in prior work as covariates. Racial/ethnic background was represented with a dummy variable (0 = non-White, 1 = White; other between-person covariates detailed below). Daily factors captured in the diary reports were also considered, including time of waking, caffeine use, daily negative affect, and perceived stress level at bedtime (participants were asked to briefly describe the most stressful event of the last hour and subjectively rate how stressful this event was, from 0 = not at all stressful to 3 = very stressful; see Adam 2006; Sladek et al. 2016). Finally, we included dummy variables indicating adherence to the protocol as predictors to test whether compliance problems influenced cortisol estimates, including whether participants used the track cap devices and whether the first two samples of the day were compliant. The dummy variable for track cap usage was not included in CAR models because these outcomes were treated as missing for individuals who did not use the track cap. Models were tested with and without these covariates.
Pubertal Status
The mean of five items from the Pubertal Development Scale assessed growth spurt in height, skin and body hair changes, breast development, and age at menarche (Petersen et al. 1988; α = .70).
Internalizing Symptoms
Current symptoms of anxiety and depressive disorders were assessed using the Schedule for Affective Disorders and Schizophrenia for school-aged children—present and lifetime version (K-SADS-PL; Kaufman et al. 1997) on a 4-point scale from 0 (no symptoms) to 3 (meets DSM-IV criteria). Given the low prevalence of internalizing symptoms (none had diagnosable depression), we created a composite to increase variance by taking the maximum of those ratings across anxiety and depressive disorders (Starr et al. 2016).
Analytic Strategy
We conducted multilevel models in Mplus 7 (Muthén and Muthén 1998–2012) using maximum likelihood estimation with robust standard errors to account for nested data (days within persons) and handle missing data. We tested a series of two-level models separately for each of four cortisol outcomes (waking cortisol, CAR, diurnal slope, diurnal AUCg). First, we conducted unconditional models with no predictors to assess between- and within-person variance for each outcome. Next, we entered active coping style as a Level 2 (between-person) predictor of intercepts (average cortisol parameters; Hypothesis 1a). Then we added day-specific covariates at Level 1 (e.g., wake time) and individual factors at Level 2 (e.g., chronic interpersonal stress) that could influence cortisol estimates or might account for associations between coping and cortisol. We then included the product of active coping style and chronic interpersonal stress to test their interaction in predicting cortisol outcomes (Hypothesis 1b). As recommended (Enders and Tofighi 2007), we centered continuous predictors at Level 1 within-person (i.e., an individual’s average score across 3 days subtracted from each daily score) and centered Level 2 predictors at the grand mean (i.e., the average for the entire sample subtracted from each individual’s score). By centering in this way, we can interpret Level 1 coefficients as estimated changes in cortisol associated with a 1-unit increase in daily coping relative to an individual’s average or typical coping score (i.e., when coping more than usual); we can interpret Level 2 coefficients as estimated changes in cortisol associated with a 1-unit increase in an individual’s coping style relative to the sample average (i.e., girls who cope more actively than other girls). We investigated significant two-way interactions using simple slopes techniques for multilevel modeling (Preacher et al. 2006). We estimated simple slopes for associations between coping and cortisol at the mean of interpersonal stress and +/−1 SD (Aiken and West 1991). We also assessed the range of interpersonal stress for which relations were significant.
We estimated multilevel models of day-to-day variation in cortisol in a similar fashion with diary-reported coping entered as a Level 1 (within-person) predictor of daily differences in cortisol, followed by models including covariates (Hypothesis 2). Based on the timing of bedtime diary reports, we tested both concurrent and lagged associations to examine whether coping more than usual during the day of or day prior to saliva sampling was associated with variation in cortisol. Likelihood ratio chi-square difference tests indicated the within-person associations between coping and cortisol did not significantly vary across individuals (ps > .08).
Results
Preliminary Analyses
Descriptive statistics and correlations are shown in Table 1. The sample mean of voluntary engagement coping was 2.34 (SD = 0.44), indicating that participants usually used these types of strategies in response to interpersonal stress between a little and some of the time. The sample mean of involuntary responses was 1.80 (SD = 0.54), indicating that participants usually responded to interpersonal stress in this way less than a little. On average, participants reported using more voluntary engagement coping in response to interpersonal stress compared to involuntary responses (M of voluntary to involuntary ratio = 1.39, where 1.00 indicates equal use of voluntary to involuntary responses, SD = 0.40); 84.9 % of participants typically used more voluntary relative to involuntary responses (participants with ratio scores > 1.00). On average, higher scores on this active coping style were associated with lower CARs, steeper diurnal slopes, and lower diurnal AUCg. Notably, individual averages of daily diary-reported engagement coping were not significantly associated with average diurnal cortisol parameters.
Table 1.
Descriptive statistics and zero-order correlations
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Waking cortisol | – | ||||||||||||||
| 2. Cortisol awakening response | −.61** | – | |||||||||||||
| 3. Diurnal cortisol slope | −.28** | .09 | – | ||||||||||||
| 4. Diurnal cortisol output (AUCg) | .31** | .15 | .51** | – | |||||||||||
| 5. Active coping style | .15 | −.23* | −.24* | −.21* | – | ||||||||||
| 6. Chronic interpersonal stress | −.17† | .11 | .05 | −.08 | −.32** | – | |||||||||
| 7. Internalizing symptoms | −.10 | .11 | .05 | .04 | −.10 | .19* | – | ||||||||
| 8. White (1 = yes) | −.01 | .01 | −.19* | −.06 | −.03 | −.10 | −.08 | – | |||||||
| 9. Pubertal status | .01 | .02 | .22* | .25** | −.06 | .16† | .17† | −.02 | – | ||||||
| 10. Age | .03 | −.03 | .15 | .13 | −.17† | .01 | .16† | .25** | .43** | – | |||||
| 11. Parent income | .16† | .01 | −.03 | .11 | .05 | −.32** | −.18* | .13 | −.17† | .05 | – | ||||
| 12. Average diary-reported coping | −.07 | −.07 | .06 | −.05 | −.02 | .12 | .19* | −.05 | .14 | .18† | −.15 | – | |||
| 13. Average diary-reported stress | −.13 | .05 | .08 | −.01 | −.03 | .10 | −.02 | −.01 | .06 | .18† | .12 | .01 | – | ||
| 14. Average diary-reported NA | −.10 | .12 | .02 | −.01 | −.26** | .35** | .17† | −.04 | .15† | .14 | −.16† | .24* | .18† | – | |
| 15. Average wake time | −.22* | −.10 | .16† | −.37** | .07 | .09 | .02 | −.29** | .06 | .11 | −.03 | .21* | .01 | .08 | – |
| Ma | 0.26 | 0.09 | −0.18 | 75.81 | 1.39 | 2.08 | 1.34 | 87.70 | 2.66 | 12.39 | 4.69 | 1.74 | 2.51 | 0.29 | 7.40 |
| SD | 0.15 | 0.20 | 0.08 | 12.25 | 0.40 | 0.49 | 0.94 | – | 0.60 | 0.76 | 1.39 | 0.43 | 0.68 | 0.33 | 1.55 |
| Minimum | 0.01 | −0.69 | −0.40 | 41.91 | 0.60 | 1.25 | 0.00 | – | 1.20 | 10.83 | 1.00 | 1.00 | 1.00 | 0.00 | 4.83 |
| Maximum | 0.94 | 0.83 | 0.04 | 122.13 | 2.95 | 3.56 | 3.00 | – | 3.70 | 15.00 | 6.00 | 2.89 | 3.89 | 1.63 | 12.01 |
Notes. N = 122 for waking cortisol, diurnal slope, and AUCg. N = 91 for CAR after excluding participants who did not adhere to saliva protocol to avoid biased estimates of the CAR. Cortisol values were transformed using the natural log function. Raw value presented for waking cortisol for descriptive purposes but log transformation used for analyses. Active coping style = proportion of voluntary engagement coping to involuntary responses (>1.00 indicates more active coping style). Parent income ranges from 1 = under $10,000/year to 6 = over $100,000/year. NA negative affect. Wake time reported in hours with decimals representing portion of hour
p < .10.
p < .05.
p < .01
Mean for continuous variables and percentage of sample for dichotomous variables
Results from unconditional models with no predictors for each of the four outcomes were used to compute intraclass correlations (ICCs), which quantify the proportion of person-level variance for nested cortisol data that was stable within individuals over 3 days: ICCwakingcort = .413, ICCCAR = .113, ICCslope = .231, ICCAUCg = .377. The residual variances for these variables (1 – ICC) indicated that day-to-day fluctuations or changes accounted for approximately 58.7 % of the variance in waking cortisol, 88.7 % for CAR, 76.9 % for diurnal slope, and 62.3 % for diurnal AUCg. These results from unconditional models demonstrated that, similar to prior work (e.g., Ross et al. 2014), these diurnal cortisol parameters differ between individuals but also within individuals across days of the study.
Individual Differences in Active Coping Style and Chronic Interpersonal Stress
In models with a single between-person predictor, individual differences in active coping style were significantly associated with steeper average diurnal cortisol slopes, γ01 = −0.044, p = .007, and lower diurnal AUCg, γ01 = −6.310, p = .013, but not cortisol levels at waking, γ01 = 0.173, p = .367. The association of active coping style with a lower CAR was marginally significant, γ01 = −0.114, p = .061. Between-person associations of coping and cortisol remained significant when adjusting for other factors, including wake time, non-compliance, race/ethnicity, pubertal status, interpersonal stress, and internalizing symptoms.
In addition, interpersonal stress significantly moderated the associations of active coping style with the diurnal slope and CAR (Table 2). The negative association between coping and diurnal slope was significant for those scoring at the mean (B = −0.041, p = .004) and 1 SD below (B = −0.074, p < .001), but not 1 SD above the mean (B = −0.008, p = .641), of chronic interpersonal stress (Fig. 1); this association was significant for those scoring below 2.28 on stress (70 % of the sample). The negative association between coping and CAR was significant for those scoring at the mean (B = −0.132, p = .032) and 1 SD above (B = −0.253, p = .001), but not 1 SD below the mean (B = −0.012, p = .883), of chronic interpersonal stress (Fig. 2); this association was significant for those scoring above 2.03 on stress (50 % of the sample). These findings were generally consistent when also adjusting for caffeine use, daily negative affect, age, parent income, and whether participants used track cap devices (for waking cortisol, diurnal slope, and AUCg), none of which significantly contributed to prediction.
Table 2.
Fixed and random effects estimates for multilevel models predicting diurnal cortisol from individual differences in active coping style, chronic interpersonal stress, and their interaction
| Fixed effects | Waking cortisol
|
Cortisol awakening response
|
Diurnal cortisol slope
|
Diurnal cortisol AUCg
|
||||
|---|---|---|---|---|---|---|---|---|
| Est. | SE | Est. | SE | Est. | SE | Est. | SE | |
| Intercept: average level, γ00 | −1.754** | 0.159 | 0.154* | 0.050 | −0.124** | 0.022 | 79.386** | 3.862 |
| Level 1 (day-specific) | ||||||||
| Wake time, γ10 | −0.122 | 0.081 | 0.030 | 0.047 | 0.009 | 0.013 | −4.210** | 0.858 |
| Non-compliance, γ20 | 0.233† | 0.123 | −0.097** | 0.037 | −0.011 | 0.014 | −0.721 | 1.875 |
| Level 2 (person-specific) | ||||||||
| Active coping style, γ01 | 0.060 | 0.213 | −0.132* | 0.062 | −0.041** | 0.015 | −8.009** | 2.970 |
| Interpersonal stress, γ02 | −0.205 | 0.136 | −0.004 | 0.039 | −0.005 | 0.012 | −5.229* | 2.453 |
| Coping × stress, γ03 | −0.010 | 0.414 | −0.246* | 0.095 | 0.068** | 0.023 | 2.045 | 5.970 |
| White race/ethnicity, γ04 | −0.063 | 0.136 | 0.014 | 0.044 | −0.050** | 0.017 | −3.142 | 3.778 |
| Pubertal status, γ05 | 0.054 | 0.085 | 0.005 | 0.031 | 0.023* | 0.010 | 5.452** | 1.898 |
| Internalizing symptoms, γ06 | −0.056 | 0.064 | 0.014 | 0.017 | 0.001 | 0.006 | 0.072 | 1.080 |
| Random effects | ||||||||
| σ2ε (L1 residual variance) | 0.365** | 0.059 | 0.089** | 0.026 | 0.009** | 0.001 | 148.177** | 35.121 |
| σ2u0 (L2 residual variance) | 0.240** | 0.084 | 0.007 | 0.005 | 0.002* | 0.001 | 77.942** | 20.559 |
Note 366 days (Level 1) nested within 122 individuals (Level 2). Wake time within-person centered and all continuous Level 2 predictors grand-mean centered. Non-compliance dummy coded (1 = not compliant with saliva sampling procedures). White race/ethnicity dummy coded (1 = White). Outcomes calculated from log-based cortisol values (μg/dl). Bold indicates significant findings in support of hypotheses
Est. partial regression coefficient estimate, SE robust standard error
p < .10,
p < .05,
p < .01
Fig. 1.
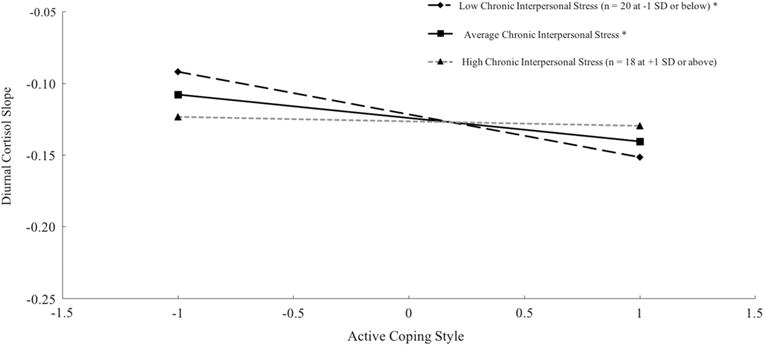
Simple slope plots of average diurnal cortisol slope [(bedtime sample – waking sample)/time awake] by active coping style (at the grand mean and +/−1 SD) at chronic interpersonal stress scores at the grand mean and +/−1 SD. Cortisol values (μg/dl) log transformed. Note that lower (more negative) values reflect steeper slopes. *p < .05
Fig. 2.
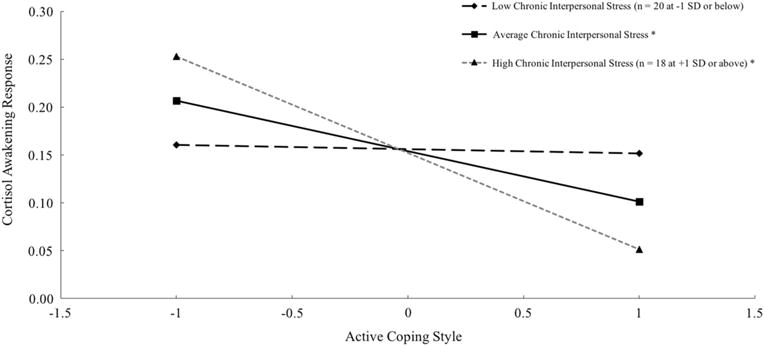
Simple slope plots of average cortisol awakening response (AUCi) by active coping style (at the grand mean and +/−1 SD) at chronic interpersonal stress scores at the grand mean and +/−1 SD. Cortisol values (μg/dl) log transformed. *p < .05
Day-to-Day Differences in Coping
There were no significant within-person associations between diary-reported engagement coping and cortisol parameters from the same day (ps > .15). However, lagging daily coping reported at bedtime to predict cortisol parameters the following day revealed that within-person increases in prior night engagement coping were associated with increases in waking cortisol, γ10 = 0.209, p = .062. This association was significant (p = .024) after adjusting for wake time, non-compliance, perceived stress level at bedtime (reported in conjunction with coping), race/ethnicity, and pubertal status (Table 3). The association remained significant when also adjusting for caffeine use, daily negative affect, internalizing symptoms, age, parent income, and compliance indicators, none of which significantly contributed to prediction. Within-person differences in prior night coping were not significantly associated with daily variation in the CAR, slope, or AUCg (ps > .18). As a check of directionality, within-person differences in cortisol parameters did not significantly predict variation in same-day coping (ps > .25).
Table 3.
Fixed and random effects estimates for multilevel models predicting diurnal cortisol from diary-reported engagement coping
| Fixed effects | Waking cortisol
|
Cortisol awakening response
|
Diurnal cortisol slope
|
Diurnal cortisol AUCg
|
||||
|---|---|---|---|---|---|---|---|---|
| Est. | SE | Est. | SE | Est. | SE | Est. | SE | |
| Intercept: average level, γ00 | −1.774** | 0.161 | 0.171** | 0.058 | 0.127** | 0.023 | 78.997** | 3.865 |
| Level 1 (day-specific) | ||||||||
| Prior night coping, γ10 | 0.241* | 0.106 | −0.096 | 0.075 | 0.010 | 0.024 | 1.861 | 2.689 |
| Wake time, γ20 | −0.131 | 0.083 | 0.035 | 0.047 | 0.008 | 0.012 | −4.304** | 0.852 |
| Non-compliance, γ30 | 0.230† | 0.124 | −0.098* | 0.042 | −0.016 | 0.003 | −1.352 | 1.898 |
| Prior night stress level, γ40 | 0.016 | 0.033 | −0.003 | 0.016 | 0.008 | 0.005 | 1.175 | 0.757 |
| Level 2 (person-specific) | ||||||||
| White race/ethnicity, γ01 | −0.035 | 0.136 | 0.012 | 0.042 | −0.047* | 0.019 | −2.211 | 3.854 |
| Pubertal status, γ02 | 0.008 | 0.095 | 0.004 | 0.034 | 0.027* | 0.010 | 5.185* | 2.010 |
| Random effects | ||||||||
| σ2ε (L1 residual variance) | 0.358** | 0.058 | 0.088** | 0.026 | 0.009** | 0.001 | 146.681** | 34.816 |
| σ2u0 (L2 residual variance) | 0.261** | 0.093 | 0.012 | 0.007 | 0.002** | 0.001 | 90.383** | 21.944 |
Note 366 days (Level 1) nested within 122 individuals (Level 2). Wake time and prior night stress level within-person centered and all continuous Level 2 predictors grand-mean centered. Non-compliance dummy coded (1 = not compliant with saliva sampling procedures). White race/ethnicity dummy coded (1 = White). Outcomes calculated from log-based cortisol values (μg/dl). Bold indicates significant findings in support of hypotheses
Est. partial regression coefficient estimate, SE robust standard error
p < .10,
p < .05,
p < .01
Alternative Models
We considered several alternative models in order to check the robustness of our findings. First, we examined whether findings involving chronic interpersonal stress were consistent when considering different interview sources (i.e., mothers and daughters separately). Highly similar to results presented in Table 2, adolescents’ chronic interpersonal stress assessed in mother interviews significantly moderated between-person associations of active coping style with the diurnal slope (γ03 = 0.065, p = .002) and CAR (γ03 = −0.218, p < .001). In contrast, chronic interpersonal stress assessed in adolescent interviews did not significantly moderate these associations, but the pattern of interactive effects remained the same (for diurnal slope: γ03 = 0.036, p = .215; for CAR: γ03 = −0.153, p = .132). Given that combining highly correlated ratings of adolescents’ chronic interpersonal stress in the past year from separate mother and daughter interviews followed convention to reduce measurement error (e.g., Rudolph et al. 2000; Stroud et al. 2016) and that we failed to find substantially different findings across interview sources, we remain confident in full results presented in Table 2 and the simple slope plots for interactions presented in Figs. 1 and 2.
Second, we conducted all analyses without three siblings of participants and one participant whose father participated with her in the study (N = 118 vs. 122) to assess whether the possibility for non-independence or characteristics of father as primary caregiver unduly influenced results. All significant findings reported above remained significant when excluding these data. Retaining these individuals in analyses is consistent with prior work using this sample (Stroud et al. 2016).
Finally, we conducted supplementary analyses to examine whether between-person associations of active coping style with diurnal cortisol parameters varied with pubertal status. None of these interactions were significant: waking cortisol (γ03 = −0.098, p = .386), CAR (γ03 = −0.028, p = .344), diurnal slope (γ03 = 0.024, p = .136), and AUCg (γ03 = −2.200, p = .323).
Discussion
A prominent gender gap in the prevalence of major depression and internalizing symptoms begins to emerge during mid-adolescence (Hankin et al. 1998; Wade et al. 2002). Among many factors, interpersonal stress and alterations in HPA axis stress physiology have been identified as potential contributors to the development of adolescent girls’ internalizing psychopathology (Doane et al. 2013; Ge et al. 2001; Klimes-Dougan et al. 2001). Various forms of interpersonal stress grow in salience during adolescence, and these stressors disproportionately impact adolescent girls’ emotional well-being compared to boys (Conley and Rudolph 2009; Hampel and Petermann 2006). Although previous research has demonstrated connections between interpersonal stress (e.g., social exclusion, loneliness) and HPA axis activity during middle childhood and adolescence (e.g., Doane and Adam 2010; Peters et al. 2011), relatively less work has focused on how adolescent girls’ psychological responses to such stressors might promote (or hinder) their physiological regulation. The extent to which early adolescent girls develop adaptive tendencies of responding to stress has been identified as a critical factor in the prevention of stress-related mental and physical health problems (Sontag et al. 2008; Miller et al. 2011). Only more recently have researchers also focused greater attention on the context of stress and coping by considering adolescents’ ability to shift their use of specific coping strategies to meet daily life demands in relation to HPA axis activity (e.g., Sladek et al. 2016).
In the present study, we provided novel contributions to extant literature by examining how individual differences in early adolescent girls’ active coping style and day-to-day (within-person) differences in active coping efforts were associated with daily HPA axis activity, a candidate biological mechanism underlying the link between interpersonal stress and internalizing psychopathology. The identification of such patterns during early adolescence, a theorized “sensitive” developmental period, both socially and biologically (Conley and Rudolph 2009; Graber and Sontag 2009; Stroud et al. 2009), may strengthen existing arguments suggesting interactive effects of stress biology and interpersonal stress in the development of psychopathology. The findings revealed that girls who more likely used voluntary engagement (active) coping in response to interpersonal stress, relative to involuntary responses (assessed via standard self-report survey), exhibited steeper diurnal cortisol slopes, lower total diurnal cortisol output, and lower cortisol awakening responses. The association between this active coping style and steeper diurnal slopes was most pronounced for girls with lower chronic interpersonal stress, whereas the association with lower cortisol awakening response was most pronounced for girls with higher chronic interpersonal stress. Regarding within-person differences (assessed via daily diaries), using active coping strategies more than usual during the day was associated with higher waking cortisol the following morning.
Prior literature has mostly focused on associations between specific forms of interpersonal stress and diurnal cortisol without considering adolescents’ capacity to respond psychologically to these stressors. For example, loneliness and social exclusion have been associated with flatter diurnal cortisol slopes among children and adolescents (Doane and Adam 2010; Peters et al. 2011). Results from the present study complement this prior work by demonstrating that early adolescent girls who more readily respond to interpersonal stress through active coping efforts (assessed with standard survey) tended to exhibit the opposite—steeper slopes—and also lower total cortisol output, indices that generally reflect adaptive physiological stress regulation. These results also complement studies that have shown associations between involuntary responses to stress and cortisol dysregulation (Foland-Ross et al. 2014; Sontag et al. 2008). Further, these findings contribute to the broader gendered perspective on the salient contributions of interpersonal stress and maladaptive coping (e.g., venting, rumination) to the development of depression in adolescent girls (Gore et al. 1993; Nolen-Hoeksema 2001). These findings also have direct implications for health promotion by showing that a voluntary, active coping style is related to adaptive physiological regulation. Overall, our findings suggest adolescent girls should be encouraged to cope actively with increasing interpersonal stress during this sensitive period in development with primary or secondary control strategies (e.g., problem-solving, seeking help from others, acceptance), while working to reduce or minimize involuntary responses (e.g., intrusive thoughts) that may heighten daily cortisol production and contribute to emotional disturbance or other disease outcomes over time.
Importantly, however, the association between active coping style in response to interpersonal stress and steeper diurnal slope was only significant for girls with average and low (but not high) levels of chronic interpersonal stress. This interactive influence of coping and stress is in line with the hypothesized stress buffering role of active coping efforts, which may be limited at higher levels of stress (Grant et al. 2003; Compas et al. 2001). This finding is also consistent with prior literature showing that girls who tend to actively respond when facing interpersonal stress are protected from poor adjustment and psychopathology (Marceau et al. 2014), but such active efforts may not prove as useful under more chronically stressful conditions (Compas et al. 1997). Based on the present findings, messages about using active coping strategies should acknowledge that the most adaptive disposition of responding to chronic stress likely includes some degree of intrapersonal flexibility in which individuals alter responses depending on level and type of stress exposure (Aldao et al. 2015). Other coping styles that do not comprise the usual “adaptive” profile, such as avoidance or other forms of disengagement coping, may be of greater use in facilitating daily physiological regulation for girls facing more chronically stressful conditions (Compas et al. 2001).
Girls with a more active coping style (i.e., greater tendency to use voluntary engagement coping rather than involuntary responses to interpersonal stress) also exhibited a lower cortisol awakening response, on average. Other studies have identified a heightened cortisol awakening response as a potential early risk marker of depression among adolescents (Vrshek-Schallhorn et al. 2013). Importantly, an active coping style in response to interpersonal stress (assessed with standard survey) was associated with a lower cortisol awakening response for girls with average or above average levels of chronic interpersonal stress, suggesting that the specific combination of more active coping tendencies in the presence of chronic interpersonal stress may produce a blunted awakening response. A blunted or flattened cortisol awakening response has been found in individuals facing chronically stressful conditions, such as “burnout” and chronic fatigue (Adam et al. 2006; Saxbe 2008). In the present study, girls who tended to use more active coping strategies in response to interpersonal stress who also had above average levels of chronic interpersonal stress were probably the most likely to routinely engage psychological and physiological resources to meet interpersonal demands. In the short term such responses may be beneficial, but a prolonged pattern of heightened responses can result in HPA hypoactivation over time (Miller et al. 2007). Zahn-Waxler et al. (2000) have argued that promoting emotion regulation and other coping strategies too early in development may encourage some girls to overregulate their behaviors and emotions without learning what types of coping strategies will be effective across situations. The interactive influence of an active coping style and chronic interpersonal stress in predicting a lower cortisol awakening response in the present study highlights the importance of providing developmentally and contextually tailored messages to girls regarding the use of active coping for dealing with interpersonal stress early in adolescence.
Taking advantage of a daily diary approach, within-person analyses revealed that waking cortisol levels were higher following days characterized by greater than usual engagement (active) coping in response to any stressors deemed relevant by the participants (i.e., not specific to interpersonal stress, per se). Utilizing particularly active coping responses to meet an array of daily demands might produce physiological benefits the following day in an adaptive carryover effect. This is consistent with the “boost” hypothesis, which suggests that higher morning cortisol levels (the cortisol awakening response or waking cortisol) are adaptive in the short-term by providing resources to help meet perceived daily demands (Adam et al. 2006). Clinicians and other stakeholders invested in supporting early adolescent girls prior to the initiation of stress-induced health problems should consider how coping functions differently across daily situations, in addition to more general differences between girls. For instance, clinicians should be encouraged to highlight diverse coping skills that adolescent girls can use to manage various stressors, rather than a “one-size-fits-all” approach. Interestingly, there was not a significant day-to-day association between engagement coping and the cortisol awakening response. It is possible that the awakening response is not as necessary in the psychophysiological process of daily preparation when there is already heightened cortisol production immediately upon waking. Consistent with expectations, day-to-day differences in engagement coping were not associated with daily variation in diurnal slope or total diurnal output, indices with relatively greater day-to-day stability (Ross et al. 2014).
This study is not without limitations. First, the sample was small, self-selected, and comprised early adolescent girls who were mostly White. Thus, generalizability may be limited. Future work should replicate these findings in a representative sample of early adolescents from diverse racial and ethnic backgrounds, during other developmental periods, and in boys. For example, examining whether these psychobiological processes are specific to adolescent girls (rather than girls and boys) will be important to further understanding of gender-specific pathways to internalizing symptoms, particularly given the abundance of conceptual models that prominently feature gender and gender-linked processes as risk factors and/or moderators of depression (Nolen-Hoeksema 2001). Second, although stability of average diurnal cortisol estimates may be limited by only three sampling days, protocol compliance was carefully considered using objective measures and diary reports of wake time. Third, the diary measures of perceived stress and coping in this study were not specific to interpersonal stress, and are thus not directly comparable to the survey assessment of coping with interpersonal stress and the interpersonal domain of the life stress interview. Future work should consider including items capturing adolescent girls’ daily interpersonal stressors and associated coping strategies using a diary approach. Finally, causal ordering of between-person associations remains unclear given the cross-sectional design. It is possible that differences in diurnal cortisol patterns somehow influence coping style, or more likely, that psychological and physiological stress responses reciprocally influence one another. For within-person associations, the temporal ordering of diary-reported coping prior to observed cortisol patterns the next day supports this direction of effect, with less support found for cortisol as a predictor of daily coping. A compelling avenue for future research will be to examine how coping early in adolescence impacts later HPA functioning, and more importantly, how subsequent changes in HPA functioning lead to the development of mental and physical health problems over time.
Conclusion
The present study provided unique contributions to the study of psychological and physiological stress and coping processes during early adolescence. Drawing upon the methodological benefits of in-person objective life stress interviews, a daily diary approach, and repeated saliva sampling outside the laboratory, results from multilevel models revealed both individual and day-to-day differences in active coping as predictors of cortisol patterns in the daily lives of early adolescent girls. These results join a growing stress and coping literature that extends biological methods beyond the laboratory to gain valuable insight into the daily landscape of stress responses during adolescence, a sensitive and dynamic period of development coinciding with a marked rise in internalizing symptoms and disorders (Ge et al. 2001; Hankin et al. 1998). This study advances available literature by highlighting both a typically active coping style and specific daily active coping efforts as resources that may allow early adolescent girls to adapt to interpersonal stress and/or prepare them to successfully face such stressors in daily life. These findings should be of interest to developmental scientists interested in how gender, stress, and coping contribute to adjustment, as well as to psychobiologists interested in physiological mechanisms of stress and coping. More importantly, this study joins prior work (e.g., Foland-Ross et al. 2014) in aiming to foster a multi-disciplinary conversation regarding the importance of encouraging adaptive forms of responding to interpersonal stress among adolescent girls. By demonstrating the underlying physiological benefits of these active coping responses (under typical but not chronically stressful conditions), results from the present study should encourage clinicians and other stakeholders (e.g., schools, healthcare providers) invested in using evidence-based forms of stress-related health promotion, which already include teaching effective coping skills (e.g., cognitive behavioral therapy). It is particularly critical to focus prevention efforts early in adolescence prior to the emergence of many long-term health problems and before adolescents develop unhealthy patterns of coping with interpersonal stress.
Acknowledgments
We thank the families who generously gave their time to participate in this project, the staff of the Williams College Youth Emotion Center, and Andrea Gierens at Biochemisches Labor at the University of Trier for technical assistance with salivary assays.
Funding This research was supported by institutional funds from Williams College. M.R.S. was supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1311230 and L.D.D. was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD079520 and a William T. Grant Foundation Scholars Award. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the funding institutions.
Biographies
Michael R. Sladek, M.A. is a doctoral student in Developmental Psychology at Arizona State University. His major research interests include social and emotional development during adolescence and young adulthood. He is interested in better understanding daily psychological and physiological stress and coping processes that have implications for adolescents’ health and well-being.
Leah D. Doane, Ph.D. is an Associate Professor of Psychology at Arizona State University. She received her doctorate in Human Development and Social Policy from Northwestern University’s School of Education and Social Policy. Her major research interests include adolescent and emerging adult development, particularly with regard to identifying and understanding psychophysiological mechanisms underlying adolescent and young adult everyday stress experiences in naturalistic settings.
Catherine B. Stroud, Ph.D. is an Assistant Professor of Psychology at Williams College. She received her doctorate in Clinical Psychology from State University of New York, Stony Brook. Her research examines stress, interpersonal relationships, and psychopathology, with a particular focus on adolescent depression.
Footnotes
Author Contributions M.R.S. performed the statistical analyses and drafted the manuscript; L.D.D. participated in the design of the larger project, consulted on the salivary cortisol collection, and helped draft the manuscript; C.B.S. designed the larger project, led and coordinated data collection, and helped draft the manuscript. All authors conceived of the study, contributed to hypothesis generation, responded to peer reviews during the revision process, and read and approved the final manuscript.
Conflict of Interest The authors declare that they have no competing interests.
Ethical Approval All procedures performed in this study involving human participants were in accordance with the ethical standards of the Institutional Review Board at Williams College, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Participants and their primary caregivers completed assent and consent forms, respectively, during a laboratory visit prior to participation.
Portions of these data were presented at the 2016 biennial meeting of the Society for Research on Adolescence and the 2016 annual meeting of the Association for Cognitive and Behavioral Therapies.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34(10):1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Aldao A, Sheppes G, Gross JJ. Emotion regulation flexibility. Cognitive Therapy and Research. 2015;39:263–278. [Google Scholar]
- Almeida DM. Resilience and vulnerability to daily stressors assessed via diary methods. Current Directions in Psychological Science. 2005;14(2):64–68. [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology. 2009;80(3):265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: More than a measure of HPA axis function. Neuroscience & Biobehavioral Reviews. 2010;35(1):97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Compas BE, Connor JK, Osowiecki D, Welch A. Effortful and involuntary responses to stress: Implications for coping with chronic stress. In: Gottlieb BH, editor. Coping with chronic stress. New York: Plenum; 1997. pp. 105–130. [Google Scholar]
- Compas BE, Connor-Smith JK, Saltzman H, Thomsen AH, Wadsworth ME. Coping with stress during childhood and adolescence: Problems, progress, and potential in theory and research. Psychological Bulletin. 2001;127(1):87–127. [PubMed] [Google Scholar]
- Conley CS, Rudolph KD. The emerging sex difference in adolescent depression: Interacting contributions of puberty and peer stress. Development and Psychopathology. 2009;21(02):593–620. doi: 10.1017/S0954579409000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor-Smith JK, Compas BE, Wadsworth ME, Thomsen AH, Saltzman H. Responses to stress in adolescence: Measurement of coping and involuntary stress responses. Journal of Consulting and Clinical Psychology. 2000;68(6):976–992. [PubMed] [Google Scholar]
- de Kloet ER. Hormones and the stressed brain. Annals of the New York Academy of Sciences. 2004;1018(1):1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- DeLongis A, Holtzman S. Coping in context: The role of stress, social support, and personality in coping. Journal of Personality. 2005;73(6):1633–1656. doi: 10.1111/j.1467-6494.2005.00361.x. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doane LD, Adam EK. Loneliness and cortisol: Momentary, day-to-day, and trait associations. Psychoneuroendocrinology. 2010;35(3):430–441. doi: 10.1016/j.psyneuen.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Zeiders KH. Contextual moderators of momentary cortisol and negative affect in adolescents’ daily lives. Journal of Adolescent Health. 2014;54(5):536–542. doi: 10.1016/j.jadohealth.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Doane LD, Mineka S, Zinbarg RE, Craske M, Griffith JW, Adam EK. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Development and Psychopathology. 2013;25(3):629–642. doi: 10.1017/S0954579413000060. [DOI] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemistry. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Dumont M, Provost MA. Resilience in adolescents: Protective role of social support, coping strategies, self-esteem, and social activities on experience of stress and depression. Journal of Youth and Adolescence. 1999;28:343–363. [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychological Methods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Flynn M, Rudolph KD. Perceptual asymmetry and youths’ responses to stress: Understanding vulnerability to depression. Cognition and Emotion. 2007;21(4):773–788. doi: 10.1080/02699930600824635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn M, Rudolph KD. The contribution of deficits in emotional clarity to stress responses and depression. Journal of Applied Developmental Psychology. 2010;31(4):291–297. doi: 10.1016/j.appdev.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Kircanski K, Gotlib IH. Coping with having a depressed mother: The role of stress and coping in hypothalamic-pituitary-adrenal axis dysfunction in girls at familial risk for major depression. Development and Psychopathology. 2014;26:1401–1409. doi: 10.1017/S0954579414001102. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001;37(3):404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Gore S, Aseltine RH, Jr, Colten ME. Gender, social-relationship involvement, and depression. Journal of Research on Adolescence. 1993;3(2):101–125. [Google Scholar]
- Graber JA, Sontag LM. Internalizing problems during adolescence. In: Lerner R, Steinberg L, editors. Handbook of adolescent psychology. 3rd. Vol. 1. New York: Wiley; 2009. pp. 3–14. (Individual bases of adolescent development). Ch. 1. [Google Scholar]
- Grant KE, Compas BE, Stuhlmacher AF, Thurm AE, McMahon SD, Halpert JA. Stressors and child and adolescent psychopathology: Moving from markers to mechanisms of risk. Psychological Bulletin. 2003;129(3):447–466. doi: 10.1037/0033-2909.129.3.447. [DOI] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hampel P, Petermann F. Perceived stress, coping, and adjustment in adolescents. Journal of Adolescent Health. 2006;38(4):409–415. doi: 10.1016/j.jadohealth.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107(1):128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Herman-Stahl M, Petersen AC. The protective role of coping and social resources for depressive symptoms among young adolescents. Journal of Youth and Adolescence. 1996;25(6):733–753. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rau U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–987. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13(03):695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosomatic Medicine. 2003;65(2):313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York, NY: Springer Publishing Company; 1984. [Google Scholar]
- Malooly AM, Flannery KM, Ohannessian CM. Coping mediates the association between gender and depressive symptomatology in adolescence. International Journal of Behavioral Development. doi: 10.1177/0165025415616202. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Zahn-Waxler C, Shirtcliff EA, Schreiber JE, Hastings P, Klimes-Dougan B. Adolescents’, mothers’, and fathers’ gendered coping strategies during conflict: Youth and parent influences on conflict resolution and psychopathology. Development and Psychopathology. 2014;27:1025–1044. doi: 10.1017/S0954579415000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving towards a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137(6):959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. Seventh. Los Angeles, CA: Muthén & Muthén; 1998. 2012. [Google Scholar]
- Nicolson NA. Measurement of cortisol. In: Luecken LJ, Gallo LC, editors. Handbook of physiological research methods in health psychology. New York, NY: Sage Publications; 2008. pp. 37–74. [Google Scholar]
- Nolen-Hoeksema S. Gender differences in depression. Current Directions in Psychological Science. 2001;10(5):173–176. [Google Scholar]
- Obradović J. How can the study of physiological reactivity contribute to our understanding of adversity and resilience processes in development? Development and Psychopathology. 2012;24:371–387. doi: 10.1017/S0954579412000053. [DOI] [PubMed] [Google Scholar]
- Peters E, Riksen-Walraven JM, Cillessen AHN, de Weerth C. Peer rejection and HPA activity in middle childhood: Friendship makes a difference. Child Development. 2011;82(6):1906–1920. doi: 10.1111/j.1467-8624.2011.01647.x. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Ptacek JT, Smith RE, Espe K, Raffety B. Limited correspondence between daily coping reports and retrospective coping recall. Psychological Assessment. 1994;6(1):41–49. [Google Scholar]
- Rohde P, Beevers CG, Stice E, O’Neil K. Major and minor depression in female adolescents: Onset, course, symptom presentation, and demographic associations. Journal of Clinical Psychology. 2009;65(12):1339–1349. doi: 10.1002/jclp.20629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KM, Murphy ML, Adam EK, Chen E, Miller GE. How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology. 2014;39:184–193. doi: 10.1016/j.psyneuen.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Development. 1999;70:660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, Burge D, Lindberg N, Herzberg D, Daley SE. Toward an interpersonal life-stress model of depression: The developmental context of stress generation. Development and Psychopathology. 2000;12:215–234. doi: 10.1017/s0954579400002066. [DOI] [PubMed] [Google Scholar]
- Saxbe DE. A field (researcher’s) guide to cortisol: Tracking HPA axis functioning in everyday life. Health Psychology Review. 2008;2(2):163–190. [Google Scholar]
- Schwartz JE, Neale J, Marco C, Shiffman SS, Stone AA. Does trait coping exist? A momentary assessment approach to the evaluation of traits. Journal of Personality and Social Psychology. 1999;77(2):360–369. doi: 10.1037//0022-3514.77.2.360. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50(7):690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek MR, Doane LD, Luecken LJ, Eisenberg N. Perceived stress, coping, and cortisol reactivity in daily life: A study of adolescents during the first year of college. Biological Psychology. 2016;117:8–15. doi: 10.1016/j.biopsycho.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatcher RB, Chi P, Li X, Zhao J, Zhao G, Ren X, et al. Associations between coping and diurnal cortisol among children affected by parental HIV/AIDS. Health Psychology. 2015;34(8):802–810. doi: 10.1037/hea0000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: A psychobiological model of social rejection and depression. Neuroscience & Biobehavioral Reviews. 2010;35(1):39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Leffingwell TR, Ptacek JT. Can people remember how they coped? Factors associated with discordance between same-day and retrospective reports. Journal of Personality and Social Psychology. 1999;76(6):1050–1061. [Google Scholar]
- Sontag LM, Graber JA, Brooks-Gunn J, Warren MP. Coping with social stress: Implications for psychopathology in young adolescent girls. Journal of Abnormal Child Psychology. 2008;36(8):1159–1174. doi: 10.1007/s10802-008-9239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr LR, Stroud CB, Li YI. Predicting the transition from anxiety to depressive symptoms in early adolescence: Negative anxiety response style as a moderator of sequential comorbidity. Journal of Affective Disorders. 2016;190:757–763. doi: 10.1016/j.jad.2015.10.065. [DOI] [PubMed] [Google Scholar]
- Stroud CB, Chen FR, Doane LD, Granger DA. Individual differences in early adolescents’ latent trait cortisol (LTC): Relation to recent acute and chronic stress. Psychoneuroendocrinology. 2016;70:38–46. doi: 10.1016/j.psyneuen.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology. 2009;21(01):47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: Social rejection versus achievement stress. Biological Psychiatry. 2002;52(4):318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Tennen H, Affleck G, Armeli S, Carney MA. A daily process approach to coping: Linking theory, research, and practice. American Psychologist. 2000;55(6):626–636. doi: 10.1037//0003-066x.55.6.626. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg RE, Craske MG, Adam EK. The cortisol awakening response predicts major depression: Predictive stability over a 4-year follow-up and effect of depression history. Psychological Medicine. 2013;43(03):483–493. doi: 10.1017/S0033291712001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TJ, Cairney J, Pevalin DJ. Emergence of gender differences in depression during adolescence: National panel results from three countries. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(2):190–198. doi: 10.1097/00004583-200202000-00013. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Klimes-Dougan B, Slattery MJ. Internalizing problems of childhood and adolescence: Prospects, pitfalls, and progress in understanding the development of anxiety and depression. Development and Psychopathology. 2000;12:443–466. [PubMed] [Google Scholar]


