Abstract
Context:
The most common genetic cause of permanent neonatal diabetes mellitus is activating mutations in KCNJ11, which can usually be treated using oral sulfonylureas (SUs) instead of insulin injections, although some mutations are SU unresponsive. In this work, we provide a report of the pancreatic islet endocrine cell composition and area in a patient with an SU-unresponsive KCNJ11 mutation (p.G334D), in comparison with age-matched controls.
Case Description:
Pancreatic autopsy tissue sections from a 2-year-old female child diagnosed with KCNJ11-related diabetes at 4 days of age and 13 age-matched controls were stained with insulin, glucagon, somatostatin, pancreatic polypeptide, and Ki67 antibodies to determine islet endocrine cell composition and area. β-cell ultrastructure was assessed by electron microscopic (EM) analysis. The patient’s pancreas (sampling from head to tail) revealed insulin-positive cells in all regions. The pancreatic β-cell (insulin) area was significantly reduced compared with controls: 0.50% ± 0.04% versus 1.67% ± 0.20%, respectively (P < 0.00001). There were no significant differences in α-cell (glucagon) or δ-cell (somatostatin) area. EM analysis revealed secretory granules with a dense core typical of mature β-cells as well as granules with a lighter core characteristic of immature granules.
Conclusions:
Our results suggest that mechanisms exist that allow preservation of β-cells in the absence of insulin secretion. It remains to be determined to what extent this reduction in β-cells may be reversible.
Précis: We examined pancreatic islet composition from a deceased patient with SU-unresponsive KCNJ11-related diabetes compared with 13 age-matched control specimens and found reduced insulin-positive cells.
Activating mutations in the genes (KCNJ11 and ABCC8) encoding the adenosine triphosphate–sensitive potassium (KATP) channel are the most common causes of permanent neonatal diabetes (1). Most of these patients can be successfully treated with high doses of oral sulfonylureas (SUs) that close KATP channels and restore insulin secretion. However, rare mutations are SU unresponsive, and these patients require lifelong exogenous insulin injections.
It has not previously been possible to study the structure of the pancreatic islets and β-cells in patients with KCNJ11-related diabetes. It has thus been unclear whether β-cells that are incapable of insulin secretion continue to synthesize insulin and what might be the fate and distribution of such cells. In this study, we report islet cell composition in the first autopsy case of a patient with SU-unresponsive KCNJ11-related diabetes in comparison with 13 age-matched control specimens.
Subjects and Methods
A full-term female baby presented at 4 days of life due to poor feeding and was found to have diabetes. C-peptide was <0.05 ng/mL, and genetic testing revealed the strongly activating p.G334D mutation in KCNJ11. The only other reported case with this mutation was nonambulatory and nonverbal at 13 years old and was SU unresponsive (2). Our patient was treated with insulin therapy (1.0 to 1.6 U/kg/d) from diagnosis until her death with reasonable glycemic control (HbA1c < 8.0%). She was enrolled in the Institutional Review Board–approved Monogenic Diabetes Registry through which detailed longitudinal clinical information is collected (3). She exhibited severe global developmental delay and was started on glyburide at 2 months of age. Despite no reduction of exogenous insulin requirements, she was initially continued on SU therapy because of theoretical potential improvement in her neurodevelopmental outcome, but this was discontinued by 22 months. Mixed meal tests performed at 8 months before/after 5 days of treatment with high-dose glyburide (1.8 mg/kg/d) revealed barely detectable C-peptides at 180 minutes both before and after glyburide (0.034 and 0.04 nmol/mL; assay limit 0.0331). She had been making slow developmental progress, including being able to stand on her own. At 24 months of age, she was found unresponsive during a nap (glucose level 220 mg/dL), and death was presumed due to central hypoventilation (previously demonstrated by polysomnography). With permission from the family, the patient’s body was preserved by the medical examiner at 4°C until postmortem examination approximately 60 hours following her death. The pancreas was fixed in formalin and had a total weight of 20 g, which is within expectations for age based on imaging studies of pancreas volume (4, 5).
Paraffin-embedded sections (5 µm) of the patient and 13 control subjects [7 males and 6 females, age range 16 to 28 months (average 22.4 ± 1.0 months) (Supplemental Table 1 (30.2KB, docx) ) obtained from the Biospecimen Bank and exempted from review by the Institutional Review Board at the University of Chicago] were stained with the indicated antibodies (Supplemental Table 2 (58.9KB, docx) ). Supplemental Table 1 (30.2KB, docx) includes all available information about the control samples, including clinical details, cause of death, and postmortem time, but information regarding the region of the pancreas from which each sample was taken is unfortunately not available.
Microscopic images were taken with an Olympus IX8 DSU spinning disk confocal microscope (Melville, NY) with imaging software StereoInvestigator (MicroBrightField, Williston, VT). Quantification of cellular composition of each islet and islet cell area was carried out using a macro custom written for Fiji/ImageJ (http://rsbweb.nih.gov/ij/) (6). MATLAB (MathWorks, Natick, MA) was used for mathematical analyses.
For electron microscopic (EM) analysis, tissue was fixed with 4% paraformaldehyde and 0.02% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) and embedded in resin. Ultrathin sections (80 nm) were stained with uranyl acetate and lead citrate. Images were taken using a Tecnai F30 microscope (FEI, Hillsboro, OR).
Results
The pancreas from the KCNJ11-related diabetes patient was divided into 11 blocks; sections from each block were stained for insulin (β-cells), glucagon (α-cells), somatostatin (δ-cells), and nuclei (4′,6-diamidino-2-phenylindole); and images were captured for large-scale computer-assisted quantification of endocrine cell area. An adjacent section was stained for insulin, glucagon, pancreatic polypeptide (PP) cells, and nuclei. Despite the 60 hours that elapsed prior to autopsy, the pancreas was histologically preserved, with virtually all sections revealing parenchyma that was nonautolyzed.
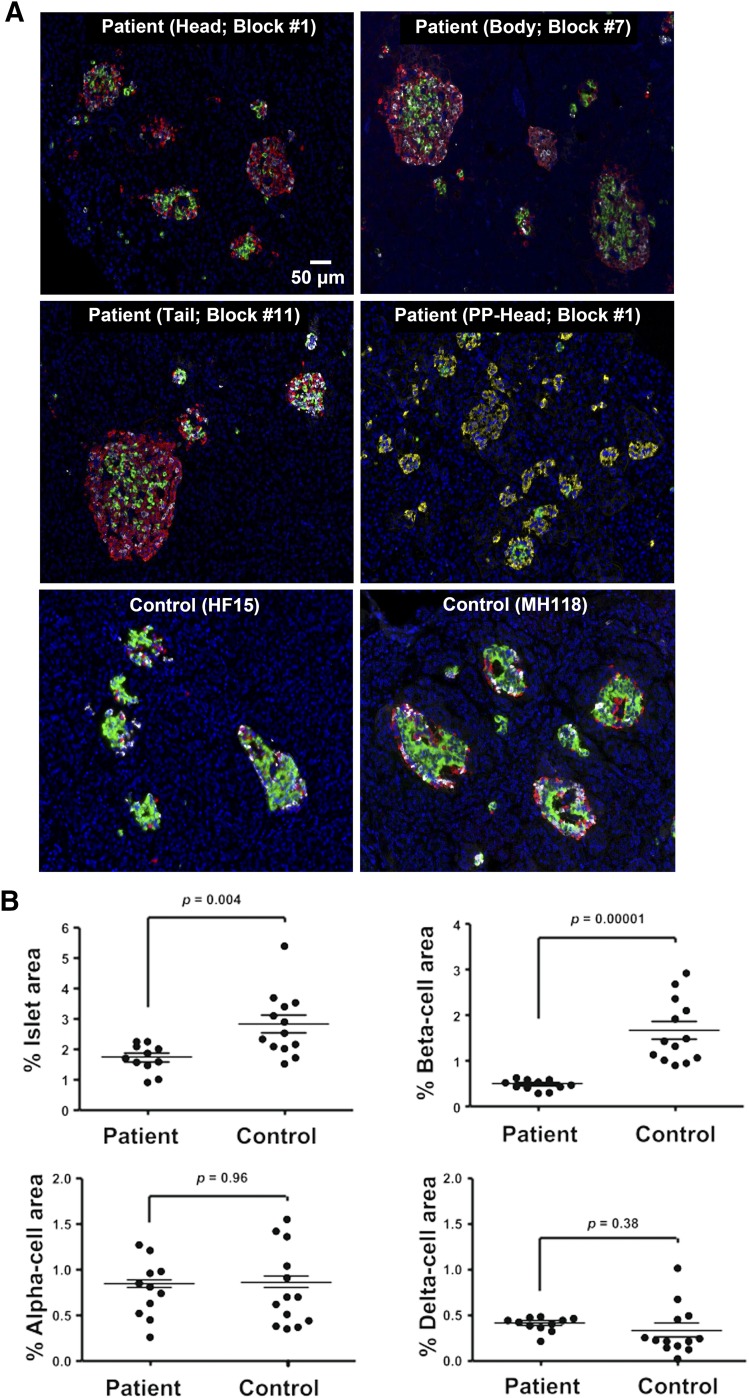
The islets in the patient had a thick mantle of α- and δ-cells but fewer β-cells in the core, whereas in control subjects the architecture was typical of large islets with mixed endocrine cell composition [Fig. 1(A)]. Insulin-, glucagon-, and somatostatin-positive cells were present in all regions (head, body, and tail) of the pancreas (Supplemental Fig. 1 (50.9MB, tif) ). PP cells were localized primarily to the head region, as noted in adults (6). None of the cells that stained for insulin were observed to also stain for any of the other hormones (glucagon, somatostatin, or PP).
Figure 1.
Histology in a 2-year-old female patient with KCNJ11 mutation p.G334D. (A) Representative islets from the head, body, and tail regions of the pancreas from the patient and two representative age-matched control subjects stained for insulin (green), glucagon (red), somatostation (white), and 4′,6-diamidino-2-phenylindole (blue). An additional panel (PP-Head) from the patient is stained for the pancreatic polypeptide (yellow), insulin (green), glucagon (white), and 4′,6-diamidino-2-phenylindole (blue). (B) Endocrine cell area. The percentage of pancreatic area that is islets, β-cells, α-cells, and δ-cells is shown for all 11 blocks in the patient and one section from each of the 13 age-matched control subjects. Horizontal lines represent mean ± SEM.
The fraction of pancreatic area comprised by the different endocrine cell types was averaged (based on the analysis of the 11 blocks in the patient) and compared with the averages of one section each from 13 age-matched controls [Fig. 1(B); Supplemental Fig. 2 (20.7MB, tif) ; Supplemental Table 1 (30.2KB, docx) ]. The islet area in the patient was reduced by 39% compared with controls (1.70% ± 0.14% versus 2.80% ± 0.30%; P = 0.004). There was a 70% reduction in β-cell area compared with controls (0.50% ± 0.04% versus 1.67% ± 0.20%; P < 0.00001). There was no significant difference in α- or δ-cell area between the patient and control samples (α-cells, 0.81% ± 0.10% versus 0.82% ± 0.12%, P = 0.96; δ-cells, 0.42% ± 0.03% versus 0.34 ± 0.08%, P = 0.38).
The contribution of large islets to the total endocrine cell area was reduced in the patient compared with the controls with large islets (>85 µm in diameter) comprising 40% of the total endocrine cell area in the patient and 70% in the controls (Supplemental Fig. 3 (3.6MB, tif) ). A reduction in β-cells was observed in all islet size classes in the patient compared with the controls. Both the proportion and total number of Ki67-positive cells were reduced compared with controls (Supplemental Fig. 4 (7.7MB, tif) ), in contrast to a reported increase in similarly aged children (7).
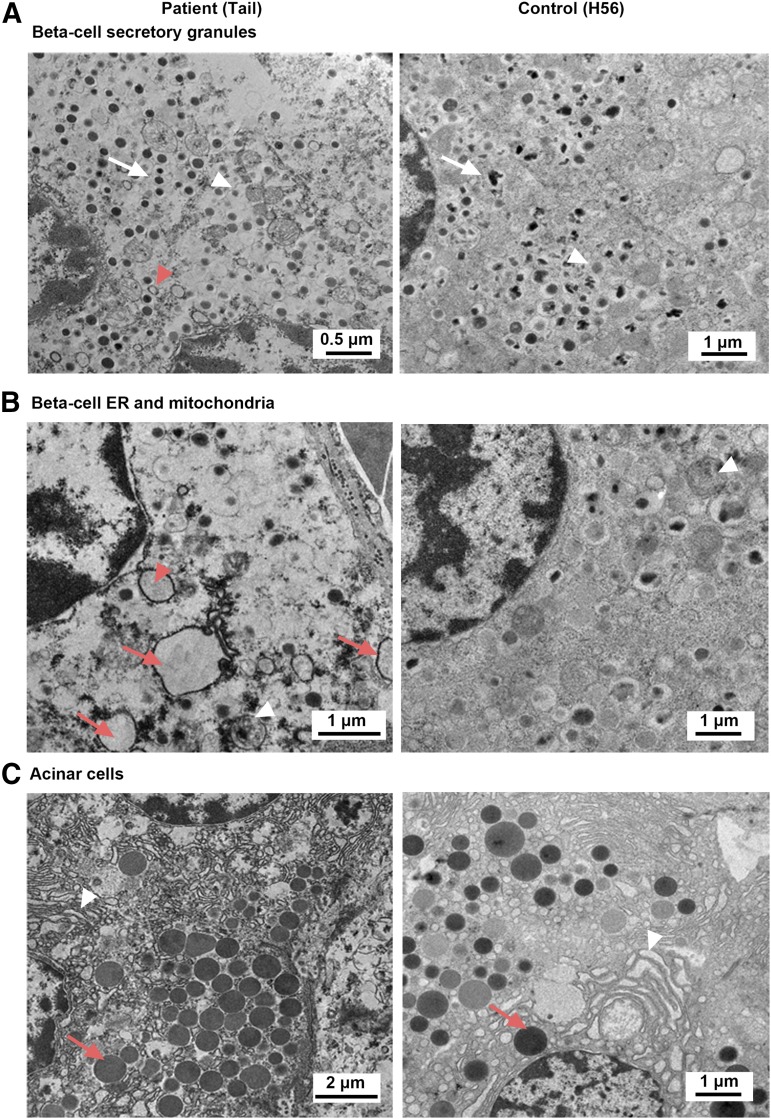
Ultrastructural analysis revealed mostly normal features, with increased altered β-cell morphology, including immature granules (light dense core), empty granules, and reduced crystalline cores (Fig. 2), in the patient compared with the control. In contrast to rodents, human insulin granules are present in various forms, including crystallines. The nuclei showed normal morphology in the patient and the control. Some β-cells from the patient showed dilated rough endoplasmic reticulum as well as partially degenerated mitochondrion, whereas no such structures were observed in the control [Fig. 2(B)]. We saw no evidence of apoptosis, such as aggregation of nuclear material or condensed chromatin.
Figure 2.
EM analysis. (A) EM ultramicrograph of a β-cell from patient and control showing the morphology of the secretory granules. Ultrastructural analysis revealed altered morphology of β-cells in the patient with immature granules (light dense core, white arrowhead), empty granules (red arrowhead), and reduced crystalline cores in the patient (white arrow) compared with the control. Note that nuclei showed normal morphology in both panels. (B) Endoplasmic reticulum and mitochondria structure in patient and control. A deteriorated β-cell contained several dilated rough endoplasmic reticulum (ER, red arrow) that were filled with flocculent material (presumably protein). One close to a nucleus (red arrowhead) appears to be directly attached to a perinuclear membrane and an underlying perinuclear space, likely to represent the conduit of protein production. A partially degenerated mitochondrion is seen in the patient (white arrowhead), whereas all mitochondria in the control appear normal (white arrowhead). (C) Pancreatic acinar tissue in patient and control. Zymogen granules (red arrow) appear normal in both patient and control.
Discussion
We report the histological analysis of a pancreas from a patient with KCNJ11-related diabetes and show that β-cells are still present but are substantially reduced in number compared with age-matched controls. Our results showing 70% decrease in β-cells are in agreement with two different mouse models of KCNJ11-related diabetes (8, 9) and a 3-week-old case with ABCC8-related diabetes (10) that reported a similar reduction in β-cells. We did not observe an increase in glucagon area as described in the ABCC8 patient. EM analysis suggested possible abnormalities of mitochondria and endoplasmic reticulum; however, despite the lack of tissue autolysis, we cannot definitively conclude whether these features are related to the postmortem time or represent KCNJ11 mutation-related pathology, as study of more cases would be necessary. Similar to a mouse model of KCNJ11-related diabetes that used EM, we did not find evidence of apoptosis (8).
This histopathological description of KCNJ11-related diabetes suggests an explanation for why some cases have been shown to be able to respond to SU therapy even after decades of exogenous insulin treatment (11). Our study provides the fascinating insight that β-cells remain present, albeit at a reduced number, even in the unusual situation in which they are unable to secrete insulin. Some studies have suggested that hyperpolarization of β-cells with agents such as diazoxide (as opposed to the presumed chronic hyperpolarization in our case due to overactive KATP channels) may protect islet cells from apoptosis (12). It remains unclear to what extent the reduction in β-cell mass may result from increased β-cell death, reduced replication (as suggested from our reduced Ki67 staining), or reduced intracellular insulin production in β-cells that may undergo dedifferentiation or transdifferentiation (13, 14). Although a mouse model was characterized by double-positive cells staining for both insulin and glucagon that were restored to normal by exogenous insulin treatment (8), we could not find double-positive cells in our patient. Overall, the reasons for the significant decrease in the number of detectable β-cells that we describe in this work are unclear, but may result from combined effects of ongoing hyperglycemia (which itself is harmful for β-cells) and/or other signals that upregulate cellular machinery that produces insulin that cannot be secreted, or the effects of hyperpolarization that may lead to decreased insulin synthesis as a result of chronically low intracellular concentrations of calcium. Further study of patients with this rare form of diabetes may clarify factors that lead to a reduction in functional β-cell mass in other forms of diabetes.
Acknowledgments
The authors are truly grateful to the family of this patient who graciously offered her organs for study in the hopes that doing so might benefit other patients in the future.
Acknowledgments
This work was supported by National Institutes of Health Grants P30DK020595, K23DK094866, R03AG042151, R03DK103096, R01DK104942, and UL1TR000430, as well as the American Diabetes Association (1-11-CT-41) and a gift from the Kovler Family Foundation.
Acknowledgments
Author contributions: S.A.W.G., D.C., D.F.S., L.H.P., J.R.W., and M.H. conceived and designed the study. S.A.W.G. and J.R.W. provided data and facilitated sample collection. M.C.Z., A.P., S.B., and M.H. performed the experiments. M.C.Z., A.P., H.Y., D.F.S., J.B.T., and M.H. performed the data analysis. S.A.W.G., D.C., and M.H. wrote the manuscript. All authors reviewed/edited the manuscript and approved the final version (except for D.F.S., who approved an earlier version prior to his death).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Deceased.
- EM
- electron microscopic
- KATP
- adenosine triphosphate–sensitive potassium
- PP
- pancreatic polypeptide
- SU
- sulfonylurea
References
- 1.Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert J-J, Holst JJ, Clark PM, Ellard S, Søvik O, Polak M, Hattersley AT; Neonatal Diabetes International Collaborative Group . Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355(5):467–477. [DOI] [PubMed] [Google Scholar]
- 2.Masia R, Koster JC, Tumini S, Chiarelli F, Colombo C, Nichols CG, Barbetti F. An ATP-binding mutation (G334D) in KCNJ11 is associated with a sulfonylurea-insensitive form of developmental delay, epilepsy, and neonatal diabetes. Diabetes. 2007;56(2):328–336. [DOI] [PubMed] [Google Scholar]
- 3.Greeley SAW, Naylor RN, Cook LS, Tucker SE, Lipton RB, Philipson LH. Creation of the Web-based University of Chicago Monogenic Diabetes Registry: using technology to facilitate longitudinal study of rare subtypes of diabetes. J Diabetes Sci Technol. 2011;5(4):879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saisho Y, Butler AE, Meier JJ, Monchamp T, Allen-Auerbach M, Rizza RA, Butler PC. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat. 2007;20(8):933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier JM, Alavi A, Iruvuri S, Alzeair S, Parker R, Houseni M, Hernandez-Pampaloni M, Mong A, Torigian DA. Assessment of age-related changes in abdominal organ structure and function with computed tomography and positron emission tomography. Semin Nucl Med. 2007;37(3):154–172. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Zielinski MC, Misawa R, Wen P, Wang T-Y, Wang C-Z, Witkowski P, Hara M. Quantitative analysis of pancreatic polypeptide cell distribution in the human pancreas. PLoS One. 2013;8(1):e55501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab. 2012;97(9):3197–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brereton MF, Iberl M, Shimomura K, Zhang Q, Adriaenssens AE, Proks P, Spiliotis II, Dace W, Mattis KK, Ramracheya R, Gribble FM, Reimann F, Clark A, Rorsman P, Ashcroft FM. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat Commun. 2014;5:4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remedi MS, Kurata HT, Scott A, Wunderlich FT, Rother E, Kleinridders A, Tong A, Brüning JC, Koster JC, Nichols CG. Secondary consequences of beta cell inexcitability: identification and prevention in a murine model of K(ATP)-induced neonatal diabetes mellitus. Cell Metab. 2009;9(2):140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busiah K, Verkarre V, Cavé H, Scharfmann R, Polak M. Human pancreas endocrine cell populations and activating ABCC8 mutations. Horm Res Paediatr. 2014;82(1):59–64. [DOI] [PubMed] [Google Scholar]
- 11.Thurber BW, Carmody D, Tadie EC, Pastore AN, Dickens JT, Wroblewski KE, Naylor RN, Philipson LH, Greeley SAW; United States Neonatal Diabetes Working Group . Age at the time of sulfonylurea initiation influences treatment outcomes in KCNJ11-related neonatal diabetes. Diabetologia. 2015;58(7):1430–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Wang S, Harvat T, Kinzer K, Zhang L, Feng F, Qi M, Oberholzer J. Diazoxide, a K(ATP) channel opener, prevents ischemia-reperfusion injury in rodent pancreatic islets. Cell Transplant. 2015;24(1):25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cinti F, Bouchi R, Kim-Muller JY, Ohmura Y, Sandoval PR, Masini M, Marselli L, Suleiman M, Ratner LE, Marchetti P, Accili D. Evidence of β-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab. 2016;101(3):1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler AE, Dhawan S, Hoang J, Cory M, Zeng K, Fritsch H, Meier JJ, Rizza RA, Butler PC. β-cell deficit in obese type 2 diabetes, a minor role of β-cell dedifferentiation and degranulation. J Clin Endocrinol Metab. 2016;101(2):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]