Abstract
Context:
Daughters of women with polycystic ovary syndrome (PCOS) are thought to be at increased risk for developing stigmata of the syndrome, but the ontogeny during puberty is uncertain.
Objective:
We phenotyped daughters (n = 76) of mothers with PCOS and daughters (n = 80) from control mothers for reproductive and metabolic parameters characteristic of PCOS.
Design, Setting, and Participants:
We performed a matched case/control study at Penn State Hershey Medical Center that included non-Hispanic, white girls 4 to 17 years old.
Intervention:
We obtained birth history, biometric, ovarian ultrasounds, whole-body dual-energy X-ray absorptiometry scan for body composition, 2-hour glucose challenged salivary insulin levels, and two timed urinary collections (12 hours overnight and 3 hours in the morning) for gonadotropins and sex steroids.
Main Outcome Measures:
We measured integrated urinary levels of adrenal (dehydroepiandrosterone sulfate) and ovarian [testosterone (TT)] steroids. Other endpoints included integrated salivary insulin levels and urinary luteinizing hormone levels.
Results:
There were no differences in detection rates or mean levels for gonadotropins and sex steroids in timed urinary collections between PCOS daughters and control daughters, nor were there differences in integrated salivary insulin levels. Results showed that 69% of Tanner 4/5 PCOS daughters vs 31% of control daughters had hirsutism defined as a Ferriman-Gallwey score >8 (P = 0.04). There were no differences in body composition as determined by dual-energy X-ray absorptiometry between groups in the three major body contents (i.e., bone, lean body mass, and fat) or in ovarian volume between groups.
Conclusions:
Matched for pubertal stage, PCOS daughters have similar levels of urinary androgens and gonadotropins as well as glucose-challenged salivary insulin levels.
Précis: We conclude that daughters of women with PCOS appear to have a reproductive and metabolic profile across the pubertal transition that is very similar to that of daughters of control mothers.
The ontogeny of polycystic ovary syndrome (PCOS) is poorly understood, although it may manifest during puberty with the development of signs of androgen excess, such as acne or hirsutism, and with infrequent menstrual cycles owing to chronic anovulation that persist after menarche (1). However, normal puberty is also characterized by a relative androgen excess (compared with prepuberty), with the awakening of the adrenal glands and the ovaries, the development of acne (which is multifactorial in etiology), and a prolonged time of irregular menses after menarche up to 1 to 2 years (2). Furthermore, normal puberty is characterized by the development of hyperinsulinemia and weight gain; these changes have also been implicated in the development of PCOS. Our work and the work of others has suggested that insulin resistance and the resulting hyperinsulinemia may be exacerbated during puberty in daughters of mothers with PCOS mothers and precede the development of the later reproductive signs and symptoms of PCOS (3). Interventions that ameliorate these metabolic abnormalities have been shown to slow or improve the development of reproductive signs and symptoms of PCOS (4).
There is a strong familial component to PCOS (5, 6). For a daughter of a woman with PCOS, a shared environment, including the intrauterine environment (7), and potentially select genes may increase the risk of developing signs and symptoms of PCOS. Recent genome-wide association studies in women with PCOS have highlighted the important role of genetic variants associated with gonadotropin action in the disorder (8–10). We sought to replicate our previous study (3) and to gain insights into the ontogeny of PCOS in daughters of women with PCOS. We hypothesized that there was a pubertal related increase in adrenal and ovarian androgens and in hyperinsulinism in daughters of women with PCOS compared with control subjects.
Materials and Methods
Subjects
A matched case-control study was conducted to examine daughters of women with and without PCOS. The definition of PCOS in mothers was based on the 1990 National Institutes of Health criteria of unexplained hyperandrogenic chronic anovulation (11). Mothers with PCOS had a history of chronic anovulation defined as intermenstrual period of ≥45 days or a total of ≤8 menses per year, and hyperandrogenism was defined either by hirsutism or hyperandrogenemia, which is defined by an elevated total or bioavailable serum TT (total TT ≥50 ng/dL or bioavailable T ≥10 ng/dL) (5, 12). Secondary causes of oligomenorrhea, including thyroid disease, prolactin excess, and congenital adrenal hyperplasia, were excluded.
Inclusion criteria for control mothers were designed to identify women without hyperandrogenism or aberrant insulin action. They were required to have a history of regular menstrual cycles (10 to 14 cycles per year), no history of hyperandrogenism (including hirsutism or androgenic alopecia), no personal or family history of type 2 diabetes (first-degree relatives), no sister or mother with PCOS, normal circulating androgen levels as noted above (i.e., total TT ≤50 ng/dL and bioavailable TT ≤10 ng/dL) (5), normal levels of 17 OH progesterone (i.e., a morning level <2 ng/mL) or a normal adrenocorticotropic hormone stimulation test if above that level, no suspicion of Cushing syndrome, and no current use of confounding medications that could affect steroid levels.
Daughters were age 4 to 17 years and Tanner stage 1 to 5 based on pubic hair distribution (2) and were the products of term delivery (≥36 weeks) singleton gestations. We did not study daughters with a preexisting diagnosis of type 1 or 2 diabetes or with a known history of PCOS in the sisters or mother of the father of the child. Daughters were not on any medications that could affect hormonal levels or glucose tolerance, such as GnRH agonists, hormonal contraceptives, insulin-sensitizing agents, statins, and many antihypertensives. We did not study daughters conceived through in vitro fertilization; nor did we study daughters of control women who themselves were conceived with the use of clomiphene citrate (because the indication may have been oligo-ovulation and latent PCOS). We studied more than one daughter from a mother if each daughter was eligible to participate.
We intentionally studied only non-Hispanic white children to minimize possible variability among ethnic and racial groups in age of onset of puberty and menarche, which will affect sex steroid levels in blood and urine (13–15). Similarly, there are racial and ethnic-related differences in insulin sensitivity, which are detectable even in children (16–18).
Study procedures
The study was approved by the Investigational Review Board at Penn State Hershey Medical Center. Patients gave written consent where age appropriate or verbal or written assent as age appropriate. Every child was able to opt out of specific procedures if they were not comfortable with participation. We obtained a brief history from the mother and the child, including medical disorders, current medications, means of conception, birth weight, gestational age, pregnancy complications, current and past medical history, and pubertal landmarks.
Height, weight, and waist and hip circumferences were recorded to the nearest 0.1 cm, 0.1 kg, and 0.1 cm, respectively. Blood pressure was determined three times in the right arm in the sitting position after a 15-minute rest, and the average value was used. Waist was measured at the level of the umbilicus, and hip circumference was measured at the widest diameter. Tanner staging, including pubic hair distribution and breast development, was conducted by a trained study coordinator. Tanner stage according to pubic hair was used to classify subjects. Hair distribution was assessed by Ferriman-Gallwey scoring (19). Acne was assessed by standardized pictorial methods (20).
We performed transabdominal ultrasound using a 2- to 5-MHz linear abdominal probe on a Philips HDI 4000 machine (Koninklijke Philips, Amsterdam, Netherlands). The uterus and ovaries were measured in three dimensions, and endometrial thickness was measured in the sagittal plane. Ovarian volume was calculated as the measure of a prolate ellipsoid. Body composition analyses, including percent body fat, was obtained (QDR4500; Hologic, Inc., Bedford, MA) as we have previously performed in adults (21). Our observed coefficient of variation (CV) is <0.7% for the day-to-day quality control scans using the manufacturer’s spine phantom (22). We also examined the R1 region (i.e., the centripetal area from the lower rib to the lateral iliac crest) for baseline distribution in body fat (23).
We collected two timed urinary specimens over a continuous 15-hour period: the first was an overnight collection prior to their study visit, and the second was a 3-hour timed collection during their study visit. We used our previously validated glucose-challenged salivary insulin levels to assess hypersinsulinism (3). Daughters consumed a modified high-carbohydrate diet for 3 days prior to the visit and presented to the visit after an overnight fast for a modified oral glucose tolerance test consisting of timed fasting and glucose-challenged salivary specimens. After collection of the fasting salivary insulin levels, oral glucose was administered (1.7 g/kg), and saliva was collected at 30-minute intervals for up to 2 hours after administration. Forty percent of our subjects weighed more than 44.1 kg (97 pounds) and therefore received the maximum 75-g dose of the oral glucose load.
Assays
Urinary luteinizing hormone (LH) and follicle-stimulating hormone (FSH) were measured at the University of Washington using modified versions of enzyme immunoassays previously described elsewhere (24). The urinary LH assay was modified to measure the intact LH molecule using an anti-α subunit polyclonal antibody (AFP5953689Rb, NIDDK NHPP; AF Parlow Library of Health Sciences, University of California, Los Angeles) as the detection antibody. LH was quantified relative to a calibration curve prepared using an intact LH reference preparation (AFP4395A, NIDDK NHPP; AF Parlow Library of Health Sciences). For both LH and FSH, the original colorometric assay format was replaced with a chemiluminescent format using a luminol substrate (SuperSignal ELISA Pico Chemiluminescent Substrate; Thermo Fisher Scientific, Waltham, MA). Urine quality control specimens were run on every plate. For the LH plates used in this study (n = 20), the intra- and interassay CVs were 9.4% and 12.1%, respectively, for the high control (14.2 pg/mL) and 18.0% and 7.0% for the low control (5.9 pg/mL). For FSH plates (n = 16), the high control (1.37 ng/mL) had intra- and interassay CVs of 4.5% and 3.1%, respectively, and the low control intra- and interassay CVs were 3.7% and 6.5%, respectively. Specific gravity measurements for each urine sample were taken with a hand-held urine specific gravity refractometer (Atago Uricon-PN; Bellevue, WA) and used to normalize hormone values for urine concentration (25). All values were standardized to a specific gravity of 1.020. The limits of detection were 0.8 pg/mL for LH and 0.08 ng/mL for FSH.
Urinary estrogens and androgens were run at the Centers for Disease Control in Atlanta, GA, using established methods. Nine steroid hormones were analyzed in urine using isotope dilution liquid chromatography coupled with tandem mass spectrometryLC-MS/MS-. The analytes measured with this method were 17-hydroxyprogesterone (17-OHP), androstenedione (AD), etiocholanolone (EC), TT, estrone (E1), estradiol (E2), estrone sulfate (ES), testosterone sulfate (TS), and dehydroepiandrosterone sulfate (DHEAS). In brief, 1.0 mL urine with 75 µL of internal standard solution (containing 3.34 ng/mL 17-OHP-13C3, 7.03 ng/mL AD-13C3, 138 ng/mL EC-d5, 1.44 ng/mL TT-13C3, 1.37 ng/mL E1-13C3, 4.0 ng/mL E2-13C3, 12.2 ng/mL ES-d4, 56.4 ng/mL TS-d3, and 78.1 ng/mL DHEAS-d5) was acidified with 200 μL of 5% formic acid and applied to solid-phase extraction using an Oasis HLB Plate (60 mg × 60 μm particle size) (Waters Inc., Milford, MA). The solid-phase extraction plate was washed with 5% formic acid in water and 4.5% ammonium hydroxide in 10% methanol in water, and the analytes were eluted with acetonitrile. The eluate was concentrated using vacuum (Genevac EZ 2.3 Elite; GeneVac Inc., Valley Cottage, NY) and analyzed by LC-MS/MS. The mass spectrometer (AB/Sciex API 5500; Triple Quad, Foster City, CA) was operated in both positive and negative electrospray ionization modes. Chromatographic separation was carried out using a C18 HPLC column (100 × 2.0 mm, 3.0 µm particle size) (Luna C18 column; Phenomenex Torrance, CA) at a flow rate of 250 µL/min (Nexera LC-30AD LC Pumps; Shimadzu Corp., Kyoto, Japan). Analytes were eluted using a gradient changing from 30% methanol in 2.5 mM ammonium bicarbonate (pH 7) in water/methanol (80:20 v/v, solvent A) to 80% methanol in solvent B over 18 minutes. To aid in the negative-mode ionization, 0.6 mM ammonium fluoride in methanol was added post column. The within-day and between-day precision expressed as percent coefficient of variation (%CV) determined with 3 different levels of urine pools ranged from 4.3% to 5.9% and from 5.1% to 10.2% for 17-OHP, 3.8% to 6.7% and 1.4% to 5.6% for AD, 10.7% to 15.7% and 6.8% to 15.3% for EC, 3.3% to 5.4% and 4.5% to 6.5% for TT, 6.8% to 9.0% and 8.6% to 10.6% for E1, 9.2% to 17.5% and 12.6% to 15.9% for E2, 5.3% to 11.5% and 9.4% to 11.4% for ES, 102% to 15.0% and 11.4% to 18.3% for TS, and 7.3% to 9.1% and 5.2% to 11.2% for DHEAS, respectively. The limits of detection determined using Taylor’s method (26) were 0.03 ng/mL for 17-OHP, 0.05 ng/mL for AD, 4.13 ng/mL for EC, 0.02 ng/mL for TT, 0.05 ng/mL for E1, 0.06 ng/mL for E2, 0.14 ng/mL for ES, 0.46 ng/mL for TS, and 12.1 ng/mL for DHEAS. The average accuracy calculated from the individual recoveries of all 9 steroids was 105.9% [95% confidence interval (CI), 101.9 to 109.9].
Saliva samples were stored at –20°C or colder to precipitate out proteoglycans in the salivary fluid. Upon thawing, samples were centrifuged at 3000 rpm for 10 minutes at room temperature to sediment the heavy proteinaceous material in the sample. The supernatant was removed, and an aliquot was pipetted into assay tubes for the insulin assay. Insulin in saliva was determined with a double antibody method using reagents obtained from Linco Research, Inc. (St. Charles, MO) (19). The assay detects free insulin, and the crossreactivity of this assay with proinsulin is <0.2%. All assays had intra- and interassay CVs <10%.
Statistical analysis
The primary prespecified endpoint of the study was hyperandrogenism, as detected by urinary levels of adrenal (DHEAS) or ovarian (TT) sex steroids as detected from the timed collections. The main secondary endpoints were the integrated salivary insulin levels from the modified oral glucose tolerance test and the urinary LH levels as determined from the timed collections. We combined the 5 Tanner stage groups into 3 groups (Group 1: Tanner 1; Group 2: Tanner 2/3; Group 3: Tanner 4/5) to reduce the number of comparisons as per our previous work (3).
Linear mixed-effects models were used to compare baseline characteristics between PCOS and control daughters within each of the 3 Tanner stage groups. These models included a random effect to control for the within-family correlation due to daughters from the same mother. The effect size from the linear mixed-effects models are reported as difference in means with associated 95% CI values. We also used a linear mixed-effects model to compare salivary insulin levels between PCOS and control daughters within each of the 3 Tanner stage groups. Due to skewness, the salivary insulin data were log-transformed, and the effect size from the linear mixed-effects models are reported as a ratio of geometric means with associated 95% CI. Given the low detectability of urinary sex steroid levels from the timed collections, comparisons of the urinary sex steroids between PCOS and control daughters within each of the 3 Tanner stage groups were simplified to Wilcoxon rank-sum tests using only the detectable values. We used recently published weight for gestational age data to determine the prevalence of girls who were small for gestational age (SGA) or large for gestational age (27). All hypothesis tests were two-sided, and all statistical analyses and graphics were performed using SAS software (version 9.4; SAS Institute, Inc., Cary, NC) or S-Plus software (version 8.2; TIBCO Software Inc., Seattle, WA).
Results
We studied 76 daughters from 60 mothers with PCOS (PCOS daughters) and 82 daughters from 53 control mothers (control daughters). Baseline characteristics of the daughters are provided in Table 1. There were no differences between PCOS and control daughters in birthweight or gestational age. We noted no difference in the prevalence of SGA infants in our overall cohorts [PCOS: n = 7 (8.9%) vs controls: n = 7 (9.3%); P = 0.92] or in large-for-gestational-age infants [PCOS: n = 4 (5.3%) vs controls: n = 6 (7.6%); P = 0.75]. Similarly, there were no differences in BMI, waist/hip ratios, blood pressure, or acne lesions between PCOS and control daughters. An increased Ferriman-Gallwey score, with a mean value consistent with hirsutism, was noted in PCOS daughters compared with control daughters at Tanner stage 4/5 (P < 0.001). Similarly, there was increased hirsutism in the PCOS mothers compared with the control mothers (Ferriman-Gallwey score: PCOS mothers, 12.3 ± 6.2 vs control mothers, 5.5 ± 4.4; P < 0.0001). We noted no differences in body composition as determined by dual-energy X-ray absorptiometry between groups in the three major body contents (i.e., bone, lean body mass, and fat). However, we noted in a subregion analysis an increased android/gynoid fat ratio in the PCOS daughters compared with control daughters in the Tanner 2/3 group (P = 0.02). We noted no difference in ovarian volume between groups. Menarche was reported as follows: Tanner 1, 1 control; 0 PCOS; Tanner 2/3, 13 (48%) controls, 4 (25%) PCOS; Tanner 4/5, 14 (87.5%) controls, 13 (100%) PCOS. Proportions were not significantly different for any of the Tanner stage groups.
Table 1.
Baseline characteristics of daughters from mothers with PCOS or control mothers without PCOS
| Tanner Stage 1 |
Tanner Stage 2/3 |
Tanner Stage 4/5 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Controla | PCOSa | Control vs PCOSb | Controla | PCOSa | Control vs PCOSb | Controla | PCOSa | Control vs PCOSb | |
| Birth data | |||||||||
| Gestational age, wk | 39.47 (1.42) [37] | 39.13 (1.51) [47] | 0.37 (−0.59 to 1.32) [0.45] | 39.52 (1.19) [27] | 39.51 (1.63) [16] | 0.03 (−1.33 to 1.40) [0.96] | 39.19 (3.53) [16] | 37.92 (4.72) [13] | 1.23 (−0.41 to 2.86) [0.14] |
| Birth weight, pounds | 7.61 (1.04) [36] | 7.38 (0.96) [47] | 0.27 (−0.27 to 0.82) [0.33] | 7.46 (1.12) [27] | 7.47 (0.84) [15] | 0.03 (−0.74 to 0.80) [0.94] | 7.31 (1.78) [16] | 6.53 (1.91) [13] | 0.64 (−0.28 to 1.56) [0.17] |
| Biometric data | |||||||||
| Age, y | 7.61 (2.60) [39] | 7.02 (1.96) [47] | 0.59 (−0.31 to 1.49) [0.20] | 13.04 (1.89) [27] | 11.46 (1.33) [16] | 1.52 (0.24 to 2.80) [0.02] | 14.35 (1.91) [16] | 15.24 (1.17) [13] | −0.91 (−2.46 to 0.65) [0.25] |
| BMI, kg/m2 | 16.59 (3.75) [39] | 17.66 (3.43) [46] | −0.63 (−2.85 to 1.60) [0.58] | 21.13 (3.87) [27] | 21.37 (5.63) [16] | −0.64 (−3.68 to 2.40) [0.68] | 25.68 (6.62) [16] | 27.56 (8.70) [13] | −3.62 (−7.34 to 0.11) [0.06] |
| Waist/hip ratio | 0.87 (0.07) [36] | 0.90 (0.07) [47] | −0.02 (−0.06 to 0.01) [0.14] | 0.85 (0.06) [27] | 0.88 (0.09) [16] | −0.03 (−0.08 to 0.01) [0.16] | 0.84 (0.08) [16] | 0.86 (0.05) [12] | −0.01 (−0.06 to 0.05) [0.78] |
| Systolic blood pressure, mm Hg | 101.74 (11.61) [38] | 100.42 (12.73) [45] | 0.69 (−4.92 to 6.29) [0.81] | 108.04 (9.20) [27] | 107.25 (12.26) [16] | 0.83 (−6.82 to 8.47) [0.83] | 114.25 (12.79) [16] | 114.85 (14.49) [13] | −1.61 (−10.95 to 7.74) [0.73] |
| Diastolic blood pressure, mm Hg | 62.74 (6.93) [38] | 62.67 (6.55) [45] | −0.94 (−4.49 to 2.61) [0.60] | 66.00 (7.72) [27] | 65.81 (7.07) [16] | 0.21 (−4.63 to 5.05) [0.93] | 67.25 (9.64) [16] | 71.08 (10.23) [13] | −3.93 (−9.85 to 1.98) [0.19] |
| Acne, total lesions | 0.21 (0.77) [39] | 0.49 (2.71) [47] | −0.53 (−2.40 to 1.34) [0.58] | 2.74 (6.16) [27] | 3.25 (5.94) [16] | −0.46 (−3.12 to 2.20) [0.73] | 4.06 (4.58) [16] | 5.42 (6.63) [12] | −1.56 (−4.86 to 1.74) [0.35] |
| Ferriman-Gallwey score | 1.14 (2.84) [36] | 1.12 (2.92) [41] | 0.10 (−1.65 to 1.85) [0.91] | 2.07 (2.64) [27] | 1.38 (2.90) [16] | 0.54 (−1.76 to 2.85) [0.64] | 6.13 (5.63) [16] | 10.62 (6.71) [13] | −5.50 (−8.32 to −2.68) [<0.001] |
| Imaging (dual-energy X-ray absorptiometry) | |||||||||
| Bone mineral content, kg | 0.89 (0.28) [31] | 0.83 (0.20) [41] | 0.06 (−0.08 to 0.20) [0.43] | 1.59 (0.37) [25] | 1.35 (0.29) [15] | 0.25 (0.06 to 0.43) [0.01] | 1.94 (0.36) [16] | 1.97 (0.24) [12] | −0.05 (−0.27 to 0.18) [0.68] |
| Lean body mass, kg | 19.01 (5.96) [31] | 18.48 (5.59) [41] | 0.34 (−2.85 to 3.52) [0.83] | 34.51 (5.93) [25] | 33.00 (7.87) [15] | 1.60 (−2.64 to 5.84) [0.46] | 40.33 (6.14) [16] | 43.73 (9.37) [12] | −4.04 (−9.14 to 1.06) [0.12] |
| Fat, kg | 7.97 (3.76) [31] | 8.49 (4.82) [41] | −0.64 (−4.83 to 3.55) [0.76] | 15.46 (6.81) [25] | 17.32 (9.10) [15] | −2.22 (−7.70 to 3.26) [0.42] | 25.48 (12.61) [16] | 26.50 (16.83) [12] | −1.89 (−8.58 to 4.81) [0.58] |
| Android/gynoid fat ratio | 0.28 (0.06) [29] | 0.32 (0.13) [37] | −0.03 (−0.09 to 0.03) [0.36] | 0.33 (0.12) [25] | 0.40 (0.13) [14] | −0.10 (−0.18 to −0.03) [0.01] | 0.37 (0.11) [16] | 0.39 (0.13) [12] | 0.02 (−0.07 to 0.11) [0.69] |
| Imaging (transabdominal ultrasound) | |||||||||
| Total ovarian volume, cm3 | 2.23 (1.57) [9] | 4.19 (3.22) [6] | −2.75 (−21.65 to 16.16) [0.77] | 14.54 (6.65) [11] | 12.92 (8.98) [9] | 1.61 (−14.48 to 17.70) [0.84] | 25.94 (34.78) [10] | 19.22 (8.88) [6] | 9.09 (−10.02 to 28.20) [0.34] |
Mean (standard deviation) [n].
Mean difference (95% CI) [P value].
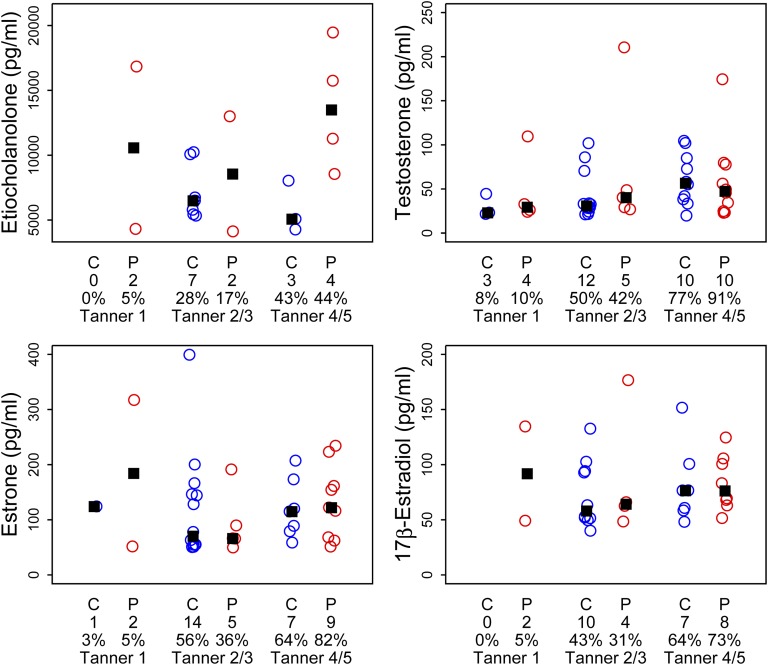
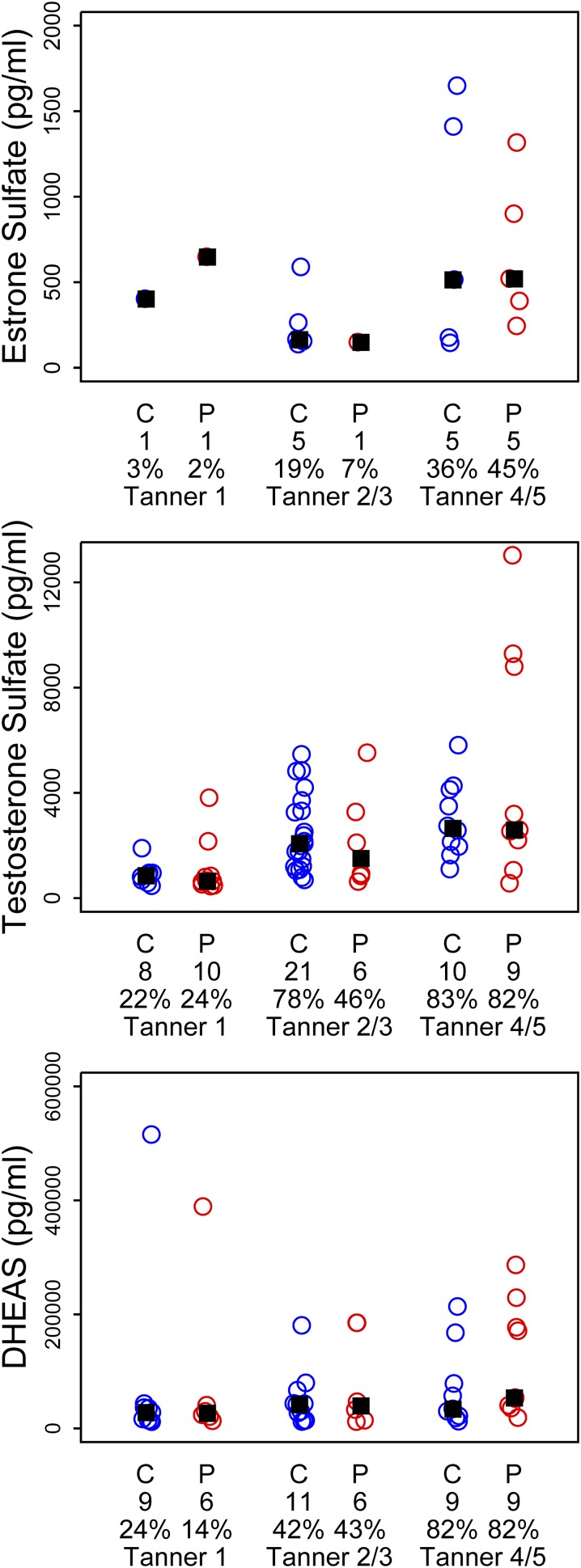
Figure 1 shows results from our LH and FSH urinary assays, both the 12-hour overnight collection and the morning 3-hour collection, from which we were able to obtain a detectable result. In general, LH was more often detectable in the 12-hour than in the 3-hour collection, with the exception of the controls in the 3-hour Tanner 4/5 group. In contrast, almost all daughters had detectable FSH levels. There were no differences between groups in median values of LH, FSH, or LH/FSH ratio. Figure 2 shows the 12-hour collection results for unconjugated estrogens (E1 and E2) and androgens (EC and TT). We had increasing detection rates of measurable unconjugated steroids with advancing puberty in both groups. There were no significant differences in median values between PCOS and control daughters at any Tanner stage. We similarly noted an increased trend toward detection of 12-hour urinary conjugated sex steroids (DHEAS, ES, and TS) with advancing puberty in both groups (Fig. 3). Again we noted no differences in median values between PCOS and control daughters by Tanner stage group. We noted similar results with the 3-hour urinary collections for unconjugated steroids (Supplemental Fig. 1 (4.4MB, tiff) ) and conjugated steroids (Supplemental Fig. 2 (4.4MB, tiff) ) (i.e., incomplete detection for all Tanner stages), a trend toward increased detection with advancing puberty, and no differences in median values between PCOS and control daughters.
Figure 1.
Results from overnight (12-h) and morning (3-h) timed urinary gonadotropin collections by diagnosis (C, daughter of control mother; P, PCOS mother), number of subjects per group (directly under the diagnosis), and percentage of detectable hormone and Tanner stage. No significant differences between groups in median levels (▪) by Tanner stage were noted.
Figure 2.
Results from overnight (12-h) urinary collections for unconjugated sex steroids by diagnosis (C, daughter of control mother; P, PCOS mother), number of subjects per group (directly under the diagnosis), and percentage of detectable hormone and Tanner stage. No significant differences between groups in median levels (▪) by Tanner stage were noted.
Figure 3.
Results from overnight (12-h) urinary collections for conjugated sex steroids by diagnosis (C, daughter of control mother; P, PCOS mother), number of subjects per group (directly under the diagnosis), and percentage of detectable hormone and Tanner stage. No significant differences between groups in median levels (▪) by Tanner stage were noted.
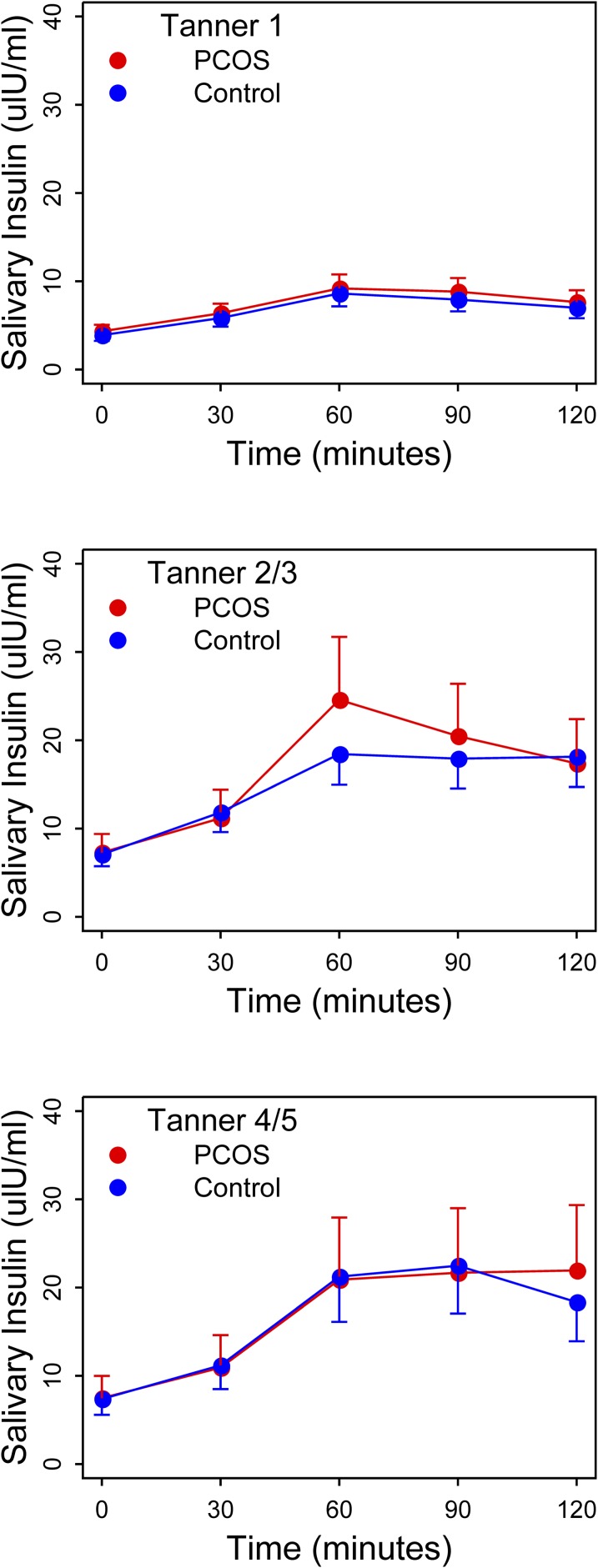
Figure 4 shows the results of the glucose-challenged salivary insulin levels over a 2-hour observation period. We noted no significant differences between PCOS daughters and control daughters at any time point; nor did we note differences in integrated area under the curve values (ratio of geometric means for Tanner stage 1 PCOS vs control daughters, 1.1; 95% CI, 0.9 to 1.4, P = 0.41; ratio of geometric means for Tanner stage 2/3 PCOS vs control daughters, 1.1, 95% CI, 0.8–1.5, P = 0.52; ratio of geometric means for Tanner stage 4/5 PCOS vs control daughters, 1.0, 95% CI, 0.7–1.5, P = 0.98).
Figure 4.
Results of salivary insulin levels from 2-h oral glucose tolerance test by diagnosis [daughter of PCOS mother (PCOS) or daughter of control mother (Control)] by Tanner stage. There were no significant differences by time point or in area-under-the-curve values between groups for any Tanner stage.
Discussion
This matched case-control study used technologies in a vulnerable prepubertal to pubertal population to study the ontogeny of signs and symptoms of PCOS in daughters of women with PCOS compared with the daughters of women without PCOS. Our study was weighted more toward daughters in the early stages of puberty, but we noted that both groups were very similar for most of the parameters we examined, with the exception of more body hair (Tanner stage 4/5) and more central abdominal fat (Tanner stage 2/3) in the daughters of women with PCOS. We were unable to replicate the finding of increased hyperinsulinism in daughters of women with PCOS in the later puberty stages (Tanner 4/5) that we noted in our previous study (3). Thus, overall we can conclude from this larger sample size that the transition through puberty for daughters of women with PCOS appears very similar to that of daughters of women without PCOS.
Our findings differ from other large studies of daughters of women with PCOS where generally there has been a higher prevalence of reproductive and metabolic abnormalities among daughters of mothers with PCOS (28). We did not note an increased prevalence of SGA or decreased birth weights in daughters of women with PCOS (29). This same research group has noted differences in glucose-challenged insulin levels in daughters of women with PCOS, including lower levels in prepubertal PCOS daughters and higher levels in postpubertal PCOS daughters (28, 30). Differences in sex steroids have also been detected commonly in daughters of women with PCOS as early as in cord blood at birth, although in this case decreased levels of androgen and estrogen were noted (31). More commonly, elevated levels of androgens have been detected in later puberty in daughters of PCOS mothers, and there also have been related elevations in LH levels (28).
There are several potential reasons for our findings. One may be that the inheritance of PCOS and PCOS-related traits is low in daughters. PCOS is a heterogeneous syndrome and likely a complex genetic syndrome without a clear Mendelian pattern of trait inheritance. Genome-wide association studies have identified a number of alleles associated with the syndrome, although each alone, or in combination, explains only a fraction of the stigmata of the syndrome (8, 9). Nonetheless, twin studies have shown that hirsutism is more highly correlated among monoamnionic twins than diamnionic twins, suggesting a strong genetic component (32). We did note increased hirsutism among the later stages of puberty in daughters of women with PCOS. This suggests that the full phenotype of PCOS may not present until well beyond the pubertal transition. The difficulties in making a diagnosis of PCOS in adolescent girls in the later stages of puberty is well documented because relative hyperandrogenism, oligomenorrhea, and polycystic ovaries all may be a part of normal puberty for girls (33). Our Tanner 2/3 group of PCOS daughters were younger than the control daughters, and this may suggest that they entered puberty at a younger age, which has been associated with the later development of PCOS (7). Our cohort was smallest in the later stages of puberty, and we did not study girls beyond puberty into adulthood. Further, our study was limited to non-Hispanic white subjects, whereas other studies included Hispanic South Americans, who may have higher rates of metabolic abnormalities than white subjects (34).
We noted many subjects who, even in the advanced stages of puberty, had levels of androgens and gonadotropins that were not quantitated. In many of these samples, levels were detected but not quantitated because they were below the level of detection. This likely is due to the markedly lower levels of these analytes in urine compared with blood, which for gonadotropins can be an order of magnitude lower (24). Therefore, they were not reported by the laboratory and were not considered in data analysis. We were unable to draw blood on all our subjects due to ethical concerns about lack of benefit from phlebotomy in this generally healthy population, so we had to rely on urinary measures instead of serum measures. The lower-than-expected values of analytes in our population may also reflect incomplete urine collections, although the 3-hour morning collections were monitored for compliance and show similar prevalences of nonquantifiable levels. Our assays of sex steroids used current Endocrine Society–recommended state-of-the-art LC-MS/MS assays (35). We interpret this similar rate of nonreportable values of androgens and gonadotropins in both groups as a sign of normal puberty.
The strengths of our study includes a robust sample size for a study of children and daughters, a systematic examination of subjects for a wide variety of metabolic and reproductive parameters (including an oral glucose challenge test performed according to a predetermined protocol), and the use of state-of-the-art imaging and assay technology.
There are several weaknesses to our study. By characterizing subjects by their pubertal stage, we may fail to detect age-related trends in pubertal development; for instance, daughters of women with PCOS may experience an earlier pubertal development than control daughters. There is, in our opinion, less bias by analyzing daughters by Tanner stage than age because Tanner staging was developed to correct for the age variation in pubertal development. It is possible that pubertal changes in serum sex hormone binding globulin mediated through insulin resistance may have effected urinary T (and other sex steroids) excretion, blurring differences between groups (36). This report is a cross-sectional study and not a longitudinal study of intraindividual changes by group, so rates of pubertal development cannot be calculated. There also is an imbalance of subjects weighted toward the earlier stages of puberty, such that smaller numbers may have led to type 2 errors in detecting differences. We did not perform additional challenge tests of pituitary, adrenal, or ovarian function, which may have demonstrated more subtle differences between groups (28, 37). We elected not to study sons because of the uncertainty of the male phenotype, especially in children. Finally, to avoid the heterogeneity of pubertal development that is associated with race and ethnicity, our study was limited to non-Hispanic white girls. Therefore, these findings cannot be extrapolated to other racial and ethnic groups without further study of these groups.
We conclude that daughters of women with PCOS appear to have a profile across the pubertal transition that is very similar to that of daughters of mothers without PCOS. Providing reassurance to the mother with PCOS about her daughter and avoiding excessive evaluation and intervention in an asymptomatic daughter may be the prudent course. Further studies should focus on larger samples sizes, greater racial and ethnic diversity, and longitudinal follow up.
Acknowledgments
We acknowledge the expert and compassionate coordination of this study by Barb Scheetz at Pennsylvania State, as well as the contributions of staff to the assays performed in this study. We thank Lumi Duke and Paul Kim (Centers for Disease Control and Prevention) for contributions to laboratory measurements of sex steroids.
Acknowledgments
This project was supported by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD), National Center for Research Resources, and the National Center for Advancing Translational Sciences at the National Institutes of Health, through Grants U54 HD034449 (Specialized Cooperative Centers Program in Reproductive & Infertility Research at VCU) and UL1 TR000127 (Pennsylvania State Clinical and Translational Institute). Partial support for this research came from a Eunice Kennedy Shriver NICHD research infrastructure grant, R24 HD042828, to the Center for Studies in Demography & Ecology at the University of Washington. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official views or positions of the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry.
Acknowledgments
Disclosure Summary: R.S.L. receives consulting fees from Euroscreen, Astra Zeneca, Clarus Therapeutics, Takeda, Kindex, Bayer, and Millendo and research funding from Ferring. S.J.E. receives research funding from AbbVie. A.R.K. reports ownership of Merck stock.
Footnotes
- 17-OHP
- 17-hydroxyprogesterone
- AD
- androstenedione
- CV
- coefficient of variation
- DHEAS
- dehydroepiandrosterone sulfate
- E1
- estrone
- E2
- estradiol
- EC
- etiocholanolone
- ES
- estrone sulfate
- FSH
- follicle-stimulating hormone
- LC-MS/MS
- liquid chromatography coupled with tandem mass spectrometry
- LH
- luteinizing hormone
- PCOS
- polycystic ovary syndrome
- SGA
- small for gestational age
- TS
- testosterone sulfate
- T
- testosterone
- TT
- total testosterone.
References
- 1.McGee WK, Bishop CV, Bahar A, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod. 2012;27(2):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanner JM. Fetus Into Man: Physical Growth From Conception to Maturity. Cambridge, MA: Harvard University Press;1989. [Google Scholar]
- 3.Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2008;93(5):1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibáñez L, Valls C, Ong K, Dunger DB, de Zegher F. Metformin therapy during puberty delays menarche, prolongs pubertal growth, and augments adult height: a randomized study in low-birth-weight girls with early-normal onset of puberty. J Clin Endocrinol Metab. 2006;91(6):2068–2073. [DOI] [PubMed] [Google Scholar]
- 5.Legro RS, Driscoll D, Strauss JF III, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA. 1998;95(25):14956–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franks S, Webber LJ, Goh M, Valentine A, White DM, Conway GS, Wiltshire S, McCarthy MI. Ovarian morphology is a marker of heritable biochemical traits in sisters with polycystic ovaries. J Clin Endocrinol Metab. 2008;93(9):3396–3402. [DOI] [PubMed] [Google Scholar]
- 7.Ibáñez L, Potau N, Ferrer A, Rodriguez-Hierro F, Marcos MV, De Zegher F. Anovulation in eumenorrheic, nonobese adolescent girls born small for gestational age: insulin sensitization induces ovulation, increases lean body mass, and reduces abdominal fat excess, dyslipidemia, and subclinical hyperandrogenism. J Clin Endocrinol Metab. 2002;87(12):5702–5705. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Zhao H, Shi Y, Cao Y, Yang D, Li Z, Zhang B, Liang X, Li T, Chen J, Shen J, Zhao J, You L, Gao X, Zhu D, Zhao X, Yan Y, Qin Y, Li W, Yan J, Wang Q, Zhao J, Geng L, Ma J, Zhao Y, He G, Zhang A, Zou S, Yang A, Liu J, Li W, Li B, Wan C, Qin Y, Shi J, Yang J, Jiang H, Xu JE, Qi X, Sun Y, Zhang Y, Hao C, Ju X, Zhao D, Ren CE, Li X, Zhang W, Zhang Y, Zhang J, Wu D, Zhang C, He L, Chen ZJ. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44(9):1020–1025. [DOI] [PubMed] [Google Scholar]
- 9.Hayes MG, Urbanek M, Ehrmann DA, Armstrong LL, Lee JY, Sisk R, Karaderi T, Barber TM, McCarthy MI, Franks S, Lindgren CM, Welt CK, Diamanti-Kandarakis E, Panidis D, Goodarzi MO, Azziz R, Zhang Y, James RG, Olivier M, Kissebah AH, Stener-Victorin E, Legro RS, Dunaif A, Dunaif A; Reproductive Medicine Network . Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun. 2015;6:7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, Bjonnes A, Broer L, Dunger DB, Halldorsson BV, Lawlor DA, Laval G, Mathieson I, McCardle WL, Louwers Y, Meun C, Ring S, Scott RA, Sulem P, Uitterlinden AG, Wareham NJ, Thorsteinsdottir U, Welt C, Stefansson K, Laven JS, Ong KK, Perry JR. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015;6:8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zawadzki JK, Dunaif A. Diagnostic Criteria for Polycystic Ovary Syndrome: Towards a Rational Approach. In Dunaif A, ed. Polycystic Ovary Syndrome. Boston, MA: Blackwell Scientific, 1995: 377–84. [Google Scholar]
- 12.Legro RS, Myers ER, Barnhart HX, Carson SA, Diamond MP, Carr BR, Schlaff WD, Coutifaris C, McGovern PG, Cataldo NA, Steinkampf MP, Nestler JE, Gosman G, Guidice LC, Leppert PC; Reproductive Medicine Network . The Pregnancy in Polycystic Ovary Syndrome study: baseline characteristics of the randomized cohort including racial effects. Fertil Steril. 2006;86(4):914–933. [DOI] [PubMed] [Google Scholar]
- 13.Frisch RE. Influences on age of menarche. Lancet. 1973;301(7810):1007. [DOI] [PubMed] [Google Scholar]
- 14.Hill P, Wynder EL, Garbaczewski L, Helman P, Hill M, Sporangisa J, Huskisson J. Diet and menarche in different ethnic groups. Eur J Cancer. 1980;16(4):519–525. [DOI] [PubMed] [Google Scholar]
- 15.Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001;108(2):347–353. [DOI] [PubMed] [Google Scholar]
- 16.Yanovski JA, Sovik KN, Nguyen TT, Sebring NG. Insulin-like growth factors and bone mineral density in African American and White girls. J Pediatr. 2000;137(6):826–832. [DOI] [PubMed] [Google Scholar]
- 17.Saad RJ, Danadian K, Lewy V, Arslanian SA. Insulin resistance of puberty in African-American children: lack of a compensatory increase in insulin secretion. Pediatr Diabetes. 2002;3(1):4–9. [DOI] [PubMed] [Google Scholar]
- 18.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in african-american children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51(10):3014–3019. [DOI] [PubMed] [Google Scholar]
- 19.Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140(7):815–830. [DOI] [PubMed] [Google Scholar]
- 20.Lookingbill DP, Egan N, Santen RJ, Demers LM. Correlation of serum 3 alpha-androstanediol glucuronide with acne and chest hair density in men. J Clin Endocrinol Metab. 1988;67(5):986–991. [DOI] [PubMed] [Google Scholar]
- 21.Legro RS, Dodson WC, Kris-Etherton PM, Kunselman AR, Stetter CM, Williams NI, Gnatuk CL, Estes SJ, Fleming J, Allison KC, Sarwer DB, Coutifaris C, Dokras A. Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100(11):4048–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd T, Lin HM, Matthews AE, Bentley CM, Legro RS. Oral contraceptive use by teenage women does not affect body composition. Obstet Gynecol. 2002;100(2):235–239. [DOI] [PubMed] [Google Scholar]
- 23.Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, Di Fede G, Rini G. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab. 2007;92(7):2500–2505. [DOI] [PubMed] [Google Scholar]
- 24.Brindle E, Miller RC, Shofer JB, Klein NA, Soules MR, O’Connor KA. Urinary beta-luteinizing hormone and beta-follicle stimulating hormone immunoenzymometric assays for population research. Clin Biochem. 2006;39(11):1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller RC, Brindle E, Holman DJ, Shofer J, Klein NA, Soules MR, O’Connor KA. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clin Chem. 2004;50(5):924–932. [DOI] [PubMed] [Google Scholar]
- 26.Taylor JK. Quality Assurance of Chemical Measurements Chelsea, MI: Lewis Publishers; 1987. [Google Scholar]
- 27.Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstet Gynecol. 2014;124(1):16–22. [DOI] [PubMed] [Google Scholar]
- 28.Sir-Petermann T, Codner E, Pérez V, Echiburú B, Maliqueo M, Ladrón de Guevara A, Preisler J, Crisosto N, Sánchez F, Cassorla F, Bhasin S. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(6):1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburú B, Gazitúa R, Recabarren S, Cassorla F. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20(8):2122–2126. [DOI] [PubMed] [Google Scholar]
- 30.Sir-Petermann T, Maliqueo M, Codner E, Echiburú B, Crisosto N, Pérez V, Pérez-Bravo F, Cassorla F. Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(12):4637–4642. [DOI] [PubMed] [Google Scholar]
- 31.Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab. 2010;95(5):2180–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91(6):2100–2104. [DOI] [PubMed] [Google Scholar]
- 33.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK, Endocrine S; Endocrine Society . Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunaif A, Sorbara L, Delson R, Green G. Ethnicity and polycystic ovary syndrome are associated with independent and additive decreases in insulin action in Caribbean-Hispanic women. Diabetes. 1993;42(10):1462–1468. [DOI] [PubMed] [Google Scholar]
- 35.Wierman ME, Auchus RJ, Haisenleder DJ, Hall JE, Handelsman D, Hankinson S, Rosner W, Singh RJ, Sluss PM, Stanczyk FZ. Editorial: The new instructions to authors for the reporting of steroid hormone measurements. J Clin Endocrinol Metab. 2014;99(12):4375. [DOI] [PubMed] [Google Scholar]
- 36.Maynar M, Caballero MJ, Mena P, Rodríguez C, Cortés R, Maynar JI. Urine excretion of androgen hormones in professional racing cyclists. Eur J Appl Physiol Occup Physiol. 1994;68(3):200–204. [DOI] [PubMed] [Google Scholar]
- 37.Maliqueo M, Sir-Petermann T, Pérez V, Echiburú B, de Guevara AL, Gálvez C, Crisosto N, Azziz R. Adrenal function during childhood and puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(9):3282–3288. [DOI] [PubMed] [Google Scholar]