Abstract
Context:
US Hispanic/Latino youth are disproportionally affected by the obesity and diabetes.
Objective:
We examined associations of adiposity measures with insulin resistance (IR) and hyperglycemia and the influences of sex and pubertal development on these associations.
Design, Setting, and Participants:
We performed a cross-sectional analysis of 1223 8- to 16-year-old Hispanic/Latino youth from a community-based study in the United States (SOL Youth).
Main Outcome Measures:
We measured IR (≥75th percentile of sex-specific Homeostatic Model Assessment of Insulin Resistance) and hyperglycemia (fasting glucose ≥100 mg/dL or hemoglobin a1c ≥5.7%).
Results:
In boys, body mass index (BMI) showed the strongest association with IR [prevalence ratio (PR), 2.10; 95% confidence interval (CI), 1.87 to 2.36 per standard deviation], which was not statistically different compared with body fat percentage (%BF) (PR, 2.03; 95% CI, 1.81 to 2.29) and waist circumference (WC) (PR, 1.89; 95% CI, 1.67 to 2.13) but was significantly stronger compared with fat mass index (FMI) (PR, 1.79; 95% CI, 1.63 to 1.96), waist-to-hip ratio (WHR) (PR, 1.32; 95% CI, 1.21 to 1.44), and waist-to-height ratio (WHtR) (PR, 1.76; 95% CI, 1.54 to 2.01) (P for difference, <0.05). In girls, %BF (PR, 2.73; 95% CI, 2.34 to 3.20) showed a significantly stronger association with IR compared with BMI (PR, 1.48; 95% CI, 1.29 to 1.70), FMI (PR, 1.71; 95% CI, 1.49 to 1.95), WC (PR, 1.96; 95% CI, 1.70 to 2.27), WHR (PR, 1.95; 95% CI, 1.70 to 2.23), and WHtR (PR, 1.79; 95% CI, 1.53 to 2.09) (P for difference, <0.003). Associations between adiposity measures and IR were generally stronger among children in puberty versus those who had completed puberty, with significant interactions for WC and WHtR in boys and for BMI in girls (P for interaction, <0.01). Adiposity measures were modestly associated with hyperglycemia (PR, 1.14 to 1.25), with no interactions with sex or pubertal status.
Conclusions:
Sex and puberty may influence associations between adiposity measures and IR in US Hispanic/Latino youth. Multiple adiposity measures are needed to better assess IR risk between boys and girls according to pubertal status.
Précis: Magnitudes of associations between adiposity measures and insulin resistance vary between US Hispanic/Latino boys and girls in different periods of pubertal development.
Childhood obesity has become a major public health problem in the United States (1). Hispanic/Latino youth are disproportionally affected by the obesity epidemic. Recent National Health and Nutrition Examination Survey [NHANES, 2010–2011 (1)] data showed that Hispanic/Latino boys and girls aged 2 to 19 years have a higher prevalence of overweight and obesity [38.9%, body mass index (BMI) ≥85th percentile], compared with non-Hispanic white (28.5%), black (35.2%), and Asian (19.5%) youth. Moreover, the prevalence of abdominal obesity was also much higher in Hispanic/Latino youth [24.2%, defined by waist circumference (WC) ≥90th percentile] compared with non-Hispanic white (18.4%) and black (17.1%) youth (2).
Concurrent with the childhood obesity epidemic has been the increasing prevalence of hyperglycemia and diabetes among youth in recent years (3). It has been well established that childhood obesity is associated with insulin resistance (IR) and diabetes (4–7). However, childhood obesity is typically measured by BMI, which has limitations as a measure of adiposity, particularly in children, whose BMI increases can reflect lean mass increases more than fat mass increases (8, 9). A number of studies have compared the relationships of IR and other cardiometabolic risk factors with different adiposity measures, including BMI, WC, waist-to-hip ratio (WHR), waist-to-height-ratio (WHtR), body fat percentage (%BF), and body fat mass index (FMI) (10–18). However, previous studies in different setting have demonstrated the relevance of different obesity indexes. For instance, in an African study, WHR as an index of central obesity was shown to overestimate the prevalence of central obesity both in the general population and when considering sex, compared with WC, which might be a better predictor (19), whereas in an European study, WHR was suggested to be the best predictor of cardiovascular events and mortality based on strengths of associations and discrimination statistics in patients with type 2 diabetes (20). However, the superiority of any 1 adiposity measure over others as a surrogate measure of metabolic risk remains unclear.
Relationships between adiposity measures and IR are further complicated by influences of race/ethnicity, sex, and pubertal development (21–25). For example, a multiethnic study of 4633 children ages 9 to 10 years from the United Kingdom reported that %BF was more strongly associated with IR in children of South Asian origin compared with those of white European origin, and some associations of adiposity measures (e.g., BMI) and IR were stronger in boys than in girls (21). Another recent study of 1278 European children aged 11 to 18 years reported that associations between adiposity measures (i.e., BMI, WC, and WHtR) and IR were dependent on pubertal development, with the strongest associations during puberty (23). However, despite a high prevalence of obesity (1, 2) and IR and hyperglycemia (7, 26, 27) in US Hispanic/Latino youth, existing studies relating obesity to IR and hyperglycemia in this population have been of narrow scope, have been mainly limited to Mexican American subjects, and have ignored potential influences of sex and pubertal development on these associations (22, 28–30).
Therefore, in this study, we aimed to examine associations of multiple adiposity measures (i.e., BMI, WC, WHR, WHtR, %BF, and FMI) with IR, hyperglycemia, and related glycemic traits among 1223 children and adolescents, aged 8 to 16 years, of diverse Hispanic/Latino backgrounds from the Hispanic Community Children's Health Study (HCHS)/Study of Latino Youth (SOL Youth).
Methods
Study population
The SOL Youth, launched in April 2011, is an ancillary study to the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) (31). The HCHS/SOL is a prospective cohort study that aims to determine the role of acculturation and risk factors in the prevalence and development of disease in Hispanic/Latino populations. A total of 16,415 self-identified Hispanic/Latino participants aged 18 to 74 years at the time of screening were recruited from 4 communities in the United States (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA) sampled during 2008 to 2011 using a 2-stage area household probability sampling design (32, 33).
Eligible for SOL Youth were 8- to 16-year-old children living with at least 1 parent or legal guardian who enrolled in the HCHS/SOL. They were brought, accompanied by their parent, to the study clinic for interview and clinical examination, which included anthropometry, laboratory, lifestyle behaviors, acculturation, medical history, and so on (31). Out of 1466 enrolled SOL Youth participants, 37 who were underweight, 32 non-Hispanic/Latinos, and 174 subjects who were missing exposures, outcomes, or covariates were excluded from our study population, resulting in 602 boys and 621 girls in the final sample. The study was approved by institutional review boards in each field center and the data coordinating center. Written informed consent was obtained from parent(s) and children.
Assessment of IR and hyperglycemia
IR was defined with a cut-off at the 75th percentile of homeostatic model assessment of insulin resistance (HOMA-IR) values (4.36 in boys and 4.52 in girls) for each sex in the current study. HOMA-IR was derived from fasting glucose and fasting insulin using an established formula: HOMA-IR = fasting glucose × (fasting insulin/6)/405 (34). Hyperglycemia was defined as fasting glucose ≥100 mg/dL or hemoglobin a1c (HbA1c) ≥5.7%. Before the clinic visit, parents were instructed to keep their children fasting for at least 10 hours (31). A fasting blood sample was taken from each participant and processed in a central laboratory at the University of Minnesota. Plasma glucose was measured on a Modular P Chemistry Analyzer (Roche Diagnostics Corp., Indianapolis, IN) using a hexokinase enzymatic method (Roche Diagnostics Corp.). Serum insulin level was measured on an Elecsys 2010 Analyzer (Roche Diagnostics Corp.) using a sandwich immunoassay method (Roche Diagnostics Corp.). HbA1c was measured with a G7 Automated HPLC Analyzer (Tosoh Bioscience, Inc., South San Francisco, CA) (35).
Assessment of adiposity measures
In the current study, we examined 6 adiposity measures: BMI, %BF, FMI, WC, WHR, and WHtR. Weight and %BF were obtained from Body Composition Analyzer TBF-300A (Tanita Corporation, Arlington Heights, IL), which applied a bioelectrical impedance method. Height, WC, and hip circumference were measured 3 times per participant and rounded to the nearest centimeter according to a standard protocol. An average of 3 measurements was used in the study for each participant (36). BMI was calculated as weight (kg) divided by height squared (m2). FMI was calculated as fat mass (kg) divided by height squared (m2). WHR was calculated as the ratio of WC (in centimeters) to hip circumference (cm), and WHtR was calculated as the ratio of WC (cm) to height (cm). General obesity is defined as BMI ≥95th percentile of the sex-specific age-standardized BMI of study population using CDC Growth Charts (37). Abdominal obesity is defined as WC ≥90th percentile for a child’s sex and age (38).
Covariates
Information on age, sex, Hispanic/Latino background, nativity (whether born in mainland United States), pubertal status, and physical activity level were collected from children with questionnaires administered in English or Spanish. Household income and parent education level were reported by parents. Pubertal development was assessed with the Pubertal Development Scale (PDS), which contains questions regarding occurrence of growth spurt, body hair, and skin change in both boys and girls; facial hair development and voice change in boys; and breast development and menstruation in girls (39). Except for menstruation (No versus Yes), the response categories for each item were: (1) not yet begun, (2) barely started, (3) definitely started, or (4) seemed complete. A mapping algorithm was used to generate a sex-specific composite score that is equivalent to Tanner staging from the preceding items (40). In the current analysis, 849 participants (70%) had data on pubertal development with all reported PDS items. Consistent with previous reported data, younger participants were more likely to have missing responses to PDS items (41).
Statistical analysis
All analyses accounted for the complex survey sampling design features, including stratification and cluster sampling, and were adjusted for nonresponse. Weighted statistics were used to describe the distribution of sociodemographic variables and clinical characteristics. For continuous variables with nonnormal distributions, weighted median and quartiles were presented. For categorical variables, unweighted frequencies and weighted proportions were used. Correlations among adiposity measures and glycemic traits were assessed using weighted Pearson correlation coefficients. In addition, we used survey linear regression to examine correlations of adiposity measures with fasting glucose, fasting insulin, HbA1c, and HOMA-IR and tested for potential interaction by sex. Adiposity measures were age standardized and transformed to sex-specific z scores [mean, 0; standard deviation (SD), 1] in regression models. Fasting insulin and HOMA-IR were log transformed before analysis. To examine associations of adiposity measures with IR and hyperglycemia, prevalence ratios (PRs) were estimated from survey Poisson regression models with robust variance estimates. Sex, Hispanic/Latino background, field center, nativity, pubertal status, and physical activity level were adjusted when appropriate. Missing pubertal status was grouped as 1 category and was included in the model. Differences in PRs for IR and hyperglycemia between adiposity measures were examined by χ2 test to compare magnitudes of associations. The analyses were performed for overall study population, by sex, by puberty status (during puberty versus after puberty), by Hispanic/Latino background (Mexican versus non-Mexican background; Caribbean versus non-Caribbean), and by annual family income (≤$20,000 versus >$20,000). Potential effect modification was assessed by incorporating an interaction terms between each adiposity measure and these covariates in the regression models. All P values were 2-sided, with a significance level at 0.05. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and SUDAAN release 11.0 (RTI International, Research Triangle Park, NC).
Results
Study characteristics
Characteristics of the study target population are shown in Table 1. The median age of our target population was 13 years for boys and 12 years for girls. The largest group was of Mexican background (48.8% of boys; 49.5% of girls), followed by those of Dominican (14.0% of boys; 13.7% of girls) and Puerto Rican (10.6% of boys; 10.1% of girls) backgrounds. Among those who reported information on pubertal development, 36.3% of boys and 53.7% of girls completed pubertal development. Overall, 29.4% of boys and 26.3% girls had general obesity, 13.0% of boys and 13.4% of girls had abdominal obesity, and 21.0% of boys and 11.7% of girls had hyperglycemia (3 subjects had diabetes, with fasting glucose >126 mg/dL and/or HbA1c >6.5%). Adiposity measures were similar between boys and girls except for %BF, which was 9.3% higher in girls. Fasting glucose level was higher in boys than in girls, whereas fasting insulin was lower in boys than in girls.
Table 1.
Characteristics of Study Target Population
| All (N = 1223) | Boys (n = 602) | Girls (n = 621) | |
|---|---|---|---|
| Age, y, median (IQR) | 12.0 (10.0–14.1) | 13.0 (10.1–14.9) | 12.0 (10.0–14.1) |
| Hispanic/Latino background, n (%) | |||
| Dominican | 151 (13.9) | 76 (14.0) | 75 (13.7) |
| Cuban | 93 (5.7) | 47 (5.0) | 46 (6.4) |
| Mexican | 577 (49.1) | 276 (48.8) | 301 (49.5) |
| Puerto Rican | 116 (10.4) | 59 (10.6) | 57 (10.1) |
| Central American | 95 (5.8) | 39 (4.8) | 56 (6.7) |
| South American | 60 (4.3) | 34 (4.8) | 26 (3.7) |
| Mixed or other Hispanic | 131 (10.8) | 71 (12.0) | 60 (9.9) |
| Nativity (within 50 states and DC), n (%) | 943 (77.8) | 464 (78.8) | 479 (76.8) |
| Parental education level < high school, n (%) | 475 (38.9) | 229 (37.4) | 246 (40.5) |
| Annual family income, n (%) | |||
| <$20,000 | 638 (52.3) | 309 (51.4) | 329 (53.2) |
| $20,000–$50,000 | 398 (31.6) | 196 (31.7) | 202 (31.4) |
| >$50,000 | 187 (16.1) | 97 (16.8) | 90 (15.4) |
| Moderate to vigorous physical activity, times/day | 7 (5–11) | 8 (5–11) | 7 (4–11) |
| Pubertal status, n (%)a | |||
| Prepuberty (Tanner stage 1) | 30 (3.5) | 20 (4.8) | 10 (2.3) |
| Puberty (Tanner stage 2–4) | 436 (51.4) | 248 (58.9) | 188 (43.9) |
| Postpuberty (Tanner stage 5) | 383 (45.1) | 153 (36.3) | 230 (53.7) |
| Field center, n (%) | |||
| Bronx | 357 (36.6) | 180 (37.6) | 177 (35.6) |
| Chicago | 286 (14.5) | 125 (13.8) | 161 (15.1) |
| Miami | 226 (13.7) | 119 (13.7) | 107 (13.7) |
| San Diego | 354 (35.2) | 178 (34.8) | 176 (35.6) |
| General obesity, n (%)b | 357 (27.8) | 195 (29.4) | 162 (26.3) |
| Abdominal obesity, n (%)c | 181 (13.2) | 97 (13.0) | 84 (13.4) |
| Hyperglycemia, n (%)d | 221 (16.5) | 138 (21.0) | 83 (11.7) |
| Insulin resistance, n (%)e | 305 (24.9) | 150 (24.9) | 155 (25.0) |
| Parental abdominal obesity, n (%)f | 859 (72.1) | 433 (73.2) | 426 (71.0) |
| Adiposity measures, median (IQR) | |||
| BMI, kg/m2 | 21.4 (18.7–25.1) | 21.4 (18.7–25.2) | 21.5 (18.6–25.1) |
| Body fat percentage, % | 25.8 (17.8–34.4) | 20.7 (15.0–29.4) | 30.0 (24.1–36.6) |
| Fat mass index, kg/m2 | 5.4 (3.4–8.5) | 4.4 (2.8–7.3) | 6.3 (4.4 –9.1) |
| Waist circumference, cm | 75.7 (68.1–85.9) | 75.1 (68.1–86.7) | 76.2 (68.1–85.1) |
| Waist-to-hip ratio | 0.9 (0.8–0.9) | 0.9 (0.8–0.9) | 0.9 (0.8–0.9) |
| Waist-to-height ratio | 0.49 (0.45–0.56) | 0.48 (0.43–0.56) | 0.50 (0.46–0.56) |
| Glycemic traits, median (IQR) | |||
| Fasting glucose, mg/dL | 92 (87.4–95.8) | 93.7 (89.7–97) | 89.6 (85.8–94.3) |
| Fasting insulin, mU/L | 74.4 (49.7–115.2) | 67.2 (46.4–108.2) | 80.5 (54.5–121.9) |
| HOMA-IR | 2.8 (1.9–4.5) | 2.6 (1.8–4.3) | 3.0 (2.0–4.6) |
| Hemoglobin A1c, % | 5.3 (5.1–5.4) | 5.3 (5.1–5.5) | 5.2 (5.1–5.4) |
Data are weighted medians (IQR) or frequencies (%).
An overall Tanner staging could not be derived for some participants due to incomplete PDS items.
General obesity is defined as BMI ≥95th percentile of the sex-specific, age-standardized BMI of study population using CDC Growth Charts.
Abdominal obesity is defined as WC ≥90th percentile for a child’s sex and age.
Hyperglycemia is defined as fasting glucose ≥100 mg/dL or HbA1c ≥5.7%.
Insulin resistance is defined as HOMA-IR >4.36 in boys and HOMA-IR >4.52 in girls.
Parental abdominal obesity is defined as WC ≥88 cm for women and ≥102 for men.
Adiposity measures and diabetes related traits
Table 2 shows weighted correlation coefficients between adiposity measures and diabetes-related traits by sex. There were high correlations among all adiposity measures in boys (r ≥ 0.84) and girls (r ≥ 0.76), except for WHR, which showed moderate correlations with BMI, %BF, and FMI (r = 0.48 to 0.56). All adiposity measures were positively correlated with fasting insulin and HOMA-IR in boys and girls, and correlation coefficients were generally similar (r = 0.59 to 0.65 in boys; r = 0.48 to 0.58 in girls), except for WHR, which showed slightly weaker correlations with fasting insulin (r = 0.47 in boys; r = 0.40 in girls) and HOMA-IR (r = 0.45 in boys; r = 0.39 in girls) compared with other adiposity measures. We found relatively weak correlations between adiposity measures and HbA1c in girls but not in boys, and no significant correlations between adiposity measures and fasting glucose were observed in boys or girls.
Table 2.
Age-adjusted Pearson correlation coefficients between adiposity measures and cardiometabolic markers in Hispanic/Latino boys and girls
| Boys |
Girls |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | %BF | FMI | WC | WHR | WHtR | BMI | %BF | FMI | WC | WHR | WHtR | |
| Adiposity measures | ||||||||||||
| %BF | 0.91 | — | — | — | — | — | 0.76 | — | — | — | — | — |
| FMI | 0.95 | 0.97 | — | — | — | — | 0.86 | 0.91 | — | — | — | — |
| WC | 0.89 | 0.85 | 0.87 | — | — | — | 0.83 | 0.85 | 0.92 | — | — | — |
| WHR | 0.55 | 0.56 | 0.56 | 0.82 | — | — | 0.48 | 0.50 | 0.51 | 0.73 | — | — |
| WHtR | 0.88 | 0.86 | 0.86 | 0.96 | 0.84 | — | 0.81 | 0.81 | 0.89 | 0.95 | 0.76 | — |
| Glycemic traits | ||||||||||||
| Fasting glucose | 0.08 | 0.08 | 0.07 | 0.07 | 0.08 | 0.08 | 0.06 | 0.10 | 0.07 | 0.09 | 0.08 | 0.04 |
| Fasting insulina | 0.65 | 0.62 | 0.61 | 0.64 | 0.47 | 0.61 | 0.50 | 0.58 | 0.56 | 0.58 | 0.40 | 0.53 |
| HOMA-IRa | 0.63 | 0.60 | 0.59 | 0.62 | 0.45 | 0.59 | 0.48 | 0.56 | 0.54 | 0.56 | 0.39 | 0.51 |
| Hemoglobin A1c | 0.09 | 0.10 | 0.09 | 0.09 | 0.08 | 0.10 | 0.20 | 0.15 | 0.17 | 0.20 | 0.21 | 0.17 |
Values are weighted to the target population and adjusted for age; all correlations are statistically significant (P < 0.05) except for correlations between adiposity measures and fasting glucose or Hemoglobin A1c in boys and correlations between adiposity measures and fasting glucose in girls.
These variables were log-transformed before analysis.
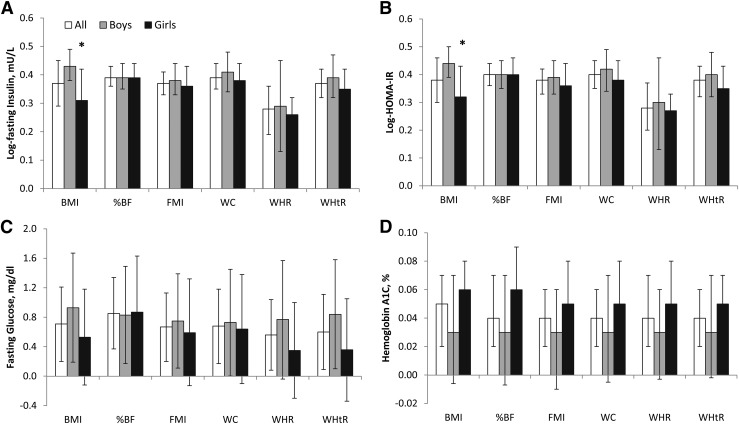
Multivariable linear regression analyses led to a similar conclusion that adiposity measures were strongly associated with fasting insulin and HOMA-IR (Fig. 1). Overall, a 1 SD increase in %BF, FMI, WC, and WHtR was associated with ∼42% to 50% (β = 0.35 to 0.40 log-units) higher fasting insulin and HOMA-IR levels, whereas a 1 SD increase in WHR was associated with ∼30% to 32% (β = 0.26 to 0.28 log-units) higher fasting insulin and HOMA-IR in both boys and girls. Associations of BMI with fasting insulin and HOMA-IR were stronger in boys compared with girls (β = 0.43 versus 0.31 log-insulin; β = 0.44 versus 0.32 log–HOMA-IR; both P for interaction, <0.05). Associations of adiposity measures with fasting glucose (β = 0.56 to 0.85 mg/dL) and HbA1c (β = 0.03% to 0.06%) were relatively modest, with no observed sex differences.
Figure 1.
Differences (95% CIs) in glycemic traits with 1 SD increment in adiposity measures in Hispanic/Latino youth. (A) Log-fasting insulin. (B) Log-HOMA-IR. (C) Fasting glucose. (D) Hemoglobin A1C. Adiposity measures are transformed to age-adjusted, sex-specific z scores. Models adjusted for sex, pubertal status, Hispanic background, field center, nativity, parental education level, annual family income, and self-reported physical activity. Error bars are 95% CIs. *P < 0.05 for interaction between sex and adiposity measures on glycemic traits.
Adiposity measures and IR
All adiposity measures were significantly associated with IR, as defined by HOMA-IR ≥75th percentile of values of each sex (Table 3). In boys, BMI showed the strongest association with IR [PR, 2.10; 95% confidence interval (CI), 1.87 to 2.36]. This PR associated with a 1 SD increase in BMI was not statistically different from the analogous PRs for %BF (PR, 2.03; 95% CI, 1.81 to 2.29) and WC (PR, 1.89; 95% CI, 1.67 to 2.13), but it was significantly stronger compared with PRs for FMI (PR, 1.79; 95% CI, 1.63 to 1.96), WHR (PR, 1.32; 95% CI, 1.21 to 1.44), and WHtR (PR, 1.76; 95% CI, 1.54 to 2.01) (all P for difference, <0.05). In girls, %BF (PR, 2.73; 95% CI, 2.34 to 3.20) showed a much stronger association with insulin resistance compared with BMI (PR, 1.48; 95% CI, 1.29 to 1.70), FMI (PR, 1.71; 95% CI, 1.49 to 1.95), WC (PR, 1.96; 95% CI, 1.70 to 2.27), WHR (PR, 1.95; 95% CI, 1.70 to 2.23), and WHtR (PR, 1.79; 95% CI, 1.53 to 2.09) (all P for difference, <0.003). Significant sex differences were observed for the associations of BMI, %BF, and WHR with IR (all P for interaction, ≤0.003). Association between BMI and IR was stronger in boys compared with girls, whereas associations of %BF and WHR with IR were stronger in girls compared with boys.
Table 3.
Prevalence ratios (95% CIs) for insulin resistance and hyperglycemia associated with 1 SD increase in adiposity measures in Hispanic/Latino youth by sex and puberty development
| All Subjects | Sex |
P Value for Interaction | Puberty Development |
P Value for Interaction | |||
|---|---|---|---|---|---|---|---|
| Boys | Girls | Puberty | Post Puberty | ||||
| Insulin resistance | |||||||
| BMI | 1.61 (1.43–1.81) | 2.10 (1.87–2.36) | 1.48 (1.29–1.70) | <0.001 | 2.26 (1.91–2.67) | 1.49 (1.25–1.78) | <0.001 |
| %BF | 2.24 (2.03–2.47) | 2.03 (1.81–2.29) | 2.73 (2.34–3.20) | 0.003 | 2.16 (1.84–2.55) | 2.08 (1.75–2.48) | 0.74 |
| FMI | 1.74 (1.60–1.90) | 1.79 (1.63–1.96) | 1.71 (1.49–1.95) | 0.59 | 1.92 (1.67–2.21) | 1.67 (1.50–1.87) | 0.13 |
| WC | 1.92 (1.75–2.11) | 1.89 (1.67–2.13) | 1.96 (1.70–2.27) | 0.68 | 2.23 (1.90–2.61) | 1.83 (1.61–2.07) | 0.08 |
| WHR | 1.40 (1.29–1.52) | 1.32 (1.21–1.44) | 1.95 (1.70–2.23) | <0.001 | 1.36 (1.19–1.56) | 1.36 (1.20–1.53) | 0.97 |
| WHtR | 1.78 (1.60–1.97) | 1.76 (1.54–2.01) | 1.79 (1.53–2.09) | 0.89 | 2.16 (1.83–2.55) | 1.73 (1.51–1.98) | 0.06 |
| Hyperglycemia | |||||||
| BMI | 1.25 (1.13–1.38) | 1.21 (1.01–1.46) | 1.28 (1.15–1.42) | 0.64 | 1.33 (1.07–1.65) | 1.27 (1.11–1.44) | 0.69 |
| BF% | 1.24 (1.08–1.42) | 1.17 (0.98–1.39) | 1.40 (1.09–1.81) | 0.26 | 1.23 (1.01–1.50) | 1.24 (0.97–1.60) | 0.96 |
| FMI | 1.21 (1.08–1.36) | 1.17 (1.00–1.37) | 1.28 (1.07–1.53) | 0.47 | 1.29 (1.08–1.53) | 1.19 (0.98–1.46) | 0.55 |
| WC | 1.24 (1.10–1.41) | 1.18 (0.99–1.40) | 1.35 (1.11–1.64) | 0.32 | 1.28 (1.04–1.56) | 1.25 (1.04–1.52) | 0.89 |
| WHR | 1.14 (1.03–1.27) | 1.08 (0.94–1.24) | 1.33 (1.05–1.68) | 0.13 | 1.14 (1.00–1.30) | 1.09 (0.92–1.29) | 0.67 |
| WHtR | 1.19 (1.05–1.35) | 1.15 (0.97–1.36) | 1.26 (1.03–1.53) | 0.52 | 1.20 (0.96–1.50) | 1.22 (1.00–1.48) | 0.90 |
Adiposity measures are transformed to age-adjusted, sex-specific z scores. Models were adjusted for sex, pubertal status, Hispanic background, field center, nativity, parental education level, annual family income, and self-reported physical activity. Prepubertal children (Tanner stage I) were excluded due to small sample size (n ≤ 50). Subjects missing Tanner staging were also excluded.
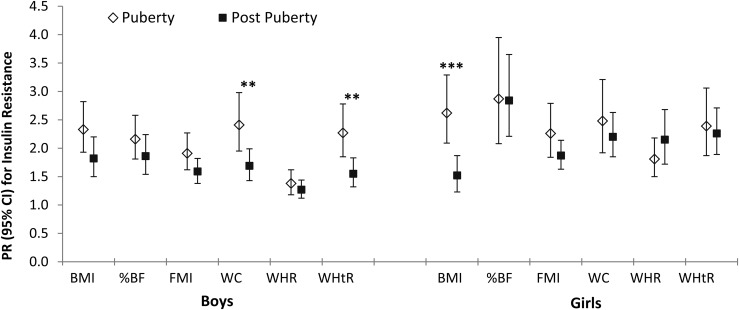
Among children and adolescents who had data on stage of pubertal development (with prepubertal children excluded because of a very small number), there was a significant interaction between BMI and puberty development on IR (P for interaction, <0.001) (Table 3). The association between BMI and IR was stronger in children during puberty compared with those who were postpubertal (PR, 2.26; 95% CI, 1.91 to 2.67 versus PR, 1.49; 95% CI, 1.25 to 1.78). We then further examined potential interactions between adiposity measures and pubertal status in boys and girls separately (Fig. 2). Associations between adiposity measures and IR were generally stronger in children during puberty compared with those who had completed pubertal development, with significant interactions for WC (PR, 2.41; 95% CI, 1.95 to 2.98 versus PR, 1.69; 95% CI, 1.43 to 1.99) and WHtR (PR, 2.27; 95% CI, 1.85 to 2.78 versus PR, 1.55; 95% CI, 1.32 to 1.83) in boys (both P < 0.01 for interaction) and for BMI in girls (PR, 2.62; 95% CI, 2.09 to 3.29 versus PR, 1.52; 95% CI, 1.23 to 1.87; P for interaction, <0.001).
Figure 2.
Prevalence ratios (95% CIs) for insulin resistance with 1 SD increment in adiposity measures by sex and pubertal status. Adiposity measures are transformed to age-adjusted, sex-specific z scores. Models adjusted for Hispanic/Latino background, field center, nativity, parental education level, annual family income, and self-reported physical activity. Prepubertal children (Tanner stage 1) were excluded owing to small sample size (n ≤ 50). Subjects missing Tanner staging were also excluded. **P < 0.01 for interaction between puberty development and adiposity measures on insulin resistance in boys. ***P < 0.001 for interaction between puberty development and adiposity measures on insulin resistance in girls.
In addition, associations between adiposity measures and IR were consistent across Hispanic/Latino groups by comparing Mexican and non-Mexican or Caribbean or non- Caribbean backgrounds (Supplemental Table 1 (23.8KB, docx) ). However, we found significant interactions between some adiposity measures and family income on IR, with stronger associations for %BF and FMI and weaker association for WHR observed in children from families with annual income >$20,000 compared with those from families with annual income ≤$20,000 (Supplemental Table 2 (23.8KB, docx) ).
As expected, the prevalence ratio of central obesity is higher in children with a history of parental central obesity compared with those without history of parental obesity, especially in boys (Supplemental Table 3 (23.8KB, docx) ). However, we did not find a significant association between history of parental central obesity and IR, although there was a trend toward a positive association.
Adiposity measures and hyperglycemia
The PRs for hyperglycemia with 1 SD difference in adiposity measures were relatively modest (range, 1.14 to 1.25), and magnitudes of associations were similar among these measures (Table 3). No significant interactions between adiposity measures and sex, pubertal status, Hispanic background, or family income were observed (Table 3; Supplemental Tables 1 and 2 (23.8KB, docx) ).
Discussion
In this population study of 1233 US Hispanic/Latino children and adolescents, we found that magnitudes of associations between some adiposity measures (e.g., BMI, %BF, and WHR) and IR were different between boys and girls. Previous studies, mostly of non-Hispanic children and adolescents, have generally shown that measures of central obesity (e.g., WC) or directly assessed fat mass (e.g., FMI) are not more strongly associated with IR or other cardiometabolic risk factors than BMI (10–18). For example, a recent study of 5235 children aged 9 to 12 years in the United Kingdom reported that BMI, WC, and fat mass were all strongly associated with IR, high blood pressure, and dyslipidemia in similar magnitudes (10). Moreover, sex differences in associations between adiposity measures and IR (generally stronger in boys compared with girls) have also been observed, but 1 adiposity measure did not seem to be superior to others in 1 sex group (10, 21). In contrast, our study found that the association between BMI and IR was stronger in boys compared with girls, whereas associations of %BF and WHR with IR were stronger in girls compared with boys. Our further analyses indicated that these associations were influenced by pubertal development differently between sexes, with relatively weaker associations between BMI and IR in postpubertal girls and weaker associations between central adiposity measures (e.g., WC, WHtR) and IR in postpubertal boys compared with those who were pubertal. Our findings suggested that influences of both sex and puberty status need to be considered in evaluating relationships between different adiposity measures and IR in Hispanic/Latino children and adolescents.
Differences between results from this study of Hispanic/Latino children in the United States compared with previous studies might reflect ethnic differences in body fat composition and distribution. For example, in the nationally representative sample of US adolescents aged 12 to 20 years (NHANES, 1999–2004), there were no differences in mean BMI by race/ethnicity, whereas %BF differed significantly among non-Hispanic white, non-Hispanic black, and Mexican American boys. In NHANES (42), both BMI and %BF differed significantly among the groups, with Mexican American girls having the highest %BF. Moreover, higher amounts of visceral adipose tissue were observed in Hispanics compared with non-Hispanic whites aged 5 to 18 years (43). Despite the differences in adiposity phenotypes (42, 43) and IR (26), several analyses using the NHANES data provided little evidence for differences in predictive capacity of BMI, WC, and FMI for identification of IR or metabolic syndrome between non-Hispanic white, non-Hispanic black, and Mexican American youth (11, 15, 18). However, NHANES does not contain data on pubertal stage and thus did not examine the potential influences of pubertal status, which we observed in the current analysis.
Consistent with a previous study in 1278 children aged 11 to 18 years from Europe (23), we also found that associations between adiposity measures and IR were stronger in children during puberty than in those who were postpubertal. Furthermore, our analyses indicated that some of the associations influenced by puberty status were different between boys and girls. The previous study of European children did not report a sex difference in this regard (23). Sex differences in body fat deposition and IR during pubertal development have been widely observed (24, 44–46). Sex differences in body composition are primarily attributable to the action of sex steroid hormones, which drive the dimorphisms during pubertal development (44, 46). During puberty, boys develop a more android shape by depositing more abdominal fat, whereas girls develop more total body fat in general (44, 46). This might help explain our results that WC and WHtR reflecting abdominal adiposity showed stronger association during puberty in boys, whereas BMI measuring general adiposity showed stronger association during puberty in girls. However, %BF measuring total body fat in general showed strong association with IR in girls independent of puberty status. Nevertheless, further studies are needed to confirm our findings and to clarify the complicated interactions of obesity measures with sex and puberty status in relation to IR.
Published data comparing associations of multiple adiposity measures with IR and hyperglycemia are limited in US Hispanic/Latino children and adolescents. Two previous studies, including 170 Mexican American boys and girls aged 13 years and 325 Mexican American youth aged 15 to 18 years, respectively, found that multiple adiposity measures showed strong associations with IR, but the magnitudes of these associations were apparently similar across measures (29, 30). In contrast, our study with a relatively larger sample including youths of Mexican and other Hispanic/Latino backgrounds provides some evidence that 1 adiposity measure may be superior to others in relation to IR, depending on sex and pubertal status. Partially consistent with our findings, a previous study of 167 Hispanic and non-Hispanic black youths aged 2 to 19 years suggested that WC and WHtR might be better indicators of IR and other cardiometabolic risk factors than BMI and WHR (22). Another small study (n = 32) suggested that specific accumulation of visceral fat in addition to overall adiposity might have a unique effect on IR in Hispanic/Latino children (28). However, those previous studies did not examine potential sex differences or take into account pubertal status (22, 28–30).
To the best of our knowledge, this is the largest study to date to examine associations of multiple adiposity measures with IR and hyperglycemia among US Hispanic/Latino children and adolescents of diverse backgrounds. However, there are several limitations to this study. First, our study was limited by the nature of cross-sectional data, and thus prospective evaluations of different adiposity measures as predictors for the development of IR and diabetes are warranted. Second, the gold standard technique for assessing IR, the hyperinsulinemic-euglycemic clamp, was not used, although HOMA-IR has been validated and acknowledged as a surrogate measure of IR in pediatric studies (47, 48). Third, because there were missing data regarding pubertal status, largely due to unreported data on PDS among younger children, we only compared youth during puberty with those post puberty. Finally, although we have included multiple measures for overall and central obesity, our study lacked data on precise measurements of regional fat deposition and distribution through computed tomography or magnetic resonance imaging. These precise measurements may help us better understand the relationship between adiposity and IR and diabetes in US Hispanic/Latino youth because potential uniqueness of visceral fat and its effect on IR have been suggested (28, 43).
In summary, our findings suggest that magnitudes of associations of different adiposity measures and IR vary between US Hispanic/Latino boys and girls and by different pubertal status. Thus, multiple adiposity measures might be needed to better assess the risk of IR between US Hispanic/Latino boys and girls according to pubertal status. Further studies are warranted to confirm our results.
Acknowledgments
The SOL Youth Study was supported by Grant R01HL102130 from the National Heart, Lung, and Blood Institute (NHLBI). The children in SOL Youth are drawn from the study of adults, The Hispanic Community Health Study/Study of Latinos, which was supported by contracts from the NHLBI to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contributed to the HCHS/SOL through a transfer of funds to NHLBI: National Center on Minority Health and Health Disparities, the National Institute of Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurologic Disorders and Stroke, and the Office of Dietary Supplements. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the National Institutes of Health. Q.Q. received a Scientist Development Award (K01HL129892) from the NHLBI.
Acknowledgments
Author contributions: Q.Q. designed the study, researched data, and wrote the manuscript. S.H. researched data and wrote the manuscript. K.M.P, J.C., L.V.H., N.S., B.T., and A.M.D. researched data, contributed to discussion, and edited/reviewed the manuscript. R.C.K and C.R.I. designed the study, contributed to discussion, and edited/reviewed the manuscript. Q.Q. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- %BF
- body fat percentage
- BMI
- body mass index
- CI
- confidence interval
- FMI
- fat mass index
- HbA1c
- hemoglobin a1c
- HCHS/SOL
- the Hispanic Community Health Study/Study of Latinos
- HOMA-IR
- Homeostatic Model Assessment of Insulin Resistance
- IR
- insulin resistance
- NHANES
- National Health and Nutrition Examination Survey
- PDS
- Pubertal Development Scale
- PR
- prevalence ratio
- SD
- standard deviation
- SOL Youth
- The Hispanic Community Children's Health Study/Study of Latino Youth
- WC
- waist circumference
- WHR
- waist-to-hip ratio
- WHtR
- waist-to-height ratio.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014;168(6):561–566. [DOI] [PubMed] [Google Scholar]
- 3.Cruz ML, Shaibi GQ, Weigensberg MJ, Spruijt-Metz D, Ball GD, Goran MI. Pediatric obesity and insulin resistance: chronic disease risk and implications for treatment and prevention beyond body weight modification. Annu Rev Nutr. 2005;25:435–468. [DOI] [PubMed] [Google Scholar]
- 4.Freedman DS, Katzmarzyk PT, Dietz WH, Srinivasan SR, Berenson GS. Relation of body mass index and skinfold thicknesses to cardiovascular disease risk factors in children: the Bogalusa Heart Study. Am J Clin Nutr. 2009;90:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin RS, Caprio S. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity [published correction appears in N Engl J Med 2002;346(22):1756]. N Engl J Med. 2002;346(11):802–810. [DOI] [PubMed] [Google Scholar]
- 6.Goran MI, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab. 2003;88(4):1417–1427. [DOI] [PubMed] [Google Scholar]
- 7.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29(11):2427–2432. [DOI] [PubMed] [Google Scholar]
- 8.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, Dietz WH, Horlick M. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes. 2005;29(1):1–8. [DOI] [PubMed] [Google Scholar]
- 9.Maynard LM, Wisemandle W, Roche AF, Chumlea WC, Guo SS, Siervogel RM. Childhood body composition in relation to body mass index. Pediatrics. 2001;107(2):344–350. [DOI] [PubMed] [Google Scholar]
- 10.Lawlor DA, Benfield L, Logue J, Tilling K, Howe LD, Fraser A, Cherry L, Watt P, Ness AR, Davey Smith G, Sattar N. Association between general and central adiposity in childhood, and change in these, with cardiovascular risk factors in adolescence: prospective cohort study. BMJ. 2010;341:c6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spolidoro JV, Pitrez Filho ML, Vargas LT, Santana JC, Pitrez E, Hauschild JA, Bruscato NM, Moriguchi EH, Medeiros AK, Piva JP. Waist circumference in children and adolescents correlate with metabolic syndrome and fat deposits in young adults. Clin Nutr. 2013;32(1):93–97. [DOI] [PubMed] [Google Scholar]
- 12.Ali O, Cerjak D, Kent JW Jr, James R, Blangero J, Zhang Y. Obesity, central adiposity and cardiometabolic risk factors in children and adolescents: a family-based study. Pediatr Obes. 2014; 9(3):e58–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller NT, Pereira MA, Buitrago-Lopez A, Rodríguez DC, Duran AE, Ruiz AJ, Rueda-Clausen CF, Villa-Roel C. Adiposity indices in the prediction of insulin resistance in prepubertal Colombian children. Public Health Nutr. 2013;16(2):248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garnett SP, Baur LA, Srinivasan S, Lee JW, Cowell CT. Body mass index and waist circumference in midchildhood and adverse cardiovascular disease risk clustering in adolescence. Am J Clin Nutr. 2007;86(3):549–555. [DOI] [PubMed] [Google Scholar]
- 15.Weber DR, Leonard MB, Shults J, Zemel BS. A comparison of fat and lean body mass index to BMI for the identification of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab. 2014;99(9):3208–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotlyarevska K, Wolfgram P, Lee JM. Is waist circumference a better predictor of insulin resistance than body mass index in U.S. adolescents? J Adolesc Health. 2011;49(3):330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khoury M, Manlhiot C, McCrindle BW. Role of the waist/height ratio in the cardiometabolic risk assessment of children classified by body mass index. J Am Coll Cardiol. 2013;62(8):742–751. [DOI] [PubMed] [Google Scholar]
- 18.Messiah SE, Arheart KL, Lipshultz SE, Miller TL. Body mass index, waist circumference, and cardiovascular risk factors in adolescents. J Pediatr. 2008;153(6):845–850. [DOI] [PubMed] [Google Scholar]
- 19.Ademolu AB, Ademolu AO, Ipadeola A, Ogbera A. Body mass index, waist circumference, waist hip ratio as predictors of obesity and abdominal obesity in Ikorodu: a community survey. Available at: http://journalofasianhealth.com/body-mass-indexwaist-circumferencewaist-hip-ratio-as-predictors-of-obesity-and-abdominal-obesity-in-ikorodua-community-survey/. Accessed 26 June 2016.
- 20.Czernichow S, Kengne AP, Huxley RR, Batty GD, de Galan B, Grobbee D, Pillai A, Zoungas S, Marre M, Woodward M, Neal B, Chalmers J, Group AC; ADVANCE Collaborative Group . Comparison of waist-to-hip ratio and other obesity indices as predictors of cardiovascular disease risk in people with type-2 diabetes: a prospective cohort study from ADVANCE. Eur J Cardiovasc Prev Rehabil. 2011;18(2):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nightingale CM, Rudnicka AR, Owen CG, Wells JC, Sattar N, Cook DG, Whincup PH. Influence of adiposity on insulin resistance and glycemia markers among U.K. children of South Asian, black African-Caribbean, and white European origin: child heart and health study in England. Diabetes Care. 2013;36(6):1712–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cossio S, Messiah SE, Garibay-Nieto N, Lopez-Mitnik G, Flores P, Arheart KL, Carrillo-Iregui A. How do different indices of obesity correlate with cardiometabolic disease risk factors in multiethnic youths? Endocr Pract. 2009;15(5):403–409. [DOI] [PubMed] [Google Scholar]
- 23.Blüher S, Molz E, Wiegand S, Otto KP, Sergeyev E, Tuschy S, L’Allemand-Jander D, Kiess W, Holl RW, Adiposity Patients Registry I; Adiposity Patients Registry Initiative and German Competence Net Obesity . Body mass index, waist circumference, and waist-to-height ratio as predictors of cardiometabolic risk in childhood obesity depending on pubertal development. J Clin Endocrinol Metab. 2013;98(8):3384–3393. [DOI] [PubMed] [Google Scholar]
- 24.Crocker MK, Stern EA, Sedaka NM, Shomaker LB, Brady SM, Ali AH, Shawker TH, Hubbard VS, Yanovski JA. Sexual dimorphisms in the associations of BMI and body fat with indices of pubertal development in girls and boys. J Clin Endocrinol Metab. 2014;99(8):E1519–E1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang RC, de Klerk N, Mori TA, Newnham JP, Stanley FJ, Landau LI, Oddy WH, Hands B, Beilin LJ. Differential relationships between anthropometry measures and cardiovascular risk factors in boys and girls. Int J Pediatr Obes. 2011;6(2-2):e271–e282. [DOI] [PubMed] [Google Scholar]
- 26.Demmer RT, Zuk AM, Rosenbaum M, Desvarieux M. Prevalence of diagnosed and undiagnosed type 2 diabetes mellitus among US adolescents: results from the continuous NHANES, 1999–2010. Am J Epidemiol. 2013;178(7):1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fagot-Campagna A, Saaddine JB, Flegal KM, Beckles GL; Third National Health and Nutrition Examination Survey . Diabetes, impaired fasting glucose, and elevated HbA1c in U.S. adolescents: the Third National Health and Nutrition Examination Survey. Diabetes Care. 2001;24(5):834–837. [DOI] [PubMed] [Google Scholar]
- 28.Cruz ML, Bergman RN, Goran MI. Unique effect of visceral fat on insulin sensitivity in obese Hispanic children with a family history of type 2 diabetes. Diabetes Care. 2002;25(9):1631–1636. [DOI] [PubMed] [Google Scholar]
- 29.McFarlin BK, Johnston CA, Tyler C, O’Connor DP, Strohacker KA, Reeves R, Jackson AS, Foreyt JP. Relation between adiposity and disease risk factors in Mexican American children. J Pediatr Gastroenterol Nutr. 2009;49(4):450–455. [DOI] [PubMed] [Google Scholar]
- 30.Rentfro AR, Nino JC, Pones RM, Innis-Whitehouse W, Barroso CS, Rahbar MH, McCormick JB, Fisher-Hoch SP. Adiposity, biological markers of disease, and insulin resistance in Mexican American adolescents, 2004-2005. Prev Chronic Dis. 2011;8(2):A40. [PMC free article] [PubMed] [Google Scholar]
- 31.Isasi CR, Carnethon MR, Ayala GX, Arredondo E, Bangdiwala SI, Daviglus ML, Delamater AM, Eckfeldt JH, Perreira K, Himes JH, Kaplan RC, Van Horn L. The Hispanic Community Children’s Health Study/Study of Latino Youth (SOL Youth): design, objectives, and procedures. Ann Epidemiol. 2014;24(1):29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavange LM, Kalsbeek WD, Sorlie PD, Avilés-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, Criqui MH, Elder JP. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M, Lavange L, Chambless LE, Heiss G. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. [DOI] [PubMed] [Google Scholar]
- 35.Collaborative Studies Coordinating Center at The University of North Carolina at Chapel Hill. Hispanic Community Health Study/Study of Latinos Manual 7 Addendum: Central Laboratory Procedures Version 1.0. 2011. https://www2.cscc.unc.edu/hchs/protocols-and-manuals. Accessed 7 December 2016.
- 36.Collaborative Studies Coordinating Center at The University of North Carolina at Chapel Hill. Hispanic Community Children's Health/Study of Latino Youth (SOL Youth) Manual 1: Field Center Procedures Version 3.1.2013. https://www2.cscc.unc.edu/hchs/Youth. Accessed 7 December 2016.
- 37.Center for Disease Control and Prevention. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years). http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. Accessed 26 March 2014.
- 38.Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007-2010. Vital Health Stat 11. 2012;Oct(252):1–48.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25204692&dopt=Abstract [PubMed] [Google Scholar]
- 39.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17(2):117–133. [DOI] [PubMed] [Google Scholar]
- 40.Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bond L, Clements J, Bertalli N, Evans-Whipp T, McMorris BJ, Patton GC, Toumbourou JW, Catalano RF. A comparison of self-reported puberty using the Pubertal Development Scale and the Sexual Maturation Scale in a school-based epidemiologic survey. J Adolesc. 2006;29(5):709–720. [DOI] [PubMed] [Google Scholar]
- 42.Dugas LR, Cao G, Luke AH, Durazo-Arvizu RA. Adiposity is not equal in a multi-race/ethnic adolescent population: NHANES 1999-2004. Obesity (Silver Spring). 2011;19(10):2099–2101. [DOI] [PubMed] [Google Scholar]
- 43.Brambilla P, Bedogni G, Moreno LA, Goran MI, Gutin B, Fox KR, Peters DM, Barbeau P, De Sim1 M, Pietrobelli A. Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int J Obes. 2006;30(1):23–30. [DOI] [PubMed] [Google Scholar]
- 44.Staiano AE, Katzmarzyk PT. Ethnic and sex differences in body fat and visceral and subcutaneous adiposity in children and adolescents. Int J Obes. 2012;36(10):1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moran A, Jacobs DR Jr, Steinberger J, Hong CP, Prineas R, Luepker R, Sinaiko AR. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48(10):2039–2044. [DOI] [PubMed] [Google Scholar]
- 46.Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16(1):10–15. [DOI] [PubMed] [Google Scholar]
- 47.Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care. 2004;27(2):314–319. [DOI] [PubMed] [Google Scholar]
- 48.Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144(1):47–55. [DOI] [PubMed] [Google Scholar]