Abstract
Context:
Among women with a single, recent pregnancy loss, daily preconception low-dose aspirin (LDA) increased the live birth rate with no effect on pregnancy loss. Ovulation is a potential mechanism underlying this effect.
Objective:
We estimated the effect of LDA on the per-cycle risk of anovulation among eumenorrheic women.
Design:
Multicenter, randomized, double-blind, placebo-controlled trial of daily LDA on reproductive outcomes. Preconception follow-up lasted 1 to 6 menstrual cycles (ClinicalTrials.gov, NCT00467363).
Setting:
Four US medical centers during 2007 to 2011.
Patients or Other Participants:
Healthy women (n = 1214), age 18 to 40, were attempting pregnancy, had regular menstrual cycles (21 to 42 days), and had a history of 1 to 2 documented pregnancy losses, ≤2 live births, and no infertility. All participants completed at least 1 menstrual cycle of follow-up; none withdrew due to adverse events.
Intervention:
Aspirin (81 mg) daily for 1 to 6 menstrual cycles.
Main Outcome Measure:
Per-cycle risk of anovulation, defined as the absence of both a positive spot-urine pregnancy test and a luteinizing hormone (LH) peak (2.5-fold increase in daily urinary LH). Hypothesis formulation preceded data collection.
Results:
Among 4340 cycles, LDA was not associated with anovulation (LDA: 13.4%, placebo: 11.1%; risk ratio = 1.16, 95% confidence interval, 0.88 to 1.52). Results were similar among women with a single, recent loss.
Conclusions:
Daily LDA had no effect on anovulation among women with a history of 1 to 2 pregnancy losses. LDA may affect fertility via other pathways, and these warrant further study.
Précis: Using daily measures of luteinizing hormone from 1214 eumenorrheic women (3430 menstrual cycles) in the EAGeR Trial, we observed no effect of low-dose aspirin vs placebo on anovulation.
Preconception daily low-dose aspirin (LDA) increased the chances of pregnancy and live birth, with no effect on pregnancy loss, among women attempting to conceive spontaneously after a single recent loss (1, 2). Yet, reasons for this effect of LDA are unclear. Possible LDA targets include an effect on preventing sporadic anovulation (3), improving tubal transport of the gametes and embryo (4), and supporting implantation (5, 6). Sporadic anovulation, defined by low luteal progesterone, has been reported to occur in 0% to 11% of cycles in large studies of eumenorrheic women age <40 (7–11).
The effect of LDA on ovulation is uncertain; available data are conflicting and largely indirect (3, 12–15). Because LDA reduces vasoconstriction and platelet aggregation, it may support ovulation by improving ovarian blood flow (16). Evidence from clinical trials is mixed as to whether LDA increases ovarian responsiveness and blood flow among women undergoing in vitro fertilization (IVF) (15, 17–19). Furthermore, self-reported use of over-the-counter analgesics during the follicular phase was associated with a lower risk of sporadic anovulation (3). In contrast, LDA’s inhibition of cyclooxygenase (COX) may be detrimental to ovulation, as the COX-2 isoform performs requisite prostaglandin synthesis for ovulation (12–14).
Given the uncertain effect of aspirin on ovulation, we evaluated the effect of LDA on sporadic anovulation among women in the Effects of Aspirin in Gestation and Reproduction (EAGeR) Trial, a fecund population with regular menstrual cycles.
Materials and Methods
Participants and study design
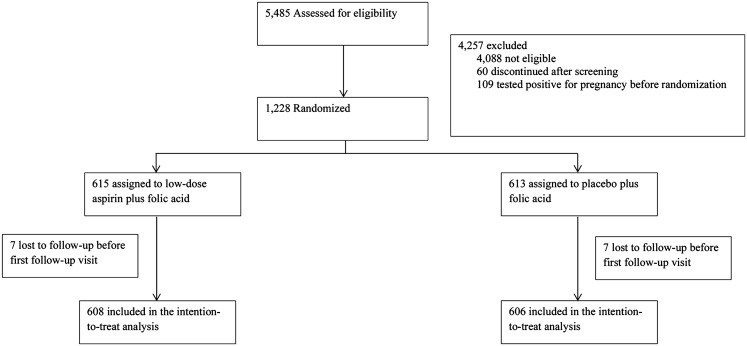
The EAGeR Trial, a block-randomized, double-blind, placebo-controlled trial of daily preconception 81 mg of aspirin to increase live birth was conducted from 2007 to 2011 at 4 US clinical centers (ClinicalTrials.gov no. NCT00467363) (20). Participants had a history of 1 to 2 pregnancy losses and were attempting to conceive spontaneously. They were 18 to 40 years old, had regular menstrual cycles, 21 to 42 days in length, and had no diagnosis of infertility, reproductive health disorder, or major medical condition (20). There were 2 strata of eligibility criteria: original (1 documented pregnancy loss at <20 weeks’ gestation in the past 12 months; ≤1 prior live birth) and expanded (1 to 2 documented pregnancy losses at any gestational age, at any time in the past; ≤2 prior live births). Of 1228 women who enrolled (615 LDA, 613 placebo), the current study excluded 14 women who withdrew before the first follow-up visit (7 LDA, 7 placebo; Fig. 1).
Figure 1.
Consolidated Standards of Reporting Trials algorithm, the EAGeR Trial (United States, 2007 to 2011).
Randomization occurred on days 2 to 4 of menses. The Data Coordinating Center used an automated algorithm with a permuted block design, consisting of blocks of 6 or 8 in random order that were defined by eligibility criteria stratum and study center. Participants took the study pill (LDA or matching placebo, 1:1) plus 400 mcg of folic acid daily for up to 6 menstrual cycles until conception and, if they became pregnant, until gestational week 36. The study protocol was approved by the institutional review board at each clinical center, and participants provided written informed consent.
Outcome assessment
Anovulation in a given menstrual cycle was a secondary outcome of the EAGeR Trial. Participants used fertility monitors (Clearblue®, Alere, Waltham, MA) daily throughout the menstrual cycle to measure luteinizing hormone (LH) and estrone-3-gluconuride concentrations in first morning urine (21) according to study instructions. The test stick measures urinary LH via a classical sandwich assay, and the monitor optically reads the intensity of the line on the test stick (21). Testing dates and times, hormone values, and associated fertility status were recorded on the devices and downloaded from the monitor’s internal memory chip at each end-of-cycle clinic visit. Computerized monitor data were checked for accuracy of dates, missed tests, and test malfunctions.
Systematic use of pregnancy tests (QuickVue™, Quidel, San Diego, CA) at home and at end-of-cycle clinic visits identified 776 pregnancies. In addition, 21 very early positive human chorionic gonadotropin (hCG) pregnancies were documented after analysis of stored urine specimens by using sequential laboratory assays for free beta hCG (initial test: catalog no. RIS0011R, BioVendor, Asheville, NC; confirmatory test: catalog no. 4221-16, Diagnostic Automation Inc., Calabasas, CA). These stored specimens included spot urine samples collected at each end-of-cycle clinic visit and first morning urine collected and frozen at home on the last 10 days of the first 2 menstrual cycles of follow-up. A cycle was considered anovulatory in the absence of both (a) an LH surge as defined below and (b) an hCG-detected pregnancy.
Algorithms to detect the LH surge
A menstrual cycle was considered to have no LH surge if there was no ≥2.5-fold increase in LH above the previous 5-day mean (9). We selected this algorithm as the primary definition out of several others that were previously compared (22) for its stringent criterion for lack of an LH surge, thus reducing the potential for a false-positive outcome and concomitant bias to the risk ratio (23). To evaluate this algorithm’s performance in our data, we estimated its percent agreement for classifying anovulation with that of 3 other algorithms (21, 24, 25), plus a summary measure indicating the cycle met the criteria of ≥2 of the algorithms. The 3 alternative algorithms defined an LH surge as follows: (a) Clearblue® fertility monitor’s detection of urinary LH concentration >30 IU/L (approximate value as the algorithm is proprietary) (21), (b) a fourfold increase in LH from the previous day’s value (24), and (c) an LH value that exceeded both the mean plus 2 times the standard deviation (SD) of the previous 5 days, and the mean plus 2 times the SD of the next 5 days (25).
Statistical analysis
Participants contributed menstrual cycles at risk for anovulation from the cycle in which they were randomized until they had an hCG-detected pregnancy, completed 6 menstrual cycles without a pregnancy, or withdrew from the study, whichever came first. Thus, multiple outcomes could be clustered within a participant. Log-binomial models estimated risk ratios (RRs) and 95% confidence intervals (CIs) for anovulation in a randomly chosen cycle from the LDA vs the placebo group. The generalized estimating equations method with an unstructured correlation matrix accounted for within-participant correlated outcomes (26). Sensitivity analyses using other correlation structures (autoregressive, independent) produced similar results (Supplemental Table 1 (16.8KB, docx) ). The per-cycle risk of anovulation increased with the number of cycles observed, indicating informative cluster size (27). We adjusted for this by weighting each observation by the inverse of the cluster size (27). Key features of the data and methods to account for them in the analysis are highlighted in Table 1.
Table 1.
Low-Dose Aspirin and Risk of Anovulation in the EAGeR Trial (United States, 2007 to 2011): Key Features of the Data and Analysis
| Data | Analysis |
|---|---|
| Clustered outcome data | |
| —1 to 6 outcomes per woman based on menstrual cycles contributed to follow-up | Accounted for within-woman correlated outcomes with generalized estimating equations extension of the log-binomial regression model |
| —Number of menstrual cycles contributed was associated with outcome | Each woman’s data were weighted to equalize the amount of data contributed to analysis |
| Missing outcome data | |
| —Multiple imputation procedure produced 20 imputed datasets with plausible values for missing outcomes | Final results were summarized from the results from 20 imputed data sets |
| Sensitivity to missing data was assessed with a secondary analysis restricted to cycles with observed outcome |
We also stratified the analysis by category of trial eligibility criteria, because LDA increased live birth only in the original stratum (1). Because LDA was associated with an increase in the cycle-specific probability of pregnancy after cycle 1 in the original stratum (28), we performed a secondary analysis of data from cycles 2 to 6 to assess a lagged treatment effect of LDA on anovulation. To assess the sensitivity to the choice of LH surge algorithm, we repeated the primary analysis with anovulation defined using, in turn, each of the alternative algorithms.
Missing data on anovulation (18% of cycles) were imputed using a multiple imputation procedure that created 20 datasets (29, 30). Reasons for missing data on anovulation in a cycle were infrequent (≤10 testing days) or nonexistent use of the fertility monitor (8% and 2% of cycles, respectively), testing malfunctions (1% of cycles), or, if no LH surge was detected, missing tests on or around the anticipated day of the LH surge (7% of cycles). The anticipated day of the LH surge was defined as 15 days before the start of menses (31). We assessed the sensitivity of our results to missing data with a secondary analysis of the cycles that had complete outcome data. SAS Software® version 9.4 (SAS Institute, Cary, NC) was used for all analyses.
Results
The 1214 women (608 LDA, 606 placebo) had mean age of 28.8 years (SD = 4.8); they tended to be white, married, used, and to have a college education and annual household income ≥$75,000 (1). Recent hormonal contraceptive use and current regular smoking were each reported by 5% of participants. Baseline characteristics were balanced by treatment group (1).
Anovulation occurred in 530 (12.2%) of 4340 cycles. Comparing the primary method of classifying anovulation with each of the alternative algorithms among cycles with valid, complete data, percent agreement ranged from 72.6% to 92.1% (Table 2). Percent agreement among the 5 algorithms ranged from 66.4% to 92.1%.
Table 2.
Comparison of a Summary Algorithm With 4 Previously Reported Algorithms To Classify Anovulation: 3565 Cycles With Complete Anovulation Data From 1164 Women in the EAGeR Trial (United States, 2007 to 2011)
| Anovulatory Cycles |
Percent Agreement With Other Algorithms |
||||||
|---|---|---|---|---|---|---|---|
| Algorithm | No. | % | Behre et al. 2000 (21)a | Park et al. 2007 (9)b | Johansson et al. 1971 (24)c | Brown 1977 (25)d | Summarye |
| Behre et al. 2000a | 627 | 18.0 | 100 | 85.9 | 66.4 | 80.4 | 88.6 |
| Park et al. 2007b | 409 | 11.5 | 85.9 | 100 | 72.6 | 85.4 | 92.1 |
| Johansson et al. 1971c | 1127 | 32.4 | 66.4 | 72.6 | 100 | 68.1 | 77.4 |
| Brown 1977d | 506 | 14.5 | 80.4 | 85.4 | 68.1 | 100 | 89.0 |
| Summarye | 649 | 18.2 | 88.6 | 92.1 | 77.4 | 89.0 | 100 |
Cycle has no “peak fertility” reading on the monitor, approximately corresponding to no urinary LH >30 IU/L (15), and has no hCG-detected pregnancy. Estimates were calculated from 3565 cycles with complete anovulation data according to both this algorithm and that of Park et al. 2007 (16).
Cycle has no increase in LH ≥2.5-fold from mean of prior 5 days (16) and has no hCG-detected pregnancy. Estimates were calculated from 3565 cycles with complete anovulation data according to this algorithm.
Cycle has no increase in LH ≥fourfold from previous day’s value (19) and has no hCG-detected pregnancy. Estimates were calculated from 3476 cycles with complete anovulation data according to both this algorithm and that of Park et al. 2007 (9).
Cycle has no LH value exceeds both (1) the sum of the mean and 2 times the SD of the prior 5 days, and (2) the sum of the mean and 2 times the SD of the next 5 days (20) and has no hCG-detected pregnancy. Estimates were calculated from 3489 cycles with complete anovulation data according to both this algorithm and that of Park et al. 2007 (9).
Cycle meets the anovulation criteria of ≥2 of the previous 4 algorithms and has no hCG-detected pregnancy.
LDA did not appreciably affect the occurrence of anovulation (13.4% LDA, 11.1% placebo; RR = 1.16, 95% CI, 0.88 to 1.52; Table 3). Stratified by category of trial eligibility criteria, the RRs were 1.06 (95% CI, 0.68 to 1.54) in the original stratum and 1.24 (95% CI, 0.86 to 1.79) in the expanded stratum. The secondary analysis of cycles 2 to 6 and an analysis restricted to cycles with complete data both produced results that were similar to those from the primary analysis (Table 3). The sensitivity analyses of LDA and anovulation using alternative algorithms to define the lack of an LH surge produced RRs from 0.96 to 1.14.
Table 3.
Low-Dose Aspirin and Risk of Anovulation: 4340 Menstrual Cycles From 1214 Women in the EAGeR Trial (United States, 2007 to 2011)
| Overall |
Cycles 2 to 6b |
Complete Outcomec |
|||||
|---|---|---|---|---|---|---|---|
| Anovulatory Cycles N (%) | RRa | 95% CIa | RRa | 95% CIa | RRa | 95% CIa | |
| —Placebo | 245 (11.1) | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| —Low-dose aspirin | 285 (13.4) | 1.16 | 0.88 to 1.52 | 1.07 | 0.80 to 1.42 | 1.14 | 0.88 to 1.47 |
| Stratified by eligibility criteria | |||||||
| Original | |||||||
| —Placebo | 101(10.1) | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| —Low-dose aspirin | 101 (11.1) | 1.06 | 0.68 to 1.54 | 0.88 | 0.55 to 1.41 | 1.08 | 0.72 to 1.63 |
| Expanded | |||||||
| —Placebo | 144 (12.0) | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| —Low-dose aspirin | 184 (15.1) | 1.24 | 0.86 to 1.79 | 1.24 | 0.85 to 1.80 | 1.19 | 0.85 to 1.66 |
Analyses were performed using log-binomial regression models with the generalized estimating equations method to account for within-participant correlated outcomes. Women were block randomized by stratum of eligibility criteria: original, women with 1 prior pregnancy loss <20 weeks’ gestation, which occurred in the 12 months before enrollment; expanded, women not eligible for the original stratum, and with 1 or 2 prior pregnancy losses of any gestational age occurring any time before enrollment.
RR and 95% CI are adjusted for number of cycles contributed to follow-up.
This analysis includes 3126 cycles from 947 women in cycles 2 to 6.
This analysis includes 3565 cycles (1164 women) with valid and complete information for determining anovulation based on test results on ≥10 days and in the middle of the cycle.
Discussion
In a randomized, controlled trial of women attempting pregnancy after 1 to 2 pregnancy losses, LDA did not affect the occurrence of anovulation overall or by trial eligibility stratum. Excluding the first cycle of follow-up to examine a lagged effect of treatment did not change the findings. Previously, the EAGeR Trial found that LDA increased pregnancies and live births among women with a single recent loss, with no effect on pregnancy loss (1, 2), raising the question of which stage of the reproductive process was affected by LDA to increase live births. The present results clarify that anovulation was not the stage affected by LDA. Instead, LDA may have a beneficial effect on fertility through other mechanisms that affect the establishment of pregnancy, including tubal transport of the gametes and embryo, embryonic development, and implantation.
These findings help elucidate the potential effect of LDA on anovulation, which was uncertain given mixed results from previous studies of COX inhibitors. Previous studies have found that indomethacin (32, 33), oral bromfenac (33), and rofecoxib (14) were associated with delayed follicular rupture in reproductive-age women with regular menses. However, over-the-counter analgesic use, most commonly ibuprofen or acetaminophen, in the follicular phase was associated with a reduced risk of sporadic anovulation among regularly cycling women (3). Differences in the drugs and dosages studied may explain the conflicting results. Also, among women undergoing IVF, the evidence is mixed from trials of LDA initiated prior to gonadotropin treatment on ovarian responsiveness (15, 17–19).
Previous, indirect evidence also supports our findings as LDA inhibits COX-1 more selectively than COX-2 (34–36), which is the critical isoform to enable ovulation (12). Indeed, COX-2-null female mice had substantial ovulatory dysfunction (12), whereas fertility was normal in COX-1-null female mice (37). Therefore, our results may be explained by LDA’s less-potent inhibition of COX-2, which allows adequate activity for ovulation. Furthermore, the present findings show that LDA does not improve ovulation among fecund women, contradicting the theory that LDA may support ovulation by blocking thromboxane A2 production by prostaglandin H2, thus decreasing platelet aggregation and vasoconstriction (38) and supporting the natural increase in uterine blood flow velocity as ovulation approaches (39). It may be that LDA improves ovulatory function among women with an ovulatory disorder, although the limited evidence on LDA initiated prior to gonadotropin treatment as an auxiliary treatment of women undergoing IVF is mixed (15, 17–19).
Considering that our data do not support an effect on ovulation, alternative targets for LDA to support the establishment of pregnancy include fertilization, embryonic development, and implantation. Evidence from IVF patients suggests that LDA may help implantation by ameliorating poor uterine blood flow (40–43). Also, LDA may have dampened overactive endometrial inflammation, which has been observed among women with a history of pregnancy loss (6, 44, 45). We speculate that women with a single, recent pregnancy loss may have responded to LDA’s actions to increase vascular perfusion and decrease inflammation due to residual changes in their endometrial vasculature (46–48). Furthermore, if LDA increased vascular perfusion in the ovary and/or fallopian tubes, this may have improved the developmental competency of the oocyte (49) and aided transport of the gametes and embryo (50), thus supporting fertilization and embryonic development.
These results represent a high standard of evidence, as they come from a randomized, double-blind, placebo-controlled trial with over 4000 cycles observed, of which 530 were anovulatory. Study retention was high (94% of women completed follow-up with respect to pregnancy), as was compliance with the fertility monitor (82% of cycles had complete, valid data) and study medication (1). The fertility monitor that measured urinary LH has demonstrated 91% accuracy in predicting ovulation determined by transvaginal ultrasound (21). The results were not sensitive to the algorithm for determining the lack of an LH surge, among 4 algorithms that had been validated with progesterone, laparoscopy, or transvaginal ultrasound (9, 21, 24, 25). Among these algorithms, the percent agreement statistics were similar to the results from a different cohort (22).
Our study also has several limitations to consider. Using daily testing of urinary LH probably misclassified some ovulatory cycles as anovulatory due to missed tests or test malfunctions around the LH surge, in addition to the inaccuracy inherent in the test (21). Indeed, the observed frequency of anovulation was higher than reports from prior studies of luteal progesterone among women with regular menstrual cycles [12.2% vs 0% to 11% (7–10)]. This misclassification would have biased our results toward the null (51), but we expect the outcome measure has acceptable validity for research purposes due to the overall high compliance, detailed data checks to maximize data completeness, and multiple imputation to address missing outcome data. In addition, no power calculation was performed—as this was a secondary outcome of the trial—and so we cannot conclude the absence of a small effect of LDA with certainty. Also, because EAGeR enrolled healthy, regularly cycling, fecund women, our results may not apply to women diagnosed with ovulatory or inflammatory disorders.
In summary, LDA did not affect sporadic anovulation among regularly cycling women with a history of 1 to 2 pregnancy losses in a randomized controlled trial. These findings clarify that ovulation was not the reproductive event affected by LDA to increase pregnancy and live birth among women who were attempting to conceive spontaneously after a single recent loss (1). Possible effects of LDA to improve tubal transport of the gametes and embryo, embryonic development, and implantation merit further study.
Acknowledgments
The authors thank Dr. Janet Townsend, Dr. Anne Lynch, and Dr. David Faraggi for contributing to study management and operations; Dr. Aijun Ye for contributing to data management; and the EAGeR participants for their outstanding commitment to the study.
Acknowledgments
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (Contract Nos. HHSN267200603423, HHSN267200603424, and HHSN267200603426).
Acknowledgments
Clinical trial registry: ClinicalTrials.gov no. NCT00467363 (April 27, 2007).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- COX
- cyclooxygenase
- EAGeR
- Effects of Aspirin in Gestation and Reproduction
- hCG
- human chorionic gonadotropin
- IVF
- in vitro fertilization
- LDA
- low-dose aspirin
- LH
- luteinizing hormone
- RR
- risk ratio.
References
- 1.Schisterman EF, Silver RM, Lesher LL, Faraggi D, Wactawski-Wende J, Townsend JM, Lynch AM, Perkins NJ, Mumford SL, Galai N. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet. 2014;384(9937):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mumford SL, Silver RM, Sjaarda LA, Wactawski-Wende J, Townsend JM, Lynch AM, Galai N, Lesher LL, Faraggi D, Perkins NJ, Schliep KC, Zarek SM, Schisterman EF. Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum Reprod. 2016;31(3):657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matyas RA, Mumford SL, Schliep KC, Ahrens KA, Sjaarda LA, Perkins NJ, Filiberto AC, Mattison D, Zarek SM, Wactawski-Wende J, Schisterman EF. Effects of over-the-counter analgesic use on reproductive hormones and ovulation in healthy, premenopausal women. Hum Reprod. 2015;30(7):1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum Reprod Update. 2006;12(4):363–372. [DOI] [PubMed] [Google Scholar]
- 5.Macklon NS, Brosens JJ. The human endometrium as a sensor of embryo quality. Biol Reprod. 2014;91(4):98. [DOI] [PubMed] [Google Scholar]
- 6.Galgani M, Insabato L, Cali G, Della Gatta AN, Mirra P, Papaccio F, Santopaolo M, Alviggi C, Mollo A, Strina I, Matarese G, Beguinot F, De Placido G, Ulianich L Regulatory T cells, inflammation, and endoplasmic reticulum stress in women with defective endometrial receptivity. Fertil Steril 2015;103(6):1579–1586.e1. [DOI] [PubMed] [Google Scholar]
- 7.Haiman CA, Pike MC, Bernstein L, Jaque SV, Stanczyk FZ, Afghani A, Peters RK, Wan P, Shames L. Ethnic differences in ovulatory function in nulliparous women. Br J Cancer. 2002;86(3):367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malcolm CE, Cumming DC. Does anovulation exist in eumenorrheic women? Obstet Gynecol. 2003;102(2):317–318. [DOI] [PubMed] [Google Scholar]
- 9.Park SJ, Goldsmith LT, Skurnick JH, Wojtczuk A, Weiss G. Characteristics of the urinary luteinizing hormone surge in young ovulatory women. Fertil Steril. 2007;88(3):684–690. [DOI] [PubMed] [Google Scholar]
- 10.Gaskins AJ, Mumford SL, Zhang C, Wactawski-Wende J, Hovey KM, Whitcomb BW, Howards PP, Perkins NJ, Yeung E, Schisterman EF; BioCycle Study Group . Effect of daily fiber intake on reproductive function: the BioCycle Study. Am J Clin Nutr. 2009;90(4):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsh EE, Shaw ND, Klingman KM, Tiamfook-Morgan TO, Yialamas MA, Sluss PM, Hall JE. Estrogen levels are higher across the menstrual cycle in African-American women compared with Caucasian women. J Clin Endocrinol Metab. 2011;96(10):3199–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91(2):197–208. [DOI] [PubMed] [Google Scholar]
- 13.Nargund G, Wei CC. Successful planned delay of ovulation for one week with indomethacin. J Assist Reprod Genet. 1996;13(8):683–684. [DOI] [PubMed] [Google Scholar]
- 14.Pall M, Fridén BE, Brännström M. Induction of delayed follicular rupture in the human by the selective COX-2 inhibitor rofecoxib: a randomized double-blind study. Hum Reprod. 2001;16(7):1323–1328. [DOI] [PubMed] [Google Scholar]
- 15.Rubinstein M, Marazzi A, Polak de Fried E. Low-dose aspirin treatment improves ovarian responsiveness, uterine and ovarian blood flow velocity, implantation, and pregnancy rates in patients undergoing in vitro fertilization: a prospective, randomized, double-blind placebo-controlled assay. Fertil Steril. 1999;71(5):825–829. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz RL, Schacky CV, Weber M, Meister W, Kotzur J, Reichardt B, Theisen K, Weber PC. Improved aortocoronary bypass patency by low-dose aspirin (100 mg daily). Effects on platelet aggregation and thromboxane formation. Lancet. 1984;1(8389):1261–1264. [DOI] [PubMed] [Google Scholar]
- 17.Lok IH, Yip SK, Cheung LP, Yin Leung PH, Haines CJ. Adjuvant low-dose aspirin therapy in poor responders undergoing in vitro fertilization: a prospective, randomized, double-blind, placebo-controlled trial. Fertil Steril. 2004;81(3):556–561. [DOI] [PubMed] [Google Scholar]
- 18.Päkkilä M, Räsänen J, Heinonen S, Tinkanen H, Tuomivaara L, Mäkikallio K, Hippeläinen M, Tapanainen JS, Martikainen H. Low-dose aspirin does not improve ovarian responsiveness or pregnancy rate in IVF and ICSI patients: a randomized, placebo-controlled double-blind study. Hum Reprod. 2005;20(8):2211–2214. [DOI] [PubMed] [Google Scholar]
- 19.Moini A, Zafarani F, Haddadian S, Ahmadi J, Honar H, Riazi K. Effect of low-dose aspirin therapy on implantation rate in women undergoing in-vitro fertilization cycles. Saudi Med J. 2007;28(5):732–736. [PubMed] [Google Scholar]
- 20.Schisterman EF, Silver RM, Perkins NJ, Mumford SL, Whitcomb BW, Stanford JB, Lesher LL, Faraggi D, Wactawski-Wende J, Browne RW, Townsend JM, White M, Lynch AM, Galai N. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: design and baseline characteristics. Paediatr Perinat Epidemiol. 2013;27(6):598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behre HM, Kuhlage J, Gassner C, Sonntag B, Schem C, Schneider HPG, Nieschlag E. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15(12):2478–2482. [DOI] [PubMed] [Google Scholar]
- 22.Lynch KE, Mumford SL, Schliep KC, Whitcomb BW, Zarek SM, Pollack AZ, Bertone-Johnson ER, Danaher M, Wactawski-Wende J, Gaskins AJ, Schisterman EF Assessment of anovulation in eumenorrheic women: comparison of ovulation detection algorithms. Fertil Steril 2014;102(2):511–518.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothman K, Greenland S, Lash T. Validity in epidemiologic studies. In: Rothman K, Greenland S, Lash T, eds. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2008:128–147. [Google Scholar]
- 24.Johansson ED, Wide L, Gemzell C. Luteinizing hormone (LH) and progesterone in plasma and LH and oestrogens in urine during 42 normal menstrual cycles. Acta Endocrinol (Copenh). 1971;68(3):502–512. [DOI] [PubMed] [Google Scholar]
- 25.Brown JB. Timing of ovulation. Med J Aust. 1977;2:780–783. [DOI] [PubMed] [Google Scholar]
- 26.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–375. [DOI] [PubMed] [Google Scholar]
- 27.Williamson JM, Datta S, Satten GA. Marginal analyses of clustered data when cluster size is informative. Biometrics. 2003;59(1):36–42. [DOI] [PubMed] [Google Scholar]
- 28.Schisterman EF, Mumford SL, Schliep KC, Sjaarda LA, Stanford JB, Lesher LL, Wactawski-Wende J, Lynch AM, Townsend JM, Perkins NJ, Zarek SM, Tsai MY, Chen Z, Faraggi D, Galai N, Silver RM. Preconception low dose aspirin and time to pregnancy: findings from the effects of aspirin in gestation and reproduction randomized trial. J Clin Endocrinol Metab. 2015;100(5):1785–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer JL. Analysis of incomplete multivariate data. Boca Raton, FL: CRC Press. doi:10.1201/9781439821862 [Google Scholar]
- 30.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. [DOI] [PubMed] [Google Scholar]
- 31.Steiner AZ, Pritchard DA, Young SL, Herring AH. Peri-implantation intercourse lowers fecundability. Fertil Steril. 2014;102(1):178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Killick S, Elstein M. Pharmacologic production of luteinized unruptured follicles by prostaglandin synthetase inhibitors. Fertil Steril. 1987;47(5):773–777. [DOI] [PubMed] [Google Scholar]
- 33.Priddy AR, Killick SR, Elstein M, Morris J, Sullivan M, Patel L, Elder M. The effect of prostaglandin synthetase inhibitors on human preovulatory follicular fluid prostaglandin, thromboxane, and leukotriene concentrations. J Clin Endocrinol Metab. 1990;71(1):235–242. [DOI] [PubMed] [Google Scholar]
- 34.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. [DOI] [PubMed] [Google Scholar]
- 35.Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101(10):1206–1218. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA. 1993;90(24):11693–11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83(3):483–492. [DOI] [PubMed] [Google Scholar]
- 38.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110(5–6):255–258. [DOI] [PubMed] [Google Scholar]
- 39.Battaglia C, Larocca E, Lanzani A, Valentini M, Genazzani AR. Doppler ultrasound studies of the uterine arteries in spontaneous and IVF stimulated ovarian cycles. Gynecol Endocrinol. 1990;4(4):245–250. [DOI] [PubMed] [Google Scholar]
- 40.Zaidi J, Campbell S, Pittrof R, Tan SL. Endometrial thickness, morphology, vascular penetration and velocimetry in predicting implantation in an in vitro fertilization program. Ultrasound Obstet Gynecol. 1995;6(3):191–198. [DOI] [PubMed] [Google Scholar]
- 41.Cacciatore B, Simberg N, Fusaro P, Tiitinen A. Transvaginal Doppler study of uterine artery blood flow in in vitro fertilization-embryo transfer cycles. Fertil Steril. 1996;66(1):130–134. [DOI] [PubMed] [Google Scholar]
- 42.Isaksson R, Tiitinen A, Cacciatore B. Uterine artery impedance to blood flow on the day of embryo transfer does not predict obstetric outcome. Ultrasound Obstet Gynecol. 2000;15(6):527–530. [DOI] [PubMed] [Google Scholar]
- 43.Haapsamo M, Martikainen H, Räsänen J. Low-dose aspirin and uterine haemodynamics on the day of embryo transfer in women undergoing IVF/ICSI: a randomized, placebo-controlled, double-blind study. Hum Reprod. 2009;24(4):861–866. [DOI] [PubMed] [Google Scholar]
- 44.Quenby S, Bates M, Doig T, Brewster J, Lewis-Jones DI, Johnson PM, Vince G. Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum Reprod. 1999;14(9):2386–2391. [DOI] [PubMed] [Google Scholar]
- 45.von Wolff M, Thaler CJ, Strowitzki T, Broome J, Stolz W, Tabibzadeh S. Regulated expression of cytokines in human endometrium throughout the menstrual cycle: dysregulation in habitual abortion. Mol Hum Reprod. 2000;6(7):627–634. [DOI] [PubMed] [Google Scholar]
- 46.Thaler I, Manor D, Itskovitz J, Rottem S, Levit N, Timor-Tritsch I, Brandes JM. Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol. 1990;162(1):121–125. [DOI] [PubMed] [Google Scholar]
- 47.Zhu BP, Rolfs RT, Nangle BE, Horan JM. Effect of the interval between pregnancies on perinatal outcomes. N Engl J Med. 1999;340(8):589–594. [DOI] [PubMed] [Google Scholar]
- 48.Renaud SJ, Cotechini T, Quirt JS, Macdonald-Goodfellow SK, Othman M, Graham CH. Spontaneous pregnancy loss mediated by abnormal maternal inflammation in rats is linked to deficient uteroplacental perfusion. J Immunol. 2011;186(3):1799–1808. [DOI] [PubMed] [Google Scholar]
- 49.Van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod. 1997;12(5):1047–1055. [DOI] [PubMed] [Google Scholar]
- 50.Eddy CA, Pauerstein CJ. Anatomy and physiology of the fallopian tube. Clin Obstet Gynecol. 1980;23(4):1177–1193. [DOI] [PubMed] [Google Scholar]
- 51.Rodgers A, MacMahon S. Systematic underestimation of treatment effects as a result of diagnostic test inaccuracy: implications for the interpretation and design of thromboprophylaxis trials. Thromb Haemost. 1995;73(2):167–171. [PubMed] [Google Scholar]