Abstract
Context:
Endometriosis results in aberrant gene expression in the eutopic endometrium (EuE) and subsequent progesterone resistance. MicroRNA (miR) microarray data in a baboon model of endometriosis showed an increased expression of miR-29c.
Objectives:
To explore the role of miR-29c in progesterone resistance in a subset of women with endometriosis.
Design:
MiR-29c expression was analyzed in the endometrium of baboons and women with or without endometriosis. The role in progesterone resistance and decidualization was analyzed by transfecting human uterine fibroblast cells with miR-29c.
Patients:
Subjects diagnosed with deep infiltrative endometriosis (DIE) by transvaginal ultrasound with bowel preparation underwent surgical excision of endometriosis. Eutopic secretory endometrium was collected pre- and postoperatively. Women with normal EuE and without DIE served as controls.
Results:
Quantitative reverse transcription polymerase chain reaction demonstrated that miR-29c expression increased, while the transcript levels of its target, FK506-binding protein 4 (FKBP4), decreased in the EuE of baboons following the induction of endometriosis. FKBP4 messenger RNA and decidual markers were statistically significantly decreased in decidualized human uterine fibroblast cells transfected with a miR-29c mimic compared with controls. Human data corroborated our baboon data and demonstrated higher expression of miR-29c in endometriosis EuE compared with normal EuE. MiR-29c was significantly decreased in endometriosis EuE postoperatively compared with preoperative tissues, and FKBP4 showed an inverse trend following radical laparoscopic resection surgery.
Conclusions:
We demonstrate that miR-29c expression is increased in EuE of baboons and women with endometriosis, which might contribute to a compromised progesterone response by diminishing the levels of FKBP4. Resection of DIE is likely to reverse the progesterone resistance associated with endometriosis in women.
Précis: MiR-29c is elevated in endometriotic eutopic endometrium resulting in decreased FKBP4 expression. These changes may contribute to progesterone resistance in a subset of women with endometriosis.
Endometriosis affects ∼10% women of reproductive age with a prevalence of 20% to 50% in infertile women and >70% in women with pelvic pain (1, 2). This gynecological disorder is characterized by the presence of ectopic endometrial tissue containing glands and stroma (3, 4). The etiology and natural history of endometriosis remain unclear. Sampson’s theory of retrograde menstruation, the most widely accepted etiology, proposes that menstrual tissue passing through the fallopian tube attaches to extrauterine peritoneum and grows under the influence of ovarian steroids (5). The actions of estrogen and progesterone are mediated through their cognate steroid receptor, which are expressed in both eutopic and ectopic endometrium (6). Gene array studies from our laboratory have demonstrated that ectopic endometriotic lesions alter eutopic endometrial gene expression as early as 1 month after disease induction in baboons, and these changes are similar to those seen in women with endometriosis (4, 7, 8). These data suggest that the eutopic endometrium (EuE) is initially characterized by a dominance of estrogen action (9, 10) and then followed by the development of progesterone resistance, reflected by decreased expression of progesterone-induced genes (7, 11, 12). The molecular mechanism responsible for the progesterone resistance remains unclear.
MicroRNA (miR) are single-stranded noncoding RNA molecules approximately 22 nucleotides in length that function as repressors of gene expression through messenger RNA (mRNA) cleavage and translational repression (13). Studies demonstrated that miR plays a role in the human reproductive physiology, and abnormal expression of miR has been observed in several diseases of the female reproductive tract, including endometriosis (14–19). Our study on miR-451 demonstrated a role of this miR in modulating cell proliferation (16). Hawkins et al. (20) have demonstrated a functional role for miR-29c dysregulating extracellular matrix genes in endometriomas. Previous work from our baboon studies demonstrated that a number of miRs are altered in the baboon model of endometriosis, including one of the most highly upregulated miRs in response to disease induction, miR-29c (16). In silico analysis demonstrated a miR-29c consensus binding site in the 3′-UTR of the FK506-binding protein 4 (FKBP4) gene, which affects embryonic implantation and decidualization (21, 22) and expression of which has been shown to be altered in the EuE of women and baboons with endometriosis (11, 23). Overexpression of miR-29c in human uterine fibroblast (HuF) cells inhibits decidualization (20), a process attenuated in endometriosis (12, 24, 25).
This study was designed to investigate the hypothesis that elevated miR-29c levels suppress FKBP4 expression in the EuE of baboons and women with endometriosis, contributing to progesterone resistance. To support our in vivo findings, a series of in vitro experiments were performed. HuF cells were transfected with a miR-29c mimic and subjected to an in vitro decidualization stimulus. FKBP4 levels, markers of progesterone action [decorin, homeobox A10 (HOXA10), and mitogen-inducible gene 6 (MIG6)], and decidualization (prolactin) were assessed. Because excision of deep infiltrative endometriosis (DIE) before in vitro fertilization improves pregnancy rates (26), we also compared miR-29c and FKBP4 expression in women with DIE pre- and postoperatively.
Materials and Methods
Induction of experimental endometriosis and collection of baboon tissues
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois, Chicago and Michigan State University. Endometriosis was experimentally induced in 3 female baboons (Papio anubis) by intraperitoneal inoculation with menstrual tissue on 2 consecutive menstrual cycles, as previously described (8, 27). In the cycle prior to the induction of endometriosis, control EuE (n = 3) was obtained at laparotomy on day 10 postovulation. Endometriosis was then induced in the same 3 animals by intraperitoneal inoculation of autologous menstrual tissue on 2 consecutive cycles. Following laparoscopic confirmation of endometriosis, EuE samples (n = 3) were obtained at 3 months postinduction of disease. At 15 to 16 months following the second inoculation, the animals (n = 3) were euthanized as required by the Institutional Animal Care and Use Committee, which permits a maximum of 4 invasive surgeries, and a necropsy was carried out to obtain all of the associated reproductive tissues within the peritoneal cavity. Samples were snap-frozen in liquid nitrogen for molecular analysis.
Patient samples
We obtained institutional review board approval from the School of Medicine of the University of São Paulo. Informed consent was obtained from patients. Patients were divided according to the result of the transvaginal ultrasound with bowel preparation (n = 26). Fifteen women with DIE underwent laparoscopic resection of the ectopic tissue, and 11 patients without DIE served as the control group. No statistical difference between the groups was observed in age, body mass index, basal follicle-stimulating hormone, basal estradiol, previous in vitro fertilization failure, and repeated abortion. Endometrial samples were collected from both groups. In the endometriosis group, samples were obtained before (Eosis preoperative) and after (Eosis postoperative) the surgery. Furthermore, ectopic tissue (Lesions) collected during the surgical intervention was also included in the analysis. Endometrial samples were obtained with a Pipelle curette (Pipelle de Cornier; Laboratoire C.C.D., Paris, France) and stored in RNAlater at −80°C. Only midsecretory phase samples were included. The groups were Eosis preoperative (n = 9), Eosis postoperative (n = 4), and control (n = 8).
Deep infiltrative endometriosis surgery
After preoperative evaluation, endometriotic lesions were laparoscopically excised. Surgeries were all performed by 2 experienced gynecological surgeons (14 and 10 years of experience) using similar techniques (i.e., scissors and diathermy) to remove all superficial and deep endometriotic lesions. These tissues were then examined histologically for the presence of disease.
Immunohistochemistry
Immunohistochemical staining for FKBP4 protein was performed using formalin-fixed, paraffin-embedded endometrial samples as previously described (11). Staining was analyzed at 20× using a Nikon (Tokyo, Japan) microscope. Assessment of staining intensity and distribution was via a semiquantitative H-score system by a single blinded observer (11, 28).
Isolation and culture of primary stromal cells
Primary eutopic endometrial stromal cells were isolated from the endometrial biopsy specimens from normally cycling women (n = 4) and women with endometriosis (n = 4) at both the University of North Carolina (Chapel Hill, NC) and the Greenville Hospital System (Greenville, SC) with informed consent (Supplemental Table 2 (18.2KB, docx) ). After trypsinization, dissociated cells were maintained and propagated in defined medium (phenol red–free RPMI 1640) with or without 10% fetal bovine serum. These cells isolated from each individual patient represent a proliferating population of nondifferentiated stromal cells and were used between passages 3 and 5 (12).
Isolation and culture of primary HuFs
HuF cells were isolated from the decidua parietalis, dissected from the placental membranes after vaginal delivery from 3 different individual subjects. Stromal cells were isolated as previously described (29). The institutional review board of Michigan State University approved these studies.
In vitro decidualization
Decidualization was induced in HuF cells (in triplicate) at 80% to 90% confluence in phenol red–free RPMI-1640 medium supplemented with 2% charcoal dextran stripped fetal bovine serum by treatment with EPC (0.5 mM dibutyryl-cAMP, 36 nM 17β-estradiol, and 1 μM medroxyprogesterone acetate in ethanol) for up to 8 days (12). Controls were treated with vehicle and the media were changed every 2 days.
RNA isolation and quantitative reverse transcription polymerase chain reaction
Total RNA was isolated using Trizol reagent (Invitrogen, Waltham, MA), and RNA quality check was performed using NanoDrop 2000 (Thermo Scientific, Waltham, MA). For measuring miR transcript levels in in vivo and in vitro samples, 100 ng total RNA was reverse transcribed using the TaqMan miR reverse transcription kit (Applied Biosystems, Foster City, CA) as per the manufacturer’s protocol and as previously described (16). For mRNA analysis, total RNA (1000 ng) was reverse transcribed using the High-Capacity cDNA Synthesis Kit (Applied Biosystems). After complementary DNA synthesis, the quantitative reverse transcription polymerase chain reaction was carried out for genes using specific primers (Supplemental Table 3 (18.2KB, docx) ) for FKBP4, decorin, HOXA10, MIG6, prolactin, and 18S (endogenous control) with SyBrGn PCR Master Mix (Applied Biosystems). All quantitative reverse transcription polymerase chain reactions were run for 40 cycles, and fold change was calculated using ∆∆Ct method (30).
3′ -UTR luciferase assay
HuF cells were seeded in triplicate and allowed to attach for 16 hours. The cells were then cotransfected with 2 plasmids and a miR mimic using Lipofectamine RNAiMax reagent (Invitrogen). 3′-UTR luciferase assay was performed using the Dual Luciferase Reporter Assay Kit (Promega, Madison, WI) and relative luciferase activity was measured according to the manufacturer’s protocol and as described previously (16). Three independent experiments were performed and data are presented as the mean ± SD.
Statistical analysis
Differences in miR and gene expression were compared following normalization against U6 and 18S, respectively. One-way analysis of variance was used to test the null hypothesis of group difference followed by Sidak’s multiple comparisons test. For in vitro studies, miR and gene expression was normalized against U6 and 18S. For data comparing only 2 groups, a nonparametric Mann-Whitney U test was used. GraphPad Prism 7.0 statistical software (GraphPad Software, La Jolla, CA) was used for analyzing the data.
Results
Induction of endometriosis leads to increased expression of miR-29c and decreased expression of its predicted target, FKBP4, in a baboon model of endometriosis
In the current study, we evaluated miR-29c expression by quantitative reverse transcription polymerase chain reaction and observed that the eutopic baboon endometrial miR-29c is upregulated ∼3-fold and ∼7-fold (P < 0.001) at 3 and 15 months after endometriosis induction, respectively [Fig. 1(A)]. To predict potential targets of miR-29c, TargetScan 6.2 software (Whitehead Institute for Biomedical Research, Boston, MA) (31, 32) resulted in 1238 potentially conserved targets, which included FKBP4. We focused on FKBP4, as we and others have demonstrated that FKBP4 plays a key role in endometrial progesterone action, and FKBP4 expression is decreased in EuE of baboons with endometriosis (11, 21, 33). FKBP4 mRNA was suppressed at 3 and 15 months in the eutopic endometrium of baboons with endometriosis compared with controls [Fig. 1(B)]. Decreased FKBP4 expression correlated with decreased expression of the progesterone-induced gene decorin (P < 0.05) in these samples [Fig. 1(C)] (34).
Figure 1.
Quantitative reverse transcription polymerase chain reaction analysis of miR-29c, FKBP4, and decorin expression in baboon tissues. (A) miR-29c expression was significantly increased in the eutopic endometrial biopsy specimens obtained during the midsecretory phase from baboons (n = 4) with endometriosis compared with controls (n = 3). Decreased expression of (B) FKBP4 and (C) decorin in the EuE of baboons following the induction of endometriosis. Data displayed as mean ± SD and analyzed by 1-way analysis of variance followed by Sidak’s multiple comparisons test (*P < 0.05). Con, without endometriosis; M, months.
FKBP4 expression is decreased in women with endometriosis
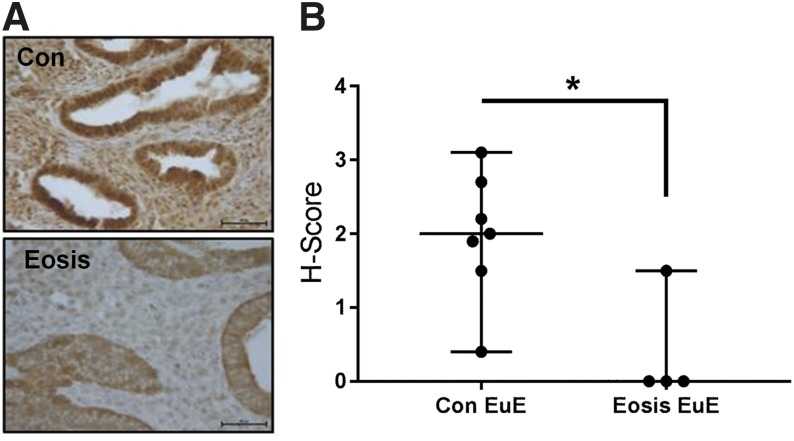
Immunohistochemistry [Fig. 2(A)] with H-score [Fig. 2(B)] analysis for FKBP4 protein in women with and without endometriosis was consistent with our previously reported baboon data (11) and in EuE of women with endometriosis (23). Although signal intensity for FKBP4 protein in both glandular epithelial cells and stromal cells decreased, this was more prominent in EuE stromal cells.
Figure 2.
Immunohistochemical and H-score analysis of FKBP4 protein expression in the eutopic endometrium of women. (A) Immunohistochemistry results show a decreased signal intensity for FKBP4 protein in both endometrial glandular epithelium and stroma of women with endometriosis compared with the women without disease. (B) Subsequent H-score analysis shows a significant decrease in FKBP4 protein expression in women with endometriosis (n = 7) compared with controls (n = 4). Data displayed as mean ± SD and analyzed by performing the Mann-Whitney test (P = 0.0152). Bar = 50 µm. Con, without endometriosis; Eosis, endometriosis.
MiR-29c regulates the expression of FKBP4 and progesterone-regulated genes in vitro
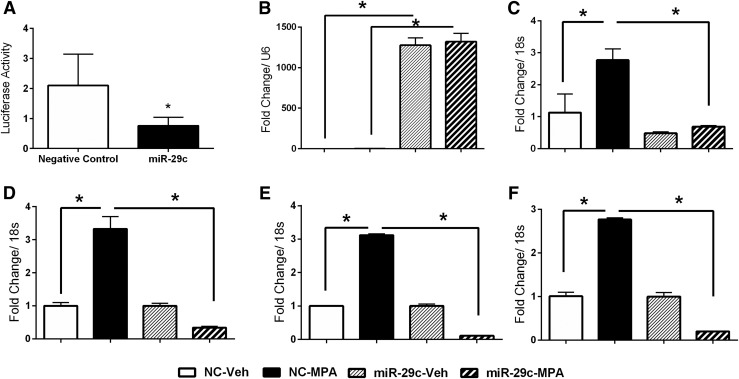
To investigate the mechanism underlying the inverse relationship of miR-29c and FKBP, we cotransfected HuF cells with a firefly luciferase expression vector containing the 3′-UTR of FKBP4, Renilla luciferase expression plasmid (internal control), and miR-29c mimic or nontargeting negative controls. Results show a significant decrease in luciferase activity in the HuF cells cotransfected with miR-29c mimic compared with the nontargeting negative controls [Fig. 3(A)], suggesting that miR-29c directly targets 3′-UTR of FKBP4. To evaluate the effect of miR-29c overexpression on FKBP4 expression and progesterone-regulated genes, we transfected HuF cells with miR-29c mimics or nontargeting negative controls and treated the cells with vehicle or progesterone (1μM medroxyprogesterone acetate) for 48 hours. Cells transfected with the miR-29c mimics demonstrated an increase in miR-29c abundance [Fig. 3(B)] compared with the cells transfected with nontargeting negative controls, confirming transfection efficiency. Progesterone treatment resulted in increased levels of FKBP4 [Fig. 3(C)], decorin [Fig. 3(D)], HOXA10 [Fig. 3(E)], and MIG6 [Fig. 3(F)] in nontargeting negative control-transfected HuF cells. In contrast, we observed a significant reduction in the progesterone-regulated genes (decorin, HOXA10, and MIG6) in HuF cells transfected with miR-29c mimic compared with the nontargeting negative control-transfected cells treated with progesterone. The known role of FKBP4 in progesterone action and its suppression by miR-29c suggests that effects of miR-29c on other progesterone target genes (decorin, HOXA10, and MIG6) are mediated by decreased FKBP4 expression. These in vitro studies support the inverse correlation observed in vivo between miR-29c and FKBP4 expression and its effect on progesterone-regulated gene expression.
Figure 3.
In vitro results shows the inverse relationship between miR-29c and FKBP4. (A) The Luciferase data are normalized with the Renilla and show that FKBP4 is a direct target of miR-29c. The Luciferase construct containing wild-type 3′-UTR of FKBP4 was cotransfected with either miR-29c mimic or nontargeting negative control in human uterine fibroblast cells along with Renilla plasmid (internal control). Quantitative reverse transcription polymerase chain reaction data suggest that overexpression of (B) miR-29c decreases (C) FKBP4 levels and attenuates the progesterone signaling as evident by the compromised expression of (D) decorin, (E) homeobox A10, and (F) mitogen-inducible gene 6. Mean values with SD are shown (*P < 0.05). miR-29c, microRNA-29c mimic-transfected cells; MPA, medroxyprogesterone acetate treatment; NC, nontargeting negative control-transfected cells; Veh, vehicle treatment.
Overexpression of miR-29c alters the in vitro decidualization response
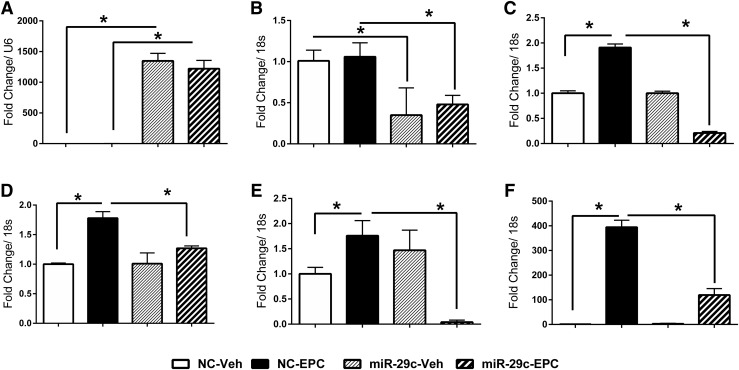
Our in vivo and in vitro data demonstrate that increased miR-29c results in decreased expression of FKBP4. To explore the possible biological effect of increased expression of miR-29c, we performed in vitro decidualization experiments using HuF cells, transfected with either miR-29c mimic or nontargeting negative controls exposed to a decidualization stimulus (EPC). Transfection with the miR-29c mimic resulted in an increased detection of miR-29c [Fig. 4(A)] and this was unaffected by EPC treatment. Treatment of nontargeting control-transfected cells with EPC also induced decorin, HOXA10, and prolactin but had little effect on FKBP4 [Fig. 4(B–F)]. Cells transfected with miR-29c mimic and EPC had reduced FKBP4 expression associated with reduced decorin, HOXA10, and MIG6 [Fig. 4(B–E)] and reduced expression of the decidualization marker, prolactin, compared with cells transfected with a nontargeting control. These data demonstrate that increased expression of miR-29c compromises the decidualization response and is likely mediated by decreased levels of FKBP4.
Figure 4.
Quantitative reverse transcription polymerase chain reaction data suggest that overexpression of miR-29c altered the decidualization response. Effect of miR-29c mimics transfection on (A) miR-29c, (B) FKBP4, (C) decorin, (D) homeobox A10, (E) mitogen-inducible gene 6, and (F) prolactin transcript levels in human uterine fibroblast cells exposed to decidualization mixture for 6 days. Mean values of 3 separate experiments with SD are shown (*P < 0.05). miR-29c, microRNA-29c mimic-transfected cells; MPA, medroxyprogesterone acetate treatment; NC, nontargeting negative control-transfected cells; Veh, vehicle treatment.
EuE stromal cells from women with endometriosis have a dampened decidualization response
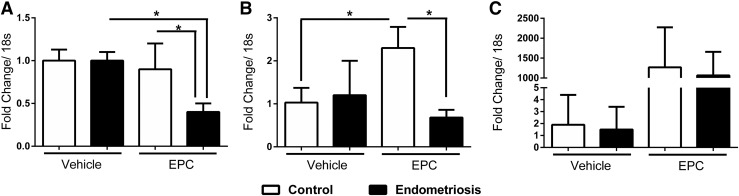
Primary stromal cells from women with and without endometriosis were exposed to the EPC. We demonstrated an increase in HOXA10 [Fig. 5(B)] and prolactin [Fig. 5(C)] expression in stromal cells collected from women without endometriosis. In contrast, we observed a significant decrease in FKBP4 [Fig. 5(A)] and HOXA10 [Fig. 5(B)] transcript levels in decidualized stromal cells from women with endometriosis compared with the stromal cells from women without endometriosis, similar to the findings in HuF cells. We also observed a decrease in prolactin [Fig. 5(C)] levels, suggesting a blunted decidualization response in endometriosis; however, this decrease was not statistically significant due to variability in the decidualization response among the stromal cells.
Figure 5.
Quantitative reverse transcription polymerase chain reaction of the decidualization response in stromal cells from women with endometriosis compared with women without endometriosis. The data show that stromal cells from women with endometriosis have lower levels of (A) FKBP4, (B) homeobox A10, and (C) prolactin when exposed to decidualization mixture compared with stromal cells from women without endometriosis. Mean values with SD are shown (*P < 0.05).
The effect of lesion excision on miR-29c and FKBP4 expression in EuE of women with endometriosis
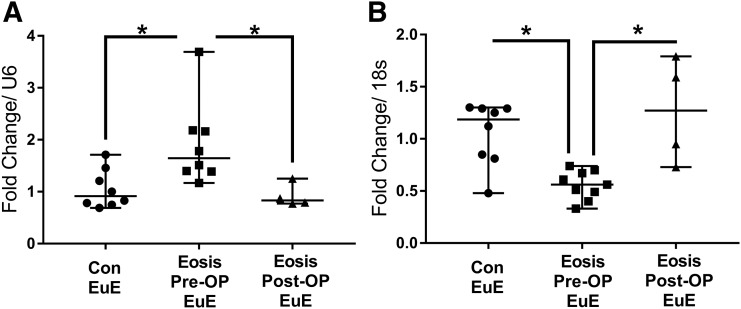
There was a significant upregulation of miR-29c and a decrease in FKBP4 mRNA expression in EuE biopsy specimens obtained in the midsecretory phase from women with endometriosis compared with controls (Fig. 6). Laparoscopic excision of DIE significantly decreased the expression of miR-29c and restored the expression of FKBP4 transcript levels in the EuE 6 months postsurgery (Fig. 6).
Figure 6.
Quantitative reverse transcription polymerase chain reaction analysis of microRNA-29c (miR-29c) expression in human EuE. miR-29c and FKBP4 expression in midsecretory phase eutopic endometrial biopsy specimens were obtained from control and women with endometriosis before and after surgery. Data are displayed as mean ± SD and analyzed by performing the Kruskal-Wallis test followed by Dunn’s multiple comparisons test (*P < 0.05). Con, without endometriosis; Eosis, endometriosis; Post-OP, after surgery; Pre-OP, before surgery.
Discussion
Endometriosis is characterized by severe cyclic chronic pelvic pain and infertility. Although the causes of endometriosis-associated infertility are not well defined, the presence of the disease results in aberrant gene expression in the EuE and the development of progesterone resistance (1, 2, 7). Rodent models suggest that FKBP4 plays a critical role in progesterone action on the endometrium necessary for embryonic implantation (35, 36). It is well established that progesterone signaling drives the decidualization process, which is crucial for implantation in both rodents and primates (37). Both human and animal studies have confirmed that aberrant progesterone action, despite normal progesterone levels, compromises fertility in the presence of endometriosis (11, 23). These observations emphasize the importance of FKBP4 as a physiological cochaperone for progesterone signaling in the uterus and suggests that progesterone resistance, a hallmark of endometriosis, can be attributed, in part, to decreased FKBP4 expression (11, 21, 23, 33, 38). We recently reported microarray data demonstrating that endometriosis induction in the baboon results in altered expression of a number of miRs, including miR-29c (16). In the current study, we focused on the expression of miR-29c and one of its predicted targets, FKBP4.
Our data suggest that HuF cells transfected with a miR-29c mimic demonstrated a significantly attenuated decidualization response. These results complement previously reported data that the overexpression of miR-29c leads to a blunted decidualization response associated with a decrease in the expression of Insulin-like Growth Factor Binding Protein-1 and WNT4 (20). This validates the observation in rodents and demonstrates that the decrease in FKBP4 in baboons and women with endometriosis is associated with impaired decidualization and embryo-maternal interactions and implantation (35).
Decorin is a member of the proteoglycan family reported to modulate the effects of the receptor tyrosine kinase family and hepatocyte growth factor receptors (39–41). Decorin expression is upregulated in stromal cells following the induction of decidualization (42), and recent reports suggest that progesterone induces the expression and secretion of decorin in endometrial cells (34). In vivo studies have also suggested that treatment with a strong progestin, dienogest, promoted the increase in decorin expression in ovarian endometriosis lesions (34). We observed increased decorin expression in progesterone-treated and decidualized HuF cells transfected with nontargeting negative control. In HuF cells transfected with miR-29c and treated with progesterone or the decidualization mixture, we observed a significant decrease in decorin expression compared with the nontargeting negative controls. These observations further support our hypothesis that increased expression of miR-29c results in a progesterone-resistant environment both in vivo and in vitro.
MIG6 expression is induced by progesterone and plays an important role in inhibiting estrogen signaling in the uterus (22, 43, 44). Our in vitro observation suggests a significant decrease in MIG6 expression in HuF cells overexpressing miR-29c and treated with either progesterone or the decidualization mixture. This observation also leads us to suggest that the increased expression of miR-29c might also be responsible for estrogen dominance observed in endometriosis pathology since MIG6 is an important inhibitor of estrogen signaling (22).
HOXA10 expression is upregulated during the window of implantation in fertile women (45), but this increase in HOXA10 is inhibited in the EuE with endometriosis (12, 46, 47). We speculate that the compromised expression of HOXA10 might be due to the increased expression of miR-29c and subsequent progesterone resistance. It has been reported that the expression of endometrial HOXA10 is restored postoperatively in gynecological pathologies such as endometriomas, leiomyomas, and hydrosalpinx (48, 49), which correlates with our postoperative finding on miR-29c, FKBP4, and HOXA10 expression in the EuE of women with DIE.
It is well established that endometriosis is one of the major causes of infertility and inflammation in women and that surgical resection of endometriotic lesions not only provides symptomatic relief but also improves fecundity (26, 50). The molecular changes in the eutopic endometrium underlying postoperative intervention remain elusive. To our knowledge, few studies suggest that surgical removal of lesions alters local and systemic proinflammatory cytokines (50, 51) and peri-implantation endometrial HOXA10 expression (48) in patients with endometriosis. In this study, we demonstrate that the excision of endometriotic lesions restores the FKBP4 expression and a progesterone-responsive environment in the eutopic endometrium via a decreased expression of miR-29c expression. These observations are also supported by the fact that 3 of 4 women in the postoperative group analyzed in this study who underwent subsequent in vitro fertilization were able to get pregnant.
There are few limitations to the current study. The sample size for the overall study is low, and particularly for the postoperative eutopic endometrial biopsy specimens, we were only able to collect 4 samples, since the other 5 patients got pregnant following surgery and assisted reproductive therapy. Therefore, we were unable to correlate mRNA changes with protein expression by immunohistochemistry or Western blot. In the future, we plan to address these limitations and focus on studies on understanding how altered miR expression contributes toward progesterone-resistant EuE of women and baboons with endometriosis.
Our results suggest that endometriosis results in a significant increase in miR-29c and decrease in FKBP4 expression in the eutopic endometrium. The association with endometriosis was further confirmed when miR-29c expression was decreased and FKBP4 expression was restored in eutopic tissues following laparoscopic lesion ablation. Combining in vivo and in vitro data, it can be inferred that the compromised expression of FKBP4 due to the increased expression of miR-29c results in blunted progesterone signaling and might contribute to the observed progesterone resistance in women with endometriosis. Furthermore, our data suggest that the infertility in the subset of women with endometriosis who fail to conceive might be due, at least in part, to a decidualization defect as a result of decreased progesterone signaling in the absence of FKBP4.
Acknowledgments
Antibodies for FKBP4 were generously provided by Dr David Smith (Mayo Clinic, Scottsdale, AZ). We thank Plavita Sharma and Samantha Bond for their technical help in carrying out the experiments.
Acknowledgments
This research was supported by the Eunice Kennedy Shriver NICHD/NIH grants R01-HD083273 to A.T.F. and R01-HD067721 to S.L.Y. and B.A.L.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DIE
- deep infiltrative endometriosis
- EPC
- estrogen + medroxyprogesterone acetate + dibutyryl-cAMP treatment
- EuE
- eutopic endometrium
- FKBP4
- FK506-binding protein 4
- HOXA10
- homeobox A10
- HuF
- human uterine fibroblast
- MIG6
- mitogen-inducible gene 6
- miR
- microRNA
- miR-29c
- microRNA-29c
- mRNA
- messenger RNA.
References
- 1.Giudice LC. Clinical practice: endometriosis. N Engl J Med. 2010;362(25):2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 3.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. [DOI] [PubMed] [Google Scholar]
- 4.Braundmeier AG, Fazleabas AT. The non-human primate model of endometriosis: research and implications for fecundity. Mol Hum Reprod. 2009;15(10):577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- 6.Jones RK, Bulmer JN, Searle RF. Immunohistochemical characterization of proliferation, oestrogen receptor and progesterone receptor expression in endometriosis: comparison of eutopic and ectopic endometrium with normal cycling endometrium. Hum Reprod. 1995;10(12):3272–3279. [DOI] [PubMed] [Google Scholar]
- 7.Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med. 2010;28(1):75–80. [DOI] [PubMed] [Google Scholar]
- 8.Afshar Y, Hastings J, Roqueiro D, Jeong JW, Giudice LC, Fazleabas AT. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biol Reprod. 2013;88(2):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gashaw I, Hastings JM, Jackson KS, Winterhager E, Fazleabas AT. Induced endometriosis in the baboon (Papio anubis) increases the expression of the proangiogenic factor CYR61 (CCN1) in eutopic and ectopic endometria. Biol Reprod. 2006;74(6):1060–1066. [DOI] [PubMed] [Google Scholar]
- 10.Hastings JM, Jackson KS, Mavrogianis PA, Fazleabas AT. The estrogen early response gene FOS is altered in a baboon model of endometriosis. Biol Reprod. 2006;75(2):176–182. [DOI] [PubMed] [Google Scholar]
- 11.Jackson KS, Brudney A, Hastings JM, Mavrogianis PA, Kim JJ, Fazleabas AT. The altered distribution of the steroid hormone receptors and the chaperone immunophilin FKBP52 in a baboon model of endometriosis is associated with progesterone resistance during the window of uterine receptivity. Reprod Sci. 2007;14(2):137–150. [DOI] [PubMed] [Google Scholar]
- 12.Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, Wu Y, Guo SW, Fazleabas AT. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007;13(5):323–332. [DOI] [PubMed] [Google Scholar]
- 13.Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development. 2005;132(21):4645–4652. [DOI] [PubMed] [Google Scholar]
- 14.Ohlsson Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update. 2009;16(2):142–165. [DOI] [PubMed] [Google Scholar]
- 15.Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, Giudice LC. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod. 2009;15(10):625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi NR, Su RW, Chandramouli GV, Khoo SK, Jeong JW, Young SL, Lessey BA, Fazleabas AT. Altered expression of microRNA-451 in eutopic endometrium of baboons (Papio anubis) with endometriosis. Hum Reprod. 2015;30(12):2881–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chill HH, Dior UP, Kogan L, Revel A. microRNAs and endometrial pathophysiology. Adv Exp Med Biol. 2015;887:143–155. [DOI] [PubMed] [Google Scholar]
- 18.Nothnick WB. Non-coding RNAs in uterine development, function and disease. Adv Exp Med Biol. 2015;886:171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laudanski P, Charkiewicz R, Tolwinska A, Szamatowicz J, Charkiewicz A, Niklinski J. Profiling of selected microRNAs in proliferative eutopic endometrium of women with ovarian endometriosis. Biomed Res Int. 2015;2015:760698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25(5):821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tranguch S, Smith DF, Dey SK. Progesterone receptor requires a co-chaperone for signalling in uterine biology and implantation. Reprod Biomed Online. 2007;14(Spec No 1):39–48. [DOI] [PubMed] [Google Scholar]
- 22.Yoo JY, Kim TH, Lee JH, Dunwoodie SL, Ku BJ, Jeong JW. Mig-6 regulates endometrial genes involved in cell cycle and progesterone signaling. Biochem Biophys Res Commun. 2015;462(4):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirota Y, Tranguch S, Daikoku T, Hasegawa A, Osuga Y, Taketani Y, Dey SK. Deficiency of immunophilin FKBP52 promotes endometriosis. Am J Pathol. 2008;173(6):1747–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85(3):564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su RW, Strug MR, Joshi NR, Jeong JW, Miele L, Lessey BA, Young SL, Fazleabas AT. Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J Clin Endocrinol Metab. 2015;100(3):E433–E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianchi PH, Pereira RM, Zanatta A, Alegretti JR, Motta EL, Serafini PC. Extensive excision of deep infiltrative endometriosis before in vitro fertilization significantly improves pregnancy rates. J Minim Invasive Gynecol. 2009;16(2):174–180. [DOI] [PubMed] [Google Scholar]
- 27.Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reprod Biol Endocrinol. 2006;4(suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48(9):876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srisuparp S, Strakova Z, Brudney A, Mukherjee S, Reierstad S, Hunzicker-Dunn M, Fazleabas AT. Signal transduction pathways activated by chorionic gonadotropin in the primate endometrial epithelial cells. Biol Reprod. 2002;68(2):457–464. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 31.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. [DOI] [PubMed] [Google Scholar]
- 32.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2008;19(1):92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117(7):1824–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ono YJ, Terai Y, Tanabe A, Hayashi A, Hayashi M, Yamashita Y, Kyo S, Ohmichi M. Decorin induced by progesterone plays a crucial role in suppressing endometriosis. J Endocrinol. 2014;223(2):203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci USA. 2005;102(40):14326–14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sánchez ER, Shou W. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol Endocrinol. 2006;20(11):2682–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905. [DOI] [PubMed] [Google Scholar]
- 38.Yang H, Zhou Y, Edelshain B, Schatz F, Lockwood CJ, Taylor HS. FKBP4 is regulated by HOXA10 during decidualization and in endometriosis. Reproduction. 2012;143(4):531–538. [DOI] [PubMed] [Google Scholar]
- 39.Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 2008;32(2):141–174. [DOI] [PubMed] [Google Scholar]
- 40.Seidler DG, Dreier R. Decorin and its galactosaminoglycan chain: extracellular regulator of cellular function? IUBMB Life. 2008;60(11):729–733. [DOI] [PubMed] [Google Scholar]
- 41.Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185(4):743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buzzio OL, Lu Z, Miller CD, Unterman TG, Kim JJ. FOXO1A differentially regulates genes of decidualization. Endocrinology. 2006;147(8):3870–3876. [DOI] [PubMed] [Google Scholar]
- 43.Kim TH, Franco HL, Jung SY, Qin J, Broaddus RR, Lydon JP, Jeong JW. The synergistic effect of Mig-6 and Pten ablation on endometrial cancer development and progression. Oncogene. 2010;29(26):3770–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeong JW, Lee HS, Lee KY, White LD, Broaddus RR, Zhang YW, Vande Woude GF, Giudice LC, Young SL, Lessey BA, Tsai SY, Lydon JP, DeMayo FJ. Mig-6 modulates uterine steroid hormone responsiveness and exhibits altered expression in endometrial disease. Proc Natl Acad Sci USA. 2009;106(21):8677–8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101(7):1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naqvi H, Mamillapalli R, Krikun G, Taylor HS. Endometriosis located proximal to or remote from the uterus differentially affects uterine gene expression. Reprod Sci. 2015;23(2):186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanatta A, Pereira RM, Rocha AM, Cogliati B, Baracat EC, Taylor HS, Motta EL, Serafini PC. The relationship among HOXA10, estrogen receptor α, progesterone receptor, and progesterone receptor B proteins in rectosigmoid endometriosis: a tissue microarray study. Reprod Sci. 2014;22(1):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Celik O, Unlu C, Otlu B, Celik N, Caliskan E. Laparoscopic endometrioma resection increases peri-implantation endometrial HOXA-10 and HOXA-11 mRNA expression. Fertil Steril. 2015;104(2):356–365. [DOI] [PubMed] [Google Scholar]
- 49.Unlu C, Celik O, Celik N, Otlu B. Expression of endometrial receptivity genes increase after myomectomy of intramural leiomyomas not distorting the endometrial cavity. Reprod Sci. 2015;23(1):31–41. [DOI] [PubMed] [Google Scholar]
- 50.Ahn SH, Edwards AK, Singh SS, Young SL, Lessey BA, Tayade C. IL-17A contributes to the pathogenesis of endometriosis by triggering proinflammatory cytokines and angiogenic growth factors. J Immunol. 2015;195(6):2591–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monsanto SP, Edwards AK, Zhou J, Nagarkatti P, Nagarkatti M, Young SL, Lessey BA, Tayade C. Surgical removal of endometriotic lesions alters local and systemic proinflammatory cytokines in endometriosis patients. Fertil Steril. 2016;105(4):968–977.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]