Abstract
Context:
Pancreatic duct glands (PDGs) have been proposed as a source of regeneration in response to exocrine pancreas injury, and thus may serve as an organ stem cell niche. There is evidence to suggest ongoing β-cell formation in longstanding type 1 diabetes (T1D), but the source is unknown.
Objective:
To investigate the PDG compartment of the pancreas in humans with T1D for evidence of an active regenerative signature (presence of progenitor cells and increased proliferation) and, in particular, as a potential source of β-cells.
Design, Setting, and Participants:
Pancreases from 46 brain dead organ donors (22 with T1D, 24 nondiabetic controls) were investigated for activation (increased proliferation) and markers of pancreatic exocrine and endocrine progenitors.
Results:
PDG cell replication was increased in T1D (6.3% ± 1.6% vs 0.6% ± 0.1%, P < 0.001, T1D vs nondiabetic), most prominently in association with pancreatic inflammation. There were increased progenitor-like cells in PDGs of T1D, but predominantly with an exocrine fate.
Conclusion:
The PDG compartment is activated in T1D consistent with a response to ongoing inflammation, and via resulting ductal hyperplasia may contribute to local obstructive pancreatitis and eventual pancreatic atrophy characteristic of T1D. However, there is no evidence of effective endocrine cell formation from PDGs.
Précis: PDGs serve as a stem cell niche in the pancreas. Replication in PDGs is increased in T1D, implying ongoing inflammation, but the majority of cells in PDGs have an exocrine rather than endocrine fate.
In longstanding type 1 diabetes (T1D), there is a near complete loss of pancreatic β-cells through autoimmune-mediated cell death (1, 2). However, several lines of evidence suggest that there may be ongoing attempted β-cell regeneration, although insufficient to be clinically meaningful. For example, even in individuals with longstanding T1D, there are still detectable insulin-expressing cells, often isolated and scattered in the exocrine pancreas or in small clusters in and around pancreatic ducts (3, 4). Consistent with this, by use of sensitive assays, residual insulin secretion is often present in individuals with longstanding T1D (5–8).
Theoretically, these observations may indicate that there are small populations of β-cells that elude immune detection or that there is ongoing new β-cell formation subject to continued autoimmune destruction. In favor of the latter explanation, autoreactive T-cells targeted to pancreatic β-cells are frequently detected many years after diabetes onset, implying sustained autoreactivity (9). In pancreas of individuals with T1D who have sufficient residual β-cells to evaluate, we previously reported immune cells in close proximity to insulin-expressing cells and an increased frequency of β-cell apoptosis (3). Also, consistent with other diseases with ongoing autoimmune-mediated inflammation, the pancreas in individuals with T1D is subject to progressive fibrotic changes and loss of volume, although this may also reveal that the target of autoimmunity in T1D includes the exocrine pancreas as well as β-cells (4, 10, 11).
Given the possibility that there may be ongoing β-cell formation in T1D, we considered potential sources of these cells. The pancreatic duct glands (PDGs) have been proposed as a potential pancreatic stem cell niche (12–14). The purpose of the present investigation was to investigate the PDG compartment of the pancreas in humans as a potential source of newly forming β-cells in T1D.
Materials and Methods
Study subjects
Pancreata were procured from brain dead organ donors by the Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD), administered by the University of Florida (Gainesville, FL) (Table 1). All procedures were in accordance with federal guidelines for organ donation and the University of Florida Institutional Review Board. Twenty-two pancreata from individuals with T1D and 24 from nondiabetic (ND) controls matched by age, sex, and body mass index (BMI) were examined in this study. The T1D and control cases were age and BMI matched (Supplemental Fig. 1 (578.1KB, tif) ). The BMI was not available in 2 T1D donors (6050 and 6068). Because the nature of the nPOD consortium is one of a shared resource, multiple publications have used sections from the same pancreata reported in this work. A full listing of the publications is available from the nPOD website http://www.jdrfnpod.org/category/publications/.
Table 1.
Characteristics of Human Subjects With and Without T1D
| T1D |
Controls |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| nPOD Case No. | Age (y) | Sex | BMI | Duration (y) | Diabetes Medications | % Ki67 in PDG Cells | nPOD Case No. | Age (y) | Sex | BMI | % Ki67 in PDG Cells |
| 6031 | 39 | M | 24.5 | 35 | Insulin | 0.51 | 6010 | 47 | F | 19.7 | 0.55 |
| 6035 | 32 | M | 27.1 | 28 | Insulin | 0.54 | 6012 | 68 | F | 23.7 | 0.57 |
| 6036 | 49 | F | 25.5 | 34 | Insulin | 2.07 | 6013 | 65 | M | 24.2 | 0.82 |
| 6040 | 50 | F | 31.6 | 20 | Insulin | 1.07 | 6015 | 39 | F | 32.2 | 0.13 |
| 6045 | 26 | M | 23.1 | 8 | Insulin | 7.04 | 6017 | 59 | F | 24.8 | 0.46 |
| 6050a | 82 | M | N/A | 58 | Insulin | 5.12 | 6021 | 72 | F | 24.5 | 0.86 |
| 6051 | 20 | M | 22.7 | 13 | Insulin | 0.33 | 6022 | 75 | M | 30.6 | 0.40 |
| 6054 | 35 | F | 30.4 | 30 | Insulin | 1.14 | 6029 | 24 | F | 22.6 | 0.11 |
| 6061 | 28 | M | 22.1 | 23 | Insulin | 2.20 | 6030 | 30 | M | 27.1 | 0.26 |
| 6062 | 11 | M | 21.9 | 6 | Insulin | 1.62 | 6034 | 32 | F | 25.2 | 0.59 |
| 6063 | 4 | M | 23.8 | 3 | Insulin | 0.82 | 6047 | 8 | M | 23.9 | 1.80 |
| 6064 | 20 | F | 22.6 | 9 | Insulin | 48.70 | 6048 | 30 | M | 20.6 | 0.49 |
| 6068a | 72 | F | N/A | 69 | Insulin | 1.08 | 6057 | 22 | M | 26.0 | 0.30 |
| 6070 | 23 | F | 21.6 | 7 | Insulin | 0.36 | 6060 | 24 | M | 32.7 | 1.14 |
| 6076 | 26 | M | 18.8 | 15 | Insulin | 0.51 | 6091 | 27 | M | 35.6 | 0.75 |
| 6085a | 89 | M | 20.3 | 84 | Insulin | 2.32 | 6096 | 16 | M | 18.8 | 0.27 |
| 6088 | 31 | M | 27.0 | 5 | Insulin | 1.12 | 6098 | 18 | M | 22.8 | 0.11 |
| 6089 | 14 | M | 26.0 | 8 | Insulin | 22.18 | 6102 | 45 | F | 35.1 | 0.38 |
| 6119 | 28 | M | 19.4 | 14 | Insulin | 1.98 | 6104 | 41 | M | 20.5 | 0.38 |
| 6128 | 34 | F | 22.2 | 31.5 | Insulin | 0.21 | 6126 | 25 | M | 25.1 | 0.29 |
| 6135 | 44 | M | 28.7 | 21 | Insulin | 6.40 | 6130 | 5 | M | 18.5 | 0.05 |
| 6138 | 49 | F | 33.7 | 41 | Insulin | 29.03 | 6131 | 24 | M | 24.8 | 0.92 |
| 6134 | 27 | M | 20.1 | 0.73 | |||||||
| 6140 | 38 | M | 21.7 | 0.78 | |||||||
| Mean | 36.6 | 24.7 | 25.6 | 6.20 | Mean | 35.9 | 25.0 | 0.55 | |||
| SEM | 4.7 | 0.9 | 4.6 | 2.55 | SEM | 4.0 | 1.0 | 0.08 | |||
Denotes T1D medalist.
Pancreas sections and staining
A standardized preparation procedure for pancreata recovered from cadaveric organ donors is used at the nPOD facility. Paraffin tissue sections from each region of pancreas (head, body, and tail) were sequentially stained at the University of California, Los Angeles, for Ki67, insulin, and alcian blue by immunohistochemistry. Slides were incubated with the following primary antibodies: mouse anti-Ki67 (1:25, 4°C overnight; Dako, Carpinteria, CA; reference M7240) and rabbit anti-insulin (1:200, 4°C overnight; Cell Signaling, Danvers, MA; catalogue 3014S). Slides were then incubated with alcian blue solution (4°C overnight; Electron Microscope Sciences, Hatfield, PA). Secondary antibodies used were biotin donkey anti-mouse (1:100; The Jackson Laboratory, Sacramento, CA), followed by peroxidase staining (Vector Peroxidase Standard PK-4000; Vector Laboratories, Burlingame, CA), and biotin donkey anti-rabbit (1:100; The Jackson Laboratory) using alkaline phosphatase for color development (Vector Laboratories).
Morphometric analysis
Whole sections of pancreas stained for insulin, Ki67, and alcian blue with hematoxylin counterstain were digitally scanned using Aperio ScanScope (Aperio Technologies, Vista, CA). Analysis was performed using Aperio ImageScope version 11.0.2.725. Three sections per case, 1 each from head, body, and tail, were analyzed, except in 1 T1D case [6031] and 7 control cases (6010, 6012, 6013, 6015, 6017, 6021, 6022) in which only head and tail sections were available. All sections were examined and quantified in a blinded manner.
Interlobular ducts were defined as ductal structures embedded in mesenchyme and possessing a PDG compartment. PDGs were identified as coiled invaginations composed of columnar epithelium arising from interlobular ducts and lying within the mesenchyme surrounding those ducts. The number of interlobular duct epithelial cells and PDG cells was counted, as was the number of those cells with nuclei staining for Ki67 and cytoplasmic staining for insulin. Small ducts, defined as intralobular ducts not embedded in mesenchyme, were comparably analyzed. Numbers of cells counted for each compartment are shown in Table 2.
Table 2.
The Numbers of Cells Counted in the PDGs and Interlobular and Intralobular Ducts in the Donors with T1D and ND Controls
| Donor Type | Head | Body | Tail | Total |
|---|---|---|---|---|
| T1D | ||||
| PDG cells, total | 43,718 | 21,443 | 29,057 | 94,218 |
| PDG cells, mean per donor (range) | 1987 (430–5780) | 1021 (152–2531) | 1321 (545–3461) | |
| Interlobular duct cells, total | 56,183 | 33,706 | 37,298 | 127,187 |
| Interlobular duct cells, mean per donor (range) | 2554 (202–7335) | 1605 (280–3628) | 1695 (310–4657) | |
| Intralobular duct cells, total | 59,923 | 48,628 | 49,681 | 158, 232 |
| Intralobular duct cells, mean per donor (range) | 2724 (752–4942) | 2316 (990–3988) | 2258 (810–3359) | |
| ND | ||||
| PDG cells, total | 32,396 | 19,185 | 22,823 | 74,404 |
| PDG cells, mean per donor (range) | 1350 (197–4835) | 1199 (117–5580) | 951 (70–2071) | |
| Interlobular duct cells, total | 53,857 | 32,859 | 37,711 | 124,427 |
| Interlobular duct cells, mean per donor (range) | 2244 (199–7997) | 2054 (436–6169) | 1571 (289–2950) | |
| Intralobular duct cells, total | 68,952 | 61,284 | 66,407 | 196,643 |
| Intralobular duct cells, mean per donor (range) | 2873 (840–4354) | 3605 (1626–4937) | 2767 (988–4303) |
For assessment of PDGs as a potential pancreatic stem cell niche
To assess the possible role of PDGs as an organ-specific stem cell niche, we evaluated PDGs for expression of known lineage defining transcription factors for endocrine and exocrine fate. We evaluated sections from the 4 T1D donors that had a high frequency of replication in the PDG compartment (6064, 6089, 6135, and 6138) and 4 age- and BMI-matched ND donors (6015, 6102, 6057, and 6098).
Slides were incubated at 4°C overnight with a cocktail of primary antibodies prepared in blocking solution (3% bovine serum albumin in Tris-buffered saline with Tween 20) at the following combinations: rabbit anti-sox9 (1:500; EMD Millipore, Billerica, MA) plus mouse anti-Nkx6.1 (1:300; DHSB, F55A10 Iowa City, IA) and rabbit anti-sox9 (1:500) plus mouse anti-GATA4 (1:50, sc-25310 X; Santa Cruz Biotechnology, Dallas, TX). The primary antibodies were detected by a cocktail of appropriate secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA); Alexa 647-conjugated donkey anti-rabbit (1:100); Cy3-conjugated donkey anti-rabbit (1:200); and fluorescein isothiocyanate–conjugated donkey anti-mouse (1:100). Slides were counterstained to mark the nuclei using a mounting medium containing 4′,6-diamidino-2-phenylindole (Vectashield; Vector Laboratories, Burlingame, CA), and sections were viewed and analyzed, as described previously (15).
To investigate the potential endocrine cell lineage of PDG cells, we evaluated Nkx2.2 as a pan-endocrine transcription factor and Nkx6.1 as a β-cell transcription factor and combined this evaluation with a cocktail of all known pancreatic hormones, as described previously (15). Sections were evaluated from 4 T1D donors (6050, 6138, 6051, and 6076) and 4 age- and BMI-matched ND donors (6015, 6021, 6029, and 6134). Slides were blocked and incubated sequentially with the same antibodies, as described previously (15).
Statistical analysis
Statistical analysis was performed using the Student’s t test or analysis of variance where appropriate (Graphpad Prism 6, La Jolla, CA). Data in graphs and tables are presented as means ± SEM. Findings were assumed statistically significant at the P < 0.05.
Results
PDGs in human pancreas
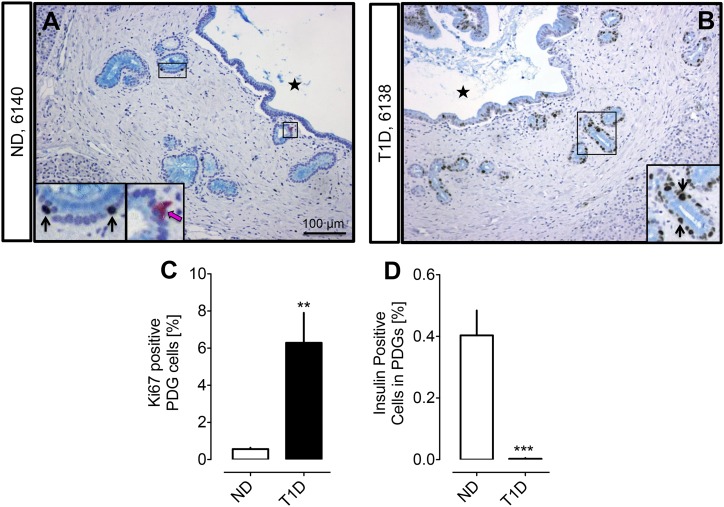
PDGs were readily identified in pancreatic sections from donors with T1D and controls (Fig. 1). The extent of PDGs per unit area was increased in pancreas of humans with T1D [Fig. 1(B)] with more PDG rings per section compared with the ND controls (71.0 ± 7.0 vs 55.6 ± 5.9 PDG rings per section, T1D vs ND, P < 0.05). The number of PDG cells/mm2 pancreas was also increased throughout the pancreas in T1D (head 14.5 ± 2.4 vs 9.7 ± 1.9, PDG cells/mm2, body 10.4 ± 1.9 vs 6.5 ± 1.2, PDG cells/mm2, tail 13.2 ± 2.0 vs 7.1 ± 0.9 PDG cells/mm2, T1D vs ND).
Figure 1.
Examples of PDG compartments in ND (A) and subjects with T1D (B). Pancreas sections were stained by immunohistochemistry for insulin (pink), Ki67 (brown), and alcian blue with hemotoxylin counterstain. Insets, magnified areas of the low-power images marked by black rectangle. Black arrows indicate Ki67-positive nuclei in PDG cells. Pink arrows indicate insulin-positive PDG cells. Black stars indicate the interlobular duct lumen. (C) The frequency of replicating cells in the epithelium of PDGs is higher in T1D compared with ND (6.3% ± 1.6% vs 0.6% ± 0.1%, T1D vs ND, **P < 0.001). (D) The frequency of insulin-positive cells in the epithelium of PDGs is higher in ND compared with T1D (0.003% ± 0.002% vs 0.40% ± 0.08%, T1D vs ND, ***P < 0.0001). White bars = ND, black bars = T1D. Scale bar, 100 μm.
However, because pancreas size is decreased in T1D, this increase in PDGs per unit cross-section of pancreas could be due to the relative loss of acinar tissue and preservation of PDGs. To address this, we normalized the number of PDG cells/mm2 to pancreatic weight in the cases where pancreatic regional weights were available (T1D: n = 11; ND: n = 10). Following this correction, there was no increase in PDG cells in T1D [484 ± 74 vs 548 ± 77 PDG cells/mm2 × pancreas weight (g), T1D vs ND, P = not significant (NS)], implying the apparent increase in PDG density in T1D may reflect a loss of pancreas volume rather than a selective expansion of the PDGs.
PDG cell replication in T1D
Because a characteristic of a stem cell niche is increased proliferation under conditions of active tissue repair, we next quantified cell replication in PDGs in T1D vs controls by use of Ki67 nuclear immunoreactivity. By this approach, there was an 11-fold increase in replication in the PDGs in T1D compared with ND controls (6.3% ± 1.6% vs 0.6% ± 0.1%, P < 0.001, T1D vs ND) [Fig. 1(C)]. However, on inspection of the data, it was apparent that PDG cell replication was markedly increased in pancreatic sections of 11 of the 22 individuals with T1D compared with ND controls, whereas in the pancreatic sections of the other 11 T1D donors the frequency of Ki67-positive PDG cells was comparable to that in ND controls. To probe the potential difference between these groups, T1D with high PDG replication and T1D with normal PDG replication, we subdivided the T1D donors into those with a mean PDG percentage positive for Ki67 of 1.2% or higher (range 1.6% to 49%) and those with a mean PDG percentage positive for Ki67 <1.2% (range 0.2% to 1.1%). This cutoff value was chosen as the mean Ki67-positive PDG percentage value in the normal PDG Ki67-positive T1D donor group and then matched that of the ND donor group (0.7% ± 0.1% vs 0.6% ± 0.1%, T1D normal vs ND, P = NS).
To investigate the potential mechanisms accounting for the difference between the high vs normal PDG cell replication groups, we next considered both technical and patient characteristics because of potential influences of the preterminal clinical condition (16). With respect to the former, order effect (nPOD pancreas donors are assigned case numbers sequentially), from the donor numbers used in this study (Table 1), it is apparent that ND and T1D donor pancreases were procured in a random fashion. Hospitalization stay was similar in both groups (6026 ± 984 vs 4915 ± 788 minutes, T1D vs ND, P = NS). The transport time (from cross clamping to processing) was, however, longer in the T1D donor group (1107 ± 58 vs 711 ± 76 minutes, T1D vs ND, P < 0.001), but this, if anything, may have negatively affected Ki67 staining, resulting in artificially low numbers (17). Length of hospitalization and transport time was not available in 3 of the T1D donors (6050, 6068, and 6085) and 3 of the ND donors (6029, 6091, and 6130). Slides were stained in batches of 10 at University of California Los Angeles, each batch including 5 T1D and 5 ND pancreas sections. The Ki67 antibody lot (Ki67 catalogue M7240, lot number 00070375) used was the same for all sections.
Within the T1D donor group, with respect to the clinical characteristics, neither age (42.7 ± 7.6 vs 29.4 ± 3.9 years, high vs normal PDG replication, P = NS), duration of diabetes (27.8 ± 7.5 vs 23.3 ± 5.7 years, high vs normal PDG replication, P = NS), gender (high: 8 males, 3 females; normal: 6 males, 5 females), nor body mass index (24.3 ± 1.4 vs 25.0 ± 1.3 kg/m2, high vs normal PDG replication, P = NS) distinguished the groups. However, in those that did have increased PDG replication, this tended to be less markedly increased with age and duration of diabetes. Within the T1D donor group, hospitalization stay was similar in both high and normal PDG replication groups (7380 ± 1490 vs 4808 ± 1247 minutes, high vs normal PDG replication groups, P = NS). The transport time (from cross clamping to processing) was similar in the high and normal T1D PDG replication groups (1032 ± 77 vs 1175 ± 87 minutes, high vs normal PDG replication, P = NS). Within the T1D donor group, length of hospitalization and transport time was not available in 2 of the donors who had high PDG replication (6050 and 6085) and in 1 of the donors with normal PDG replication (6068).
Having excluded technical or case ascertainment bias to account for large variance in PDG cell replication in T1D, we then turned to the possibility that pancreatic inflammation in T1D might account for the difference because the PDG compartment has been shown to respond with increased proliferation in response to experimentally induced pancreatitis (13, 18). Interestingly, of the four donors with the highest PDG cell replication (6045, 6064, 6089, and 6138), all had regions of discernable acute pancreatitis and 1 donor (6064) also had insulitis and peri-insulitis. Notably, none of these individuals had known clinical pancreatitis documented in life, consistent with accumulating data that subclinical subacute chronic pancreatitis is common in both T1D and T2D, the origin of which remains unknown (19). These findings are consistent, therefore, with the PDG compartment being activated by pancreatic inflammation, whether originating from the exocrine compartment or with sufficient intensity from the islet compartment and surrounds.
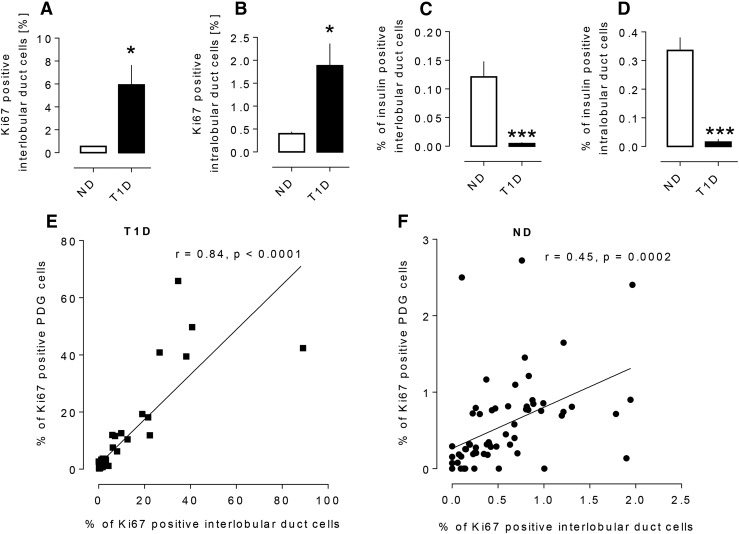
Interlobular and intralobular duct cell replication
It has previously been reported that, under conditions of increased PDG proliferation, the adjacent epithelium in interlobular pancreatic ducts is also increased (13, 18). Replication of interlobular duct epithelial cells as evaluated by Ki67 was increased 10-fold in T1D when compared with ND cases (5.9% ± 1.7% vs 0.6% ± 0.1% Ki67 positive, P < 0.01, T1D vs ND) [Fig. 2(A)]. The increased frequency of Ki67-positive pancreatic epithelial cells correlated with that in PDG cells in both individuals with T1D and ND controls [Fig. 2(E) and 2(F)].
Figure 2.
The frequency of replicating cells in the epithelium of interlobular duct cells (5.9 ± 1.7 vs 0.6 ± 0.1% Ki67-positive cells, T1D vs ND, *P < 0.01) (A) and intralobular duct cells (1.9 ± 0.5 vs 0.4 ± 0.0% Ki67-positive cells, *P < 0.01, T1D vs ND) (B) in T1D and ND subjects. The frequency of insulin-positive cells in the epithelium of interlobular ducts (0.005 ± 0.002 vs 0.12 ± 0.03% insulin-positive cells T1D vs ND, ***P < 0.0001) (C) and intralobular ducts (0.02 ± 0.01 vs 0.34 ± 0.05% insulin-positive cells, T1D vs ND, ***P < 0.0001) (D) in T1D and ND subjects. The relationship between frequency of Ki67 positivity in interlobular duct cells and in PDG cells from subjects with T1D (E) and ND subjects (F). The percentage of Ki67-positive cells in the PDGs (the number of Ki67+ PDG cells/total number of PDG cells) and the percentage of Ki67-positive cells in the interlobular pancreatic ducts (the number of Ki67+ interlobular duct cells/total number of interlobular duct cells) show a positive correlation in both the T1D donors (r = 0.84, P < 0.0001) and the ND donors (r = 0.45, P = 0.0002). Data were present as mean ± SEM, n = 22 donors for T1D, the data points representing 22 sections of head of pancreas, 21 sections of body of pancreas, and 22 sections of tail of pancreas; n = 24 donors for ND, the data points representing 24 sections of head of pancreas, 16 sections of body of pancreas, and 24 sections of tail of pancreas. White bars, ND, black bars, T1D.
The percentage of intralobular duct cells positive for Ki67 was also increased in T1D when compared with ND cases (1.9% ± 0.5% vs 0.4% ± 0.0% Ki67-positive cells, P < 0.01, T1D vs ND) [Fig. 2(B)]. Taken together, based on Ki67 immunoreactivity, there is increased replication throughout the ductal tree in T1D, most prominent in the PDG compartment, and then adjacent interlobular duct epithelium, but still present even in the smaller intralobular ducts.
Insulin-expressing cells in PDGs and pancreatic duct cells
Having established that the putative pancreatic stem cell niche, the PDG compartment, has the increased cell proliferation anticipated in a stem cell niche engaged in repair, we then sought to investigate whether the PDG compartment might be the source of newly forming β-cells in T1D. Insulin immune-reactive cells, although infrequent, were detected in PDGs [Fig. 1(A)]. However, the frequency of these insulin-expressing PDG cells as a percentage of PDG epithelial cells was decreased in T1D (0.003% ± 0.002% vs 0.40% ± 0.08%, T1D vs ND, P < 0.0001) [Fig. 1(D)]. Likewise, the percentage of insulin immune-reactive interlobular duct cells (0.005% ± 0.002% vs 0.12% ± 0.03% T1D vs ND, P < 0.0001) and intralobular duct cells (0.02% ± 0.01% vs 0.34% ± 0.05%, T1D vs ND, P < 0.0001) was decreased in T1D vs controls [Fig. 2(C) and 2(D)].
Although these data argue against the PDG compartment being an effective source of new β-cells in T1D, it is plausible that there are activated endocrine progenitors within the PDG compartment, but that any insulin-expressing cells thus formed are rapidly removed through the β-cell–specific autoimmune surveillance. Therefore, we next examined PDGs for evidence of potential progenitor cells.
Pancreatic progenitors in PDGs
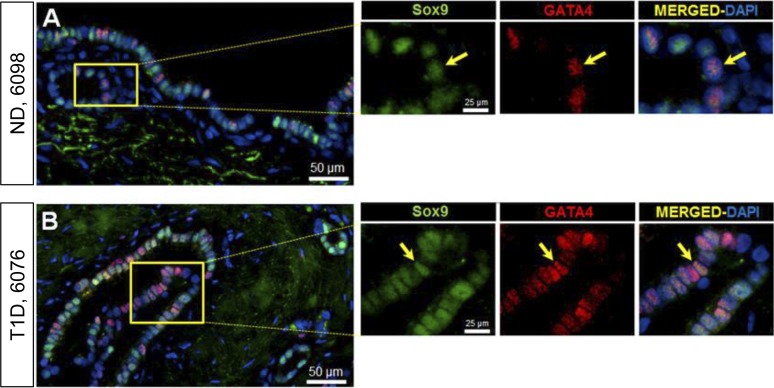
Resident stem cell pools in the adult tissues emulate the progenitor cells that gave rise to the organ during embryogenesis (20). To investigate whether PDGs recapitulate embryonic pancreatic progenitors, we selected transcription factors that mark early pancreatic progenitors: GATA4, which predominantly marks an acinar fate (21), and Sox9, which predominantly marks a pancreatic duct cell fate (21) (Fig. 3). Cells expressing these transcription factors were commonly present in PDGs, and comparable in T1D and ND controls: Sox9 (68.02% ± 7.05% vs 66.78% ± 3.25% of cells, T1D vs ND, P = NS) and GATA4 (49.6% ± 8.4% vs 37.5% ± 11.3% of cells, T1D vs ND, P = NS). Sox9/GATA4 double-positive cells (34.7% ± 11.6% vs 35.8% ± 10.6% of cells, T1D vs ND, P = NS) were also common. These findings are consistent with the PDG compartment serving as a source of pancreatic duct cells, and plausibly acinar cell regeneration.
Figure 3.
Examples of PDG cells expressing Sox9-GATA4 in pancreas from an adult ND (A) and in an adult T1D donor (B). Individual layers stained for Sox9 (green) and GATA4 (red) are shown along with merged (with 4′,6-diamidino-2-phenylindole) images. Insets, higher magnification of individual layers as marked by the yellow square in the low-power images. Yellow arrows indicate a PDG cell costaining for Sox9 and GATA4. Scale bars, 50 µm (for low-power images) and 25 µm (for insets).
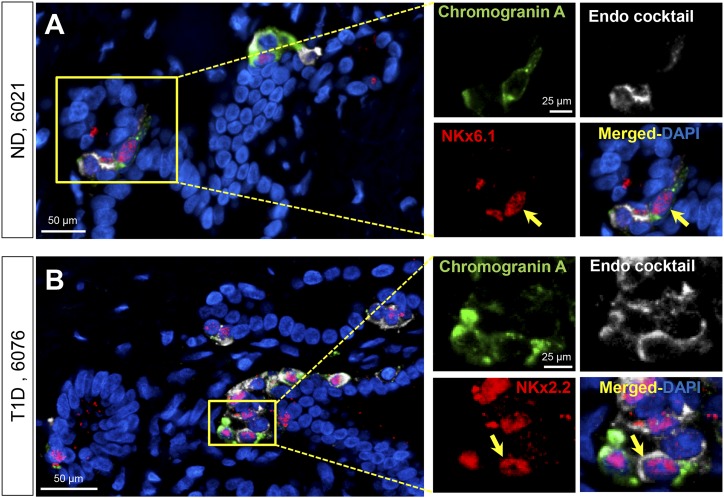
To probe the PDG compartment as a potential source of endocrine cells, we also examined PDGs for cells expressing Nkx6.1, a transcription factor that first appears in the pancreatic bud (21, 22) but is later restricted to the β-cell lineage, and Nkx2.2, a transcription factor that marks pancreatic cells with an endocrine lineage. We further refined this evaluation by simultaneously immunostaining PDGs with these transcription factors and for known islet hormones by use of an endocrine cocktail of antibodies. We identified very occasional NKx6.1-positive/hormone cocktail positive or NKx2.2-positive/hormone cocktail-positive cells in PDGs in both ND and T1D donors (Fig. 4), but these were too infrequent to quantify and much less frequent than the GATA4 or Sox9-expressing PDG cells. Taken together these findings lend support to the proposal that the PDG compartment may serve as a source of exocrine pancreas regeneration in response to pancreatic injury, but there is no evidence that it serves as a meaningful source of endocrine cells.
Figure 4.
Examples of PDG cells expressing chromogranin A, NKx6.1, and endocrine cocktail (insulin, glucagon, somatostatin, pancreatic polypeptide, and ghrelin) in pancreas from an adult ND donor (A) and chromogranin A, NKx2.2, and endocrine cocktail in an adult T1D donor (B). Individual layers stained for NKx6.1/NKx2.2 (red), endocrine cocktail (insulin, glucagon, somatostatin, pancreatic polypeptide, and ghrelin) (white), and chromogranin A (green) are shown along with the merged (with 4′,6-diamidino-2-phenylindole) image. Insets, higher magnification of individual layers as marked by the yellow square in the low-power images. Yellow arrows indicate a PDG cell costaining for Nkx6.1, chromogranin A, and a cocktail of all the endocrine hormones (A) or Nkx2.2, chromogranin A, and a cocktail of all the endocrine hormones (B). Scale bars, 50 µm (for low-power images) and 25 µm (for insets).
Discussion
The PDG compartment has been proposed as a potential pancreatic stem cell niche, based on its anatomic properties and its proliferative response to experimentally induced pancreatic injury (12). We investigated the PDG compartment as a potential source of newly forming β-cells. These studies, inevitably limited by the constraints of evaluating human tissue, support the concept that the PDG compartment may serve as a source of regeneration for the exocrine pancreatic ductal tree; however, the PDG compartment does not appear to be a significant source of endocrine cells.
PDGs have been recognized for many years (23, 24), and their extent in human pancreas has recently been delineated (14). Like Carpino et al., we have noted that the PDG compartment is present in head, body, and tail regions, thus extending throughout the length of the organ (14). Attention was drawn to PDGs because they proliferate in response to acute injury and express genes characteristic of early pancreatic endoderm (12, 13). Similar glandular structures (peribiliary glands) are present as crypt-like outpouchings off the biliary tree and have been reported to bear multiprogenitor cells with potential ductal epithelium or endocrine fate (25, 26)
Although new β-cells can form in adult humans, for example during pregnancy (27), the source of these new β-cells is unknown. It was proposed that new endocrine cells form from pancreatic ducts in rats based on the intimate relationship between islets and adjacent ducts (ductulo-insular complexes) (28). However, most (29–34), but not all (35), lineage-tracing studies have ruled out pancreatic duct or acinar cells as precursors of β-cells in postnatal mice. Although it is plausible that lineage studies focused on exocrine ducts were negative because ducts are a committed derivative of PDG pluripotential cells, to date there is no known specific marker defined that could be used to lineage trace the fate of cells arising from PDGs. As such, the role of PDGs as a pancreatic stem cell niche remains speculative, although the current study supports the possibility that the PDG compartment serves as a source of ductal epithelium and possibly acinar cells following pancreatic injury.
It is possible that insulin-expressing cells produced in the PDGs in the setting of T1D might be selectively removed by the autoimmune response, and through epitope spreading any potential precursors of β-cells might also be eliminated (36). With this in mind, we did examine the PDG compartment for any evidence of increased immune cell infiltrate in T1D, but did not find a consistent difference. Arguably, however, given the generally low immune infiltrate in humans with T1D, there could be such a difference that we were unable to find in the available samples studied.
An area of unresolved interest is why pancreata in longstanding T1D are decreased in volume and mass in comparison with ND controls (37). Consistent with that data, exocrine function is also impaired in T1D (38). Hypotheses that have been put forward to account for these findings include loss of the growth-promoting properties of insulin and subclinical pancreatitis (19). Chronic inflammation is a characteristic of autoimmune-mediated disease states in which the target of autoreactive T-cells is replenished, such as gut epithelium in ulcerative colitis and synovial epithelium in rheumatoid arthritis (39, 40). Under those conditions, the chronic inflammation results in accumulating fibrosis and distortion of the architecture of the underlying targeted tissue. The duration of detectable autoantibodies characteristic of T1D and the decline in β-cell function long precede diabetes onset (41). A prolonged period of insulitis preceding diabetes onset may lead to cytokine-mediated proliferation of the PDGs and pancreatic duct tree in the affected lobes. The high frequency of replication noted in PDGs in the current study was not accompanied by an increase in apoptosis, PDG cell apoptosis by terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end labeling being rarely detected in even PDGs with the highest detected Ki67 (data not shown). As in the case of epithelia derived from the gut stem cell niche, it appears that the excess epithelial cells derived from the PDG are either sloughed off into the ductal lumen or contribute to ductal hyperplasia distorting and obstructing ducts. The latter is known to lead to local areas of pancreatitis and likely subsequent atrophy of the affected region of acinar tissue (18, 42). This would be consistent with the findings of a distorted exocrine ductal tree with stenosis, tortuosity, and obstruction documented in patients with long-term autoimmune-mediated diabetes, but not those who developed diabetes after short-term islet inflammation (43). An alternative hypothesis previously advanced is that autoimmunity directed toward β-cells in T1D is only 1 facet of the autoimmune process, with autoimmunity also directed toward the exocrine pancreas. Autoimmune pancreatitis is well described, but does not typically involve the islets (44).
The present studies have limitations, in large part because they are exclusively undertaken in human pancreas and are therefore inevitably cross-sectional. Although brain-dead organ donors have the advantage of being transplant grade, this does not mean they are unaffected by the final illness of the donor who was most likely subject to numerous potent therapies. Because of the nature of procurement of organ donors, there is also necessarily a limited prior clinical history in comparison with a formal clinical trial. Although the study of human pancreas has obvious advantages in terms of potential clinical relevance, given our primary goal in this study, there is no means to undertake the gold standard lineage-tracing approach required to properly define a stem cell niche and its derivatives. Also, by definition, studies of human pancreas are cross-sectional and necessarily descriptive.
In conclusion, the PDG compartment of the pancreas and the ductal tree in T1D are characterized by increased proliferation consistent with a role in pancreatic duct and possibly acinar cell regeneration, but without evidence of effective β-cell regeneration.
Acknowledgments
This work was performed with the support of the nPOD, a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation International. Organ Procurement Organizations partnering with nPOD provide research resources, which are listed at www.jdrfnpod.org/our-partners.php. We appreciate the editorial assistance of Bonnie Lui from the Larry L. Hillblom Islet Research Center at University of California, Los Angeles.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant DK077967 and Larry Hillblom Foundation Grant 2014-D-001-NET (to P.C.B.).
Acknowledgments
Author contributions: A.S.M.M. and A.E.B. performed the studies, undertook the microscopy, and performed the morphological analysis. A.S.M.M., A.E.B., and P.C.B. researched data, wrote, reviewed, and edited the manuscript, and contributed to the discussion.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- ND
- nondiabetic
- nPOD
- Network for Pancreatic Organ Donors with Diabetes
- NS
- not significant
- PDG
- pancreatic duct gland
- T1D
- type 1 diabetes.
References
- 1.Atkinson MA, von Herrath M, Powers AC, Clare-Salzler M. Current concepts on the pathogenesis of type 1 diabetes: considerations for attempts to prevent and reverse the disease. Diabetes Care. 2015;38(6):979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4(2):110–125. [DOI] [PubMed] [Google Scholar]
- 3.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48(11):2221–2228. [DOI] [PubMed] [Google Scholar]
- 4.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14(10):619–633. [DOI] [PubMed] [Google Scholar]
- 5.Sjöberg S, Gunnarsson R, Gjötterberg M, Lefvert AK, Persson A, Ostman J. Residual insulin production, glycaemic control and prevalence of microvascular lesions and polyneuropathy in long-term type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987;30(4):208–213. [DOI] [PubMed] [Google Scholar]
- 6.Madsbad S. Prevalence of residual B cell function and its metabolic consequences in type 1 (insulin-dependent) diabetes. Diabetologia. 1983;24(3):141–147. [DOI] [PubMed] [Google Scholar]
- 7.Davis AK, DuBose SN, Haller MJ, Miller KM, DiMeglio LA, Bethin KE, Goland RS, Greenberg EM, Liljenquist DR, Ahmann AJ, Marcovina SM, Peters AL, Beck RW, Greenbaum CJ; T1D Exchange Clinic Network . Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2015;38(3):476–481. [DOI] [PubMed] [Google Scholar]
- 8.Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, Bonner-Weir S, King GL. Residual insulin production and pancreatic ß-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59(11):2846–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, Roep BO, von Herrath MG. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Löhr M, Klöppel G. Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia. 1987;30(10):757–762. [DOI] [PubMed] [Google Scholar]
- 11.Campbell-Thompson ML, Kaddis JS, Wasserfall C, Haller MJ, Pugliese A, Schatz DA, Shuster JJ, Atkinson MA. The influence of type 1 diabetes on pancreatic weight. Diabetologia. 2016;59(1):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strobel O, Rosow DE, Rakhlin EY, Lauwers GY, Trainor AG, Alsina J, Fernández-Del Castillo C, Warshaw AL, Thayer SP. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology. 2010;138(3):1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi J, Liss AS, Sontheimer A, Mino-Kenudson M, Castillo CF, Warshaw AL, Thayer SP. Pancreatic duct glands (PDGs) are a progenitor compartment responsible for pancreatic ductal epithelial repair. Stem Cell Res. 2015;15(1):190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpino G, Renzi A, Cardinale V, Franchitto A, Onori P, Overi D, Rossi M, Berloco PB, Alvaro D, Reid LM, Gaudio E. Progenitor cell niches in the human pancreatic duct system and associated pancreatic duct glands: an anatomical and immunophenotyping study. J Anat. 2016;228(3):474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Md Moin AS, Dhawan S, Shieh C, Butler PC, Cory M, Butler AE. Increased hormone-negative endocrine cells in the pancreas in type 1 diabetes. J Clin Endocrinol Metab. 2016;101(9):3487–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.In’t Veld P, De Munck N, Van Belle K, Buelens N, Ling Z, Weets I, Haentjens P, Pipeleers-Marichal M, Gorus F, Pipeleers D. Beta-cell replication is increased in donor organs from young patients after prolonged life support. Diabetes. 2010;59(7):1702–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan BA, Hollister-Lock J, Bonner-Weir S, Weir GC. Reduced Ki67 staining in the postmortem state calls into question past conclusions about the lack of turnover of adult human β-cells. Diabetes. 2015;64(5):1698–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gier B, Matveyenko AV, Kirakossian D, Dawson D, Dry SM, Butler PC. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras(G12D) mouse model [published correction appears in Diabetes 2012; 61(8):2195]. Diabetes. 2012;61(5):1250–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foulis AK, Stewart JA. The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia. 1984;26(6):456–461. [DOI] [PubMed] [Google Scholar]
- 20.Van de Casteele M, Leuckx G, Baeyens L, Cai Y, Yuchi Y, Coppens V, De Groef S, Eriksson M, Svensson C, Ahlgren U, Ahnfelt-Rønne J, Madsen OD, Waisman A, Dor Y, Jensen JN, Heimberg H. Neurogenin 3+ cells contribute to β-cell neogenesis and proliferation in injured adult mouse pancreas. Cell Death Dis. 2013;4:e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jennings RE, Berry AA, Kirkwood-Wilson R, Roberts NA, Hearn T, Salisbury RJ, Blaylock J, Piper Hanley K, Hanley NA. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 2013;62(10):3514–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conrad E, Stein R, Hunter CS. Revealing transcription factors during human pancreatic β cell development. Trends Endocrinol Metab. 2014;25(8):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taguchi M, Yamaguchi T, Otsuki M. Induction of PDX-1-positive cells in the main duct during regeneration after acute necrotizing pancreatitis in rats. J Pathol. 2002;197(5):638–646. [DOI] [PubMed] [Google Scholar]
- 24.Taguchi M, Otsuki M. Co-localization of nestin and PDX-1 in small evaginations of the main pancreatic duct in adult rats. J Mol Histol. 2004;35(8-9):785–789. [DOI] [PubMed] [Google Scholar]
- 25.Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, Berloco PB, Cantafora A, Wauthier E, Furth ME, Inverardi L, Dominguez-Bendala J, Ricordi C, Gerber D, Gaudio E, Alvaro D, Reid L. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54(6):2159–2172. [DOI] [PubMed] [Google Scholar]
- 26.Carpino G, Cardinale V, Onori P, Franchitto A, Berloco PB, Rossi M, Wang Y, Semeraro R, Anceschi M, Brunelli R, Alvaro D, Reid LM, Gaudio E. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat. 2012;220(2):186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, Butler PC. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53(10):2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas: a possible recapitulation of embryonic development. Diabetes. 1993;42(12):1715–1720. [DOI] [PubMed] [Google Scholar]
- 29.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. [DOI] [PubMed] [Google Scholar]
- 30.Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol. 2010;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopinke D, Brailsford M, Shea JE, Leavitt R, Scaife CL, Murtaugh LC. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development. 2011;138(3):431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopp JL, Dubois CL, Hao E, Thorel F, Herrera PL, Sander M. Progenitor cell domains in the developing and adult pancreas. Cell Cycle. 2011;10(12):1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kushner JA, Weir GC, Bonner-Weir S. Ductal origin hypothesis of pancreatic regeneration under attack. Cell Metab. 2010;11(1):2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest. 2007;117(4):971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA. 2008;105(50):19915–19919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol. 2007;7(12):988–994. [DOI] [PubMed] [Google Scholar]
- 37.Fonseca V, Berger LA, Beckett AG, Dandona P. Size of pancreas in diabetes mellitus: a study based on ultrasound. Br Med J (Clin Res Ed). 1985;291(6504):1240–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larger E, Philippe MF, Barbot-Trystram L, Radu A, Rotariu M, Nobécourt E, Boitard C. Pancreatic exocrine function in patients with diabetes. Diabet Med. 2012;29(8):1047–1054. [DOI] [PubMed] [Google Scholar]
- 39.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365(18):1713–1725. [DOI] [PubMed] [Google Scholar]
- 40.Lundy SK, Sarkar S, Tesmer LA, Fox DA. Cells of the synovium in rheumatoid arthritis. T lymphocytes. Arthritis Res Ther. 2007;9(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taplin CE, Barker JM. Autoantibodies in type 1 diabetes. Autoimmunity. 2008;41(1):11–18. [DOI] [PubMed] [Google Scholar]
- 42.Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol. 2008;1(4):306–316. [PMC free article] [PubMed] [Google Scholar]
- 43.Nakanishi K, Kobayashi T, Miyashita H, Okubo M, Sugimoto T, Murase T, Hashimoto M, Fukuchi S, Kosaka K. Exocrine pancreatic ductograms in insulin-dependent diabetes mellitus. Am J Gastroenterol. 1994;89(5):762–766. [PubMed] [Google Scholar]
- 44.Deshpande V, Gupta R, Sainani N, Sahani DV, Virk R, Ferrone C, Khosroshahi A, Stone JH, Lauwers GY. Subclassification of autoimmune pancreatitis: a histologic classification with clinical significance. Am J Surg Pathol. 2011;35(1):26–35. [DOI] [PubMed] [Google Scholar]