Abstract
Context:
Estrogens influence many physiological processes in mammals, including reproduction. Estrogen peripheral actions are mainly mediated through estrogen receptors (ERs) α and β, encoded by ESR1 and ESR2 genes, respectively.
Objective:
The study's aim was to describe a family in which 3 members presented with estrogen insensitivity.
Design and Setting:
Clinical evaluation and genetic and mutational analysis were performed in an academic medical center.
Patients and Interventions:
An ESR1 mutation was identified in 2 sisters and 1 brother, originating from a consanguineous Algerian family, who did not enter puberty and presented with delayed bone maturation consistent with estrogen insensitivity. The 2 sisters had enlarged multicystic ovaries. Hormonal evaluation as well as genetic and mutational analysis were performed.
Results:
Hormonal evaluation revealed extremely high plasma 17β-estradiol (>50-fold normal range) associated with elevated gonadotropin levels (greater than threefold normal range), highly suggestive of estrogen resistance. The 3 affected patients carried a homozygous mutation of a highly conserved arginine 394 for which histidine was substituted through an autosomal recessive mode of transmission. Structural and functional analysis of the mutant ERα revealed strongly reduced transcriptional activity and the inability to securely anchor the activating hormone, estradiol, compared with wild-type ERα. A group of other potential ER activating ligands were tested, but none overcame the estrogen insensitivity in these patients.
Conclusion:
Description and analysis of this family of patients with mutant ERα provide additional clinical findings toward identification and characterization of what was previously thought to be a highly rare clinical condition.
Precis: Herein we describe a family in which 3 members had loss-of-function mutation of the ERS1 gene. Clinical evaluation and genetic and mutational analysis were performed.
Estrogens control many physiological processes in mammals, including female reproductive tract development, breast development, reproduction, cardiovascular health, and bone integrity. Estrogen actions are mainly mediated through estrogen receptors (ERs) α and β, which belong to the steroid receptor superfamily (1, 2) and are encoded by ESR1 and ESR2 genes, respectively. These nuclear receptors are known to act as transcriptional regulators through their direct interaction with specific coregulators (3). To date, only 2 patients with ESR1 mutations have been reported, 1 man (4) and 1 girl (5). Their clinical phenotypes were in accordance with the Esr1 knockout mouse model previously described (6, 7). Female Esr1 knockout mice are infertile as a result of anovulation that is associated with multicystic ovaries without corpora lutea and hypoplastic uteri (6), and have no pubertal mammary gland development (8). Male Esr1 knockout mice have reduced testis weight and are infertile, with testicular dysmorphogenesis and inactive sperm (6, 9). Herein, we report cases of familial loss-of-function ESR1 mutation in multiple members of a large Algerian consanguineous family.
Methods
Hormonal evaluation
Hormonal parameters were measured by radioimmunoassay (Immunotech; Beckman Coulter, Marseille, France, for 17β-estradiol, testosterone, and dehydroepiandrosterone sulfate; and Access; Beckman Coulter, Brea, CA, for luteinizing hormone and follicle-stimulating hormone). In the proband, a serum steroid profile was obtained by isotopic dilution-liquid chromatography–tandem mass spectrometry. Plasma concentrations of inhibin A, inhibin B, and anti-Müllerian hormone (AMH) were measured by enzyme immunometric assays (Oxford Bio-Innovation, Kidlington, UK, for inhibins; and Immunotech; Beckman Coulter, for AMH). Sex hormone–binding globulin was measured by IRMA (Cisbio International, Codolet, France).
Genetic and mutational analysis
Informed consent for DNA sequence analysis was obtained. Leukocyte DNA sequence was analyzed with the use of polymerase chain reaction assays for protein-coding exons in ESR1 (GenBank accession number, NM_000125.3). Specific primers used are presented in Supplemental Table 1 (14.8KB, docx) . The putative mutation frequency was searched in the National Heart, Lung, and Blood Institute Exome Sequencing Project database (Exome Variant Server 2012, National Heart, Lung, and Blood Institute GO Exome Sequencing Project, Seattle, WA; http://evs.gs.washington.edu/EVS/, accessed February 2013) and in the new Exome Aggregation Consortium (ExAC) Browser Database (http://exac.broadinstitute.org), which includes more than 120,000 alleles. To assess the possible functional effect of ERα amino acid variant, PolyPhen-2 v.2.2.5 (http://genetics.bwh.harvard.edu/pph2/) and Sorting Intolerant From Tolerant (SIFT; http://sift.jcvi.org/) software were used.
Structural and functional studies of the ESR1 mutation
The identified R394H mutation was introduced within the human ER (hER) α ligand-binding domain (Protein Data Bank identification code 1GWR; www.rcsb.org/pdb) using PyMOL software (www.rcsb.org/pdb) (10). The orientation of the histidine side chain was adjusted using the PyMOL rotamer function. The mutation was inserted by means of site-directed mutagenesis using the QuickChange XLII Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) into a nonmutated ESR1 clone, VP16-ERα (Addgene, Cambridge, MA). Human embryonic kidney 293T (HEK293T) cells were transiently transfected with wild-type (WT) or mutant ESR1 plasmids and a luciferase reporter construct containing 3 estrogen-response elements (EREs), 3xERE-TATA-luc (Addgene). Transfection efficiency was adjusted by cotransfection with a plasmid encoding β-galactosidase regulated by a cytomegalovirus promoter. Transcriptional activity of WT and mutated ERα was also evaluated after treatment of 5 nM with other ER ligands [ethinyl estradiol, clomiphene, diethylstilbestrol (DES), raloxifene, and tamoxifen]. Western blot analysis was also performed, as described previously (11), to evaluate WT and mutant receptor expression, with an anti-ER antibody (sc-543; Santa Cruz Biotechnology, Santa Cruz, CA).
Results
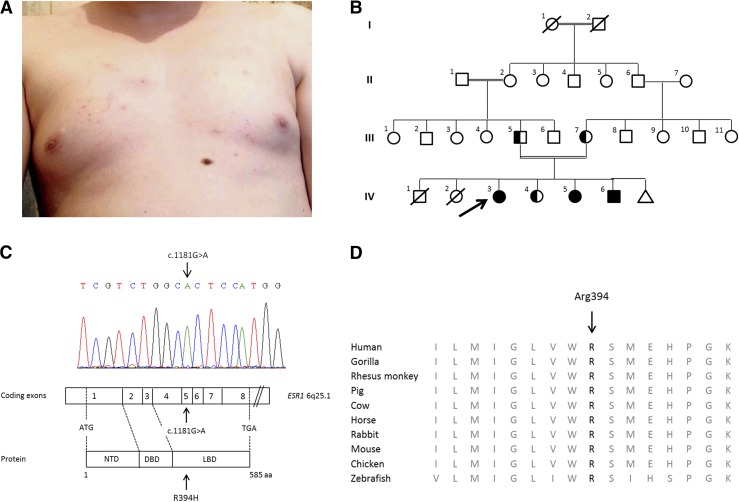
The proband (family member IV-3), a 25-year-old, 46,XX woman, was referred because of primary amenorrhea. Her clinical characteristics are reported in Table 1. She had Tanner stage I breast development with mild adipomastia and chest acne (Fig. 1A; photograph taken with patient’s approval). Pelvic ultrasonography showed enlarged bilateral multicystic ovaries with a small uterus and thin endometrium. Initial hormonal evaluation indicated an extremely high plasma 17β-estradiol level of 9476 pmol/L (normal range for early follicular phase, 120 to 300 pmol/L) that was associated with elevated gonadotropin levels [luteinizing hormone, 24 IU/L (normal range, 2 to 8 IU/L), follicle-stimulating hormone, 13 IU/L (normal range, 2 to 10 IU/L)]. This clinical and biological presentation strongly suggested an estrogen-resistance/insensitivity syndrome. Her thyrotropin level was 2.18 mIU/L (normal range, 0.4 to 4.5). The family’s pedigree is presented in Fig. 1B.
Table 1.
Clinical Characteristics, Hormonal Parameters, and Imaging of the 3 Affected Family Members
| Family Member | ||||
|---|---|---|---|---|
| IV-3 (Proband) | IV-5 (Affected Sister) | IV-6 (Affected Brother) | Normal Range | |
| Clinical characteristics | ||||
| Age, y | 25 | 21 | 18 | |
| Height, cm | 156 | 175 | 163 | |
| Weight, kg | 64 | 70 | 63 | |
| BMI, kg/m2 | 26.3 | 22.9 | 23.7 | |
| Birth weight, g | 2200 | 3300 | 2600 | |
| Puberty onset, y | ─ | ─ | ─ | |
| Tanner stage | P5B1 | P5B1 | P2G1 | |
| Hormonal parameters | ||||
| 17β-estradiol level, pmol/L | 9476 | 9233 | 218 | F (EFP): 120–300; M: 10–50 |
| LH level, IU/L | 24 | 18 | 13 | F (EFP): 2–8; M: 2–8 |
| FSH level, IU/L | 13 | 15 | 56 | F (EFP): 2–10; M: 2-10 |
| Testosterone level, nmol/L | 6.5 | 7.3 | 3 | F (EFP): 0.3–1.5; M: 12.1–29.5 |
| DHEAS, µmol/L | 1.4 | 1.7 | 1.4 | F (EFP): 0.95–10.9; M: 2.2–12.2 |
| Imaging | ||||
| Ovarian ultrasonography | Multicystic ovaries | Multicystic ovaries | NA | |
| Bone age evaluation, y | 13 at 19 years old | 13 at 21 years old | 11 at 18 years old | |
Abbreviations: BMI, body mass index; DHEAS, dehydroepiandrosterone sulfate; EFP, early follicular phase; F, female; FSH, follicle-stimulating hormone; LH, luteinizing hormone; M, male; NA, not applicable.
Figure 1.
Phenotypic features of the proband, pedigree of the consanguineous family, and ESR1 sequence analysis. (A) Absence of breast development (Tanner stage I), mild adipomastia, and the presence of chest acne are shown from examination of the proband at age 25 years. (B) The pedigree of the proband (arrow) and her family. The double line indicates consanguinity. The triangle indicates miscarriage. Circles denote female family members; squares denote male family members. Solid symbols illustrate homozygous affected patients, and half-solid symbols represent family members who were heterozygous for ERS1 mutation. (C) The results of sequencing of the ESR1 gene in affected members of the proband’s family. Nucleotides are labeled with 1-letter symbols at the top of the chromatogram, with the corresponding double-stranded nucleotide sequences below. The arrow shows the homozygous mutant genotype resulting in a nucleotide change from G to A at complementary DNA nucleotide 1181 (GenBank accession number, NM_000125.3). Below, the 8 coding exons of the ESR1 gene and the corresponding domains of ERα protein are schematically represented. Arrows indicate the nucleotide (c.1181G>A) and residue (p.Arg394His) changes in affected patients. (D) Alignment of the Arg (R) 394 residue in ERα protein of other species, indicating complete conservation among species. DBD, DNA binding domain; LBD, ligand binding domain; NTD, N-terminal domain.
The proband’s affected 21-year-old sister (IV-5) had no breast development and primary amenorrhea. The proband’s affected 46,XY brother (IV-6) had Tanner stage I gonadal development at 18 years of age with a cryptorchid right testis. His left testis volume was hypoplastic to <1 mL. The 3 affected patients (IV-3, IV-5, and IV-6) presented with marked delayed bone maturation for their chronological age.
The parents (III-5 and III-7) were healthy first cousins. The mother underwent menarche at 15 years of age, and the father achieved puberty at 15 years of age. One of the proband’s sisters (IV-4) had normal puberty and underwent menarche at 14 years of age.
Circulating steroid precursors were measured by liquid chromatography–tandem mass spectrometry in the proband (Table 2). Progesterone, 17-hydroxyprogesterone, testosterone, androstenedione, and dihydrotestosterone were elevated. By contrast, adrenal steroids, including aldosterone, cortisol, dehydroepiandrosterone, 11β-hydroxyandrostenedione, 11-deoxycortisol, and 21-deoxycortisol were within the normal range. The sex hormone–binding globulin level was very low, which suggested the absence of a hepatic effect of estrogen. AMH was normal in the proband, whereas inhibin A and B were significantly elevated. AMH [4.3 pmol/L (normal range, 10 to 364 pmol/L)] and inhibin B [5 pg/mL (normal range, >100 pg/mL)] were low in the brother in conjunction with a low testosterone level.
Table 2.
Proband’s Detailed Hormonal Evaluation
| IV-3 (Proband) | Normal Range | |
|---|---|---|
| Steroid evaluation by LC-MS/MS or GCMS | ||
| Estradiol, pmol/La | 6771 | 120–300 |
| Estrone, pmol/La | 6416 | 65–400 |
| Testosterone, nmol/L | 3.5 | 0.3–1.5 |
| Androstenedione, nmol/L | 18.5 | 1.7–5.3 |
| Progesterone, nmol/L | 14.3 | 0.3–6.5 |
| Aldosterone, pmol/L | 69 | 50–600 |
| Cortisol, nmol/L | 323 | 160–610 |
| Cortisone, nmol/L | 41 | 22–45 |
| 17-OH progesterone, nmol/L | 27.4 | 0.3–2.7 |
| 11-deoxycortisol, nmol/L | 0.69 | < 6 |
| 21-deoxycortisol, nmol/L | Undetectable | < 0.9 |
| 11β-OH androstenedione, nmol/L | 6.7 | 1.5–11.5 |
| Dihydrotestosterone, nmol/L | 2.1 | < 1 |
| DHEA, nmol/L | 23.6 | 7–38 |
| Gonadic peptides | ||
| Inhibin A, pg/mLb | 516 | 10–90 |
| Inhibin B, pg/mLb | 376 | 60–195 |
| AMH, pmol/Lb | 19 | 14–48 |
| Transport protein | ||
| SHBG, nmol/Lc | 6 | 40–80 |
Abbreviations: DHEA, dehydroepiandrosterone; GCMS, gas chromatography-mass spectrometry; LC-MS/MS: liquid chromatography coupled to tandem mass spectrometry; SHBG, sex hormone–binding globulin; 11β-OH androstenedione, 11β-hydroxyandrostenedione; 17-OH progesterone, 17-hydroxyprogesterone.
By GCMS.
Plasma concentrations of inhibin A, inhibin B, and AMH were measured by enzyme immunometric assays [Oxford Bio-Innovation reagents for inhibins; Immunotech reagents (Beckman Coulter) for AMH].
SHBG was measured by IRMA (Cisbio International).
Genetic analysis revealed a homozygous missense mutation in the fifth coding exon of ESR1 in all affected individuals (Fig. 1C). The 3 affected family members were homozygous for a change from guanine to adenine at complementary DNA nucleotide 1181 (c.1181G>A), leading to the substitution of histidine for arginine at residue 394 of the ligand-binding domain of ERα (p.Arg394His), a pivotal residue completely conserved among species (Fig. 1D). Three unaffected related subjects, the proband’s parents (III-5 and III-7) and 1 sister (IV-4), were heterozygous for this mutation. The R394H mutation was absent in Exome Variant Server and ExAC databases and predicted to be deleterious according to the SIFT score and PolyPhen-2.
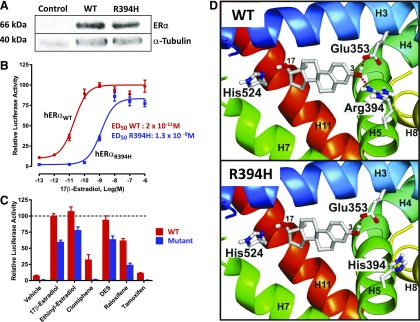
Western blot analysis revealed that both WT and mutant ERα were expressed at similar protein levels in transfected HEK293T cells (Fig. 2A). Transactivation of an ERE-dependent luciferase reporter gene in transiently transfected HEK293T cells showed a highly reduced sensitivity of R394H ERα mutant to 17β-estradiol stimulation (Fig. 2B), with an ED50 ∼65-fold higher than that of the WT ERα (1300 vs 20 pM, respectively). Potential activating ligands were evaluated for their ability to partially restore estrogen sensitivity. Known ERα ligands bearing (ethinyl estradiol, DES, and raloxifene) or lacking (clomiphene and tamoxifen) hydroxyl groups at the C3 position were tested for their ability to increase transcriptional activity of the R394H mutant. None of the compounds was found to be more efficient than 17β-estradiol in activating the mutant receptor (Fig. 2C).
Figure 2.
Functional analysis of the ESR1 mutation. (A) A representative western blot analysis of whole cell lysates detecting the presence of WT and mutant ER after transient transfection in HEK293T cells. Control corresponds to untransfected HEK293T cells. Expression of α-tubulin was also analyzed as a control. (B) 17β-estradiol dose-response curves of transactivation of ERE-luciferase (ERE upstream of luciferase) reporter constructs in transiently transfected HEK293T cells, revealing greatly reduced sensitivity of the ERα mutant compared with WT ERα. 17β-estradiol dose-response curves (0.1 pM to 1 µm) were generated in sextuplicate. Experiments were repeated 3 times. (C) The transcriptional activity of ERα ligand–activated R394H mutant (blue) never reached that of the WT ERα (red), regardless of the ligand (5 nM) indicated in the x-axis. Results are mean ± standard error of the mean of 3 independent ERE-luciferase reporter assays performed in sextuplicate. Dotted line represents the 100% maximal transcriptional activity of WT ERα. Statistical comparison between WT and R394H ERα mutant reveals significant difference for each experimental condition. (D) Estradiol within the ligand-binding cavity of the WT (upper panel) and the R394H mutant hERα (lower panel). From the N- to the C-terminus of the domain, the secondary structure elements are represented as ribbons with a blue-green-yellow color gradient. Only residues involved in estradiol anchoring are shown. Red dashed lines indicate hydrogen bonds between estradiol and the hERα residues. The figures were generated using PyMOL software (www.rcsb.org/pdb).
The structural consequence of the R394H mutation was determined by substituting histidine for arginine at position 394. In the WT hERα receptor, estradiol is anchored in the binding pocket by 3 hydrogen bonds (Glu353 and Arg394 anchor the C3 hydroxyl groups and His524 anchors the C17; Fig. 2D, upper panel). His394 in the mutant hERα is unable to properly anchor the estradiol, which is consistent with the altered response of the mutated receptor to its ligand (Fig. 2D, lower panel).
Discussion
We describe a consanguineous family in which there was a high incidence of pubertal failure. Two female family members and a male family member had markedly elevated serum E2 levels, associated with elevated gonadotropin levels, which were strongly suggestive of an estrogen-resistance/insensitivity syndrome. These 3 affected patients were found to have a homozygous missense ESR1 mutation affecting an essential and highly conserved arginine residue in helix H5 of the ERα ligand-binding domain. Interestingly, the arginine residue in ERα is also conserved among other species and in androgen and mineralocorticoid receptors. The mutations of the analog arginine in androgen and mineralocorticoid receptors have been previously associated with androgen-insensitivity syndrome and decreased sensitivity to aldosterone, respectively (12, 13). Herein, we demonstrate that the R394H ESR1 mutation is deleterious in vitro. The unaffected parents and sister are heterozygous for this mutation, which indicates that this disorder is transmitted as an autosomal recessive trait. Thus, 1 normal allele of ESR1 appears to be sufficient to achieve puberty and fertility in humans as well as in mouse models. Indeed, the heterozygous proband’s parents have proven their fertility.
The 2 affected sisters had no breast development, illustrating the fact that ERα activity is absolutely required for mammary gland development. Impaired negative estrogen feedback accounts for the elevated gonadotropin levels (7, 14), and for ovarian cyst formation, which is also described in genetic disorders of steroidogenesis (15, 16). Ovarian cyst occurrence also requires the intraovarian actions of ERβ, the most prevalent estrogen receptor in the ovary (17). Interestingly, the ovarian phenotype of our patients differed from polycystic ovarian syndrome, given that cysts were larger than accumulating small follicles seen in the majority of patients with polycystic ovarian syndrome. Hormonal analysis in the affected proband revealed high levels of E2 and estrogen precursors, including testosterone, but normal levels of adrenal steroids. These results confirm the ovarian origin of estradiol and testosterone in the proband and her sister. Normal AMH levels suggest that the proband had normal ovarian reserve, whereas elevated levels of inhibin A and B are consistent with gonadotropin stimulation of an otherwise normal ovary. The male patient (IV-6) had a low testosterone level, probably as a result of his cryptorchidism, which was not reported in the sole male with an ESR1 mutation to date (4). On the basis of his low inhibin B level, it is very likely that spermatogenesis may not occur in our patient. A relationship between cryptorchidism or retractable testes and ESR1 mutation has been reported in male Esr1 knockout mice, consistent with the patient’s phenotype (18, 19).
Estrogens play a critical role in pubertal growth and epiphyseal maturation in both sexes. The first description of a male patient with an ESR1 mutation revealed that estrogen bone effects involved ERα, because this 28-year-old man presented with markedly delayed skeletal maturation and osteoporosis (4). Likewise, the woman previously reported with an ESR1 mutation also had open epiphyses at the age of 18 years (5). Our observations are in accordance with these previous findings, given that all 3 patients’ bone ages were delayed relative to their chronological age. Surprisingly, the proband was of normal height (target height, 155 cm), whereas her sister was tall. The proband presented with intrauterine growth retardation, as opposed to her sister, who had a normal birth weight. Our hypothesis relies on the potential link between birth weight and final height (20), or the difference between the sisters, with respect to the extent of the phenotypic penetrance of this particular ESR1 mutation.
It cannot be excluded that high levels of circulating estrogens may exert some functional influence through signaling via ERβ and G protein-coupled estrogen receptor-1, although genomic or nongenomic estrogen-dependent effects, respectively (21–23), might partially explain the variability in phenotypes. Long-term consequences of high circulating estrogen levels associated with ERα resistance could contribute to cardiovascular disorders and bone alterations. To partially restore estrogen responsiveness in patients with ESR1 mutations, various ER ligands were analyzed (as potential therapeutic options) for their ability to increase transcriptional activity of the mutated receptor. Because of the structural determinant defect in the ligand-binding domain of R394H ERα, we identified pharmacological compounds containing a C3 hydroxyl group that would more stably interact with and thus activate ERα. Although ethinyl estradiol, DES, and raloxifene were more efficient activators than other compounds, none of them was found to be a stronger ERα transactivator than 17β-estradiol. An alternative therapeutic approach to activate this defective ERα receptor would be the use of transcriptional coactivator small molecules that stabilize the ligand/receptor complex to allow activation. This might represent an attractive therapeutic option in the future, as recently reported for cancer therapy (24). Finally, description and analysis of this family of patients with mutant ER have provided additional clinical findings toward identification and characterization of what was previously thought to be a highly rare clinical condition.
Acknowledgments
We thank Dr Sylvie Brailly-Tabard for her help in determining inhibin and AMH measurements and Dr Franck Giton for measuring estrone. We thank the patients and all of the members of the family described in this article.
Acknowledgments
This work was supported by grants from INSERM and Université Paris Sud. V.B. was a recipient of a fellowship (Poste d’accueil-Plan Cancer 2014-2019) from INSERM. K.S.K. received funding from the Division of Intramural Research, National Institute of Environmental Health Sciences/National Institutes of Health (grant no. 1ZIAES70065).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMH
- anti-Müllerian hormone
- DES
- diethylstilbestrol
- ER
- estrogen receptor
- ERE
- estrogen-response elements
- HEK293T
- human embryonic kidney 293T
- hER
- human estrogen receptor
- WT
- wild type.
References
- 1.Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA. 1985;82(23):7889–7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93(12):5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasgupta S, Lonard DM, O’Malley BW. Nuclear receptor coactivators: master regulators of human health and disease. Annu Rev Med. 2014;65:279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–1061. [DOI] [PubMed] [Google Scholar]
- 5.Quaynor SD, Stradtman EW Jr, Kim H-G, Shen Y, Chorich LP, Schreihofer DA, Layman LC. Delayed puberty and estrogen resistance in a woman with estrogen receptor α variant. N Engl J Med. 2013;369(2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90(23):11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. [DOI] [PubMed] [Google Scholar]
- 8.Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia. 1997;2(4):323–334. [DOI] [PubMed] [Google Scholar]
- 9.Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137(11):4796–4805. [DOI] [PubMed] [Google Scholar]
- 10.Wärnmark A, Treuter E, Gustafsson J-A, Hubbard RE, Brzozowski AM, Pike ACW. Interaction of transcriptional intermediary factor 2 nuclear receptor box peptides with the coactivator binding site of estrogen receptor alpha. J Biol Chem. 2002;277(24):21862–21868. [DOI] [PubMed] [Google Scholar]
- 11.Bouilly J, Veitia RA, Binart N. NOBOX is a key FOXL2 partner involved in ovarian folliculogenesis. J Mol Cell Biol. 2014;6(2):175–177. [DOI] [PubMed] [Google Scholar]
- 12.Komori S, Kasumi H, Sakata K, Tanaka H, Hamada K, Koyama K. Molecular analysis of the androgen receptor gene in 4 patients with complete androgen insensitivity. Arch Gynecol Obstet. 1998;261(2):95–100. [DOI] [PubMed] [Google Scholar]
- 13.Fagart J, Wurtz JM, Souque A, Hellal-Levy C, Moras D, Rafestin-Oblin ME. Antagonism in the human mineralocorticoid receptor. EMBO J. 1998;17(12):3317–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology. 2008;149(11):5328–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito Y, Fisher CR, Conte FA, Grumbach MM, Simpson ER. Molecular basis of aromatase deficiency in an adult female with sexual infantilism and polycystic ovaries. Proc Natl Acad Sci USA. 1993;90(24):11673–11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ten Kate-Booij MJ, Cobbaert C, Koper JW, de Jong FH. Deficiency of 17,20-lyase causing giant ovarian cysts in a girl and a female phenotype in her 46,XY sister: case report. Hum Reprod. 2004;19(2):456–459. [DOI] [PubMed] [Google Scholar]
- 17.Couse JF, Yates MM, Sanford R, Nyska A, Nilson JH, Korach KS. Formation of cystic ovarian follicles associated with elevated luteinizing hormone requires estrogen receptor-beta. Endocrinology. 2004;145(10):4693–4702. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson KM, Tong SY, Washburn T, Lubahn DB, Eddy EM, Hutson JM, Korach KS. Morphometric study of the gubernaculum in male estrogen receptor mutant mice. J Androl. 1996;17(2):91–95. [PubMed] [Google Scholar]
- 19.Bartlett JE, Washburn T, Eddy EM, Korach KS, Temelcos C, Hutson JM. Early development of the gubernaculum and cremaster sac in estrogen receptor knockout mice. Urol Res. 2001;29(3):163–167. [DOI] [PubMed] [Google Scholar]
- 20.Lee PA, Chernausek SD, Hokken-Koelega ACS, Czernichow P; International Small for Gestational Age Advisory Board . International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24-October 1, 2001. Pediatrics. 2003;111(6 Pt 1):1253–1261. [DOI] [PubMed] [Google Scholar]
- 21.Herynk MH, Fuqua SAW. Estrogen receptor mutations in human disease. Endocr Rev. 2004;25(6):869–898. [DOI] [PubMed] [Google Scholar]
- 22.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153(7):2953–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Yu Y, Chow D-C, Yan F, Hsu C-C, Stossi F, Mancini MA, Palzkill T, Liao L, Zhou S, Xu J, Lonard DM, O’Malley BW. Characterization of a steroid receptor coactivator small molecule stimulator that overstimulates cancer cells and leads to cell stress and death. Cancer Cell. 2015;28(2):240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]