Abstract
Context:
In humans, dietary vs intraindividual determinants of macronutrient oxidation preference and the role of the sympathetic nervous system (SNS) during short-term overfeeding and fasting are unclear.
Objective:
To understand the influence on metabolic changes of diet and SNS during 24 hours of overfeeding.
Design, Setting, Participants, and Interventions:
While residing on a clinical research unit, 64 participants with normal glucose regulation were assessed during energy balance, fasting, and four 24-hour overfeeding diets, given in random order. The overfeeding diets contained 200% of energy requirements and varied macronutrient proportions: (1) standard (50% carbohydrate, 20% protein, and 30% fat); (2) 75% carbohydrate; (3) 60% fat; and (4) 3% protein.
Main Outcome Measures:
Twenty-four–hour energy expenditure (EE) and macronutrient oxidation rates were measured in an indirect calorimeter during the dietary interventions, with concomitant measurement of urinary catecholamines and free cortisol.
Results:
EE decreased with fasting (−7.7% ± 4.8%; P < 0.0001) and increased with overfeeding. The smallest increase occurred during consumption of the diet with 3% protein (2.7% ± 4.5%; P = 0.001) and the greatest during the diet with 75% carbohydrate (13.8 ± 5.7%; P < 0.0001). Approximately 60% of macronutrient oxidation was determined by diet and 20% by intrinsic factors (P < 0.0001). Only urinary epinephrine differed between fasting and overfeeding diets (Δ = 2.25 ± 2.9 µg/24h; P < 0.0001). During fasting, higher urinary epinephrine concentrations correlated with smaller reductions in EE (ρ = 0.34; P = 0.01).
Conclusions:
Independent from dietary macronutrient proportions, there is a strong individual contribution to fuel preference that remains consistent across diets. Higher urinary epinephrine levels may reflect the importance of epinephrine in maintaining EE during fasting.
Précis: Diet and intrinsic factors influenced fuel preference during 24 hours of fasting and consumption of 4 different overfeeding diets. Higher fasting urinary epinephrine correlated with smaller energy expenditure reductions.
Energy intake above or below energy expenditure (EE) leads to weight gain or weight loss, respectively (1). Variations in the physiologic responses to perturbations in energy balance (EB) exist within the human population (2–4) and may influence ability to maintain weight stability. Overfeeding studies in humans have shown that EE increase in response to short-term excess intake is mostly determined by the thermic effect of food and the cost of energy storage (1, 5–7). In the initial preplanned analysis of the first 20 participants in this study, we demonstrated that EE consistently and reproducibly increases in humans when a diet with 200% of the daily energy requirements is given (7). Several studies have shown marked interindividual variation in the EE responses to fasting and overfeeding (2, 7–11), and this individual variation has been shown to contribute to differences in weight change over time, with less favorable EE responses to energy excess or deficit (i.e., thrifty vs spendthrift phenotypes) leading to weight gain (3, 4).
Independent of EE, a higher respiratory quotient (RQ), an indicator of the carbohydrate-to-fat oxidation ratio, during EB predicts both greater subsequent food intake (12) and future weight gain (13). The primary determinant of this subsequent food intake is a higher carbohydrate oxidation (carbox) during EB, as opposed to lower lipid oxidation (lipox) (14). It is unknown to what extent macronutrient oxidation (MO) variations during differing diets are a result of consumed macronutrient percentages vs intraindividual factors. In our initial cohort, the changes in EE were mainly driven by the percentage of protein in the diet (7); however, the initial sample size was too small to fully assess the effects of diet on MO. Subsequently, a threefold larger cohort was recruited to determine the impact of differing effects of fat and carbohydrate content on the EE and MO response.
The sympathetic nervous system (SNS) is activated during overfeeding in rodent models (15–19). In humans, the role of the SNS during short-term energy excess (7, 20–23) is less clear. Our group has previously shown that underfeeding and low-protein overfeeding lead to an increase in plasma catecholamines the morning following the diet (7); however, urinary concentrations that reflect concurrent changes during consumption of overfeeding diets have yet to be evaluated. The importance of the hypothalamic-pituitary-adrenal (HPA) axis in the development of pathologic obesity (Cushing syndrome) in humans is well documented (24), but its role in the response to overfeeding is unknown. In humans, any associations between changes in the hormones of the SNS and the HPA axis and the EE response to short-term overfeeding have yet to be elucidated.
We studied EE and MO changes during 24 hours of overfeeding with diets of differing macronutrient content in 64 healthy participants. We hypothesized that (1) there would be a strong individual contribution as a result of intrinsic factors to the relative degree of carbohydrate-to-lipid oxidation, even if the content of the diet changed, and (2) that the macronutrient content of each overfeeding diet would lead to differential activation of the SNS and HPA axis.
Methods
Participants
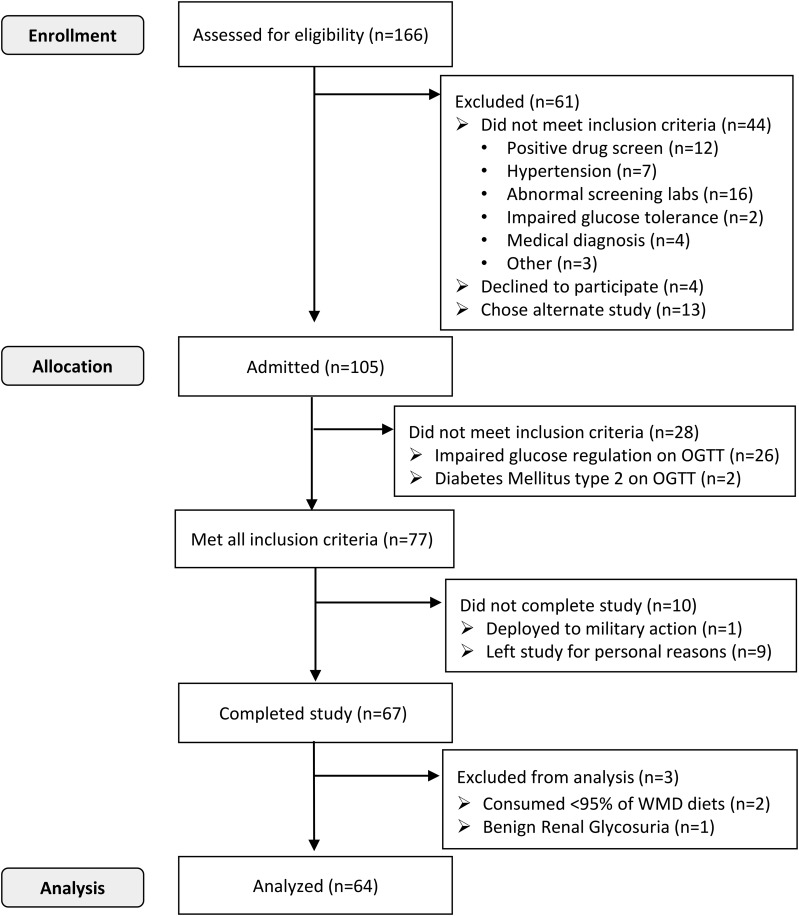
A total of 166 adults, 18 to 54 years of age, were recruited from the general Phoenix, Arizona, area between 2008 and 2015. Of these, 105 participants were considered healthy by medical history, physical examination, electrocardiogram, and basic laboratory measures. These individuals were admitted to the clinical research unit and placed on a weight-maintaining diet (WMD) composed of 50% carbohydrates, 30% fat, and 20% protein. After 3 days on the WMD, the final evaluation for inclusion into the study, a 75-g oral glucose tolerance test (OGTT), was performed. Only participants with normal glucose regulation (25) continued in the study. Sixty-four participants completed the study (Fig. 1). Body composition was determined by dual-energy X-ray absorptiometry (DPX-L, version 7.53.002; Lunar Radiation, Madison, WI). Body weight was measured daily upon first awakening. Energy content for the WMD was calculated for each individual using previously described sex-specific equations (26); the provided WMD was then adjusted by adding or subtracting 200 kcal/d as needed to maintain a stable body weight within ±1%. No further adjustments were made to the WMD once dietary interventions began. The mean coefficient of variation of the participants’ body weight before the seven 24-hour EE (24hEE) assessments was 1.05% [95% confidence interval, 0.92% to 1.18%].
Figure 1.
Flow diagram of volunteer inclusion and participation in study.
Participants were restricted to light activities while on the clinical research unit and were required to consume at least 95% of every meal (2 participants excluded; Fig. 1). Plasma glucose concentrations during the OGTT were determined using an enzymatic oxygen-rate method (Glucose Analyzer 2; Beckman Coulter, Brea, CA; GM9 Glucose Analyser; Analox, London, UK). Plasma insulin was measured using an automated immunoenzymometric assay (Tosoh Bioscience, Tessenderlo, Belgium). All participants provided written informed consent before beginning the study. The Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases approved this study.
Diets
Meals were provided at 7:00 am, 11:00 am, 4:00 pm, and 7:00 pm. To precisely determine 24hEE during EB, 24hEE was measured in each subject while he or she consumed a eucaloric diet (50% carbohydrate, 30% fat, 20% protein) on 2 separate occasions. For the first occasion, energy needs were estimated at approximately 80% of the WMD to compensate for reduced activity while within the calorimeter (27). The 24hEE result from the first chamber was used as the energy intake for the second assessment. This second assessment was considered the baseline EB comparator. The 24hEE from the EB measurement was doubled to determine the energy content of the overfeeding diets. Following determination of the baseline EE, 24hEE was measured in each subject during fasting and during consumption of 4 separate overfeeding diets. During fasting, participants only had access to noncaloric drinks. Each dietary intervention, including fasting, was given for only 24 hours, in random order, and with a 3-day WMD period between diets. The macronutrient composition of the overfeeding diets was determined using Food Processor software (ESHA Research, Salem, Oregon) and were as follows: (1) standard, with 50% carbohydrate, 30% fat, and 20% protein; (2) low protein, with 51% carbohydrate, 46% fat, and 3% protein; (3) high fat, with 20% carbohydrate, 60% fat, and 20% protein; and (4) high carbohydrate, with 75% carbohydrate, 5% fat, and 20% protein (Supplemental Tables 1–3 (218.3KB, docx) ). Food was prepared by our metabolic kitchen, and any uneaten food was returned to the kitchen for weighing to determine actual food intake per day; on average, 99.5% (95% confidence interval, 99.3% to 99.7%) of the overfeeding diets were consumed by the participants. If less than 95% of an overfeeding diet was consumed, its data were excluded from the analysis (n = 4 chambers excluded: 2 low-protein, 1 high-carbohydrate, and 1 standard overfeeding diets).
EE measurements
Whole-room indirect calorimetry was used to measure 24hEE, as previously described (7, 27). Details are provided in the Supplemental Material and Methods section. Deviation from energy balance was calculated by subtracting 24hEE from energy intake. The percent change in 24hEE from baseline was calculated by subtracting the 24hEE during EB from the 24hEE of each intervention diet, dividing the result by the 24hEE during EB, and expressing the result as a percentage, that is: (EEIntervention diet − EEEB) / EEEB × 100. Similar equations were used to calculate the percent change of carbox and lipox, for example: (carboxIntervention diet − carboxEB) / carboxEB × 100.
Hormonal measurements
Urine was collected during each 24hEE measure and assessed for catecholamine concentrations (epinephrine, norepinephrine, dopamine, metanephrine, and normetanephrine) to evaluate SNS activity. In a subset of the first 37 participants, urinary free cortisol (UFC), as an index of the HPA axis, was analyzed. A 24-hour urine collection was obtained during each 24hEE measurement. Details are provided in the Supplemental Material and Methods section.
Statistics
Alpha was set at 0.01, given the number of analyses performed and to account for 5 differing dietary interventions. Analyses were performed using SAS software (SAS 9.3, Enterprise guide version 5.1; SAS Institute, Cary, NC). Data are expressed as means ± standard deviations, except for skewed data, which are expressed as median and interquartile range. Some 24-hour urinary catecholamines (normetanephrine, total metanephrine, epinephrine, and norepinephrine) were log transformed to meet the assumptions of linear regression (i.e., homoscedasticity and normal distribution of residuals). Differences between groups were assessed with Student t test or 1-way analysis of variance. Differences in the distribution of race between sexes were assessed using χ2 analysis. Correlations between continuous variables were evaluated by either Pearson (r) or Spearman (ρ) correlation coefficients for normally or nonnormally distributed data, respectively. When appropriate, the correlations were partially adjusted for age, sex, fat mass (FM), fat-free mass (FFM), and spontaneous physical activity (SPA).
Associations between 24hEE measures and hormones were evaluated with mixed models to account for repeated measures using a compound symmetry covariance structure and including the following variables as fixed effects: age, race, sex, percentage body fat (%fat), SPA, and diet. Adjustment for multiple comparisons was performed using the Tukey-Kramer method. To assess factors explaining EE and MO variations, mixed models were used to account for repeated measures, diets, age, sex, SPA, FM, and FFM, as well as baseline measures when appropriate. To assess the contribution of dietary intervention vs individual differences in MO, linear regression models that included all feeding assessments were created with either carbox or lipox as the dependent variable and individual and diet as the 2 explanatory factors. Participants were classified as having a thrifty or spendthrift phenotype on the basis of a median split of the EE response to fasting, as described previously (3). A greater decrease in EE with fasting was considered thrifty. Sensitivity analyses that excluded females were performed for all the previously mentioned analyses and led to similar results (data not shown).
Results
Subject characteristics
Characteristics of the 64 participants (51 males, 13 females) are shown in Table 1.
Table 1.
Baseline Participant Characteristics During Eucaloric Feeding
| Variable | All (N = 64) | Males (n = 51) | Females (n = 13) | P |
|---|---|---|---|---|
| Race | 14 AA, 18 W, 13 H, 19 NA | 9 AA, 16 W, 10 H, 17 NA | 5 AA, 4 W, 1 H, 2 NA | 0.33 |
| Age, y | 36.7 ± 10.0 (18.2, 54.1) | 37.5 ± 10.3 (18.2, 54.1) | 33.8 ± 8.7 (20.4, 45.5) | 0.24 |
| Weight, kg | 78.3 ± 12.3 (47.5, 107.8) | 79.5 ± 11.2 (56.0, 104.8) | 73.7 ± 16.3 (47.5, 107.8) | 0.14 |
| Height, cm | 173.0 ± 7.3 (156.8, 196.4) | 174.9 ± 6.7 (161.0, 196.4) | 165.6 ± 4.4 (156.8, 170) | < 0.0001 |
| BMI, kg/m2 | 26.2 ± 4.0 (17.8, 39.1) | 26.0 ± 3.6 (18.3, 36.9) | 26.8 ± 5.5 (17.8, 39.1) | 0.62 |
| Percentage body fat | 27.7 ± 10.1 (6.9, 53.8) | 24.6 ± 8.0 (6.9, 38.3) | 39.8 ± 8.3 (24.2, 53.8) | < 0.0001 |
| FM, kg | 22.2 ± 10.2 (4.9, 56.9) | 20.0 ± 8.3 (4.9, 36.0) | 30.5 ± 12.8 (13.6, 56.9) | 0.014 |
| FFM, kg | 56.2 ± 9.4 (33.9, 79.4) | 59.4 ± 7.2 (46.9, 79.4) | 43.3 ± 4.7 (33.9, 50.9) | < 0.0001 |
| Fasting glucose, mg/dL | 91.4 ± 5.1 (80, 99) | 91.6 ± 5.3 (80, 99) | 90.6 ± 4.1 (84, 98) | 0.52 |
| 2-h glucose, mg/dL | 104.6 ± 19.4 (65, 138) | 104.2 ± 20.0 (65, 138) | 106.3 ± 17.5 (80, 132) | 0.73 |
| Fasting insulin, µU/mL | 7.0 ± 3.6 (2.0, 18.8) | 6.7 ± 3.6 (2.0, 18.8) | 7.9 ± 3.6 (3.2, 17.2) | 0.32 |
| 2-h insulin, µU/mL | 48.1 ± 36.2 (3.9, 184.5) | 47.5 ± 38.4 (3.9, 184.5) | 50.6 ± 26.2 (9.8, 92.0) | 0.8 |
| TSH, mU/L | 1.35 ± 0.74 (0.44, 3.59) | 1.38 ± 0.84 (0.44, 3.59) | 1.28 ± 0.46 (0.62, 1.93) | 0.8 |
| EB, kcal/da | 26.1 ± 78.0 (−194, 225) | 27.3 ± 78.7 (−194, 225) | 21.3 ± 78.2 (−81, 169) | 0.81 |
| 24-h EE, kcal/d | 2031 ± 281 (1502, 2810) | 2090 ± 267 (1573, 2810) | 1802 ± 213 (1502, 2290) | 0.0006 |
| TEF% | 7.8 ± 4.8 (−4.3, 20.9) | 7.5 ± 4.6 (−4.3, 17.0) | 9.0 ± 5.4 (−2.1, 20.9) | 0.31 |
Minimum and maximum values are provided in parentheses.
Abbreviations: AA, African American; BMI, body mass index; H, Hispanic; NA, Native American; TEF%, thermic effect of food in percent (calculated by subtracting fasting 24hEE from 24hEE during feeding and dividing by energy consumed, all in kilocalories per day); TSH, thyrotropin; W, white.
Food intake minus energy expenditure during EB diet.
EE response to intervention diets
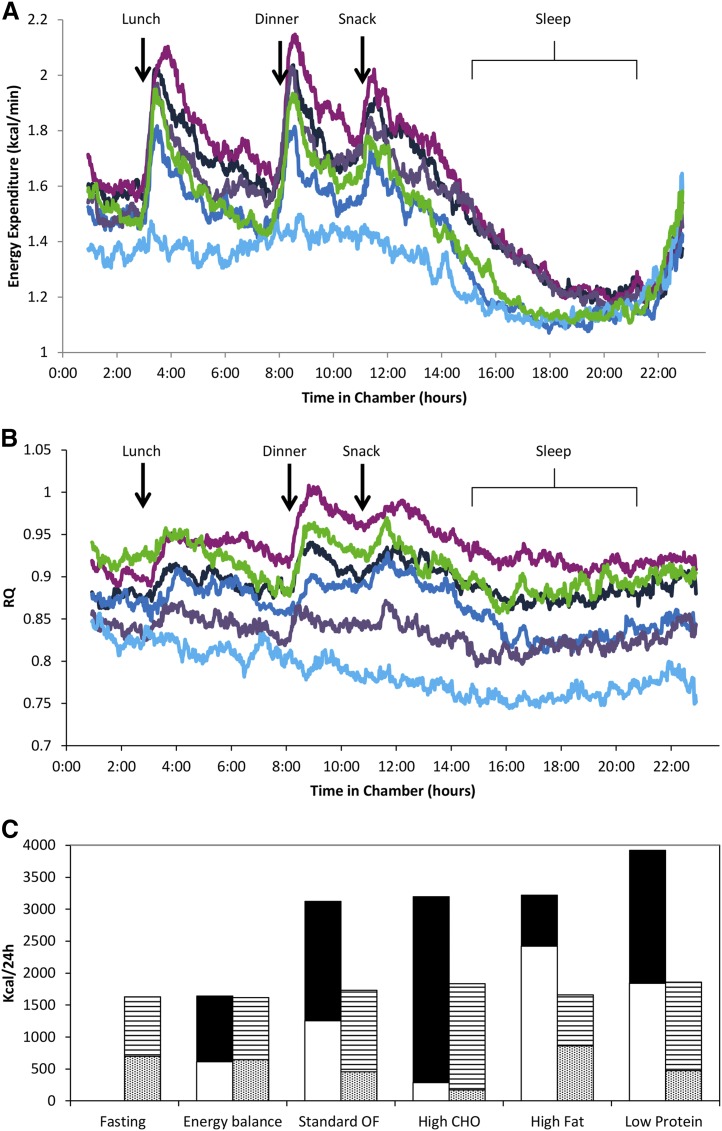
When compared with 24hEE during EB, 24hEE decreased with fasting and increased with overfeeding (Fig. 2A; Table 2). The percentage EE changes during fasting and all overfeeding diets were correlated (the correlation coefficient r ranged from 0.33 to 0.50; all P ≤ 0.008; Supplemental Fig. 1 (218.3KB, docx) ), except for the correlation between the percentage EE changes during the high-carbohydrate and fasting diets (r = 0.29; P = 0.02; n = 62). Sleeping EE after both fasting and low-protein overfeeding were similar to that following EB (Fig. 2A), but there were increases of almost 12% (P < 0.0001) during overfeeding diets with 20% protein content (Table 2). As expected (3, 4), the average individual EE response to dietary interventions (∆EE = 5.4% ± 3.7%) was negatively correlated with %fat (ρ = −0.32; P = 0.01). When individuals were categorized as spendthrift vs thrifty phenotypes, no differences in age, sex, %fat, FFM, FM, or EE were noted. The spendthrift group had a greater increase in EE during the high-fat diet (9.8% vs 6.0%; P = 0.003), but not during the other overfeeding diets. Sleeping EE did not differ between groups.
Figure 2.
Graphic of (A) EE and (B) related RQ of each different dietary intervention and of (C) mean carbohydrate (CHO) and lipid intake and oxidation. (A) EE/min and (B) RQ data series over 23.25 hours during all dietary interventions and compared with EB (shown in dark blue). The EE/min and temporal RQ during overfeeding (OF) are shown with the standard OF diet (n = 64; 50% CHO, 30% fat, and 20% protein) in black; the high-CHO OF diet (n = 63; 75% CHO, 5% fat, and 20% protein) in fuchsia; the high-fat OF diet (n = 63; 20% CHO, 60% fat, and 20% protein) in purple; and the low-protein OF diet (n = 62; 51% CHO, 46% fat, and 3% protein) in green. The EE/min and RQ during fasting (n = 64) are shown in light blue. The 0:00 time period indicates entry into the respiratory chamber (∼1 hour after consuming breakfast); lunch was given at the 3-hour mark, dinner at the 8-hour mark, and a snack at the 11-hour mark. Participants were asked to be in bed from the 15-hour mark to at least the 21-hour mark in the chamber, and to limit unnecessary activity throughout the 24-hour period. All trajectories were different from all other trajectories (P < 0.0001). (C) Graphic representation of mean for CHO intake in black, lipid intake in white, carbox in horizontal stripes, and lipox in dots during the different diets. Overall, despite the increase in MO following the respective increase in the amount of each specific macronutrient ingested, the amount of energy burned was less than energy ingested. Note that for high-fat OF, on average, all of the CHO given was oxidized.
Table 2.
EE and Macronutrient Measures During Eucaloric Feeding, Consumption of 4 OF Diets With Varied Macronutrient Content, and Fasting
| Variable | EB (n = 64) | Standard OF (n = 63) | High-CHO OF (n = 62) | High-Fat OF (n = 63) | Low-Protein OF (n = 62) | Fasting (n = 64) |
|---|---|---|---|---|---|---|
| 24-h EE, kcala | 2031 ± 281 (1502, 2810) | 2239 ± 334b (1530, 3175) | 2312 ± 333b (1573, 3339) | 2187 ± 312b (1532, 3114) | 2086 ± 298c (1453, 2895) | 1869 ± 238b (1426, 2655) |
| Change in 24hEE, %a | N/A | 10.2 ± 5.5b (−0.7, 23.8) | 13.8 ± 5.7b (1.3, 31.1) | 7.7 ± 5.2b (−7.2, 18.8) | 2.7 ± 4.5d (−6.9, 13.2) | −7.7 ± 4.8b (−19.3, 4.6) |
| SLEEP, kcale | 1597 ± 221 (1131, 2227) | 1782 ± 275b (1215, 2442) | 1789 ± 276b (1173, 2442) | 1787 ± 281b (1208, 2530) | 1626 ± 239 (1071, 2226) | 1591 ± 205 (1129, 2103) |
| Change in SLEEP, %e | N/A | 11.5 ± 9.7b (−2.0, 63.4) | 11.88 ± 8.77b (−3.7, 47.1) | 11.80 ± 9.46b (−9.8, 54.5) | 1.77 ± 6.35 (−10.7, 19.4) | −0.07 ± 6.90 (−14.5, 17.5) |
| DIT, kcal/da | 163 ± 110 (−74, 446) | 372 ± 145b (103, 649) | 445 ± 152b (120, 809) | 320 ± 131b (65, 674) | 218 ± 108c (7, 484) | N/A |
| TEF, % | 7.8 ± 4.8 (−4.3, 20.2) | 9.1 ± 3.0 (2.8, 17.5) | 11.3 ± 3.5b (3.3, 21.0) | 7.9 ± 2.7 (1.5, 14.8) | 5.4 ± 2.4b (0.2, 11.7) | N/A |
| SPA, % | 5.7 ± 3.4 (0.6, 16.1) | 6.26 ± 3.34 (0.7, 14.5) | 6.50 ± 3.94c(0.7, 17.0) | 5.80 ± 3.57 (0.5, 16.1) | 5.85 ± 3.90 (0.5, 20.3) | 5.21 ± 3.27 (0.5, 18.3) |
| Intake, kcal/24h | 2057 ± 280 (1508, 2921) | 4012 ± 599b (2609, 5615) | 3957 ± 582b (2604, 5620) | 4021 ± 554b (2914, 5377) | 4034 ± 577b (2913, 5625) | N/A |
| EB, kcal/24h | 25 ± 79 (−180, 225) | 1775 ± 321b (539, 2640) | 1640 ± 370b (331, 2391) | 1840 ± 292b (1323, 2505) | 1942 ± 317b (1371, 2864) | −1867 ± 238b (−2655, −1426) |
| Food quotient | 0.87 | 0.87 | 0.94 | 0.78 | 0.86 | N/A |
| RQa | 0.86 ± 0.03 (0.80, 0.93) | 0.89 ± 0.04f (0.82, 0.97) | 0.94 ± 0.04 (0.82, 1.02) | 0.83 ± 0.04f (0.75, 0.9) | 0.91 ± 0.05f (0.8, 1.00) | 0.79 ± 0.03 (0.71, 0.9) |
| Sleeping RQ | 0.83 ± 0.05 (0.67, 1.02) | 0.87 ± 0.05b (0.77, 0.97) | 0.91 ± 0.06b (0.76, 1.05) | 0.81 ± 0.04 (0.73, 0.89) | 0.88 ± 0.05b (0.75, 0.99) | 0.76 ± 0.03b (0.69, 0.87) |
| CHO intake, kcal/24ha | 1030 ± 140 (756, 1463) | 1870 ± 279 (1216, 2617) | 2905 ± 427 (1911, 4125) | 796 ± 110 (577, 1065) | 2078 ± 297 (1500, 2897) | N/A |
| CHO oxidation, kcal/24h | 982 ± 228 (550, 1466) | 1281 ± 296b (653, 1884) | 1655 ± 366b (884, 2379) | 796 ± 270b (211, 1483) | 1389 ± 381b (573, 2167) | 445 ± 203b (−105, 1121) |
| Fat intake, kcal/24ha | 613 ± 83 (449, 870) | 1252 ± 187 (814, 1752) | 285 ± 42 (187, 405) | 2421 ± 334 (1754, 3237) | 1844 ± 264 (1331, 2571) | N/A |
| Fat oxidation, kcal/24h | 652 ± 293 (106, 1555) | 449 ± 320b (−109, 1130) | 171 ± 379b (−613, 1355) | 862 ± 349b (304, 1934) | 473 ± 396b (−200, 1390) | 1119 ± 284b (350, 1838) |
| Protein intake, kcal/24ha | 411 ± 56 (302, 584) | 891 ± 133 (579, 1247) | 764 ± 112 (503, 1085) | 804 ± 111 (583, 1075) | 113 ± 16 (82, 158) | N/A |
| Protein oxidation, kcal/24h | 373 ± 92 (42, 557) | 474 ± 106b (175, 684) | 457 ± 124b (185, 735) | 489 ± 146b (17, 767) | 215 ± 54b (23, 322) | 279 ± 72b (78, 415) |
Data are shown as mean ± standard deviations, with minimum and maximum in parentheses. Comparisons were performed using mixed models to account for repeated measures using a compound symmetry covariance structure and including the variables age, race, sex, percentage body fat, SPA, and diet. P values were corrected for multiple comparisons using the Tukey-Kramer method. Diet-induced thermogenesis (DIT) was calculated by subtracting fasting 24hEE from 24hEE during feeding. TEF% was calculated by dividing DIT by energy consumed in kcal and expressing the result as a percentage. Food quotient was calculated as follows: food quotient = [1.0 × (% carbohydrate / 100)] + [0.707 × (% fat / 100)] + [0.80 × (% protein / 100)]. Overfeeding (OF) diets, given for 24 hours and as 200% of energy requirements, had the following macronutrient compositions: standard diet (n = 64) included 50% carbohydrate (CHO), 30% fat, and 20% protein; high-CHO diet (n = 63) included 75% CHO, 5% fat, and 20% protein; high-fat diet (n = 63) included 20% CHO, 60% fat, and 20% protein; and low-protein diet (n = 62) included 51% CHO, 46% fat, and 3% protein.
Abbreviations: N/A, not applicable; SLEEP, sleeping EE; TEF, thermic effect of food.
All results differed from one another (P ≤ 0.01), except for 24hEE during standard vs high-fat OF diets (P = 0.023).
P < 0.0001 compared with EB.
P ≤ 0.01 compared with EB.
P ≤ 0.001 compared with EB.
SLEEP was extrapolated to 24 hours for comparison.
P < 0.01 compared with food quotient.
MO during dietary interventions
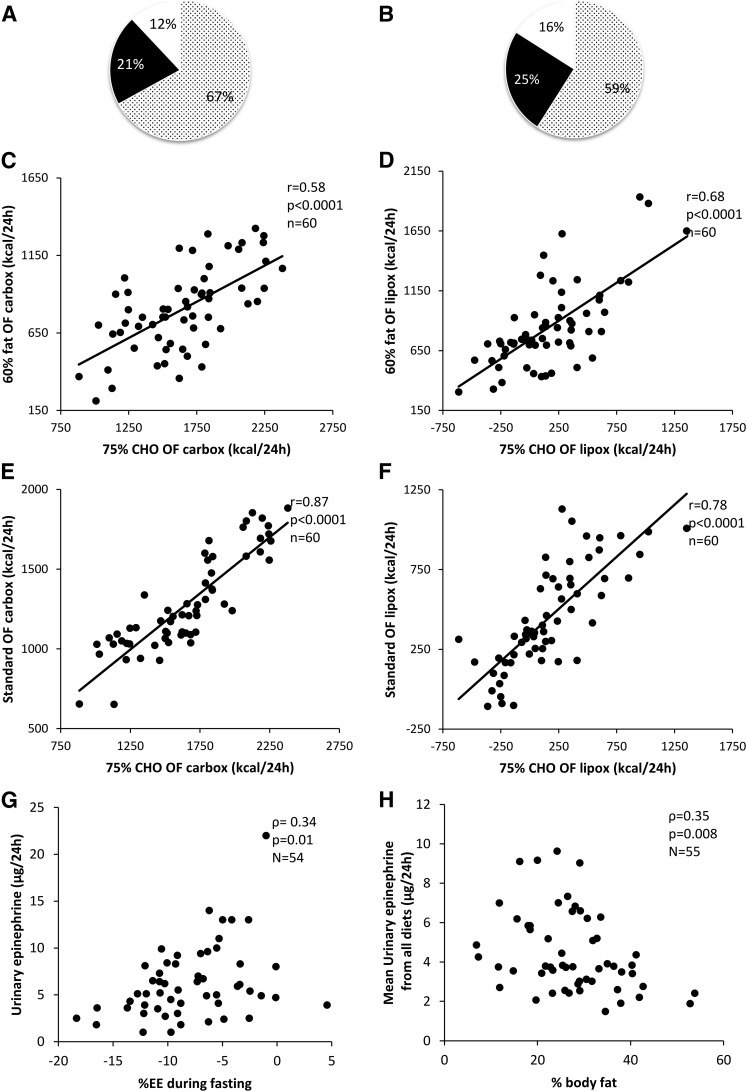
The macronutrient composition of the differnt overfeeding diets explained the majority of the variance between individuals (67% and 59% of variance in carbox and lipox resulted from differences in consumed diets; partial r2 = 0.67 and 0.59, respectively). However, intraindividual factors from a within-subject effect accounted for an additional 21% and 25% of the variance in carbox and lipox (Fig. 3A and 3B, respectively). Despite large differences in the quantitative amount of MO between diets, the ranking of each individual’s carbox and lipox during the overfeeding diets was consistent with a high intraindividual correlation of carbox (r = 0.45 to 0.87; all P ≤ 0.0002) and lipox (r = 0.48 to 0.78; all P < 0.0001) between differing diets (Fig. 3C, 3D, 3E, and 3F) and remained so after adjusting for age, sex, FFM, and FM.
Figure 3.
Pie-chart representing the contribution of diet in white/black dots, intraindividual factors on (A) carbox and (B) lipox rates in black, and undefined physiological factors in white. Intraindividual correlation between (C) carbox and (D) lipox during the high-fat (60%) and high-carbohydrate (75%; high-CHO) diets and between (E) carbox and (F) lipox during the 75% CHO and standard overfeeding (OF) diets. All correlations were adjusted for age, sex, FM, and FFM. Correlation between (G) 24-hour urinary epinephrine concentration and percent EE change during fasting, indicating possible role of epinephrine in thrifty vs spendthrift phenotypes. (H) Correlations between average 24-hour urinary epinephrine concentration during all diets and percent body fat.
Except for the high-carbohydrate diet, overfeeding with the other diets resulted in a higher-than-expected mean RQ, as compared with the calculated food quotient (P < 0.0001), indicating a preference for utilizing the carbohydrates in the diets prior to the lipids (Table 2). In mixed-model analysis, RQ increased during the high-carbohydrate, standard, and low-protein overfeeding diets but decreased during high-fat overfeeding and fasting, as compared with EB (Fig. 2B; Table 2). The mean carbohydrate and lipid intake and oxidation rates are illustrated in Fig. 2C.
In a mixed model (Supplemental Table 4 (218.3KB, docx) ) to identify carbox determinants, diet composition (P < 0.0001) and carbox during EB (β = 65 kcal/d during overfeeding per 100 kcal/d increase during EB; P < 0.0001) were determinants of carbox. Age, sex, race, SPA, FFM, and FM were not determinants of carbox during overfeeding or fasting. The main determinants of lipox response to overfeeding were diet composition (P < 0.0001) and lipox during EB (β = 69 kcal/d per increase in 100 kcal/d during EB; P < 0.0001). Percentage body fat was not a determinant of any of the MO rates, when replacing FM and FFM in the model. Fasting or 2-hour glucose and insulin during the OGTT were not determinants of any of the MO during overfeeding or fasting. When comparing spendthrift with thrifty groups, there was no difference in MO or percent change in carbox or lipox.
Urinary catecholamines and free cortisol
Concentrations of urinary catecholamines and urinary free cortisol are shown in Table 3. Compared with EB, urinary epinephrine was lower during low-protein overfeeding (median [lower quartile, upper quartile], 3.4 [25.3, 36.4] vs 4.2 [20.9, 37.7] µg/24h; P < 0.001) and higher during fasting (5.3 [22.9, 42.9] vs 4.2 [20.9, 37.7] µg/24h; P < 0.001). In general, urinary catecholamine concentration was similar during the fed state, despite diet differences, but was greater than that observed in the fasting state (Table 3). There was no diet order effect on the catecholamine results. There was no difference in UFC between diets (P = 0.37; Table 3).
Table 3.
Concentrations of Urinary Hormones During the Different Diets
| Hormone | EB | Standard OF | High-CHO OF | High-Fat OF | Low-Protein OF | Fasting |
|---|---|---|---|---|---|---|
| UFC, µg/24h | 31.0 (20.9–37.7) | 32.9 (22.1–48.1) | 30.4 (22.2–40.4) | 32.5 (23.7–45.5) | 30.8 (25.3–36.4) | 30.6 (22.9–42.9) |
| Metanephrine, µg/24h | 124 (87–173) | 127 (86–166) | 104 (73–144) | 115 (89–174) | 98 (77–136)a,b | 120 (85–168) |
| Normetanephrine, µg/24h | 219 (159–305)b | 219 (172–297)b | 176 (127–252) | 235 (152–314)c | 207 (136–256) | 169 (126–208)a |
| Total metanephrine, µg/24h | 354 (267–460) | 343 (275–470) | 292 (203–366) | 365 (245–502) | 329 (223–392) | 309 (219–407) |
| Dopamine, µg/24h | 251 (191–322) | 256 (185–325)d | 219 (181–312) | 246 (170–295) | 228(163–283) | 215 (166–277) |
| Epinephrine, µg/24h | 4.2 (2.6–6.1)d | 3.6 (2.4–5.2)c | 3.6 (2.6–4.8)c | 3.4 (2.4–5.0)c | 3.4 (2.6–4.5)c,e | 5.3 (3.8–8.0)e |
| Norepinephrine, µg/24h | 24.9 (15.7–34.0) | 22.6 (17.7–32.8) | 22.3 (16.3–26.7) | 19.6 (13.1–29.1) | 25.4 (19.1–32.6) | 20.2 (15.4–27.9) |
Values are expressed as medians, with interquartile ranges in parentheses. In the analysis, n = 37 for UFC; n = 55 for all urinary catecholamines. Overfeeding (OF) diets, given for 24 hours and as 200% of energy requirements, had the following macronutrient compositions: standard diet included 50% carbohydrate (CHO), 30% fat, and 20% protein; high-CHO diet included 75% CHO, 5% fat, and 20% protein; high-fat diet included 20% CHO, 60% fat, and 20% protein; and low-protein diet included 51% CHO, 46% fat, and 3% protein. Urinary catecholamines were measured using high-performance liquid chromatography for epinephrine, norepinephrine and dopamine, and liquid chromatography–tandem mass spectrometry stable isotope dilution analysis for metanephrine, normetanephrine, and total metanephrine by the commercial Mayo Clinic laboratory. UFC was measured using an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) by the National Institute of Diabetes and Digestive and Kidney Diseases Clinical Core Laboratory in Bethesda, MD.
Abbreviations: CHO, carbohydrate; OF, overfeeding.
P ≤ 0.01, compared with EB.
P ≤ 0.01, compared with fasting.
P < 0.0001, compared with fasting.
P < 0.001, when compared with fasting.
P < 0.001, when compared with EB.
Urinary catecholamines during the overfeeding diets did not correlate with the percent changes in EE; however, urinary epinephrine correlated with percent changes in fasting EE (ρ = 0.36; P = 0.008; Fig. 3G), such that the higher the urinary epinephrine concentration, the smaller the reduction in EE with fasting. When comparing thrifty vs spendthrift phenotypes, epinephrine (median [lower quartile, upper quartile]; thrifty, 3.39 [2.30, 5.17] vs spendthrift, 4.37 [2.97, 6.55] µg/24h; P = 0.0004) and dopamine (223.7 ± 85.4 vs 256.0 ± 85.8 µg/24h; P = 0.001) were lower in the thrifty group using the average during all dietary interventions. UFC did not differ by diet between thrifty and spendthrift groups (median [lower quartile, upper quartile], 30.24 [22.9, 41.0] vs 32.4 [24.1, 42.9] µg/24h; P = 0.63). The average urinary epinephrine across all individual measurements correlated with %fat (ρ = −0.35; P = 0.008; Fig. 3H), but the other catecholamines and UFC did not. The RQ and MO during fasting and overfeeding diets did not consistently correlate with changes in urinary catecholamines or UFC.
Discussion
In 64 adults, we evaluated the role of diet vs individual variation in the EE response to overfeeding, including the oxidative preference for carbohydrates vs lipids. We demonstrated that EE increased the most with a diet containing a high percentage of carbohydrates and an adequate percentage of protein. The lowest EE increase, despite consumption of equivalent excess energy, occurred with a low-protein (3%) overfeeding diet. As expected, the macronutrient content of the diet strongly influenced the primary source of fuel used by the body (i.e., MO) during overfeeding. However, there was also a large and significant intraindividual component to fuel preference. We did not find a role for the HPA axis in the response to overfeeding in this study. Urinary epinephrine concentration was increased during fasting, and higher urinary epinephrine concentrations were associated with smaller declines in fasting EE and lower %fat.
EE increases with overfeeding and decreases during fasting (7, 9, 11, 28). We confirmed earlier findings that the percent increase in EE with overfeeding is negatively associated with %fat (3, 4), and extended these findings, given that we now demonstrate that the increases in EE with overfeeding depend, in part, on the macronutrient composition of the diet. For the development of obesity, the concept of a thrifty vs spendthrift phenotype was hypothesized to explain why some individuals may gain weight more easily than others (2). Recently, in individuals with obesity, those who were thrifty (defined as those with a greater EE decrease during fasting) lost less weight in a carefully monitored inpatient weight loss program compared with individuals defined as spendthrift (i.e., those better able to maintain EE during fasting) (3). Our group has also shown that individuals characterized as thrifty gained more weight than those who were spendthrift at 6 months after initial evaluation (4). In addition, the EE responses to both low-protein and high-carbohydrate overfeeding were associated with weight gain, independent of EE changes with fasting. However, these associations were in opposite directions, i.e., a greater EE increase with a low-protein overfeeding diet was associated with less weight gain, but a greater increase with the high-carbohydrate overfeeding diet was associated with greater weight gain (4). Moreover, participants who were phenotypically characterized as having both a smaller EE response to low-protein overfeeding and a higher EE response to high-carbohydrate overfeeding gained the most weight. In this current study, we demonstrate that spendthrift individuals (those with a greater ability to maintain EE during fasting) also have a greater increase in EE during high-fat overfeeding. This finding may contribute to the mechanisms by which these individuals are protected from weight gain in modern society.
In prior studies, RQ and carbox measured during EB were found to be determinants of weight gain (13, 14). We found that the average changes in RQ and MO during overfeeding are highly dependent on the proportion of macronutrients consumed. Of note, on average, RQ was not greater than 1 during the high-carbohydrate overfeeding diet, which indicates that de novo lipogenesis did not routinely occur (29). Instead, the RQ of the high-carbohydrate diet was similar to the predicted food quotient, which implies that the excess energy consumed was transformed into heat, glycogen deposition, or fecal energy loss; the former possibility is supported by the higher thermic effect of food measured during this diet. Importantly, there was also a consistent intraindividual preservation of MO preference, as denoted by the strong correlations between the different diets. For example, participants with higher carbox during EB also had relatively higher carbox during the overfeeding diets. Given that increased RQ predicts weight gain (13), intrinsic preference to metabolize carbohydrates may identify individuals who tend to overconsume in the setting of increased food availability, possibly because of an increased sensitivity to glycogen depletion (30). Alternatively, high RQ may be a marker for individuals predisposed to de novo lipogenesis in the setting of continual intake of large amounts of carbohydrates.
Increases in serum norepinephrine have been reported during overfeeding in some (20, 22) but not all (21, 23) studies. We noticed no difference in 24-hour urinary norepinephrine concentrations during consumption of the different diets. However, normetanephrine, the major urinary metabolite of norepinephrine, was increased during high-fat overfeeding, standard overfeeding, and EB compared with fasting. As expected, urinary epinephrine concentrations were higher during fasting (31). Notably, urinary epinephrine concentrations were negatively associated with the decrease in 24hEE during fasting and %fat, indicating that the adrenal medulla may have a role in preventing reductions in EE with energy restriction, as well as body weight regulation. In particular, this epinephrine response may help to explain why spendthrift individuals are resistant to weight gain. In rats, the SNS is implicated in regulation of EE during feeding through mechanisms including brown adipose tissue activation, but norepinephrine is the key neurotransmitter in that response. The finding that %fat was negatively associated with urinary epinephrine concentrations is supported by a previous report (32). Epinephrine is primarily considered to be part of the hormonal response to acute stress. If fasting can be considered a state of stress, this may explain the observed epinephrine increase during fasting. Increased adrenergic activity during fasting may increase EE through increased heart rate or increased lipolysis (33). Difference in epinephrine concentrations was a key finding between thrifty and spendthrift individuals in our study, and may help to explain why spendthrift individuals lose more weight during energy restriction (3).
Most of our participants were men, despite our efforts to recruit both sexes, and we had a high proportion of overweight and obese individuals. However, our results were similar in subset analyses that excluded women. Our study population is ethnically diverse, and given the high prevalence of overweight and obese individuals in the general population (34), our findings are generalizable. Although we only have catecholamine and UFC measurements for a subset of participants, this subset did not differ from the larger group. The diets were given for only 1 day. However, overeating to such a large extent may often occur over a brief period (i.e., a holiday or special event). Although our results are cross-sectional in nature, we have a large cohort of participants for this unique, intensive, inpatient study. Our large cohort allowed for adjustment for multiple variables, and strengthens our intraindividual findings. Long-term follow-up of this cohort is currently ongoing.
In conclusion, we have demonstrated that, similar to EE changes, substrate oxidation rates with 24 hours of fasting and overfeeding are macronutrient specific, but there is also an intrinsic capability of the individual to preferentially use carbohydrate or fat, independent of the overfeeding diet type. This was supported by the consistently strong correlations of carbox and lipox between all the differing diets. We have confirmed that urinary epinephrine concentration is elevated with fasting and is negatively associated with percent changes in fasting EE; epinephrine may be a factor in EE phenotypes that we have previously found to be associated with weight change. These results are further indication of distinct individual phenotypes, which may predict response to energy restriction or fuel preference and influence body weight regulation.
Acknowledgments
We sincerely appreciate the help and dedication of the nursing and dietary staff of the Clinical Research Unit of the National Institute of Diabetes and Digestive and Kidney Diseases in Phoenix, Arizona, in the care of the participants. Most of all, we thank the volunteers for their participation in the study. Data from this research protocol were previously published when the first sample size goal (i.e., for repeatability) was reached (7).
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Clinical trial registry: ClinicalTrials.gov no. NCT00523627 (registered 30 August 2007).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 24hEE
- 24-hour energy expenditure
- carbox
- carbohydrate oxidation
- EB
- energy balance
- EE
- energy expenditure
- FFM
- fat-free mass
- FM
- fat mass
- HPA
- hypothalamic-pituitary-adrenal
- lipox
- lipid oxidation
- MO
- macronutrient oxidation
- OGTT
- 75-g oral glucose tolerance test
- RQ
- respiratory quotient
- SNS
- sympathetic nervous system
- SPA
- spontaneous physical activity
- UFC
- urinary free cortisol
- WMD
- weight-maintaining diet
- %fat
- percentage body fat.
References
- 1.Apfelbaum M, Bostsarron J, Lacatis D. Effect of caloric restriction and excessive caloric intake on energy expenditure. Am J Clin Nutr. 1971;24(12):1405–1409. [DOI] [PubMed] [Google Scholar]
- 2.Weyer C, Vozarova B, Ravussin E, Tataranni PA. Changes in energy metabolism in response to 48 h of overfeeding and fasting in Caucasians and Pima Indians. Int J Obes Relat Metab Disord. 2001;25(5):593–600. [DOI] [PubMed] [Google Scholar]
- 3.Reinhardt M, Thearle MS, Ibrahim M, Hohenadel MG, Bogardus C, Krakoff J, Votruba SB. A human thrifty phenotype associated with less weight loss during caloric restriction. Diabetes. 2015;64(8):2859–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlögl M, Piaggi P, Pannacciuli N, Bonfiglio SM, Krakoff J, Thearle MS. Energy expenditure responses to fasting and overfeeding identify phenotypes associated with weight change. Diabetes. 2015;64(11):3680–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welle SL, Seaton TB, Campbell RG. Some metabolic effects of overeating in man. Am J Clin Nutr. 1986;44(6):718–724. [DOI] [PubMed] [Google Scholar]
- 6.Rising R, Alger S, Boyce V, Seagle H, Ferraro R, Fontvieille AM, Ravussin E. Food intake measured by an automated food-selection system: relationship to energy expenditure. Am J Clin Nutr. 1992;55(2):343–349. [DOI] [PubMed] [Google Scholar]
- 7.Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. J Clin Endocrinol Metab. 2013;98(7):2791–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poehlman ET, Tremblay A, Fontaine E, Després JP, Nadeau A, Dussault J, Bouchard C. Genotype dependency of the thermic effect of a meal and associated hormonal changes following short-term overfeeding. Metabolism. 1986;35(1):30–36. [DOI] [PubMed] [Google Scholar]
- 9.Bouchard C, Tremblay A, Després JP, Nadeau A, Lupien PJ, Thériault G, Dussault J, Moorjani S, Pinault S, Fournier G. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322(21):1477–1482. [DOI] [PubMed] [Google Scholar]
- 10.Miller DS, Mumford P. Gluttony. 1. An experimental study of overeating low- or high-protein diets. Am J Clin Nutr. 1967;20(11):1212–1222. [DOI] [PubMed] [Google Scholar]
- 11.Norgan NG, Durnin JV. The effect of 6 weeks of overfeeding on the body weight, body composition, and energy metabolism of young men. Am J Clin Nutr. 1980;33(5):978–988. [DOI] [PubMed] [Google Scholar]
- 12.Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher daily energy expenditure and respiratory quotient, rather than fat free mass, independently determine greater ad libitum overeating. J Clin Endocrinol Metab. 2015;100(8):3011–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259(5 Pt 1):E650–E657. [DOI] [PubMed] [Google Scholar]
- 14.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86(3):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landsberg L, Young JB. Fasting, feeding and regulation of the sympathetic nervous system. N Engl J Med. 1978;298(23):1295–1301. [DOI] [PubMed] [Google Scholar]
- 16.Young JB, Landsberg L. Stimulation of the sympathetic nervous system during sucrose feeding. Nature. 1977;269(5629):615–617. [DOI] [PubMed] [Google Scholar]
- 17.Young JB, Saville E, Rothwell NJ, Stock MJ, Landsberg L. Effect of diet and cold exposure on norepinephrine turnover in brown adipose tissue of the rat. J Clin Invest. 1982;69(5):1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz JH, Young JB, Landsberg L. Effect of dietary fat on sympathetic nervous system activity in the rat. J Clin Invest. 1983;72(1):361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walgren MC, Young JB, Kaufman LN, Landsberg L. The effects of various carbohydrates on sympathetic activity in heart and interscapular brown adipose tissue of the rat. Metabolism. 1987;36(6):585–594. [DOI] [PubMed] [Google Scholar]
- 20.Katzeff HL, O’Connell M, Horton ES, Danforth E Jr, Young JB, Landsberg L. Metabolic studies in human obesity during overnutrition and undernutrition: thermogenic and hormonal responses to norepinephrine. Metabolism. 1986;35(2):166–175. [DOI] [PubMed] [Google Scholar]
- 21.Kush RD, Young JB, Katzeff HL, Danforth E Jr, Garrow JS, Scheidegger K, Ravussin E, Howard BV, Sims EA, Horton ES, Landsberg L. Effect of diet on energy expenditure and plasma norepinephrine in lean and obese Pima Indians. Metabolism. 1986;35(12):1110–1120. [DOI] [PubMed] [Google Scholar]
- 22.McCargar LJ, Clandinin MT, Fawcett DM, Johnston JL. Short-term changes in energy intake and serum insulin, neutral amino acids, and urinary catecholamine excretion in women. Am J Clin Nutr. 1988;47(6):932–941. [DOI] [PubMed] [Google Scholar]
- 23.Welle S, Campbell R. Effect of overeating on plasma and urinary concentrations of norepinephrine. J Clin Endocrinol Metab. 1984;59(3):531–534. [DOI] [PubMed] [Google Scholar]
- 24.Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271(2 Pt 1):E317–E325. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl):S8–S16. [DOI] [PubMed] [Google Scholar]
- 26.Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr. 1991;53(6):1368–1371. [DOI] [PubMed] [Google Scholar]
- 27.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78(6):1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhardt M, Schlogl M, Bonfiglio S, Votruba SB, Krakoff J, Thearle MS. Lower core body temperature and greater body fat are components of a human thrifty phenotype. Int J Obes. 2015;40 (5):754-760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jéquier E. Carbohydrates as a source of energy. Am J Clin Nutr. 1994;59(3, Suppl):682S–685S. [DOI] [PubMed] [Google Scholar]
- 30.Flatt JP. The difference in the storage capacities for carbohydrate and for fat, and its implications in the regulation of body weight. Ann N Y Acad Sci. 1987;499:104–123. [DOI] [PubMed] [Google Scholar]
- 31.Young JB, Rosa RM, Landsberg L. Dissociation of sympathetic nervous system and adrenal medullary responses. Am J Physiol. 1984;247(1 Pt 1):E35–E40. [DOI] [PubMed] [Google Scholar]
- 32.Peterson HR, Rothschild M, Weinberg CR, Fell RD, McLeish KR, Pfeifer MA. Body fat and the activity of the autonomic nervous system. N Engl J Med. 1988;318(17):1077–1083. [DOI] [PubMed] [Google Scholar]
- 33.Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest. 1987;79(1):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]