Abstract
Context:
Areal bone mineral density (BMD) is lower, particularly at the spine, in low-weight women with anorexia nervosa (AN). However, little is known about vertebral integral volumetric BMD (Int.vBMD) or vertebral strength across the AN weight spectrum, including “atypical” AN [body mass index (BMI) ≥18.5 kg/m2].
Objective:
To investigate Int.vBMD and vertebral strength, and their determinants, across the AN weight spectrum
Design:
Cross-sectional observational study
Setting:
Clinical research center
Participants:
153 women (age 18 to 45): 64 with low-weight AN (BMI <18.5 kg/m2; 58% amenorrheic), 44 with atypical AN (18.5≤BMI<23 kg/m2; 30% amenorrheic), 45 eumenorrheic controls (19.2≤BMI<25 kg/m2).
Measures:
Int.vBMD and cross-sectional area (CSA) by quantitative computed tomography of L4; estimated vertebral strength (derived from Int.vBMD and CSA)
Results:
Int.vBMD and estimated vertebral strength were lowest in low-weight AN, intermediate in atypical AN, and highest in controls. CSA did not differ between groups; thus, vertebral strength (calculated using Int.vBMD and CSA) was driven by Int.vBMD. In AN, Int.vBMD and vertebral strength were associated positively with current BMI and nadir lifetime BMI (independent of current BMI). Int.vBMD and vertebral strength were lower in AN with current amenorrhea and longer lifetime amenorrhea duration. Among amenorrheic AN, Int.vBMD and vertebral strength were associated positively with testosterone.
Conclusions:
Int.vBMD and estimated vertebral strength (driven by Int.vBMD) are impaired across the AN weight spectrum and are associated with low BMI and endocrine dysfunction, both current and previous. Women with atypical AN experience diminished vertebral strength, partially due to prior low-weight and/or amenorrhea. Lack of current low-weight or amenorrhea in atypical AN does not preclude compromise of vertebral strength.
Précis: Vertebral volumetric BMD and strength are impaired in women with low-weight and “atypical” anorexia nervosa (currently normal weight), with current and prior low weight and amenorrhea likely factors.
Impaired bone density is a prevalent medical complication of anorexia nervosa (AN) which can lead to debilitating fractures (1, 2). Low-weight women with AN have an elevated risk of overall, vertebral, and forearm fractures (3, 4). The spine is the skeletal site of greatest bone loss as assessed by areal bone mineral density (BMD) (1). About 70% of low-weight women with AN have low areal BMD at the AP spine, and more than 80% have low areal BMD at the lateral spine by dual-energy x-ray absorptiometry (DXA) (1). However, DXA methodology has limitations in measuring BMD and predicting fracture risk, as it is influenced by ratios of fat to lean tissues and is thus subject to inaccuracies (5, 6), particularly in the setting of weight fluctuations such as in eating disorders (6). As evidence of this, adolescents and young women with AN whose areal BMD values are normal or only modestly reduced have a higher fracture prevalence at all skeletal sites combined compared with healthy females with comparable BMDs (7), suggesting that factors besides areal BMD contribute to fracture risk.
Thus, better characterization of bone quality and strength in AN is needed, particularly at the spine where bone loss as assessed by DXA is greatest in low-weight individuals with AN; however, techniques to do so were limited in the past. High-resolution peripheral quantitative computed tomography (CT) can assess bone microarchitecture at the extremities, but not the spine. Recently it became possible to estimate vertebral compressive strength using data obtained from quantitative CT (QCT). In contrast to DXA, which measures areal BMD, QCT measures volumetric BMD (vBMD) and is not subject to potential errors that may arise with DXA due to assumptions about bone size or body composition. Using principles of engineering beam theory, measurements of vBMD and cross-sectional area (CSA) of a vertebral body obtained from QCT can be used to estimate vertebral strength (8–10).
We have shown that vertebral vBMD and strength as measured by QCT are reduced in low-weight women with AN (11). However, it is unknown whether women with “atypical” AN—a new diagnostic category included in the recently published Diagnostic and Statistical Manual of Mental Disorders (fifth edition; DSM-5) – are also characterized by low vertebral BMD. “Atypical” AN does not require a strict weight cut-off or amenorrhea. Thus, there is now a diagnosed spectrum of AN that includes individuals who are low-weight and, with the advent of DSM-5, a new category of “atypical” AN, characterized by the same psychiatric phenotype, including restrictive eating behaviors and disordered body image, but without low weight.
We have recently published that vertebral vBMD and strength as estimated by QCT are lower in women with low-weight AN compared with controls (11). However, vertebral vBMD and vertebral strength in women with atypical AN, and the comparisons to those measures in low-weight AN and controls, have not been reported to date. Furthermore, the determinants of vertebral vBMD and strength across the AN weight spectrum, including atypical AN, have not been published previously. We hypothesized that (a) women with low-weight AN and atypical AN, regardless of current weight, would have impaired vertebral vBMD and strength, and (b) impaired vertebral vBMD and strength would be associated with low-weight and endocrine dysfunction, both current and previous.
Materials and Methods
Subjects
This was a cross-sectional study of 153 women (64 with low-weight AN, 44 with atypical AN, and 45 healthy lean controls), ages 18 to 45 years, who received QCT including the L4 vertebra during prior research protocols at the Massachusetts General Hospital. All protocols were approved by Partners Institutional Review Board. Written informed consent was obtained from all participants. Low-weight AN subjects had a body mass index (BMI) <18.5 kg/m2 and met DSM-5 diagnostic criteria for AN. Atypical AN subjects had BMI ≥ 18.5 kg/m2 and met DSM-5 diagnostic criteria for atypical AN. Subjects with low-weight AN and atypical AN were eumenorrheic, amenorrheic, or receiving exogenous estrogens and progestins (E+P). Lean controls had BMI >18.5 kg/m2 and <25 kg/m2, were eumenorrheic, and were not receiving exogenous estrogens or progestins. Subjects with diabetes mellitus, renal disease, liver disease, active substance abuse, or use of medications that affect bone metabolism were excluded. Trabecular vBMD (11–14) has been reported in a subset of subjects. Integral vBMD (Int.vBMD), which includes both trabecular and cortical compartments, and estimated vertebral strength have been reported in low-weight AN and control subjects only (11). Trabecular vBMD, Int.vBMD, and estimated vertebral strength have not been reported for any atypical AN subjects. Associations of Int.vBMD and vertebral strength with hormone data, prior BMI, and amenorrhea have not been reported for any subjects previously.
Volumetric BMD, CSA, and vertebral strength
Imaging parameters and methods have been published previously (11, 13). Briefly, L4 vBMD (g/cm3) was determined by QCT of the L4 vertebral body with the use of a calibration phantom, as reported previously (11). T scores and Z scores of L4 trabecular vBMD were calculated using reference mean vBMDs of young females and of age-matched females (15), respectively. A single investigator (C.M.G.), who was blinded to the group status of the participants, outlined the contours of L4 vertebral bodies to determine L4 CSA (cm2) and L4 Int.vBMD, (g/cm3), which represents total vBMD (trabecular and cortical compartments).
Estimated vertebral compressive strength (“vertebral strength”) was calculated using the linear combination of Int.vBMD and CSA, as published previously (8, 9, 11). This approach has been validated in vitro with human cadavers (16). This method uses engineering beam theory principles, which assume that the vertebral body is loaded in compression, and that vertebral strength is proportional to the structural rigidity of the vertebral body’s weakest cross-section. Structural rigidity relies on bone size and elastic modulus (9, 10), which was estimated using Int.vBMD:
Vertebral strength was estimated using the following equation (8), derived from compression testing of human cadaveric vertebrae:
Biochemical analyses
Testosterone and estradiol levels were assessed in women with low-weight AN and atypical AN who were currently amenorrheic and who were not receiving exogenous estrogens. (Analyses were limited to amenorrheic women because in women receiving ethinyl estradiol, estradiol levels do not reflect estrogen action, and in women with spontaneous menses, 1 serum sample does not reflect integrated estrogen levels through the menstrual cycle.) Serum total and free testosterone levels were available in 44 of 50 amenorrheic subjects with low-weight AN and atypical AN. Total testosterone levels were measured in 14 of 44 samples by high-pressure liquid chromatography with tandem mass spectrometric detection (HPLC-MS/MS; Esoterix Endocrinology, Calabasas Hills, CA) with a sensitivity of 2.5 ng/dL. Total testosterone levels had been run previously in 30 of 44 samples using column chromatography (Esoterix Endocrinology). Total testosterone (T) levels assessed by the 2 assays have a high degree of agreement: Total T by column chromatography = 1.0467 × (total T by HPLC-MS/MS) – 2.9547 (R2 = 0.9909) (per correspondence with Donald Walt Chandler, PhD, Executive Director at Endocrine Sciences Laboratory, Esoterix Division of LabCorp, Calabasas Hills, CA).
Using this equation, total testosterone levels measured using column chromatography were adjusted to be comparable to total testosterone levels measured by HPLC-MS/MS. Percent free testosterone was measured by equilibrium dialysis (Esoterix Endocrinology). Serum estradiol was available in 30 of 50 amenorrheic AN subjects who were not taking exogenous estrogen. Estradiol was measured using a radioimmunoassay kit (Diagnostic System Laboratories, Webster, TX) with a sensitivity of 2.2 pg/mL. Of the 108 low-weight and atypical AN subjects, serum dehydroepiandrosterone sulfate (DHEA-S) and insulin-like growth factor (IGF)-1 levels were available in 31 and 92 subjects, respectively. DHEA-S was measured by a radioimmunoassay kit (Diagnostic System Laboratories) with a sensitivity of 2.5 μg/dl. IGF-1 was measured by the Immulite 2000 immunoassay analyzer (Siemens Health Care, Malvern, PA). All assays had coefficients of variation <10%. Hormones were not measured at a specific time of day.
Other variables
Menstrual histories were obtained during interviews. We classified the current menstrual characteristics of all low-weight AN and atypical AN subjects as follows: (a) “amenorrhea” (no menses during the 3 months preceding their QCT scans), (b) “spontaneous menses” (had menses during the prior 3 months and were not receiving exogenous E+P), or (c) “E+P” (receiving exogenous systemically absorbed E+P (e.g., oral contraceptives, estrogen-containing patch, or vaginal ring) for at least 1.5 months prior to their scans). In addition, we assessed lifetime duration of amenorrhea (excluding months pregnant or receiving exogenous E+P), nadir lifetime BMI during adulthood, and illness duration (months since diagnosis of an eating disorder by a clinical provider); data were available in 83, 95, and 96 of the 108 low-weight and atypical AN subjects, respectively.
Statistical analysis
JMP Statistical Discovery Software, version 10 Professional (SAS Institute Inc, Cary, NC) was used for statistical analyses. Variables were assessed for normality using the Shapiro–Wilk test, and, if nonnormal, underwent log transformation. All analyses have been adjusted for age. Two-tailed P values of <0.05 were considered statistically significant. Continuous variables were compared between the 3 groups with Fisher’s least significant difference test, controlling for age, using standard least squares regression. If overall P between groups was <0.05, pairwise comparisons were performed. Additional adjustment for multiple comparisons was not indicated because of the use of a preliminary test of significance with 3 groups (17). To test for associations, standard least squares regression models, controlling for age, were constructed and P values and partial correlation coefficients are reported. Results are based on the data for individuals for whom data were available; missing data were not imputed. In low-weight and atypical AN subjects with current amenorrhea, 2 multivariable models were constructed using stepwise regression modeling. Dependent variables were Int.vBMD and vertebral strength. Independent variables entered into the models were (a) serum testosterone, serum estradiol, age, and BMI in a model (to determine the independent contributions of the different gonadal steroids, testosterone and estradiol), and (b) serum testosterone, serum IGF-1, age, and BMI in another model (to determine whether IGF-1 was a significant determinant).
Results
Clinical characteristics
Controls were slightly older (mean age 28.0 years) than low-weight AN (25.7 years) and atypical AN (24.6 years) (P = 0.04 and P = 0.005, respectively) (Table 1). Mean age did not differ between low-weight AN and atypical AN. All analyses have been adjusted for age. By study design, BMI differed between groups (P < 0.0001) (Table 1). BMIs ranged from 13.3 to 18.49 kg/m2 in low-weight AN, 18.5 to 23.0 kg/m2 in atypical AN, and 19.2 to 24.9 kg/m2 in controls. Mean nadir lifetime BMI was lower in women with low-weight AN (14.9 kg/m2) compared with women with atypical AN (16.8 kg/m2) (P < 0.0001) (Table 1). Women with atypical AN experienced a wide range of nadir lifetime BMIs (11.3 to 19.9 kg/m2). Nine women with atypical AN (20%) had never experienced BMI <18.5 kg/m2 during adulthood; 6 of these women had never been amenorrheic. The percentage of women with current amenorrhea was lower among women with atypical AN (29.5%) compared with women with low-weight AN (57.8%) (Table 1). Compared with atypical AN, low-weight AN had longer mean durations of amenorrhea and of illness (P ≤ 0.009) (Table 1).
Table 1.
Clinical Characteristics
| Low-Weight AN (N = 64) | Atypical AN (N = 44) | Controls (N = 45) |
P Value |
||||
|---|---|---|---|---|---|---|---|
| Overall ANOVA | Low-Weight AN vs Controls | Atypical AN vs Controls | Low-Weight AN vs Atypical AN | ||||
| Age, y | 25.7 ± 5.7 | 24.6 ± 5.6 | 28.0 ± 6.5 | 0.017 | 0.044 | 0.005 | NS |
| Height, cm | 164.6 ± 6.8 | 164.5 ± 7.5 | 166.4 ± 6.6 | NS | — | — | — |
| Weight, kg | 46.2 ± 5.0 | 52.9 ± 5.1 | 61.4 ± 6.5 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| BMI, kg/m2 | 17.0 ± 1.1 | 19.6 ± 1.0 | 22.1 ± 1.7 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| L4 trabecular BMD, g/cm3 | 131.9 ± 29.0 | 147.1 ± 31.8 | 158.6 ± 27.5 | <0.0001 | <0.0001 | 0.034 | 0.013 |
| L4 T score | −1.7 ± 1.1 | −1.1 ± 1.2 | −0.7 ± 1.1 | <0.0001 | <0.0001 | 0.070 | 0.009 |
| L4 Z score | −1.7 ± 1.1 | −1.1 ± 1.2 | −0.5 ± 1.1 | <0.0001 | <0.0001 | 0.010 | 0.020 |
| Nadir lifetime BMI, kg/m2 | 14.9 ± 1.8 | 16.8 ± 2.0 | — | — | — | — | <0.0001 |
| Duration of illness, mo | 94 ± 81 | 51 ± 72 | — | — | — | — | 0.002 |
| Lifetime duration of amenorrhea, mo | 43 ± 51 | 20 ± 26 | 0 ± 0 | <0.0001 | <0.0001 | 0.001 | 0.008 |
| Current menstrual characteristics, % of subjects | <0.0001 | <0.0001 | <0.0001 | 0.013 | |||
| Amenorrhea | 57.8% | 29.5% | 0% | ||||
| Spontaneous menses | 23.4% | 43.2% | 100% | ||||
| E+P | 18.8% | 27.3% | 0% | ||||
| Vigorous activity, h/wk | 5.2 ± 7.4 | 3.6 ± 4.0 | 5.0 ± 4.4 | NS | — | — | — |
Results are reported as means ± standard deviation.
Abbreviations: E+P, exogenous systemically absorbed estrogens and progestins received; NS, not significant.
Volumetric BMD, CSA, and vertebral strength
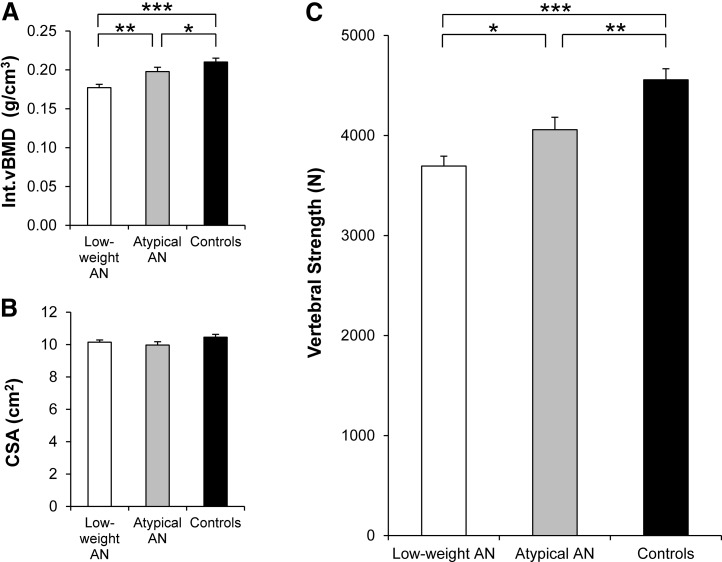
Trabecular vBMD was lowest in low-weight AN, highest in controls, and intermediate in atypical AN (P < 0.0001) (Table 1). Int.vBMD [Fig. 1(A)] was lowest in low-weight AN, highest in controls, and intermediate in atypical AN (P < 0.0001). In contrast, CSA [Fig. 1(B)] did not differ between low-weight AN, atypical AN, and controls. Vertebral strength is a function of Int.vBMD and CSA, and CSA did not differ between groups; therefore, the pattern of vertebral strength [Fig. 1(C)] was similar to that of Int.vBMD—lowest in low-weight AN, highest in controls, and intermediate in atypical AN (P < 0.0001). In addition, Int.vBMD and estimated vertebral strength of the 9 women with atypical AN who had never experienced BMI <18.5 kg/m2 during adulthood did not differ significantly from controls and were higher compared with low-weight AN.
Figure 1.
Int.vBMD, CSA, and vertebral strength in low-weight AN, atypical AN, and controls. (A) Int.vBMD was lowest in low-weight AN, highest in controls, and intermediate in atypical AN. (B) CSA did not differ between the 3 groups. (C) Vertebral strength was lowest in low-weight AN, highest in controls, and intermediate in atypical AN. Results are reported as means ±standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.0001.
Determinants of Int.vBMD and vertebral strength in low-weight AN and atypical AN
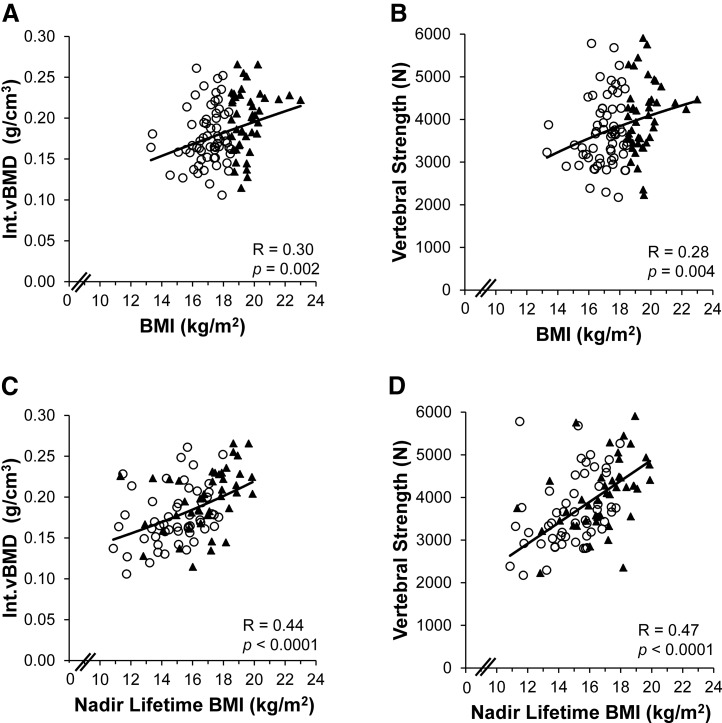
Among all women with low-weight AN and atypical AN combined, BMI was a positive determinant of Int.vBMD [R = 0.30, P = 0.002; Fig. 2(A)] and therefore also vertebral strength [R = 0.28, P = 0.004; Fig. 2(B); Supplemental Table 1 (16.7KB, docx) ], as vertebral strength is a function of Int.vBMD. Moreover, nadir lifetime BMI was a strong positive determinant of Int.vBMD [R = 0.44, P < 0.0001; Fig. 2(C)] and hence also vertebral strength [R = 0.47, P < 0.0001; Fig. 2(D); Supplemental Table 1 (16.7KB, docx) ], which remained significant after controlling for current BMI. In contrast, illness duration was negatively associated with Int.vBMD (R = –0.26, P = 0.007) and therefore with vertebral strength (R = –0.23, P = 0.02) (Supplemental Table 1 (16.7KB, docx) ). Furthermore, lifetime duration of amenorrhea was negatively associated with Int.vBMD (R = –0.27, P = 0.01) and therefore with vertebral strength (R = –0.24, P = 0.02) (Supplemental Table 1 (16.7KB, docx) ). After adjusting for BMI, associations for durations of illness and amenorrhea with respect to Int.vBMD remained significant and with respect to vertebral strength trended toward significance. Within-group associations (within the low-weight AN group and atypical AN group individually) are reported in Supplemental Table 1 (16.7KB, docx) and Supplemental Figures 1 and 2 (42.6KB, pdf) .
Figure 2.
Associations of current BMI and nadir lifetime BMI with Int.vBMD and vertebral strength in AN. Among all women with low-weight AN and atypical AN combined, Int.vBMD and vertebral strength were positively associated with (A, B) current BMI and with (C, D) nadir lifetime BMI. Regression lines were log-transformed with respect to (A, C) Int.vBMD and with respect to (A, B) current BMI. ○, low-weight AN; ▲, atypical AN.
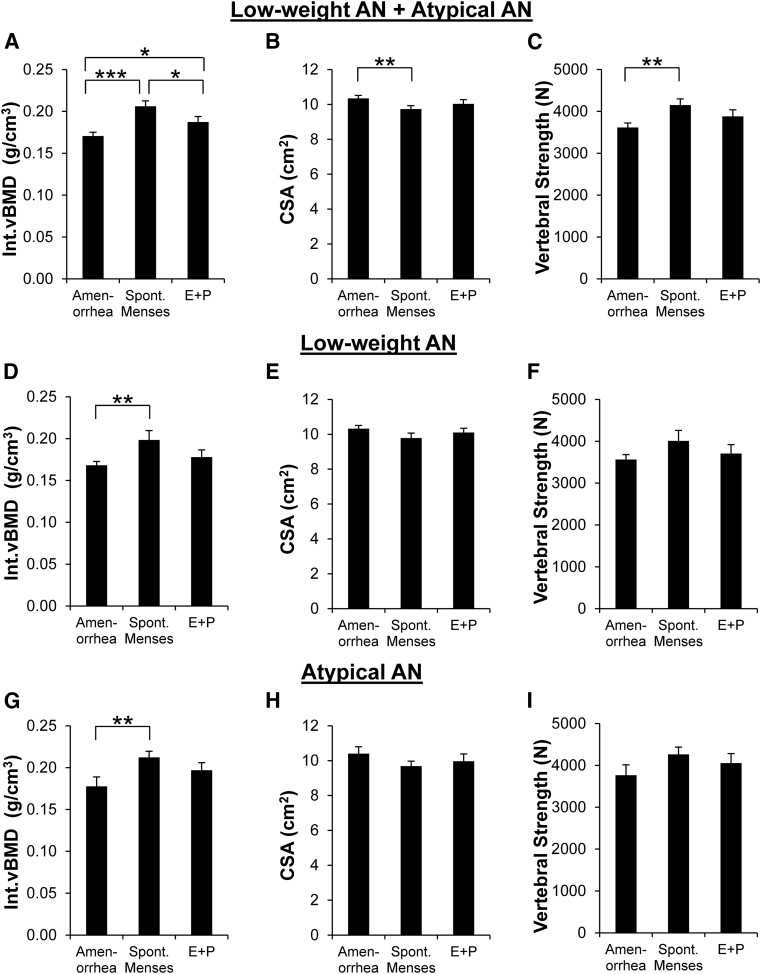
Among all women with low-weight AN and atypical AN combined, Int.vBMD [Fig. 3(A)] was lowest in those with current amenorrhea (N = 50), highest in those with spontaneous menses (N = 34), and intermediate in those receiving E+P (N = 24) (P < 0.0001). After controlling for BMI, those with current amenorrhea (P < 0.0001) and those receiving E+P (P = 0.03) still had lower Int.vBMD compared with those with spontaneous menses. CSA [Fig. 3(B)] was higher in amenorrheic subjects than those with spontaneous menses (P = 0.006), which remained significant after adjusting for BMI. Vertebral strength [Fig. 3(C)] was lower in those with current amenorrhea compared with those with spontaneous menses (P = 0.003); differences were not significant after adjusting for BMI. For AN subjects with current amenorrhea, with spontaneous menses, and receiving E+P, median heights were 166.05, 164.25, and 164.50 cm, respectively, and mean heights were 164.79, 164.76, and 163.63 cm, respectively; differences were not significant. Results within the low-weight AN group and atypical AN group individually are reported in Fig. 3(D–I).
Figure 3.
Int.vBMD, CSA, and vertebral strength in AN by current menstrual characteristics. Among all women with low-weight AN and atypical AN combined, (A) Int.vBMD was lowest in those with current amenorrhea (N = 50), highest in those with spontaneous menses currently (N = 34), and intermediate in those receiving E+P currently (N = 24). (B) CSA was higher in AN with current amenorrhea than AN with spontaneous menses currently. (C) Vertebral strength was lower in AN with current amenorrhea compared with AN with spontaneous menses currently. When analyzing the low-weight AN group individually, (D) Int.vBMD was lower in those with current amenorrhea (n = 37) compared with those with spontaneous menses (n = 15) and did not differ from those receiving E+P (n = 12); (E) CSA and (F) vertebral strength did not differ by menstrual characteristics. The same pattern was seen in the atypical AN group individually; (G) Int.vBMD was lower in atypical AN subjects who had current amenorrhea (n = 13) compared with those with spontaneous menses (n = 19) and did not differ from those receiving E+P (n = 12), and (H) CSA and (I) vertebral strength did not differ by menstrual characteristics. Results are reported as means ±SEM. Amenorrhea, current amenorrhea; Spont. Menses, spontaneous menses currently; E+P, receiving systemically absorbed E+P currently. *P < 0.04; **P < 0.01; ***P < 0.0001.
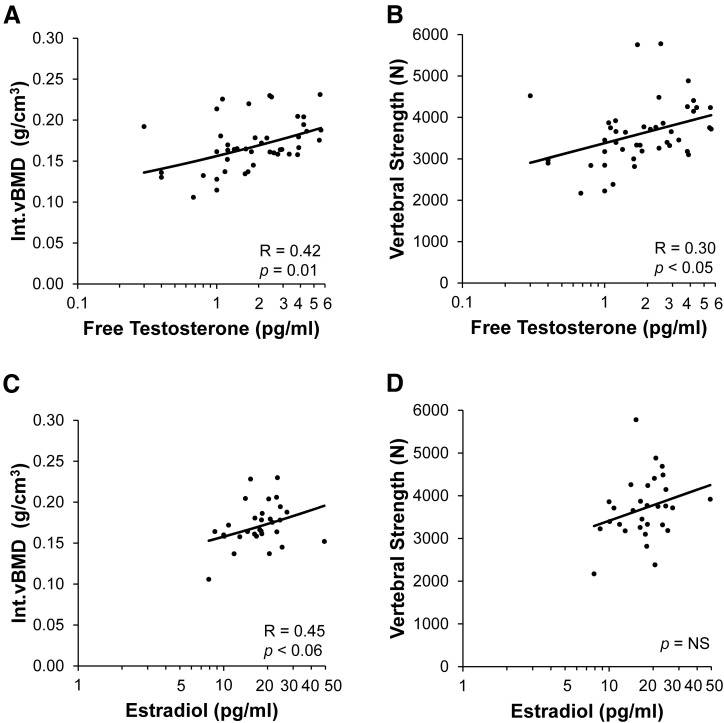
Among women with low-weight AN and atypical AN with current amenorrhea, we analyzed serum testosterone and estradiol levels to determine the relative contributions of estrogens and androgens to vertebral strength. Among those with current amenorrhea, free testosterone was positively associated with Int.vBMD [R = 0.42, P = 0.01; Fig. 4(A)] and therefore also with vertebral strength [R = 0.30, P < 0.05; Fig. 4(B)]. After controlling for BMI, the association of free testosterone with Int.vBMD remained significant (P = 0.01) and with vertebral strength trended toward significance (P < 0.06). Total testosterone had a positive association with Int.vBMD (R = 0.49, P = 0.003), including after controlling for BMI, and trended toward a positive association with vertebral strength (R = 0.31, P < 0.09), which did not persist after controlling for BMI. Among currently amenorrheic subjects with estradiol levels available, estradiol trended toward a positive association with Int.vBMD [R = 0.45, P < 0.06; Fig. 4(C)], which became significant after adjusting for BMI (R = 0.46, P < 0.05). Estradiol was not associated with vertebral strength [Fig. 4(D)]. Within the subset of amenorrheic women who had both testosterone and estradiol levels available (29 of 50), in stepwise regression models controlling for estradiol, age and BMI, free testosterone remained significantly associated with Int.vBMD (P = 0.001) and therefore with vertebral strength (P = 0.01) and explained about 33% and 21% of the variability in Int.vBMD and vertebral strength, respectively. Estradiol was not significantly associated with Int.vBMD or vertebral strength in multivariable models controlling for testosterone and age. In a stepwise regression model controlling for estradiol, age and BMI, total testosterone remained significantly associated with Int.vBMD (P = 0.003) and explained about 28% of its variability.
Figure 4.
Associations of free testosterone and estradiol levels with Int.vBMD and vertebral strength in AN with current amenorrhea. Among women with low-weight AN and atypical AN with current amenorrhea, (A, B) Int.vBMD and vertebral strength were positively associated with free testosterone levels. Among AN subjects with current amenorrhea, (C) Int.vBMD trended toward a positive association with estradiol levels, and (D) vertebral strength was not associated with estradiol levels. Values of (A, B) free testosterone and (C, D) estradiol are displayed on log-transformed axes. Regression lines were log-transformed with respect to (A, C) Int.vBMD, (A, B) free testosterone, and (C, D) estradiol. In multivariable models controlling for estradiol, age, and BMI among amenorrheic AN subjects, free testosterone remained significantly associated with vertebral strength (P = 0.01) and explained about 21% of the variability in vertebral strength, whereas estradiol was not associated with vertebral strength.
DHEA-S was positively associated with Int.vBMD (R = 0.53, P = 0.01) and therefore also with vertebral strength (R = 0.55, P = 0.005) among low-weight and atypical AN, which remained significant after controlling for BMI. IGF-1 trended toward positive associations with Int.vBMD (R = 0.18, P = 0.07) and vertebral strength (R = 0.20, P = 0.06) among low-weight and atypical AN, which did not persist after controlling for BMI. Among low-weight and atypical AN not taking exogenous E+P, IGF-1 was positively associated with Int.vBMD (R = 0.28, P = 0.02) and thus also with vertebral strength (R = 0.25, P = 0.04); neither association remained significant after adjusting for BMI. Among low-weight AN and atypical AN subjects with current amenorrhea, when IGF-1 level, free testosterone level, age, and BMI were entered into stepwise regression models, IGF-1 determined 18% of the variability of Int.vBMD and vertebral strength (R2 = 0.18, P < 0.02, for both) and free testosterone trended toward accounting for more than 25% of the variability of both Int.vBMD and vertebral strength (cumulative R2 = 0.28, P < 0.06, for Int.vBMD; cumulative R2 = 0.27, P < 0.07, for vertebral strength).
Discussion
We are the first to report that vertebral Int.vBMD and estimated vertebral strength, which is a function of Int.vBMD, are impaired across the AN weight spectrum—in both low-weight AN and atypical AN. We show that women with atypical AN have diminished Int.vBMD and hence vertebral strength compared with controls, but not as low as in women with low-weight AN. We demonstrate that the extent of impairment of Int.vBMD and hence vertebral strength across the AN spectrum is related to low-weight and amenorrhea, both current and previous. In addition, our data suggest that free testosterone and IGF-1 levels may be positive determinants of Int.vBMD and therefore vertebral strength, independent of BMI. Thus, past and present low-weight and endocrine dysfunction can have detrimental effects on skeletal health across the AN weight spectrum.
Low-weight women with AN have an elevated risk of overall fractures and vertebral fractures, and the spine is the site of the greatest bone loss assessed by DXA in such women (1). Although we have recently reported Int.vBMD and vertebral strength using QCT for women with low-weight AN, these have not been reported for women with atypical AN (in whom BMI is ≥18.5 kg/m2 and amenorrhea is not required), who may have been expected to have normal skeletal health. However, we found that Int.vBMD and estimated vertebral strength were lowest in low-weight AN, intermediate in atypical AN, and highest in controls. As estimated vertebral strength is a function of Int.vBMD and CSA, and CSA did not differ between the low-weight AN, atypical AN, and control groups, the differences in vertebral strength between these groups were driven by differences in Int.vBMD. Int.vBMD and therefore vertebral strength were lower in atypical AN than controls, despite normal weight and lack of amenorrhea in many women with atypical AN. A possible explanation is that most women with atypical AN had experienced amenorrhea and/or low weight in the past. The 9 women with atypical AN who had never experienced BMIs<18.5 kg/m2 during adulthood had similar Int.vBMD and vertebral strength as controls, supporting the argument that prior low-weight is a major contributor to impaired bone health in atypical AN. Therefore, providers should consider prior low weight and prior amenorrhea that may be detrimental to skeletal health. Moreover, our data are consistent with the hypothesis that women with low-weight AN as well as those with atypical AN (despite currently normal BMIs) have impaired vertebral strength. Our findings are consistent with previous reports that women with AN who have partial weight recovery (achieving 80% to 90% of ideal body weight), some of whom likely would have been diagnosed with atypical AN had it been a diagnostic criterion at the time, may experience some improvement in areal BMD, but not complete normalization (2, 18, 19). However, this is the first report of vBMD by QCT in women with the new “atypical AN” diagnosis or hormonal determinants of vertebral strength in any women with AN—low-weight, recovered, or “atypical.”
AN is frequently complicated by amenorrhea, which appears to contribute to the impaired BMD seen in this disorder. Women with low-weight AN who are amenorrheic have lower spine areal BMD than their eumenorrheic counterparts with comparable BMIs (20). For women with atypical AN, the relationships of amenorrhea with vertebral BMD and vertebral strength have not been reported previously. We demonstrate that women with low-weight AN and atypical AN who are currently amenorrheic have lower Int.vBMD and hence lower vertebral strength, and that the extent of impairment of Int.vBMD and therefore vertebral strength across the AN weight spectrum is related to both current amenorrhea and lifetime duration of amenorrhea. We also investigated whether the relationship of current amenorrhea with impaired Int.vBMD and vertebral strength in AN is mediated by deficiency of estrogens, androgens, or both. We demonstrate that among currently amenorrheic women with low-weight AN and atypical AN, serum free testosterone is positively associated with Int.vBMD and hence vertebral strength, independent of serum estradiol and BMI. This suggests that serum testosterone is a positive determinant of vertebral vBMD and strength in amenorrheic women with low-weight AN and atypical AN, independent of serum estradiol. This is consistent with prior findings that low-dose testosterone administration leads to increases of bone formation markers in low-weight AN (12) but is in contrast to results of a subsequent study that found no increase in BMD following testosterone administration in women with low-weight AN (21). In contrast to Int.vBMD and vertebral strength, mean CSA was higher in AN subjects with current amenorrhea than AN subjects with spontaneous menses. A possible explanation is that median height was higher in amenorrheic AN subjects than AN subjects with spontaneous menses, although this difference was not statistically significant. Because CSA reflects bone size and is positively related to height, the higher median height in amenorrheic subjects could potentially contribute to a higher CSA in amenorrheic subjects. Another possible explanation for the higher CSA in AN with current amenorrhea is that the lower vBMD in amenorrheic subjects may lead to an adaptive biomechanical response of periosteal bone formation, causing a net outward expansion of the vertebra and a higher mean CSA. As vertebral strength is a function of vBMD and CSA, a higher CSA could potentially attenuate the loss of vertebral strength caused by a lower vBMD. Bruno et al. (9) and Srinivasan et al. (22) found that men had lower vBMD and higher CSA at the spine (9) and hip (22), respectively, compared with women in large cohorts of men and women matched for areal BMD. The opposing directions of the vBMD and CSA served to lessen the difference in bone strength between men and women that would have occurred if vBMD or CSA alone had differed between groups. These findings suggest that vBMD and CSA are interrelated and potentially adaptive. Further investigation is needed to elucidate this possible adaptive response.
Moreover, we demonstrate that Int.vBMD and accordingly vertebral strength in low-weight AN and atypical AN are related not only to current health status, but also historical characteristics. Int.vBMD and vertebral strength are more impaired in women who have experienced lower prior BMIs and longer durations of amenorrhea and of illness during their lifetimes. A key finding of the current study is that women with atypical AN are subject to impaired skeletal health despite having BMI ≥18.5 kg/m2 currently, likely from having experienced low BMIs and amenorrhea previously. Lower nadir lifetime BMI is significantly associated with Int.vBMD and vertebral strength in the low-weight AN and atypical AN groups combined, and in low-weight AN and atypical AN groups individually, after controlling for current BMI. This highlights the long-lasting effects of prior low-weight on skeletal health for both low-weight AN as well as atypical AN, despite currently normal BMIs in the latter. Moreover, although fewer than 30% of women with atypical AN in our cohort were currently amenorrheic, the majority of women with atypical AN had experienced amenorrhea some time during their lives, and the lifetime duration of amenorrhea among women with atypical AN was a negative determinant of Int.vBMD and of estimated vertebral strength. These results suggest that women with atypical AN with current BMIs in the “normal” range are heavily influenced by the amenorrhea they have experienced throughout their lifetime. Our current findings are consistent with our prior report that longer lifetime durations of amenorrhea and of illness are associated with impaired hip strength in women with low-weight AN (23). Our present findings suggest that both prior low-weight and prior amenorrhea have long-lasting effects on bone, and therefore current weight in the “normal” range and lack of amenorrhea in women with atypical AN should not in themselves be reassuring with regard to skeletal health.
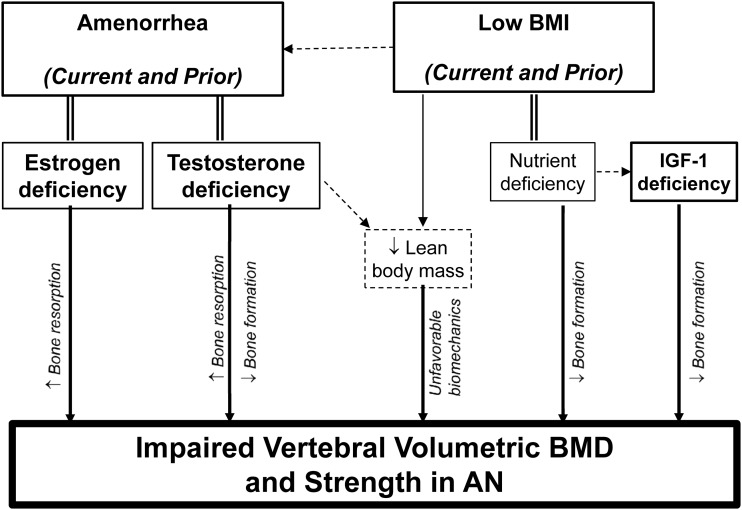
Therefore, the results of the current study highlight the importance of both current and past clinical characteristics and endocrine dysfunction on bone health in AN. The fact that the many of the women with atypical AN had experienced low BMIs and amenorrhea in the past, yet had lower prevalence of current amenorrhea, higher nadir lifetime BMIs, and shorter lifetime durations of amenorrhea and of illness compared with low-weight AN, likely accounts for the finding that vertebral Int.vBMD and estimated strength in atypical AN are diminished compared with controls but not as impaired as in low-weight AN. Figure 5 shows a conceptual model depicting the potential roles of prior and present low-weight and endocrine dysfunction on the impairment of vertebral vBMD and estimated vertebral strength in AN. Low BMI, both current and prior, can directly impact bone health through multiple mechanisms. Low BMI reflects nutrient deficiency, which can lead to decreased bone formation. Moreover, lower body weight leads to a lower degree of biomechanical loading on the bones. Body weight (particularly from lean body mass) provides biomechanical loading on bones that can stimulate increases in BMD and bone strength (24, 25); conversely a lack of biomechanical loading may lead to lower BMD and bone strength. Moreover, amenorrhea is a sign of estrogen deficiency, which can lead to increased bone resorption and thereby impairment of bone vBMD and strength. Low BMI can predispose to amenorrhea, but the fact that both current and prior amenorrhea are associated with lower Int.vBMD after adjusting for BMI in our study suggests that amenorrhea is a negative determinant of Int.vBMD independent of BMI. In addition, our finding that amenorrheic women with low-weight and atypical AN who have lower levels of free testosterone experience lower Int.vBMD independent of estradiol and of BMI implies that amenorrhea in AN may reflect not only estrogen deficiency, but also a relative testosterone deficiency. Testosterone may contribute to bone strength indirectly by increasing lean body mass and thereby increasing biomechanical loading (26). Furthermore, testosterone has been shown in men to contribute to bone strength directly by stimulating bone formation and inhibiting bone resorption (27–30). Conversely, our results suggest that a relative deficiency of testosterone in amenorrheic women with AN may contribute to decreased bone formation in AN, which would be consistent with the previously mentioned report of testosterone administration in women with low-weight AN leading to increases in bone formation (12). Reports from other groups suggest that there may be an estradiol threshold necessary for the maintenance of skeletal health in men (27–30). Our findings imply that among amenorrheic women with AN, there may be a testosterone threshold necessary for the maintenance of skeletal health. In addition, in stepwise models including both IGF-1 and testosterone levels among currently amenorrheic women with low-weight AN and atypical AN, we noted positive associations of both IGF-1 and testosterone with Int.vBMD and estimated vertebral strength, independent of BMI. AN and other states of malnutrition are characterized by resistance to growth hormone, leading to deficiency of IGF-1 (31), which can contribute to decreased bone formation. Our results suggest that the anabolic effects of IGF-1 play an important role in the skeletal health in amenorrheic women with low-weight AN and atypical AN.
Figure 5.
Conceptual model of impaired skeletal health in AN. Conceptual model depicting the determinants of impaired vertebral integral volumetric BMD and vertebral strength in low-weight AN and atypical AN.
Limitations of this study include the lack of prospective fracture data due to its cross-sectional design. However, vertebral strength estimated using Int.vBMD and CSA is highly correlated with measured breaking force from cadaveric studies (16). Moreover, some of the associations with Int.vBMD and with vertebral strength that were significant among the combined AN groups (low-weight AN group and atypical AN group combined) were not significant within the individual groups; thus, we are not able to state definitively that these variables are significant determinants of Int.vBMD and vertebral strength within each individual group. A possible explanation for the fact that some determinants were significant in the combined group but not the individual groups is that that the variables in the individual groups clustered (with little range), particularly with regard to BMI (per study design), and thus supports the view of atypical AN and low-weight AN as being part of a continuous AN spectrum. We postulate that because low-weight AN and atypical AN make up a spectrum of AN, our findings among the combined AN groups likely apply to individuals within both individual groups (within the low-weight AN group as well as the atypical AN group). Also, total testosterone levels were measured using 2 different assays; however, there is a high degree of agreement between the 2 assays, and testosterone levels have been adjusted to make the levels using the 2 assays comparable. In addition, hormone data were not available in all subjects with low-weight AN and atypical AN. In particular, because we limited the analysis of testosterone and estradiol levels to women with current amenorrhea and because these hormone levels were missing in some atypical AN subjects with current amenorrhea, our findings regarding the relative contributions of testosterone and estradiol among all amenorrheic AN subjects are not definitive. Also, because there were only 13 women with atypical AN who had current amenorrhea, this limited our ability to power to make definite conclusions in this specific subgroup. Hormone levels were not available in most control subjects, and thus hormone data for controls are not reported. Finally, it would have been informative to examine the subset of atypical AN subjects who never had low-weight and amenorrhea in the past; however, there were only 6 such women in our study, limiting our ability to generate meaningful conclusions regarding this subgroup.
In conclusion, Int.vBMD and estimated vertebral strength, which was modeled using Int.vBMD and CSA, are impaired in both low-weight AN and atypical AN, a new diagnostic category that does not require a strict weight cutoff or amenorrhea. Despite having currently normal BMIs (as well as eumenorrhea for many), women with atypical AN experience diminished vertebral BMD and strength, partially due to previously low BMIs and prior amenorrhea. Int.vBMD and therefore also vertebral strength are most severely impaired in women with AN who currently are low-weight, amenorrheic, and have lower testosterone and IGF-1 levels. Therefore, our results highlight the importance of BMI and endocrine dysfunction as determinants of vertebral bone density and estimated strength across the AN spectrum. Furthermore, women with lower prior BMIs (even if currently normal-weight) and longer lifetime durations of amenorrhea and of illness are also at risk for vertebral strength impairment. Thus, in addition to current health status, these historical characteristics are important determinants of skeletal health. Lack of current low weight or amenorrhea in women with atypical AN does not preclude significant compromise of vertebral strength.
Acknowledgments
This work was supported by the following National Institutes of Health grants: T32 DK 007028, K24 HL092902, RO1 HL 077674, R01 MH083657, R01 DK052625, RO3-DK59297, M01-RR01066-27S1, M01-RR01066, UL1 RR025758, 8UL1 TR000170, 1UL1TR001102, K23 RR-23090, R01 AR053986, F31AG041629, and K24 HD071843.
Acknowledgments
Disclosure Summary: J.M.G. received royalties from Sunrise River Press for his book Answers to Anorexia. The remaining authors have nothing to disclose.
Footnotes
- AN
- anorexia nervosa
- BMD
- bone mineral density
- BMI
- body mass index
- CSA
- cross-sectional area
- CT
- computed tomography
- DHEA-S
- dehydroepiandrosterone sulfate
- DSM-5
- Diagnostic and Statistical Manual of Mental Disorders (fifth edition)
- DXA
- dual-energy X-ray absorptiometry
- E+P
- estrogens and progestins
- HPLC
- high-pressure liquid chromatography
- IGF
- insulin-like growth factor
- Int.vBMD
- integral volumetric bone mineral density
- MS
- mass spectrometric
- QCT
- quantitative computed tomography
- vBMD
- volumetric bone mineral density.
References
- 1.Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005;165(5):561–566. [DOI] [PubMed] [Google Scholar]
- 2.Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. The clinical course of osteoporosis in anorexia nervosa: a longitudinal study of cortical bone mass. JAMA. 1991;265(9):1133–1138. [PubMed] [Google Scholar]
- 3.Lucas AR, Melton LJ III, Crowson CS, O’Fallon WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc. 1999;74(10):972–977. [DOI] [PubMed] [Google Scholar]
- 4.Vestergaard P, Emborg C, Støving RK, Hagen C, Mosekilde L, Brixen K. Fractures in patients with anorexia nervosa, bulimia nervosa, and other eating disorders; a nationwide register study. Int J Eat Disord. 2002;32(3):301–308. [DOI] [PubMed] [Google Scholar]
- 5.Bolotin HH. A new perspective on the causal influence of soft tissue composition on DXA-measured in vivo bone mineral density. J Bone Miner Res. 1998;13(11):1739–1746. [DOI] [PubMed] [Google Scholar]
- 6.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27(1):119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faje AT, Fazeli PK, Miller KK, Katzman DK, Ebrahimi S, Lee H, Mendes N, Snelgrove D, Meenaghan E, Misra M, Klibanski A. Fracture risk and areal bone mineral density in adolescent females with anorexia nervosa. Int J Eat Disord. 2014;47(5):458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouxsein ML, Melton LJ, III, Riggs BL, Muller J, Atkinson EJ, Oberg AL, Robb RA, Camp JJ, Rouleau PA, McCollough CH, Khosla S. Age- and sex-specific differences in the factor of risk for vertebral fracture: a population-based study using QCT . J Bone Miner Res. 2006;21(9):1475–1482. [DOI] [PubMed] [Google Scholar]
- 9.Bruno AG, Broe KE, Zhang X, Samelson EJ, Meng CA, Manoharan R, D'Agostino J, Cupples LA, Kiel DP, Bouxsein ML. Vertebral size, bone density, and strength in men and women matched for age and areal spine BMD. J Bone Miner Res. 2014;29(3):562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopperdahl DL, Morgan EF, Keaveny TM. Quantitative computed tomography estimates of the mechanical properties of human vertebral trabecular bone . J Orthop Res. 2002;20(4):801–805. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann KN, Bruno AG, Bredella MA, Schorr M, Lawson EA, Gill CM, Singhal V, Meenaghan E, Gerweck AV, Eddy KT, Ebrahimi S, Koman SL, Greenblatt JM, Keane RJ, Weigel T, Dechant E, Misra M, Klibanski A, Bouxsein ML, Miller KK. Vertebral strength and estimated fracture risk across the BMI spectrum in women. J Bone Miner Res. 2015;31(2):281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller KK, Grieco KA, Klibanski A. Testosterone administration in women with anorexia nervosa. J Clin Endocrinol Metab. 2005;90(3):1428–1433. [DOI] [PubMed] [Google Scholar]
- 13.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Harrington LM, Breggia A, Rosen CJ, Miller KK. Determinants of bone mineral density in obese premenopausal women. Bone. 2011;48(4):748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring). 2011;19(1):49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genant HK, Cann CE, Pozzi-Mucelli RS, Kanter AS. Vertebral mineral determination by quantitative CT: clinical feasibility and normative data. J Comput Assist Tomogr. 1983;7(3):554. [Google Scholar]
- 16.Crawford RP, Cann CE, Keaveny TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003;33(4):744–750. [DOI] [PubMed] [Google Scholar]
- 17.Marcus R, Peritz E, Gabriel KR. On closed testing procedures with special reference to ordered analysis of variance. Biometrika. 1976;63(3):655–660. [Google Scholar]
- 18.Dominguez J, Goodman L, Sen Gupta S, Mayer L, Etu SF, Walsh BT, Wang J, Pierson R, Warren MP. Treatment of anorexia nervosa is associated with increases in bone mineral density, and recovery is a biphasic process involving both nutrition and return of menses. Am J Clin Nutr. 2007;86(1):92–99. [DOI] [PubMed] [Google Scholar]
- 19.Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, Lockhart P, Cord J, Herzog DB, Katzman DK, Klibanski A. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab. 2008;93(4):1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller KK, Grinspoon S, Gleysteen S, Grieco KA, Ciampa J, Breu J, Herzog DB, Klibanski A. Preservation of neuroendocrine control of reproductive function despite severe undernutrition. J Clin Endocrinol Metab. 2004;89(9):4434–4438. [DOI] [PubMed] [Google Scholar]
- 21.Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, Herzog D, Klibanski A. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab. 2011;96(7):2081–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasan B, Kopperdahl DL, Amin S, Atkinson EJ, Camp J, Robb RA, Riggs BL, Orwoll ES, Melton LJ, Keaveny TM, Khosla S. Relationship of femoral neck areal bone mineral density to volumetric bone mineral density, bone size, and femoral strength in men and women. Osteoporos Int. 2012;23(1):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachmann KN, Fazeli PK, Lawson EA, Russell BM, Riccio AD, Meenaghan E, Gerweck AV, Eddy K, Holmes T, Goldstein M, Weigel T, Ebrahimi S, Mickley D, Gleysteen S, Bredella MA, Klibanski A, Miller KK. Comparison of hip geometry, strength, and estimated fracture risk in women with anorexia nervosa and overweight/obese women. J Clin Endocrinol Metab. 2014;99(12):4664–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho-Pham LT, Nguyen UD, Nguyen TV. Association between lean mass, fat mass, and bone mineral density: a meta-analysis. J Clin Endocrinol Metab. 2014;99(1):30–38. [DOI] [PubMed] [Google Scholar]
- 25.Rauch F, Schoenau E. The developing bone: slave or master of its cells and molecules? Pediatr Res. 2001;50(3):309–314. [DOI] [PubMed] [Google Scholar]
- 26.Clarke BL, Khosla S. Androgens and bone. Steroids. 2009;74(3):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106(12):1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khosla S. New insights into androgen and estrogen receptor regulation of the male skeleton. J Bone Miner Res. 2015;30(7):1134–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab. 2003;88(1):204–210. [DOI] [PubMed] [Google Scholar]
- 30.Ucer S, Iyer S, Bartell SM, Martin-Millan M, Han L, Kim HN, Weinstein RS, Jilka RL, O'Brien CA, Almeida M, Manolagas SC. The effects of androgens on murine cortical bone do not require ar or eralpha signaling in osteoblasts and osteoclasts. J Bone Miner Res. 2015;30(7):1138–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fazeli PK, Klibanski A. Determinants of GH resistance in malnutrition. J Endocrinol. 2014;220(3):R57–R65. [DOI] [PMC free article] [PubMed] [Google Scholar]