Abstract
Context:
The effects of caloric restriction (CR) on in vivo muscle mitochondrial function in humans are controversial.
Objective:
We evaluated muscle mitochondrial function and associated transcriptional profiles in nonobese humans after 12 months of CR.
Design:
Individuals from an ancillary study of the CALERIE 2 randomized controlled trial were assessed at baseline and 12 months after a 25% CR or ad libitum (control) diet.
Setting:
The study was performed at Pennington Biomedical Research Center in Baton Rouge, LA.
Participants:
Study participants included 51 (34 female subjects, 25 to 50 years of age) healthy nonobese individuals randomized to 1 of 2 groups (CR or control).
Intervention:
This study included 12 months of a 25% CR or ad libitum (control) diet.
Main Outcomes:
In vivo mitochondrial function [maximal ATP synthesis rate (ATPmax), ATPflux/O2 (P/O)] was determined by 31P-magnetic resonance spectroscopy and optical spectroscopy, and body composition was determined by dual-energy X-ray absorptiometry. In a subset of individuals, a muscle biopsy was performed for transcriptional profiling via quantitative reverse transcription polymerase chain reaction and microarrays.
Results:
Weight, body mass index (BMI), fat, and fat-free mass (P < 0.001 for all) significantly decreased at month 12 after CR vs control. In vivo ATPmax and P/O were unaffected by 12 months of CR. Targeted transcriptional profiling showed no effects on pathways involved in mitochondrial biogenesis, function, or oxidative stress. A subgroup analysis according to baseline P/O demonstrated that a higher (vs lower) P/O was associated with notable improvements in ATPmax and P/O after CR.
Conclusions:
In healthy nonobese humans, CR has no effect on muscle mitochondrial function; however, having a “more coupled” (versus “less coupled”) phenotype enables CR-induced improvements in muscle mitochondrial function.
Précis: Long-term caloric restriction in healthy nonobese humans increases mitochondrial efficiency but only in those with near-normal mitochondrial function at baseline.
Mitochondrial changes (content and function) are at the heart of a wide range of age-related diseases (1, 2). Loss of mitochondria occurs in many of these maladies, but defects in the remaining mitochondria are emerging as key players in diabetes (3) and aging-related dysfunctions (4, 5). Caloric restriction (CR), without malnutrition, is a well-known dietary intervention that consistently extends life span when initiated in both young and old animals (6, 7). The CR-mitigated effects on lifespan are suggested to occur via increases in mitochondrial biogenesis/content and subsequent remodeling, whereby mitochondria become more efficient [i.e., they increase the proportion of respiration coupled to ATP production and reduce overall oxygen consumption, thereby increasing coupling, or ATP turnover (ATPflux)/O2 (P/O)] (8, 9) and produce less reactive oxygen species (ROS) (10). Parts of this hypothesis, however, have been challenged by recent studies. One study demonstrated that although lifelong CR preserved muscle mitochondrial function in mice, it did so by protecting the integrity and function of existing mitochondrial cellular components rather than by increasing biogenesis (11). In the CALERIE phase 1 study, however, we demonstrated that 6 months of 25% CR in healthy humans increased the expression of genes involved in mitochondrial biogenesis and mitochondrial DNA (mtDNA) copy number (12). The activity of key mitochondrial enzymes of the tricarboxylic acid cycle, β-oxidation, and the electron transport system, conversely, were unchanged (12), leaving many unanswered questions regarding the effects of CR on muscle-specific mitochondrial function in humans. Taken together, these data lead to the question of whether duration (chronic versus short-term) is a key determinant of CR-induced changes in muscle mitochondrial mass and function. To date, there have been no randomized controlled trials that have examined the effects of long-term CR on muscle mitochondrial function in humans. We conducted an ancillary study to the CALERIE 2 randomized controlled trial to examine the effects of 12 months of 25% CR on in vivo skeletal muscle mitochondrial energetics, mitochondrial content, and markers of oxidative stress. We hypothesized that 12 months of 25% CR would induce the biogenesis of mitochondria and that these mitochondria would be capable of efficient bioenergetics in vivo and have reduced oxidative stress. We anticipated that individuals with a higher mitochondrial content and/or higher P/O at baseline might not respond to the CR as favorably as those with a lower mitochondrial content and/or lower P/O.
Materials and Methods
Study design
CALERIE 2 (13) was a 2-year, multicenter, parallel-group, randomized controlled trial that recruited healthy individuals to receive an intervention aimed to reduce energy intake by 25% (CR) or to maintain habitual energy intake on an ad libitum basis (control). The present institutional review board–approved study was offered only to the 80 individuals enrolled in the parent study at Pennington Biomedical Research Center (PBRC). All participants provided written informed consent. After baseline assessments, participants were randomized to adhere to a diet that provided 25% fewer calories (CR) or ad libitum control calorie intake (control) according to a 2:1 allocation in favor of the CR group. Randomization was stratified by study site, sex, and BMI. Ancillary testing included an extension of the inpatient stay to perform the muscle magnetic resonance spectroscopy (MRS) studies at baseline and after 1 (12 months) and 2 (24 months) years of intervention.
Participants
Men and women aged 20 to 50 years and 20 to 47 years, respectively, with BMI between 22.0 and 27.9 kg/m2 at the screening visit were included.
Study interventions
The CR intervention targeted a sustained 25% restriction of energy intake prescribed on the basis of energy requirements determined from two 2-week doubly labeled water measures at baseline (14). The goal for the intervention was adherence to a mathematically predicted weight loss trajectory that reached 15.5% below baseline after month 12 of intervention. Adherence to 25% CR was fostered by the provision of meals for the first 27 days of the study and regular group and individual meetings with trained interventionists. Participants randomized to the control group were advised to continue their current diets on a completely ad libitum basis. Energy intake (EI) during the trial was calculated between baseline and month 12 by the intake/balance method derived from total daily energy expenditure and the changes in energy content of fat and fat-free mass. The actual reduction in EI (%CR) achieved during each interval was defined as: %CR = 100 × (EI at baseline – EI during interval)/EI at baseline (15).
Anthropometrics
Weight was measured (5200; Scale Tronix, White Plains, NY) in the morning after an overnight fast and voiding. Body composition was measured by dual-energy X-ray absorptiometry (QDR 4500A; Hologic, Bedford, MA) according to a standardized protocol. Scans were analyzed at a centralized reading center (University of California, San Francisco, CA) using Hologic software version Apex 3.3.
Blood analyses
Fasting blood samples were analyzed at the CALERIE central biochemistry laboratory at the University of Vermont or at PBRC.
Cardiorespiratory fitness
Peak oxygen uptake (V̇O2peak) was measured using the Cornell incremental treadmill test (8).
Magnetic resonance and optical spectroscopy
MRS was performed on a 3T GE Signa Excite (General Electric, Milwaukee, WI).
Extra- and intramyocellular lipid
Extramyocellular lipid (EMCL) and intramyocellular lipid (IMCL) contents of the soleus and tibialis anterior muscles were measured by 1H-MRS using the PRESS technique (16). Areas of interest were expressed in arbitrary units per voxel area relative to internal water (×100). Coefficients of variation (CVs) were ±17.9% for EMCL and ±6.3% for IMCL (16).
Mitochondrial function (O2 uptake, ATPflux, ATPmax, P/O)
Resting O2 uptake was measured in vastus lateralis muscle by optical spectroscopy (Horiba Jobin Yvon, Edison, NJ) (17). The CV for O2 uptake is ±13.7%. ATP turnover (ATPflux) and mitochondrial capacity (ATPmax) were measured in the vastus lateralis by 31P-MRS (18), with respective CVs of ±11.0% and ±9.0%. Mitochondrial coupling was calculated as the ratio of ATPflux to O2 uptake to yield P/O. The CV is ±6% (18). In a cross-sectional analysis, we divided the CR group into 2 groups based on a median split of their P/O (uncoupled, n = 13; coupled, n = 10).
Muscle biopsy
A muscle biopsy (Bergstrom technique) (19) was performed at the same site (vastus lateralis) as the 31P and optical spectroscopy measurements.
DNA
Total DNA was isolated from ∼10 mg of skeletal muscle tissue using the DNeasy kit (Qiagen, Valencia, CA). Relative amounts of mtDNA and nuclear DNA were determined by real-time quantitative polymerase chain reaction (20).
RNA
Total RNA was isolated from ∼20 mg of skeletal muscle tissue using RNeasy Fibrous Tissue kit (Qiagen). Primer-probe sets were predesigned using Single Tube Taqman® Gene expression assays (Life Technologies, Grand Island, NY). Quantitative reverse transcription polymerase chain reaction reactions were performed using Taqman Fast Virus 1-step reaction mix Standard protocol (Life Technologies). Data were normalized by dividing the target genes by the geometric mean of internal control genes.
Near–whole-genome transcriptome analysis was performed using Sentrix Human-6 V2 Expression BeadChip (Illumina Inc., San Diego, CA). Data were initially log2 normalized and batch-corrected using Partek software (St. Louis, MO). A delta value was computed for each gene (month 12 to baseline). Gene set enrichment analysis (GSEA) (21) was run in GenePattern (22) using Reactome gene sets from GSEA and mSigDB (23). Important pathways and enriched categories with P < 0.05 were identified and analyzed. Per-group descriptive statistics were computed using the “psych” library in R (https://cran.r-project.org/package=psych) (24).
Protein
Protein contents of complexes III and IV were quantified using the Odyssey IR imaging system (LI-COR Biosciences, Lincoln, NE) (25). Protein adducts of 4-hydroxy-2-nonenal (4-HNE) were determined as using a polyclonal anti–4-HNE antibody (Calbiochem, San Diego, CA). Samples were normalized to total protein (LI-COR Biosciences).
Statistical methods
This study was powered on the ability to detect a significant change in maximal ATP synthesis (ΔATPmax) from baseline and to detect differences in ΔATPmax between the 2 groups (control versus CR). Anticipating that most PBRC study participants would participate in the study (n = 80, 2:1 randomization), we assumed a 10% dropout/biopsy refusal rate (based on phase 1 protocol), which translated into 45 completers in the CR group and 23 completers in the control group at month 12. Based on our preliminary data, 24 participants would provide 80% power to detect a +15% difference in ATPmax.
All analyses were performed using SAS v9.4 (SAS, Cary, NC) and with significance at α = 0.05. Owing to an insufficient amount of follow-up data at month 24, only participants having ATPmax or P/O values at both baseline and month 12 were included in the analyses. Baseline and month 12 data in all outcomes were investigated using general linear mixed models for repeated measures. Included were fixed effects for treatment group, time, and the treatment group × time interaction. P values for post hoc tests were adjusted by false-discovery rate. For the subgroup analysis, ANCOVA with baseline as covariate was used to compare the mean change between predefined uncoupled versus coupled groups from baseline to month 12. All clinical and in vivo data are presented as mean ± SD. Biopsy-derived data are expressed as mean ± SEM.
Results
Study participants and throughput
Out of 80 eligible participants, 48 completed baseline and month 12 (CR: n = 32; control: n = 16) measurements for the primary endpoint of ATPmax. Due to failure of the optical spectroscopy measurements (O2 uptake) in some individuals, 38 participants completed baseline and month 12 (CR: n = 26; control: n = 12) measurements for mitochondrial coupling (P/O), a key secondary endpoint for the study; however, 3 of these participants did not have ATPmax due to other technical difficulties with the 31P-spectroscopy measurements. Final data analyses were therefore performed on 51 participants in total (including all participants having ATPmax or P/O at both baseline and month 12; 34 female subjects, 39 ± 7 years), of which 33 participants were randomized to the 25% CR intervention and 18 participants to the control group. The 51 participants were objectively selected on the basis of adherence to their assigned treatment groups whereby the criteria for adherence was >5% weight loss at month 12 for CR participants (0 excluded) and ≤5% weight loss at month 12 for control participants. One participant was excluded; however, this participant lacked follow-up ATPmax data at month 12, so this exclusion did not affect the final analyses. Per design, all participants were healthy, with 25 participants (49.0%) being normal weight and 26 participants (51.0%) being overweight (25 to 27.9 kg/m2).
Weight, body composition, and cardiorespiratory fitness
Participants in each group did not differ at baseline for age, weight, blood pressure, and blood lipids and glucose. Insulin tended to be lower in the CR group at baseline (P = 0.06) (Table 1). There was a significant time effect and a significant group × time interaction for reductions in weight, body mass index (BMI), fat mass, total %body fat, total lean body mass, and leg lean body mass within the CR group (P < 0.001 for all) (Table 2). Cardiorespiratory fitness (VO2peak, unadjusted for body weight) was significantly reduced from baseline to month 12 in the CR group (P = 0.02) (Table 2); however, this difference was abrogated when adjusted for body weight (Table 2). Diastolic blood pressure (P = 0.04), insulin (P = 0.02), total cholesterol (P = 0.02), and low-density lipoprotein cholesterol (P = 0.008) were significantly reduced from baseline to month 12 in the CR group but did not differ from control (Table 2).
Table 1.
Baseline Characteristics of Study Participants
| Parameter | Control Group (n = 18) | CR Group (n = 33) | P Value |
|---|---|---|---|
| Age, y (mean ± SD) | 38.8 ± 5.7 | 38.6 ± 7.4 | 0.92 |
| Sex, ratio (male/female) | 7/11 | 10/23 | 0.54 |
| Race, ratio (African American/mixed European descent) | 4/14 | 8/25 | 0.41 |
| Anthropometrics, mean ± SD | |||
| Weight, kg | 70.7 ± 8.2 | 71.8 ± 9.2 | 0.65 |
| BMI, kg/m2 | 25.1 ± 1.7 | 25.3 ± 1.7 | 0.77 |
| Plasma lipids, mean ± SD | |||
| Total cholesterol, mg/dL | 169.4 ± 7.3 | 163.1 ± 5.4 | 0.49 |
| HDL cholesterol, mg/dL | 49.7 ± 11.8 | 47.9 ± 10.6 | 0.60 |
| LDL cholesterol, mg/dL | 102.2 ± 26.4 | 96.3 ± 24.5 | 0.43 |
| Plasma glucose and insulin, mean ± SD | |||
| Glucose, mg/dL | 84.6 ± 5.1 | 82.2 ± 5.7 | 0.15 |
| Insulin, μIU/mL | 6.5 ± 2.4 | 5.1 ± 2.4 | 0.06 |
| Blood pressure, mean ± SD | |||
| Systolic, mm Hg | 111.7 ± 9.9 | 114.8 ± 10.1 | 0.30 |
| Diastolic, mm Hg | 73.2 ± 8.3 | 74.6 ± 7.0 | 0.52 |
SI conversion factors: To convert glucose values from mg/dL to mmol/L, multiply by 0.0555; to convert insulin values from μIU/mL to pmol/L, multiply by 6.945; to convert total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol values from mg/dL to mmol/L, multiply by 0.0259.
Abbreviation: SD, standard deviation.
Table 2.
Analysis of Age, Weight, Body Composition, Cardiorespiratory Fitness, and Metabolic Panel
| Control Group (n = 18) |
CR Group (n = 33) |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Age, Weight, Body Composition, and Cardiorespiratory Fitness | Baseline | Month 12 | Baseline | Month 12 | Group | Time | Group × Time |
| Age, y | 38.8 ± 5.7 | – | 38.6 ± 7.4 | – | 0.92 | 1.00 | 1.00 |
| Weight, kg | 70.7 ± 8.2 | 70.8 ± 8.3 | 71.8 ± 9.2 | 63.0 ± 8.6a,b | 0.19 | <0.001 | <0.001 |
| BMI, kg/m2 | 25.1 ± 1.7 | 25.2 ± 1.6 | 25.2 ± 1.7 | 22.1 ± 1.7a,b | 0.004 | <0.001 | <0.001 |
| Fat mass, kg | 22.1 ± 3.9 | 22.2 ± 4.3 | 23.8 ± 4.9 | 17.6 ± 4.7a,b | 0.29 | <0.001 | <0.001 |
| Total body fat, % | 31.7 ± 6.4 | 31.6 ± 6.4 | 33.3 ± 6.3 | 27.9 ± 6.6a | 0.57 | <0.001 | <0.001 |
| Lean body mass, kg | 48.5 ± 9.5 | 48.7 ± 9.3 | 48.0 ± 8.6 | 45.6 ± 8.0a | 0.48 | <0.001 | <0.001 |
| Leg lean body mass, kg | 16.2 ± 3.5 | 16.4 ± 3.4 | 16.1 ± 2.9 | 15.3 ± 2.9a | 0.56 | <0.001 | <0.001 |
| VO2peak, L/min | 2.5 ± 0.8 | 2.5 ± 0.9 | 2.4 ± 0.6 | 2.2 ± 0.7a | 0.34 | 0.09 | 0.09 |
| VO2peak, mL/kg BW/min | 34.9 ± 8.6 | 34.8 ± 9.8 | 33.0 ± 6.2 | 34.5 ± 8.2 | 0.62 | 0.29 | 0.24 |
| Metabolic panel | |||||||
| Blood pressure | |||||||
| Systolic, mm Hg | 112 ± 10 | 114 ± 7 | 115 ± 10 | 113 ± 12 | 0.74 | 0.95 | 0.16 |
| Diastolic, mm Hg | 73.2 ± 8.3 | 73.4 ± 7.1 | 74.6 ± 7.0 | 71.5 ± 8.2a | 0.90 | 0.15 | 0.10 |
| Glucose, mg/dL | 84.6 ± 5.1 | 82.9 ± 3.7 | 82.2 ± 5.7 | 80.6 ± 4.6 | 0.08 | 0.02 | 0.93 |
| Insulin, μIU/ml | 6.5 ± 2.4 | 6.5 ± 2.5 | 5.1 ± 2.4 | 4.0 ± 2.0a | <0.001 | 0.23 | 0.25 |
| Total cholesterol, mg/dL | 169.4 ± 36.0 | 164.9 ± 26.4 | 163.1 ± 28.2 | 152.4 ± 26.3a | 0.24 | 0.02 | 0.32 |
| HDL cholesterol, mg/dL | 49.7 ± 11.8 | 49.4 ± 9.5 | 48.0 ± 10.6 | 50.3 ± 10.0 | 0.89 | 0.32 | 0.21 |
| LDL cholesterol, mg/dL | 102.2 ± 26.4 | 98.8 ± 23.0 | 96.3 ± 24.4 | 86.8 ± 21.7a | 0.18 | 0.02 | 0.22 |
Data are mean ± standard deviation for all participants. Only participants who complied with the intervention protocols and completed the intervention were included. SI conversion factors: to convert glucose values from mg/dL to mmol/L, multiply by 0.0555; to convert insulin values from μIU/mL to pmol/L, multiply by 6.945; to convert total, HDL, and LDL cholesterol values from mg/dL to mmol/L, multiply by 0.0259.
Abbreviations: BW, body weight; leg lean body mass, sum of lean body mass for right and left legs; SD, standard deviation.
Within a group, significant difference from baseline to month 12.
Between groups, significant difference at month 12.
Muscle lipid content and mitochondrial function
There was a significant group × time interaction for in vivo EMCL of the tibialis anterior (P = 0.04) and a similar pattern for IMCL of the tibialis anterior, although it did not reach significance (P = 0.07) (Table 3). These data suggest that the time-induced increases in EMCL and IMCL of the tibialis anterior observed in the control group were attenuated by 12 months of caloric restriction in the CR group. IMCL of the soleus was 38% lower in the CR group at baseline (P = 0.0008) and, after adjusting for these baseline differences, the change in IMCL soleus from baseline to month 12 between the CR and the control groups was significant (−0.0006 ± 0.0003 versus 0.0004 ± 0.0004 arbitrary units, P = 0.04, for CR versus control, respectively). In vivo muscle ATP synthesis rates (ATPmax) did not differ significantly by group or time (0.89 ± 0.34 to 0.88 ± 0.30 mM/s and 0.80 ± 0.26 to 0.78 ± 0.18 mM/s, baseline to month 12 for CR versus control, respectively). Similarly, resting ATPflux, O2 uptake, and P/O did not differ significantly by group or time (Table 3).
Table 3.
Analysis of In Vivo Muscle Lipid Content and Mitochondrial Function
| Control Group (n = 18) |
CR Group (n = 33) |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | Month 12 | Baseline | Month 12 | Group | Time | Group × Time | |
| Muscle lipid content | |||||||
| Extramyocellular lipid, AU | |||||||
| Tibialis anterior | 0.0088 ± 0.0055 | 0.0102 ± 0.0089 | 0.0089 ± 0.0056 | 0.0072 ± 0.0039 | 0.37 | 0.82 | 0.04 |
| Soleus | 0.0066 ± 0.0056 | 0.0065 ± 0.0031 | 0.0045 ± 0.0023 | 0.0051 ± 0.0025 | 0.04 | 0.64 | 0.42 |
| Intramyocellular lipid, AU | |||||||
| Tibialis anterior | 0.0016 ± 0.0012 | 0.0021 ± 0.0013 | 0.0014 ± 0.0009 | 0.0013 ± 0.0008b | 0.05 | 0.33 | 0.07 |
| Soleus | 0.0029 ± 0.0020 | 0.0027 ± 0.0023 | 0.0018 ± 0.0009a | 0.0015 ± 0.0009b | <0.001 | 0.46 | 0.92 |
| Muscle mitochondrial function | |||||||
| Maximal ATP synthesis (ATPmax), mM/s | 0.80 ± 0.26 | 0.78 ± 0.18 | 0.89 ± 0.34 | 0.88 ± 0.30 | 0.17 | 0.72 | 0.92 |
| Resting ATPflux, μM/s | 5.26 ± 1.15 | 5.51 ± 0.93 | 5.14 ± 1.41 | 5.00 ± 1.13 | 0.30 | 0.77 | 0.44 |
| Resting oxygen uptake, μM/s | 2.13 ± 0.42 | 2.11 ± 0.27 | 2.18 ± 0.44 | 2.13 ± 0.36 | 0.70 | 0.55 | 0.89 |
| Resting ATPflux: O (P/O) | 1.28 ± 0.39 | 1.34 ± 0.32 | 1.22 ± 0.29 | 1.21 ± 0.33 | 0.24 | 0.73 | 0.68 |
Data are mean ± standard error of the mean for all participants. Only participants who complied with the intervention protocols and completed the intervention were included.
Abbreviation: AU, arbitrary units.
Between groups, significant difference at baseline.
Between groups, significant difference at month 12.
Targeted and global gene expression profiling
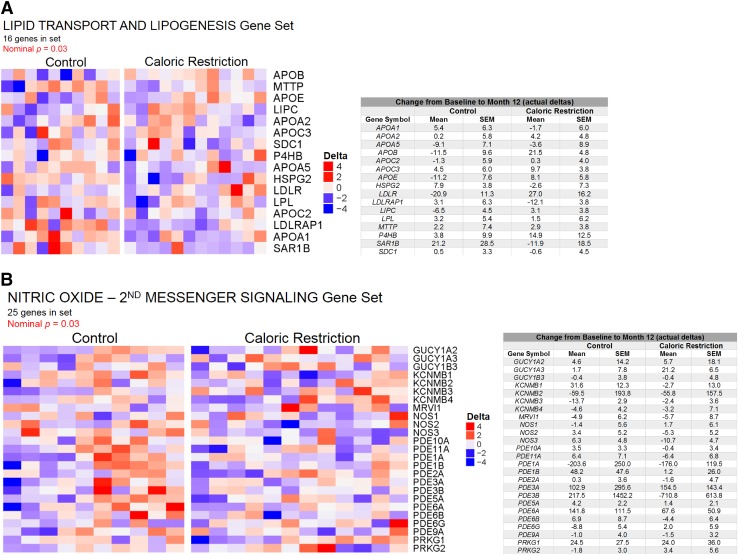
Using a panel of well-known markers of mitochondrial biogenesis, function, and oxidative stress, we profiled muscle tissue in a subset of individuals (CR: n = 15; control: n = 8) due to tissue limitations and/or biopsy refusal. No significant group effects were observed for the 29 genes measured (Table 4). Several genes (ACSL1, CS, HADH, PPARA, GSTK1, NQO1, PPM1L, and SOD2) had a significant time effect, being lower at month 12 (versus baseline) in the control group only. SIRT1 had a significant group × time interaction, decreasing from baseline to month 12 in the control group and increasing from baseline to month 12 in the CR group (P = 0.03). Protein contents of markers for complexes III and IV of the electron transport system showed no significant group or time effects; however, mtDNA copy number, a marker of mitochondrial content, had a significant time effect, being lower at month 12 (versus baseline) in the CR group (P = 0.003) (Table 4). 4-HNE protein adducts, a marker of lipid peroxidation, did not differ by group or time (Table 4). Using GSEA of microarray data for a global transcriptional analysis, we found a significant downregulation of genes involved in lipid transport and lipogenesis from baseline to month 12 in the CR group (P = 0.03) (Fig. 1A), which aligns with the reduced in vivo IMCL at month 12 in CR versus control (Table 3). GSEA also revealed a significant downregulation of genes related to nitric oxide (NO) stimulation of second messenger signaling from baseline to month 12 in the CR group (P = 0.03) (Fig. 1B). The majority of these genes were phosphodiesterases, suggesting an upregulation of second messengers (e.g., cGMP, cAMP) in the CR group at month 12.
Table 4.
Targeted Profiling of Muscle Mitochondrial Function and the Oxidative Stress Response After 12 Months of Caloric Restriction
| Control Group (n = 8) |
CR Group (n = 15) |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | Month 12 | Baseline | Month 12 | Group | Time | Group × Time | |
| Muscle mitochondrial biogenesis and function | |||||||
| ACACB | 1.50 ± 0.21 | 1.32 ± 0.15 | 1.21 ± 0.11 | 1.21 ± 0.07 | 0.22 | 0.39 | 0.39 |
| ACSL1 | 0.635 ± 0.059 | 0.537 ± 0.056a | 0.585 ± 0.052 | 0.557 ± 0.049 | 0.85 | 0.01 | 0.13 |
| CD36 | 0.778 ± 0.047 | 0.680 ± 0.037 | 0.661 ± 0.076 | 0.594 ± 0.074 | 0.32 | 0.07 | 0.72 |
| CPT1B | 1.83 ± 0.14 | 1.48 ± 0.15 | 1.61 ± 0.16 | 1.98 ± 0.33 | 0.62 | 0.95 | 0.14 |
| CS | 0.528 ± 0.035 | 0.454 ± 0.030a | 0.470 ± 0.038 | 0.448 ± 0.030 | 0.53 | 0.01 | 0.11 |
| HADH | 0.713 ± 0.051 | 0.634 ± 0.055 | 0.672 ± 0.067 | 0.599 ± 0.057 | 0.67 | 0.03 | 0.94 |
| MLYCD | 0.848 ± 0.097 | 0.652 ± 0.068 | 0.673 ± 0.072 | 0.686 ± 0.078 | 0.52 | 0.09 | 0.06 |
| NAMPT | 0.993 ± 0.078 | 0.854 ± 0.084 | 0.866 ± 0.061 | 1.370 ± 0.435 | 0.52 | 0.57 | 0.32 |
| NRF1 | 0.581 ± 0.043 | 0.559 ± 0.040 | 0.537 ± 0.028 | 0.592 ± 0.038 | 0.91 | 0.50 | 0.11 |
| PPARA | 0.482 ± 0.043 | 0.429 ± 0.039 | 0.463 ± 0.041 | 0.420 ± 0.029 | 0.79 | 0.01 | 0.77 |
| PPARD | 2.48 ± 0.26 | 2.47 ± 0.44 | 2.06 ± 0.18 | 2.30 ± 0.26 | 0.31 | 0.71 | 0.67 |
| PPARGC1A | 10.79 ± 0.88 | 8.92 ± 1.27 | 9.19 ± 0.73 | 10.19 ± 1.09 | 0.89 | 0.63 | 0.12 |
| PPARGC1B | 0.651 ± 0.063 | 0.700 ± 0.079 | 0.648 ± 0.054 | 0.635 ± 0.048 | 0.64 | 0.71 | 0.52 |
| PRKAA1 | 5.54 ± 0.73 | 5.46 ± 0.66 | 4.39 ± 0.26 | 5.87 ± 1.10 | 0.68 | 0.42 | 0.36 |
| PRKAA2 | 0.537 ± 0.042 | 0.587 ± 0.042 | 0.544 ± 0.042 | 0.565 ± 0.035 | 0.90 | 0.11 | 0.50 |
| PRKAG3 | 0.467 ± 0.036 | 0.473 ± 0.048 | 0.436 ± 0.038 | 0.413 ± 0.035 | 0.41 | 0.72 | 0.55 |
| SCD | 0.131 ± 0.030 | 0.225 ± 0.061 | 0.249 ± 0.054 | 0.227 ± 0.065 | 0.41 | 0.49 | 0.28 |
| SIRT1 | 0.950 ± 0.120 | 0.786 ± 0.080 | 0.791 ± 0.135 | 0.919 ± 0.104 | 0.94 | 0.77 | 0.03 |
| SLC25A4 | 0.715 ± 0.052 | 0.723 ± 0.070 | 0.745 ± 0.069 | 0.688 ± 0.074 | 0.97 | 0.74 | 0.66 |
| UCP3 | 4.86 ± 0.34 | 3.89 ± 0.44 | 3.75 ± 0.33 | 3.99 ± 0.45 | 0.35 | 0.25 | 0.07 |
| Muscle mitochondrial content | |||||||
| Complex III protein | 0.172 ± 0.019 | 0.143 ± 0.010 | 0.174 ± 0.015 | 0.176 ± 0.014 | 0.39 | 0.27 | 0.27 |
| Complex IV protein | 0.0332 ± 0.006 | 0.0310 ± 0.005 | 0.0448 ± 0.003 | 0.0405 ± 0.006 | 0.05 | 0.56 | 0.84 |
| mtDNA copy number | 1800 ± 88 | 1580 ± 64 | 1935 ± 95 | 1535 ± 100a | 0.76 | <0.01 | 0.23 |
| Muscle mitochondrial oxidative stress response | |||||||
| CPEB2 | 0.639 ± 0.031 | 0.612 ± 0.065 | 0.598 ± 0.036 | 0.560 ± 0.021 | 0.28 | 0.38 | 0.88 |
| GPX1 | 1.66 ± 0.11 | 1.27 ± 0.17 | 1.51 ± 0.13 | 1.76 ± 0.36 | 0.55 | 0.81 | 0.26 |
| GPX7 | 0.710 ± 0.050 | 0.704 ± 0.085 | 0.676 ± 0.033 | 0.648 ± 0.017 | 0.35 | 0.66 | 0.79 |
| GSTK1 | 0.976 ± 0.045 | 0.831 ± 0.062 | 0.973 ± 0.055 | 0.865 ± 0.027 | 0.77 | 0.01 | 0.69 |
| HMOX1 | 8.98 ± 0.69 | 6.77 ± 1.61 | 11.64 ± 1.78 | 17.54 ± 5.44 | 0.12 | 0.64 | 0.32 |
| NQO1 | 0.0639 ± 0.007 | 0.0501 ± 0.006 | 0.0601 ± 0.005 | 0.0562 ± 0.005 | 0.89 | 0.01 | 0.15 |
| OGG1 | 0.287 ± 0.020 | 0.261 ± 0.017 | 0.259 ± 0.018 | 0.277 ± 0.014 | 0.81 | 0.66 | 0.02 |
| PPM1L | 0.947 ± 0.097 | 0.803 ± 0.071 | 0.850 ± 0.091 | 0.778 ± 0.055 | 0.59 | 0.03 | 0.44 |
| SOD2 | 0.561 ± 0.053 | 0.445 ± 0.049a | 0.515 ± 0.049 | 0.473 ± 0.035 | 0.89 | 0.002 | 0.12 |
| 4-HNE protein adducts | 2.55 ± 0.71 | 1.64 ± 0.30 | 2.19 ± 0.44 | 2.56 ± 0.67 | 0.68 | 0.58 | 0.23 |
Data are mean ± standard error of the mean for all participants. Only participants who complied with the intervention protocols and completed the intervention were included. Italics indicate mRNA expression. See Materials and Methods for details on the individual methods related to this table.
Abbreviations: ACACB, acetyl-CoA carboxylase β; ACSL1, acyl-CoA synthetase long-chain family member 1; CPEB2, cytoplasmic polyadenylation element binding protein 2; CPT1B, carnitine palmitoyltransferase 1B; CS, citrate synthase; GPX1, glutathione peroxidase 1; GPX7, glutathione peroxidase 7; GSTK1, glutathione S-transferase κ-1; HADH, hydroxyacyl-CoA dehydrogenase; HMOX1, heme oxygenase 1; MLYCD, malonyl-CoA decarboxylase; NAMPT, nicotinamide phosphoribosyltransferase; NQO1, NAD(P)H quinone dehydrogenase 1; NRF1, nuclear respiratory factor 1; OGG1, 8-oxoguanine DNA glycosylase; PPARA, peroxisome proliferator-activated receptor α; PPARD, peroxisome proliferator activated receptor δ; PPARGC1A, peroxisome proliferator-activated receptor, γ, coactivator 1 α; PPARGC1B, peroxisome proliferator-activated receptor, γ, coactivator 1 β; PPM1L, protein phosphatase, Mg2+/Mn2+ dependent 1L; PRKAA1, protein kinase AMP-activated catalytic subunit α 1; PRKAA2, protein kinase AMP-activated catalytic subunit α 2; PRKAG3, protein kinase AMP-activated catalytic subunit γ 3; qRT-PCR, quantitative reverse transcription polymerase chain reaction (Western blotting); SCD, stearoyl-CoA desaturase; SIRT1, sirtuin 1; SLC25A4, solute carrier family 25 member 4/adenine nucleotide transporter 1; SOD2, superoxide dismutase 2, mitochondrial; UCP3, uncoupling protein 3.
Within group, significant difference from baseline to month 12.
Figure 1.
Global gene expression profiling. GSEA of Illumina transcription arrays in muscle tissue mRNA was performed in a subset of individuals owing to tissue limitations and/or biopsy refusal. In the Reactome gene set list, 2 sets were significantly different between groups: (A) lipid transport and lipogenesis and (B) NO-second messenger signaling, which were downregulated in the CR group from baseline to month 12 (CR, n = 12; control, n = 10). Heat map of NOS pathway was generated via the ComplexHeatmap library in R Bioconductor (https://github.com/jokergoo/ComplexHeatmap) using a per-gene scaling metric for delta expression changes (see Materials and Methods).
Subgroup analysis of in vivo mitochondrial coupling (P/O)
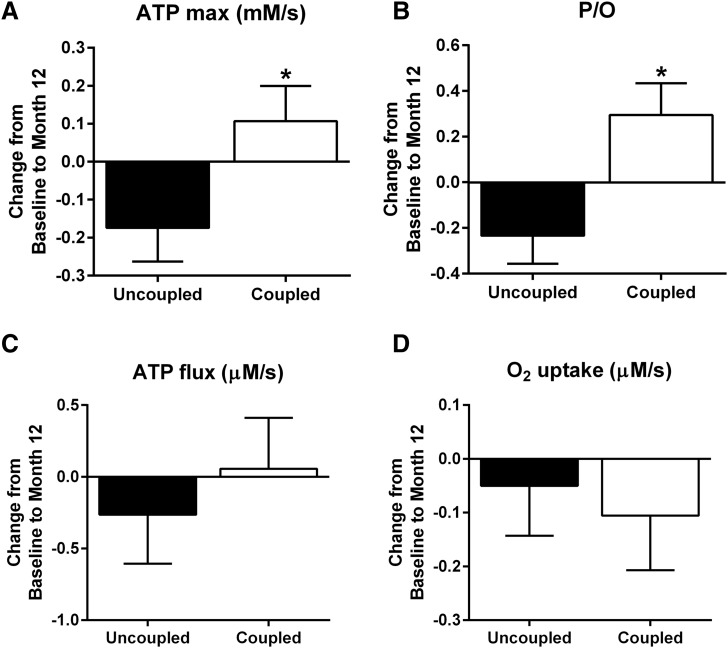
In a cross-sectional analysis of the CALERIE 2 participants at baseline, we found that in vivo P/O revealed a novel signature of mitochondrial health in the muscle of these healthy nonobese individuals (26). We applied this same median split of baseline P/O (uncoupled versus coupled) to the analysis of changes in in vivo mitochondrial function in response to 12 months of CR (CR group: uncoupled, n = 13; coupled, n = 10). The coupled group (higher P/O) significantly improved ATPmax (P < 0.05) (Fig. 2A) and P/O (P < 0.05) (Fig. 2B) after 12 months of CR versus the uncoupled group (lower P/O). ATPflux (P = 0.46) (Fig. 2C) and O2 uptake (P = 0.76) (Fig. 2D) were unaffected by 12 months of CR in either group (uncoupled versus coupled).
Figure 2.
Subgroup analysis of in vivo mitochondrial function after CR. (A) Maximal ATP synthesis rate (ATPmax), (B) resting ATP flux/O2 uptake or P/O, (C) resting ATPflux, and (D) O2 uptake were measured by 31P-MRS and optical spectroscopy in a cohort of healthy nonobese individuals separated by their in vivo mitochondrial coupling (P/O) (coupled versus uncoupled) after 12 months of CR (excluding individuals from the control group) (uncoupled, n = 13; coupled, n = 10). *P < 0.05.
Discussion
CR results in an attenuation of age-induced increases in fat mass, slows metabolic rate, reduces systemic oxidative stress (8, 27), and improves insulin sensitivity (28). To date, the majority of CR-mitigated health benefits have been proven in model systems ranging from C. elegans to nonhuman primates (29). Although CR has a multitude of metabolic benefits to the organism as a whole, its tissue-specific effects vary and are less clear in humans. Our results indicate that long-term CR (12 months) has no impact on muscle mitochondrial content or function or on oxidative stress in healthy nonobese humans. Only those individuals having “more coupled” mitochondria at baseline benefited from CR in terms of improvements in muscle mitochondrial ATP synthesis rates and coupling. It is important to note that only younger, healthy participants were studied, and thus whether CR could affect these subjects further on their way to disease differently remains to be answered.
Mitochondrial changes (content/function) lie at the heart of age-related diseases (1, 2). Loss of mitochondria occurs in many of these maladies, but defects in the remaining mitochondria are emerging as key players in T2D (3) and aging-related dysfunctions (4, 5). Recent results suggest that these defects may trigger the cascade of events that lead to cell death and muscle fiber loss (30), accounting for up to half the decline in muscle size with age (31). We show that 12 months of CR is highly effective in terms of reducing body weight, specifically fat mass, and that this adaptation occurs at the expense of total and leg (muscle) lean body mass. It is imperative to understand the effects of CR on certain aspects of muscle quality (myocellular lipid content, mitochondrial function) because there is an overall loss of muscle mass.
Muscle ectopic lipid deposition occurs with obesity and aging and has been implicated in the development of T2D (32). Reductions in IMCL content via diet, exercise, or combined interventions are effective at improving insulin sensitivity and muscle mitochondrial function (33). Despite the well-documented CR-mitigated improvements in body weight, energy expenditure (8), and insulin sensitivity (28), the effects of CR on myocellular lipid in humans are unknown. We show for the first time that 12 months of CR significantly reduces EMCL of the tibialis anterior and soleus and IMCL of the soleus muscles compared with controls. These small reductions in myocellular lipid are within range of those known to improve insulin sensitivity and oxidative capacity (34). In support of these findings, GSEA of muscle tissue revealed a substantial reduction in genes related to lipid transport and lipogenesis after CR. These CR-induced reductions in muscle lipid content and markers of lipogenesis paralleled substantial reductions in serum cholesterol, collectively pointing toward an improved lipid metabolism profile in nonobese humans after long-term CR.
We know little about the nature and extent of muscle mitochondrial dysfunction in vivo and the effects of potentially potent treatment strategies (e.g., long-term CR) on muscle mitochondrial dysfunction in humans. In aged mice, chronic CR preserved ex vivo muscle mitochondrial function without increasing mitochondrial biogenesis (11), yet we have previously shown in healthy humans that 6 months of CR increased mitochondrial biogenesis and content without affecting mitochondrial enzyme activities (12). These data lead to the question of whether long-term CR in humans will improve muscle mitochondrial content and/or function. Recent innovations in in vivo spectroscopy from our laboratory permit quantitative measurements of mitochondrial O2 uptake and ATP fluxes to yield mitochondrial phosphorylation capacity (ATPmax) and coupling efficiency (P/O) (18, 35–37). Here we exploited these unique advantages and insights and evaluated the effects of CR on in vivo muscle mitochondrial function in nonobese humans. Contrary to our hypothesis, 12 months of 25% CR had no impact on in vivo muscle ATPmax or P/O. In parallel, at the tissue level, targeted transcriptional profiles of markers of mitochondrial biogenesis and function were also unchanged after CR. mtDNA copy number was significantly reduced after CR, yet the contents of 2 proteins in the electron transport system were unchanged. These data are unexpected in the context of findings from our previous CALERIE phase 1 study in which we demonstrated an increase in mtDNA copy number and markers of mitochondrial biogenesis after 6 months of 25% CR in healthy humans (12). The lack of an impact of 12 months of 25% CR on protein markers of mitochondrial content, coupled with a substantial decrease in mtDNA, suggests to us that a longer duration (>6 months) of CR in healthy humans elicits a negative feedback loop whereby the initial signals of short-term CR that induce formation of new mitochondria subsequently “normalize” after a longer period (12 months), resulting in our observed lack of change in mitochondrial content. This supposition needs to be experimentally tested in a prospective randomized controlled trial such as this one with serial muscle biopsies at more frequent intervals to determine the time course of CR effects on mitochondrial biogenesis and content. GSEA did reveal that a small subset of genes related to nitric oxide signaling, phosphodiesterases in particular, were significantly downregulated after CR, which suggests an upregulation of second messengers such as cGMP and cAMP. This upregulation has implications for enhancing downstream events (lipolysis, fatty acid mobilization, etc.). Without functional data to corroborate this finding, however, it is difficult to conclude that CR has such an effect and requires further investigation.
Oxidative damage is a prime candidate for the cause of mitochondrial defects and is a key element of the mitochondrial theory of aging (38). ROS are produced by mitochondrial respiration (39), causing oxidative damage that accumulates with age and leads to mitochondrial defects in several tissues (40). After 12 months of CR, we found no substantial effects on transcriptional markers of oxidative stress or content of 4-HNE [marker of lipid peroxidation known to result from accumulation of ROS (41)] in muscle tissue of our healthy individuals. Collectively, these data support a recent report in which 16 weeks of CR in obese adults increased peripheral insulin sensitivity, yet this improvement was not accompanied by changes in ex vivo muscle mitochondrial oxidative capacity or oxidant emissions (28).
Based on a previous cross-sectional analysis of our cohort, we found that in vivo P/O revealed a novel signature of mitochondrial health in their muscle (26). Here we explored a subgroup analysis in which we divided the CR group into “coupled” and “uncoupled” based on a median split of their baseline P/O and compared the effects of the CR intervention on in vivo mitochondrial function. To our surprise, individuals having a higher baseline P/O (coupled) experienced a substantial improvement in ATPmax and P/O with CR; whereas, individuals with the lower baseline P/O (uncoupled) experienced decreased ATPmax and P/O after the CR intervention.
We demonstrate for the first time in a randomized controlled trial that long-term CR has no impact on in vivo muscle mitochondrial function or oxidative stress in healthy nonobese humans and that only individuals having “more coupled” mitochondria at baseline benefited from CR in terms of improvements in muscle mitochondrial ATP synthesis rates and coupling. Although this CR intervention was effective at reducing body weight and fat mass, improving serum lipid profiles, and reducing muscle ectopic lipid deposition (particularly EMCL) in younger, healthier individuals, whether CR could affect those further on their way to disease differently remains to be answered.
Acknowledgments
We thank the study participants; Kori Murray of Pennington Biomedical Research Center for help with the MRS and optical spectroscopy measurements; James Rochon, William Krauss, and Manjushri Bhapkah of the CALERIE data coordinating center; and Xiaohui “Vicky” Guo of Sanford Burnham Prebys Medical Discovery Institute for help with the GSEA.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 AG030226 (S.S.R. and E.R.) and U01 AG020478 (E.R.). Additional support for this work was supplied by Pennington/Louisiana NORC Grant P30 DK072476 and Louisiana Clinical and Translational Science Center Grant U54 GM104940.
Author contributions: L.M.S. analyzed and interpreted the data and wrote the manuscript. F.Y., A.H., A.E., S.R.C., M.E.G., C.S., and H.H.C. assisted during the acquisition, analysis and interpretation of data and revised the manuscript. L.M.R., K.E.C., and M.-E.H. contributed to the design of the study, analyzed and interpreted the data, and edited the manuscript. S.R.S. and E.R. designed the study, analyzed and interpreted the data, and edited the manuscript. All authors approved the final version of the manuscript. L.M.S. and S.R.S. are responsible for the integrity of the work as a whole.
Acknowledgments
Clinical trial registry: ClinicalTrials.gov no. NCT00427193, NCT02695511 (registered 24 January 2007).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 4-HNE
- 4-hydroxy-2-nonenal
- ATP
- adenosine triphosphate
- ATPmax
- maximal ATP synthesis rate
- BMI
- body mass index
- CR
- caloric restriction
- CV
- coefficient of variation
- EI
- energy intake
- EMCL
- extramyocellular lipid
- GSEA
- gene set enrichment analysis
- IMCL
- intramyocellular lipid
- mtDNA
- mitochondrial DNA
- MRS
- magnetic resonance spectroscopy
- PBRC
- Pennington Biomedical Research Center
- P/O
- ATPflux/O2
- ROS
- reactive oxygen species.
References
- 1.Chabi B, Adhihetty PJ, Ljubicic V, Hood DA. How is mitochondrial biogenesis affected in mitochondrial disease? Med Sci Sports Exerc. 2005;37(12):2102–2110. [DOI] [PubMed] [Google Scholar]
- 2.Nicholls D. Mitochondrial bioenergetics, aging, and aging-related disease. Sci Aging Knowledge Environ. 2002;2002:pe12. [DOI] [PubMed] [Google Scholar]
- 3.Parish R, Petersen KF. Mitochondrial dysfunction and type 2 diabetes. Curr Diab Rep. 2005;5(3):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harper ME, Bevilacqua L, Hagopian K, Weindruch R, Ramsey JJ. Ageing, oxidative stress, and mitochondrial uncoupling. Acta Physiol Scand. 2004;182(4):321–331. [DOI] [PubMed] [Google Scholar]
- 5.Marcinek DJ, Schenkman KA, Ciesielski WA, Lee D, Conley KE. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol. 2005;569(Pt 2):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Effects of short- and medium-term calorie restriction on muscle mitochondrial proton leak and reactive oxygen species production. Am J Physiol Endocrinol Metab. 2004;286(5):E852–E861. [DOI] [PubMed] [Google Scholar]
- 7.Harper ME, Monemdjou S, Ramsey JJ, Weindruch R. Age-related increase in mitochondrial proton leak and decrease in ATP turnover reactions in mouse hepatocytes. Am J Physiol. 1998;275(2 Pt 1):E197–E206. [DOI] [PubMed] [Google Scholar]
- 8.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E; Pennington CALERIE Team . Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295(13):1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, Blackwell TK. Dietary restriction involves NAD⁺-dependent mechanisms and a shift toward oxidative metabolism. Aging Cell. 2014;13(6):1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsey JJ, Harper ME, Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free Radic Biol Med. 2000;29(10):946–968. [DOI] [PubMed] [Google Scholar]
- 11.Lanza IR, Zabielski P, Klaus KA, Morse DM, Heppelmann CJ, Bergen HR III, Dasari S, Walrand S, Short KR, Johnson ML, Robinson MM, Schimke JM, Jakaitis DR, Asmann YW, Sun Z, Nair KS. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab. 2012;16(6):777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E; CALERIE Pennington Team . Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4(3):e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochon J, Bales CW, Ravussin E, Redman LM, Holloszy JO, Racette SB, Roberts SB, Das SK, Romashkan S, Galan KM, Hadley EC, Kraus WE, Group CS; CALERIE Study Group . Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci. 2011;66(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redman LM, Kraus WE, Bhapkar M, Das SK, Racette SB, Martin CK, Fontana L, Wong WW, Roberts SB, Ravussin E, Group CS; CALERIE Study Group . Energy requirements in nonobese men and women: results from CALERIE. Am J Clin Nutr. 2014;99(1):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E, Pieper C, DeLany JP, Kraus WE, Rochon J, Redman LM, Group CS; CALERIE Study Group . Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. Am J Physiol Endocrinol Metab. 2012;302(4):E441–E448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR, Look AARG; Look AHEAD Adipose Research Group . Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring). 2006;14(1):73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergström J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35(7):609–616. [PubMed] [Google Scholar]
- 18.Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA. 2007;104(3):1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conley KE, Amara CE, Bajpeyi S, Costford SR, Murray K, Jubrias SA, Arakaki L, Marcinek DJ, Smith SR. Higher mitochondrial respiration and uncoupling with reduced electron transport chain content in vivo in muscle of sedentary versus active subjects. J Clin Endocrinol Metab. 2013;98(1):129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54(7):1926–1933. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuehn H, Liberzon A, Reich M, Mesirov JP. Using GenePattern for gene expression analysis. Curr Protoc Bioinformatics. 2008; Chapter 7:Unit 7.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1(6):417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revelle W. Procedures for personality and psychological research, Northwestern University, Evanston, IL: https://CRAN.R-project.org/package=psych Version = 1.6.9. Accessed October 12, 2016. [Google Scholar]
- 25.Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab. 2010;298(1):E117–E126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparks LM, Redman LM, Conley KE, Harper ME, Hodges A, Eroshkin A, Costford SR, Gabriel ME, Yi F, Shook C, Cornnell HH, Ravussin E, Smith SR. Differences in mitochondrial coupling reveal a novel signature of mitohormesis in muscle of healthy individuals. J Clin Endocrinol Metab. 2016;jc20162742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E; Pennington CALERIE Team . Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92(3):865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson ML, Distelmaier K, Lanza IR, Irving BA, Robinson MM, Konopka AR, Shulman GI, Nair KS. Mechanism by which caloric restriction improves insulin sensitivity in sedentary obese adults. Diabetes. 2016;65(1):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanza IR, Nair KS. Mitochondrial function as a determinant of life span. Pflugers Arch. 2010;459(2):277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heerdt BG, Houston MA, Wilson AJ, Augenlicht LH. The intrinsic mitochondrial membrane potential (Deltapsim) is associated with steady-state mitochondrial activity and the extent to which colonic epithelial cells undergo butyrate-mediated growth arrest and apoptosis. Cancer Res. 2003;63(19):6311–6319. [PubMed] [Google Scholar]
- 31.Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34(12):809–824. [DOI] [PubMed] [Google Scholar]
- 32.Dubé J, Goodpaster BH. Assessment of intramuscular triglycerides: contribution to metabolic abnormalities. Curr Opin Clin Nutr Metab Care. 2006;9(5):553–559. [DOI] [PubMed] [Google Scholar]
- 33.Amati F, Dubé JJ, Shay C, Goodpaster BH. Separate and combined effects of exercise training and weight loss on exercise efficiency and substrate oxidation. J Appl Physiol (1985). 2008;105(3):825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moro C, Bajpeyi S, Smith SR. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab. 2008;294(2):E203–E213. [DOI] [PubMed] [Google Scholar]
- 35.Amara CE, Marcinek DJ, Shankland EG, Schenkman KA, Arakaki LS, Conley KE. Mitochondrial function in vivo: spectroscopy provides window on cellular energetics. Methods. 2008;46(4):312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conley KE, Esselman PC, Jubrias SA, Cress ME, Inglin B, Mogadam C, Schoene RB. Ageing, muscle properties and maximal O(2) uptake rate in humans. J Physiol. 2000;526(Pt 1):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(Pt 1):203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. [DOI] [PubMed] [Google Scholar]
- 39.Richter C. Reactive oxygen and DNA damage in mitochondria. Mutat Res. 1992;275(3-6):249–255. [DOI] [PubMed] [Google Scholar]
- 40.Sastre J, Pallardó FV, Viña J. The role of mitochondrial oxidative stress in aging. Free Radic Biol Med. 2003;35(1):1–8. [DOI] [PubMed] [Google Scholar]
- 41.Mason SA, Morrison D, McConell GK, Wadley GD. Muscle redox signalling pathways in exercise: role of antioxidants. Free Radic Biol Med. 2016;98:29–45. [DOI] [PubMed] [Google Scholar]