Abstract
Context:
Although sex hormone–binding globulin (SHBG) and testosterone (T) have been inversely associated with risk of diabetes, few studies have examined dihydrotestosterone (DHT), a more potent androgen than T, in older adults, whose glycemic pathophysiology differs from younger adults.
Objective:
To determine the associations of SHBG, T, and DHT with insulin resistance and incident diabetes in older adult men.
Design:
In a prospective cohort study, we evaluated baseline levels of SHBG, T, and DHT using liquid chromatography–tandem mass spectrometry among 852 men free of diabetes and cardiovascular disease in the Cardiovascular Health Study in 1994.
Main Outcome:
Insulin resistance estimated by Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) and insulin sensitivity estimated by the Gutt index in 1996, and incident diabetes (n = 112) ascertained over a mean follow-up of 9.8 years.
Results:
In linear regression models adjusted for demographics, alcohol consumption, current smoking, body mass index, and other androgens, SHBG [HOMA-IR 0.30 units lower per doubling; 95% confidence interval (CI), 0.08 to 0.52; P = 0.01] and total DHT (HOMA-IR 0.18 units lower per doubling; 95% CI, 0.06 to 0.30; P = 0.01), but not free T (P = 0.33), were inversely associated with insulin resistance. In corresponding Cox proportional hazards models, total DHT was again inversely associated with risk of diabetes (adjusted hazard ratio per doubling, 0.69; 95% CI, 0.52 to 0.92; P = 0.01), but SHBG (hazard ratio, 1.09; 95% CI, 0.74 to 1.59; P = 0.66) and free T (hazard ratio, 1.15; 95% CI, 0.92 to 1.43; P = 0.23) were not.
Conclusions:
Among older men, higher levels of DHT were inversely associated with insulin resistance and risk of diabetes over the ensuing 10 years, whereas levels of T were not. Future studies are still needed to clarify the role of SHBG in risk of diabetes in this population.
Précis: In a cohort study of 852 older men, SHBG and dihydrotestosterone, but not free testosterone, were inversely associated with HOMA-IR. Only dihydrotestosterone was inversely associated with diabetes risk.
Much like the epidemic of diabetes in younger populations, both the incidence and prevalence of diabetes have risen in older adults in recent years (1). Despite this growing problem, potential causes and mechanisms of diabetes have yet to be fully elucidated.
Recently, interest has grown in the relationship between sex steroid hormone levels and diabetes. To date, several studies have looked at sex hormone–binding globulin (SHBG), a circulating binding protein produced by the liver that has binding sites for sex steroid hormones, including testosterone (T), dihydrotestosterone (DHT), and estradiol, in both men and women. For example, the Massachusetts Male Aging Study, a sample of 1156 men aged 40 to 70 years, found odds ratios for incident diabetes of 1.58 for a 1 standard deviation decrease in free (unbound) T (4 ng/dL) and 1.89 for SHBG (16 nmol/L) (2). Similarly, in middle-aged Finnish men, the lowest quartiles of total T, free T, and SHBG were associated with odds ratios of 2.3, 1.7, and 4.3, respectively, for developing diabetes (3). In a nested case-control study from the Multiple Risk Factor Intervention Trial studying men aged 35 to 57 years, lower levels of total and free T and SHBG were significantly associated with incident diabetes; however, these associations were weakened with adjustment for initial glucose and body mass index (BMI) (4).
Despite this reasonably consistent evidence on SHBG and T, few studies have examined DHT, which is produced from conversion of T by 5-α-reductase and a more potent androgen than T. This partly reflects difficulty in measuring DHT levels, which are approximately 10-fold lower than circulating T levels. Furthermore, most studies have focused on middle-aged adults, with limited data on older adults. Because younger and older adults differ in their pathophysiologic risk factors for diabetes, risk factors for younger and middle-aged adults may differ in their associations with diabetes in older adults (5–11).
In our previous analyses of older men free of cardiovascular disease (CVD) in the Cardiovascular Health Study (CHS), total and free T were not associated with incident CVD or all-cause mortality, whereas total DHT and calculated free DHT had nonlinear associations with both outcomes, suggesting that DHT—as a more potent androgen than T—may have particularly strong associations with outcomes (12). Therefore, we studied the relationships between T, SHBG, and DHT with incident risk of diabetes in older men. Based on previous studies, we hypothesized that higher levels of these androgens would be associated with less insulin resistance and lower risk of diabetes (13–16).
Methods
Participants
The CHS is a multicenter, longitudinal study that began in 1989 to examine risk factors for CVD (including diabetes) in older adults (17). Participants were recruited from 4 sites (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania). Requirements for eligibility included age ≥65, being noninstitutionalized, and expecting to stay in the area for at least 3 years. In 1989 to 1990, 5201 participants enrolled, and in 1992 to 1993, 687 additional African Americans were recruited into the study. Clinic examinations alternated with telephone calls every 6 months for the first 10 years; apart from a clinic visit in 2005, telephone calls were continued semiannually thereafter. The study sample consisted of men participating in the CHS 1994 clinical examination who had no history of CVD (myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, heart failure, or stroke) and no history of diabetes or insulin resistance at that visit. The study was approved by institutional review boards at each field center and the CHS Coordinating Center. All participants provided written informed consent.
Hormone levels and assays
Blood work for these analyses was drawn from nonfasting men at the 1994 CHS annual clinic examination. The time of day of collection was not reliably recorded; however, most samples were collected before 12:00 pm. The frozen serum samples were then transported to the CHS Central Laboratory in Vermont, where they were stored at −70°C. In 2010, frozen samples were shipped on dry ice to the VA Puget Sound Health Care System (A.M.M.) for androgen assays.
All assays were conducted in duplicate, and the average value was used in these analyses. Total T and DHT were measured simultaneously using a liquid chromatography–tandem mass spectrometry assay (18). The lower limit of detection for total T was 1.0 ng/dL, with an intra-assay coefficient of variation (CV) of 4.9% and an interassay CV of 5.1%. The CDC Hormone Standardization Program certified the assay. The lower limit of detection for DHT was 0.02 ng/mL, with an intra-assay CV of 5.9% and an interassay CV of 6.2%. SHBG was assayed using a commercial radioimmunoassay kit with a time-resolved fluoroimmunoassay (Delfia; Perkin Elmer, Norton, OH). The lower limit of detection for SHBG was 0.5 nmol/L, with an intra-assay CV of 1.4% and an interassay CV of 6.6% at 31 nmol/L.
We calculated free T by the Mazer method (19). We used the Mazer formula because it allowed calculation of both free T and free DHT. In a sample of 74 participants who had longitudinal serum samples available from 1989, 1994, and 1996, the intraclass correlation coefficients for 3 hormone measures over a 7-year period were 0.82 for total T and 0.79 for total DHT, confirming their stability over time.
Outcomes
Our primary outcomes were incident diabetes and insulin resistance. Diabetes was ascertained using fasting glucose measurements, incident use of hypoglycemic medications, and Medicare claims data for inpatient (hospital, skilled nursing facility, or home health) and outpatient (outpatient or carrier) health care services. Glucose was measured on blood samples drawn in 1994 (nonfasting), 1996, 1998, and 2005, and a diagnosis of diabetes was given for fasting levels >125 mg/dL or nonfasting levels >199 mg/dL. Medications were reviewed at yearly visits (by telephone or in person), and those using hypoglycemic medications were also deemed to have diabetes (20). Finally, Medicare claims were reviewed for billing codes for diabetes (International Classification of Diseases, Ninth Revision 250.xx); a diagnosis of diabetes required at least 2 inpatient or 3 outpatient or (≥1 inpatient and ≥1 outpatient) Medicare claims during a 2-year time period, which provides a positive predictive value of ~90% in the CHS based on fasting glucose measurements conducted in 1992.
For measurement of insulin resistance, we used data collected at the 1996 CHS visit, at which measures of glucose and insulin were available in both the fasting state and 2 hours after a 75-g oral glucose tolerance test. We estimated insulin resistance with both Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) (21), which relies on fasting measures, and the Gutt index (22), which also includes postload measures.
Covariates
Methods for data collection and protocols have been previously documented (12). The covariates were determined by known risk factors for diabetes. Most covariates, including site, age, sex, alcohol consumption, smoking status, weight, and waist circumference, were measured at the 1994 visit. Some covariates, including height, lipids, and estimated glomerular filtration rate-cystatin, were measured at the 1992 visit.
Statistical analysis
We provide descriptive characteristics according to tertiles of total DHT with corresponding tests of trend and Spearman correlation coefficients among all measured and calculated androgens (Supplemental Table 1 (14.6KB, docx) ). To determine the association of androgens with insulin resistance and sensitivity, we used linear regression models adjusting initially for age, sex, race, clinic site, alcohol consumption, current smoking, and BMI and then mutually adjusted for other hormones. In most cases, we present results for SHBG, free T, and total DHT; we also examined total T and free DHT in sensitivity analyses. We explored the functional form for all 3 androgens using general additive model plots; in all cases, transformation to the log2 appeared reasonably linear, and we therefore present increases or decreases in HOMA-IR or the Gutt index per doubling of each androgen.
We used the Cox proportional hazards model to evaluate the association of androgens with incident diabetes. We present adjusted hazard ratios and 95% confidence intervals using models adjusted for the same covariates as in the linear regression models previously described. To ensure the robustness of our results, we also tested further adjustment for waist circumference, lipids, and estimated glomerular filtration rate-cystatin.
Where androgens were associated with risk of diabetes, we tested for interactions with multiplicative cross-product terms by age (dichotomized at age 75 years), race, and BMI (dichotomized at 25 kg/m2); where interactions were present, we conducted stratified analyses as previously mentioned.
Results
Baseline characteristics
Of the 1922 men who attended the 1994 CHS visit, we excluded 794 with prevalent CVD, 200 with prevalent diabetes, 69 with unknown diabetes status, and 7 without follow-up beyond 1994, leaving 852 men in the analytic sample. Table 1 presents the characteristics of these participants according to tertiles of total DHT. As expected, BMI levels were lower among individuals with higher levels of total DHT, with little difference by age, race, or health status. We observed the expected positive correlations of total androgens with each other and with SHBG (Supplemental Table 1 (14.6KB, docx) ).
Table 1.
Baseline Characteristics According to Tertile of DHT
| Characteristic | Total DHT,
ng/mL |
P trend | ||
|---|---|---|---|---|
| ≤0.35 | >0.35−0.52 | >0.52 | ||
| n | 285 | 292 | 275 | |
| Total dihydrotestosterone, ng/dLa | 0.2 ± 0.1 | 0.4 ± 0.0 | 0.7 ± 0.2 | <0.001 |
| Free dihydrotestosterone, ng/dLa | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.2 | <0.001 |
| SHBG, nmol/L | 54 ± 27 | 65 ± 22 | 80 ± 30 | <0.001 |
| Total testosterone, ng/dL | 270 ± 135 | 398 ± 101 | 532 ± 177 | <0.001 |
| Free testosterone, ng/dL | 4.0 ± 2.0 | 5.1 ± 1.3 | 5.9 ± 2.3 | <0.001 |
| Age, years | 77 ± 5 | 76 ± 5 | 76 ± 5 | 0.24 |
| African American, % | 14 | 10 | 15 | 0.68 |
| BMI, kg/m2 | 27.6 ± 3.7 | 26.4 ± 3.5 | 25.2 ± 3.2 | <0.001 |
| Systolic blood pressure, mm Hg | 134 ± 20 | 133 ± 19 | 129 ± 19 | 0.001 |
| Triglycerides, mg/dL | 149 ± 85 | 127 ± 62 | 110 ± 51 | <0.001 |
| High-density lipoprotein cholesterol, mg/dL | 47 ± 11 | 48 ± 13 | 50 ± 11 | 0.001 |
| Low-density lipoprotein cholesterol, mg/dL | 116 ± 31 | 117 ± 31 | 117 ± 32 | 0.80 |
| Fasting glucose, mg/dL | 103.3 ± 17.1 | 100.2 ± 18.3 | 98.9 ± 16.1 | 0.002 |
| Fasting insulin, IU/mLb | 15.1 ± 6.6 | 13.2 ± 7.8 | 12.3 ± 4.9 | <0.001 |
| Physical activity, kcal/wk | 1860 ± 1811 | 1969 ± 2000 | 1931 ± 1929 | 0.66 |
| eGFR-cystatin, mL/min/1.73 m2 | 72 ± 17 | 72 ± 16 | 75 ± 17 | 0.05 |
| Current smoker, % | 8 | 11 | 10 | 0.48 |
| Antihypertensives, % | 51 | 42 | 38 | 0.002 |
| Lipid-lowering medication, % | 6 | 5 | 3 | 0.19 |
| ≥7 alcoholic drinks per week, % | 21 | 22 | 16 | 0.17 |
| Self-reported health good/excellent, % | 40 | 40 | 41 | 0.91 |
Variables are shown as mean ± SD or as otherwise indicated.
Abbreviation: eGFR-cystatin, estimated glomerular filtration rate by cystatin.
Calculated.
Measured at 1996 examination.
Androgens and insulin sensitivity/prevalent insulin resistance
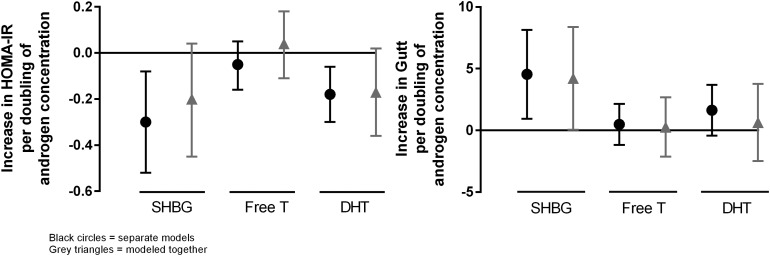
In Fig. 1, we present the associations of SHBG, free T, and total DHT with HOMA-IR and the Gutt index of insulin sensitivity. Free T was not associated with either index in any of the models (P ≥ 0.33).
Figure 1.
The left panel shows the change in HOMA-IR per doubling of androgen concentration (n = 791), and the right panel shows the change in Gutt index per doubling of androgen concentration (n = 670). Models were adjusted for demographics, alcohol consumption, smoking status, and BMI. The black circles represent separate models, and the gray triangles represent models modeled together.
Both SHBG and total DHT were inversely associated with HOMA-IR when adjusted for demographics, alcohol consumption, smoking status, and BMI (P = 0.01 for both). With further adjustment for each other, the coefficient for total DHT was virtually unchanged but estimated less precisely with wider confidence intervals (P = 0.08). In contrast, the association with SHBG was attenuated by approximately one-third (P = 0.11).
These findings were similar but not identical for Gutt-estimated insulin sensitivity, which was estimated in ~120 fewer individuals. SHBG was associated with better insulin sensitivity, both initially (P = 0.01) and with mutual adjustment (P = 0.05). Total DHT was associated only with a trend toward better insulin sensitivity initially (P = 0.12) and was attenuated by ~60% with mutual adjustment (P = 0.69) in this subset of participants. The significant association of total DHT with HOMA-IR persisted even when restricted to the smaller subset of participants with available Gutt measurements.
Androgens and incident diabetes
Over a median of 9.8 years, we ascertained 112 incident cases of diabetes, representing 13.86 cases per 1,000 person years. Table 2 provides adjusted hazard ratios for risk of incident diabetes per doubling of each androgen. As with insulin resistance, we observed no statistically significant relationship between free T levels and risk of diabetes in any model. Total T was also examined in sensitivity analyses and was not associated with risk of diabetes (Supplemental Table 2 (14.6KB, docx) ).
Table 2.
Associations Between Androgens and Risk of Incident Diabetes
| Model Number | Hazard Ratio
(95% confidence intervals) Per Doubling of Androgen
Concentration |
||
|---|---|---|---|
| SHBG | Free T | Total DHT | |
| Model 1 | 0.88 (0.62–1.24) | 0.95 (0.83–1.09) | 0.79 (0.67–0.94) |
| Model 2 | 1.09 (0.74–1.59) | 1.15 (0.92–1.43) | 0.69 (0.52–0.92) |
Model 1 adjusts for age, race, site, alcohol consumption, current smoking status, and BMI, and model 2 adjusts for model 1 covariates and the other androgens.
Concordant with our results on insulin resistance, higher total DHT was strongly associated with 21% lower risk of diabetes in initial models (P = 0.009). Further adjustment for waist circumference, lipids, and kidney function did not meaningfully attenuate this result (adjusted hazard ratio, 0.82; 95% confidence interval, 0.68 to 0.98; P = 0.03). Similarly, adjustment for other hormones did not attenuate the association (P = 0.01). Analysis of free DHT yielded similar results, showing an association between higher levels of free DHT and lower risk of diabetes even with adjustment for waist circumference, lipids, and kidney function (Supplemental Table 2 (14.6KB, docx) ). In contrast with both total and free DHT, SHBG was not associated with significantly lower risk of diabetes in initial models (P = 0.46) or with adjustment for other androgens (P = 0.66).
Given the inverse association of total DHT with risk of diabetes, we examined interaction with age, BMI, and race. Although we observed no interaction with age and race (data not shown), there was a borderline interaction with BMI >25 kg/m2 (P = 0.04). Among men with lower BMI, total DHT was unassociated with risk (adjusted hazard ratio, 1.08; 95% confidence interval, 0.72 to 1.62; P = 0.72). Among overweight and obese men, total DHT was particularly strongly associated with lower risk (adjusted hazard ratio, 0.69; 95% confidence interval, 0.57 to 0.85; P < 0.001).
Discussion
In this cohort of older men free of CVD and diabetes, baseline levels of DHT were strongly associated with lower risk of diabetes and with less insulin resistance by HOMA-IR. In contrast, we found no statistically significant relationship of SHBG levels or T levels with risk of diabetes in this cohort.
To our knowledge, this is the first study showing an association between low levels of DHT and risk of developing diabetes, in part because of the technical difficulty of measuring the relatively low levels of DHT in circulation. In contrast with most previous studies, our cohort excluded men with known CVD. Despite this, overall, our results are consistent with previous literature showing a relationship between DHT and insulin resistance (23). Mather et al. (23) found an inverse association between both T and DHT and fasting glucose levels in middle-aged men. Furthermore, Vandenput et al. (15) demonstrated that DHT levels were negatively associated with insulin resistance and fat mass in an elderly male cohort. Of note, the 5α-reductase 1 and 2 inhibitor dutasteride increases hepatic insulin resistance and hepatic lipid accumulation, strongly implicating low DHT as a causal risk factor for diabetes (24).
Although our results show an inverse relationship with DHT levels and insulin resistance based on HOMA-IR modeling, we did not observe a statistically significant association between higher levels of DHT and better insulin sensitivity based on the Gutt model. In our Gutt model, higher DHT exhibited a slight trend toward better insulin sensitivity that was further attenuated when adjusted for the other hormones. This may reflect the play of chance among the smaller cohort used for Gutt modeling (n = 670 for Gutt vs n = 791 for HOMA-IR). Alternatively, DHT may affect hepatic insulin resistance (as reflected in HOMA-IR) to a greater degree than peripheral insulin sensitivity (as reflected in the Gutt index). The effect of complete 5α-reductase inhibition on hepatic insulin resistance concords with this hypothesis. Regardless of the mechanism, DHT was clearly and strongly associated with lower risk of incident diabetes.
Previous work regarding associations between SHBG with insulin resistance and risk of developing diabetes has been inconsistent. For example, some studies have shown an association between low SHBG and increased risk of diabetes (16), whereas others have not (23). Notably, we did observe an association between low SHBG levels and increased insulin resistance; however, this did not extend to the relationship between low SHBG levels and risk of diabetes.
Interestingly, we were unable to show an association between T levels and risk of diabetes or insulin resistance. This result is consistent with some previous data, such as Vandenput et al. (15). On the other hand, other previous work, including National Health and Nutrition Examination Survey III (25), has found an association in the general male population between low levels of free T and diabetes. Because DHT was not measured in most studies of T itself, and given the correlation of DHT with T, our results suggest that the observed relationship of T with glycemia in previous studies (at least in older men) may reflect DHT rather than T per se.
Several mechanisms have been proposed to explain the links between androgens and both insulin resistance and diabetes. For example, Vandenput et al. (15) clearly showed an association between both DHT and T with fat mass in both young male and elderly male cohorts. Experimental studies in male mice lacking the androgen receptor support a role for androgens in late-life fat accumulation (26). Together, these data suggest that androgens reduce fat mass; thus, higher levels of androgens protect against insulin resistance and reduce risk of diabetes. On the other hand, studies such as National Health and Nutrition Examination Survey III and ours have associated androgens with lower risk of diabetes independent of adiposity, implying that androgens may independently influence insulin resistance (25). Other potential mechanisms include effects of androgen on skeletal muscle insulin resistance, β-cell function, or central energy regulation (27). Further studies are necessary to clarify this relationship and the mechanisms by which androgens may prevent diabetes.
Although this study shows an inverse association between DHT and risk of developing diabetes, we acknowledge several limitations. Our data come from an elderly male cohort and thus may not generalize to a younger male population; strong evidence also suggests that androgens have different effects on glucose metabolism in men and women (28). Further, there is a growing body of evidence that estrogen levels also impact insulin resistance, and our study lacks data regarding estrogen levels. Our DHT levels are limited to 1 sample draw and, given diurnal variations, would have been strengthened by multiple timed draws within a given day or repeated over time. Moreover, time of day for blood collection was not recorded; however, most samples (65%) were collected before 12:00 pm and were likely fasting. The lack of a standardized blood sampling time may introduce nondifferential measurement error that could bias the results toward the null. However, the circadian fluctuation in T levels is blunted in older men, and DHT levels do not exhibit significant circadian variation (29).
In this study of older men, we found a clear association of low DHT levels with higher insulin resistance and higher risk of diabetes. This negative association remained even after adjusting for covariates known to contribute to diabetes risk and modifiers such as binding proteins (SHBG). More research is needed to further elucidate the mechanisms by which DHT may be modifying diabetes risk and implications for potential androgen therapy.
Acknowledgments
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295, R01HL094555, and R01HL091952 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurologic Disorders and Stroke. Additional support was provided by R01AG023629 from the National Institute on Aging, the Veterans Affairs Research Service, the Veterans Affairs Epidemiology Research and Information Center, and the Veterans Affairs Geriatric Research, Education and Clinical Center. A full list of principal CHS investigators and institutions can be found at www.chs-nhlbi.org.
Acknowledgments
Disclosure Summary: A.M.M. has received research support from AbbVie, GSK, and BHR Pharmaceuticals and has served on advisory boards for AbbVie, Endo, Ligand, and Lipocine Pharmaceuticals. The remaining authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CHS
- Cardiovascular Health Study
- CI
- confidence interval
- CV
- coefficient of variation
- CVD
- cardiovascular disease
- DHT
- dihydrotestosterone
- HOMA-IR
- Homeostatic Model Assessment of Insulin Resistance
- SHBG
- sex hormone–binding globulin
- T
- testosterone.
References
- 1.Kramarow E, Lubitz J, Lentzner H, Gorina Y. Trends in the health of older Americans, 1970-2005. Health Aff (Millwood). 2007;26(5):1417–1425. [DOI] [PubMed] [Google Scholar]
- 2.Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23(4):490–494. [DOI] [PubMed] [Google Scholar]
- 3.Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27(5):1036–1041. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, Shaten J, Stern MP, Smith GD, Kuller L. Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;143(9):889–897. [DOI] [PubMed] [Google Scholar]
- 5.Borkan GA, Hults DE, Gerzof SG, Robbins AH. Comparison of body composition in middle-aged and elderly males using computed tomography. Am J Phys Anthropol. 1985;66(3):289–295. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher D, Ruts E, Visser M, Heshka S, Baumgartner RN, Wang J, Pierson RN, Pi-Sunyer FX, Heymsfield SB. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279(2):E366–E375. [DOI] [PubMed] [Google Scholar]
- 7.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB.. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985). 2001;90(6):2157–2165. [DOI] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26(2):372–379. [DOI] [PubMed] [Google Scholar]
- 9.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. [DOI] [PubMed] [Google Scholar]
- 10.Meneilly GS. Diabetes in the elderly. Med Clin North Am. 2006;90(5):909–923. [DOI] [PubMed] [Google Scholar]
- 11.Scheen AJ. Diabetes mellitus in the elderly: insulin resistance and/or impaired insulin secretion? Diabetes Metab. 2005;31 Spec No 2:5S27–5S34. [DOI] [PubMed] [Google Scholar]
- 12.Shores MM, Biggs ML, Arnold AM, Smith NL, Longstreth WT Jr, Kizer JR, Hirsch CH, Cappola AR, Matsumoto AM. Testosterone, dihydrotestosterone, and incident cardiovascular disease and mortality in the cardiovascular health study. J Clin Endocrinol Metab. 2014;99(6):2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361(12):1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vikan T, Schirmer H, Njølstad I, Svartberg J. Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type 2 diabetes in men. Eur J Endocrinol. 2010;162(4):747–754. [DOI] [PubMed] [Google Scholar]
- 15.Vandenput L, Mellström D, Lorentzon M, Swanson C, Karlsson MK, Brandberg J, Lönn L, Orwoll E, Smith U, Labrie F, Ljunggren O, Tivesten A, Ohlsson C. Androgens and glucuronidated androgen metabolites are associated with metabolic risk factors in men. J Clin Endocrinol Metab. 2007;92(11):4130–4137. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Zhang A, Yang S, Wang Y, Goswami R, Zhou H, Zhang Y, Wang Z, Li R, Cheng Q, Zhen Q, Li Q. Combined effects of sex hormone-binding globulin and sex hormones on risk of incident type 2 diabetes. J Diabetes. 2016;8(4):508–515. [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. [DOI] [PubMed] [Google Scholar]
- 18.Kalhorn TF, Page ST, Howald WN, Mostaghel EA, Nelson PS. Analysis of testosterone and dihydrotestosterone from biological fluids as the oxime derivatives using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(19):3200–3206. [DOI] [PubMed] [Google Scholar]
- 19.Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74(6):512–519. [DOI] [PubMed] [Google Scholar]
- 20.Smith NL, Psaty BM, Heckbert SR, Tracy RP, Cornell ES. The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol. 1999;52(2):143–146. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 22.Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, Schneiderman N, Skyler JS, Marks JB. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000;47(3):177–184. [DOI] [PubMed] [Google Scholar]
- 23.Mather KJ, Kim C, Christophi CA, Aroda VR, Knowler WC, Edelstein SE, Florez JC, Labrie F, Kahn SE, Goldberg RB, Barrett-Connor E; Diabetes Prevention Program . Steroid sex hormones, sex hormone-binding globulin, and diabetes incidence in the diabetes prevention program. J Clin Endocrinol Metab. 2015;100(10):3778–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazlehurst JM, Oprescu AI, Nikolaou N, Di Guida R, Grinbergs AE, Davies NP, Flintham RB, Armstrong MJ, Taylor AE, Hughes BA, Yu J, Hodson L, Dunn WB, Tomlinson JW. Dual-5α-reductase inhibition promotes hepatic lipid accumulation in man. J Clin Endocrinol Metab. 2016;101(1):103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, Dobs A, Basaria S, Golden SH, Platz EA. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) [published correction appears in Diabetes Care. 2007;30(6):1683]. Diabetes Care. 2007;30(2):234–238. [DOI] [PubMed] [Google Scholar]
- 26.Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54(4):1000–1008. [DOI] [PubMed] [Google Scholar]
- 27.Navarro G, Allard C, Xu W, Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring). 2015;23(4):713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–1299. [DOI] [PubMed] [Google Scholar]
- 29.Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94(3):907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]