Abstract
Context:
Heterozygous mutations in the aggrecan gene (ACAN) cause autosomal dominant short stature with accelerated skeletal maturation.
Objective:
We sought to characterize the phenotypic spectrum and response to growth-promoting therapies.
Patients and Methods:
One hundred three individuals (57 females, 46 males) from 20 families with autosomal dominant short stature and heterozygous ACAN mutations were identified and confirmed using whole-exome sequencing, targeted next-generation sequencing, and/or Sanger sequencing. Clinical information was collected from the medical records.
Results:
Identified ACAN variants showed perfect cosegregation with phenotype. Adult individuals had mildly disproportionate short stature [median height, −2.8 standard deviation score (SDS); range, −5.9 to −0.9] and a history of early growth cessation. The condition was frequently associated with early-onset osteoarthritis (12 families) and intervertebral disc disease (9 families). No apparent genotype–phenotype correlation was found between the type of ACAN mutation and the presence of joint complaints. Childhood height was less affected (median height, −2.0 SDS; range, −4.2 to −0.6). Most children with ACAN mutations had advanced bone age (bone age − chronologic age; median, +1.3 years; range, +0.0 to +3.7 years). Nineteen individuals had received growth hormone therapy with some evidence of increased growth velocity.
Conclusions:
Heterozygous ACAN mutations result in a phenotypic spectrum ranging from mild and proportionate short stature to a mild skeletal dysplasia with disproportionate short stature and brachydactyly. Many affected individuals developed early-onset osteoarthritis and degenerative disc disease, suggesting dysfunction of the articular cartilage and intervertebral disc cartilage. Additional studies are needed to determine the optimal treatment strategy for these patients.
Heterozygous ACAN mutations cause short stature with bone age acceleration and premature growth cessation and are frequently associated with early-onset osteoarthritis and degenerative disc disease.
Longitudinal bone growth occurs at the growth plate and is the result of proliferation, hypertrophy, and matrix production by chondrocytes. This process, chondrogenesis, requires that growth plate chondrocytes integrate a complex network of signals from endocrine and paracrine systems and inputs from cell–cell and cell–matrix interactions. Consequently, growth failure can be caused by mutations in any 1 of the many genes that directly or indirectly affect the growth plate chondrocytes and the process of growth plate chondrogenesis (1). It is thus not surprising that the molecular cause of short stature remains undiagnosed in a large fraction of affected children. Recent implementation of exome sequence analysis, however, has started to identify genetic diagnoses in subgroups of patients with idiopathic short stature, including heterozygous mutations in the aggrecan gene (ACAN) causing autosomal dominant short stature with advanced bone age (BA) (2, 3). ACAN mutations are sometimes associated with early-onset osteoarthritis (OA) but no, or only minor, skeletal findings suggestive of skeletal dysplasia (2–4).
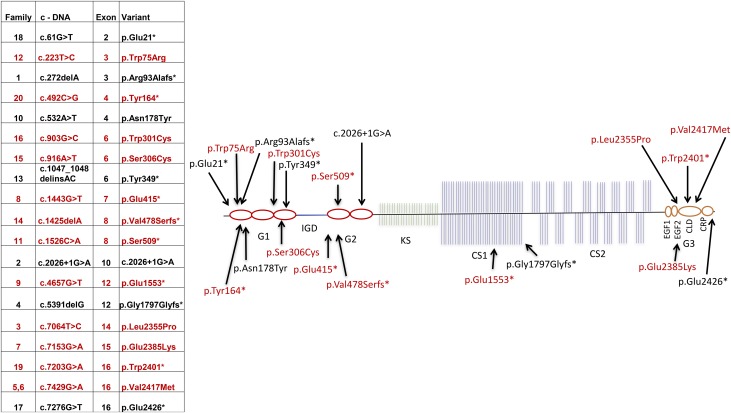
Aggrecan is a proteoglycan and important component of extracellular matrix, critical to the structure and function of growth plate cartilage and other cartilaginous tissues (5). The aggrecan core protein consists of a large, centrally located glycosaminoglycan attachment region flanked by 2 N-terminal and 1 C-terminal globular domains (G1 to G3; Fig. 1). Aggrecan forms large proteoglycan aggregates by interaction of the G1 domain with hyaluronan and cartilage link protein. The G2 domain is highly conserved during evolution, but its biological function is so far unknown. In contrast, the G3 domain contains a C-type lectin-binding domain (CLD) critical to the interaction with other extracellular proteoglycans, including tenascins and fibulins (4, 6) (Fig. 1).
Figure 1.
Structure of ACAN and the locations of pathogenic sequence variants. Aggrecan consists of a core protein, including 3 globular domains (G1 to G3), with the G3 domain including a C-type lectin-binding (CLD) multifunctional domain, plus 4 interglobular domains, a keratan-sulfate (KS), and 2 chondroitin-sulfate (CS1, CS2) attachment domains. The G1 region is encoded by exons 3 to 6, the interglobular domain region by exon 7, the G2 region by exons 8 to 10, the glycosaminoglycan (GAG; KS-CS1-CS2) attachment region by exons 11 to 12, and the G3 region by exons 13 to 19. The tabular data show the location of the sequence variant found in each family and the predicted change in amino acid sequence. The mutation in family 2 involved a splice site. The asterisk indicates a stop codon; and the red font, families with joint disease. fs, frame shift.
Since our 2014 report of heterozygous ACAN mutations in patients with short stature and advanced BA (2), multiple new families with this condition have been identified. To better characterize the phenotypic spectrum, associated conditions, and response to growth-promoting therapies in individuals with heterozygous ACAN mutations, an international cohort of 103 individuals from 20 different families with heterozygous ACAN mutations has been assembled and are reported in the present study.
Patients and Methods
Subjects
The institutional review board of Cincinnati Children’s Medical Center (Cincinnati, OH), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Bethesda, MD), Charles University in Prague and University Hospital in Motol (Prague, Czech Republic), Hospital Universitario La Paz (Madrid, Spain), and regional ethical review board of Umeå University (Umeå, Sweden) and Karolinska Institutet (Stockholm, Sweden) approved the present study. All the participants or their legal guardians provided written informed consent. All the subjects’ height and weight data were plotted on the Centers for Disease Control and Prevention growth charts (7), and z-scores for height, weight, and sitting height index (SHI) were calculated using the Third National Health and Nutrition Examination Survey growth data (8). The clinical information was collected on a standardized data form (Supplemental Table 1 (59.7MB, pdf) ) for all subjects. Birth length, birth weight, and head circumference were plotted according to the subject’s week of gestation or age (7, 9). The evaluation of puberty was assessed according to the methods described by Marshall and Tanner (10, 11), and BA was assessed according to the method of Greulich and Pyle (12) by a single reader of the films, who was kept unaware of the age and identification of the individuals. Five of the families with heterozygous ACAN mutations causing either autosomal dominant short stature and advanced BA (families 1 to 4) (2, 3) or familial osteochondritis dissecans (FOD) (family 5) with early-onset OA and disproportional short stature (4, 13) have been previously reported.
Sequencing
ACAN (MIM 155760, NM_013227.3) is located on chromosome 15q26 and encodes aggrecan, which contains 2530 amino acids with a calculated mass of 254 kDa (14) (Fig. 1). The ACAN mutations in families 1 to 5 were previously identified by either exome sequencing or linkage analysis (2–4). The proband of family 6 presented with short stature and early-onset OA and was found to be a carrier of the same mutation as the affected members in family 5. The DNA of additional members of family 6 was subsequently sequenced, confirming that the mutation cosegregated with the FOD phenotype (4, 13). Additional families were identified by Sanger sequencing, exome sequencing, or by targeted next-generation sequencing at various clinical and research laboratories around the world. All candidate variants were confirmed via Sanger sequencing and were shown to segregate with the skeletal phenotype in all available family members.
Variant analysis
None of the variants reported were present in the Exome Aggregation Consortium database. All 7 missense variants were predicted to be damaging by all 4 in silico prediction tools, CADD (complete annotation dependent depletion; available at http://cadd.gs.washington.edu/), SIFT (scale-invariant feature transform), PolyPhen, and MutationTaster (15). The level of conservation of the affected amino acids was determined using GerpN. Protein function was summarized using information found in the UniProt database (available at www.uniprot.org; NP_037359.3).
Results
Cohort characteristics
In total, 103 mutation-positive individuals (57 females, 46 males) from 20 different families with dominantly inherited short stature were identified and included in the present study. Their age ranged from 1.3 to 86 years (median, 15 years), including 33 children (age <18 years; 17 females, 16 males) and 70 adults (age ≥18 years; 40 females, 30 males; Fig. 2; Supplemental Fig. 1 (59.7MB, pdf) ). Most of the probands (18 of 20) were identified in pediatric endocrine or genetics clinics and had been evaluated for short stature with normal endocrine evaluation results and no or only subtle findings on skeletal studies, except for advanced BA. Two of the probands (families 13 and 16) were evaluated for skeletal dysplasia soon after birth. The proband of family 13, owing to prenatally identified proportionate short stature and facial dysmorphism (frontal bossing, midface hypoplasia, anteverted ears, brachydactyly), and the proband of family 16 was identified because of short limbs at birth.
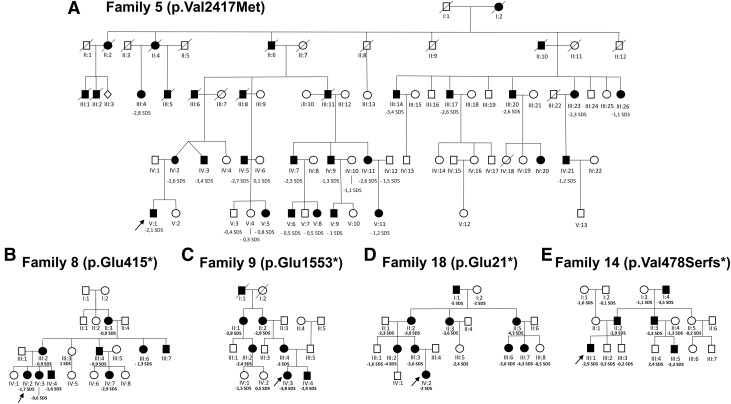
Figure 2.
Pedigrees of the studied families: (A) Family 5. (B) Family 8. (C) Family 9. (D) Family 18. (E) Family 14. The arrows indicate the proband. Individuals carrying heterozygous ACAN mutations are indicated by solid symbols, and unaffected individuals by open symbols. The height SDS of each individual is indicated.
ACAN variants
We identified 19 different ACAN mutations (11 truncating, 7 missense, 1 splice site) in the 20 different pedigrees (Fig. 1; Supplemental Fig. 1 (59.7MB, pdf) ). Five of the pathogenic sequence variants have been reported previously (2–4, 13). In silico analysis predicted that all 7 missense variants were deleterious (Supplemental Table 2 (59.7MB, pdf) ). A second family with FOD due to the p.Val2417Met mutation (4) (family 6) was identified and likely represented a branch of the original FOD family (4) (family 5), because they originated from the same region in northern Sweden. The identified mutations were widely scattered over the gene (Fig. 1).
Heterozygous ACAN mutations affect linear growth and cause adult short stature
Of 33 children, 32 were born at full term. At birth, the affected children tended to have a birth weight SDS and birth length SDS in the lower part of the normal range [median birth weight (n = 29), −0.7 SDS; range, −4.1 to 0.5 SDS; median birth length (n = 29), −1.5 SDS; range, −3.8 to 1.1 SDS; Fig. 3].
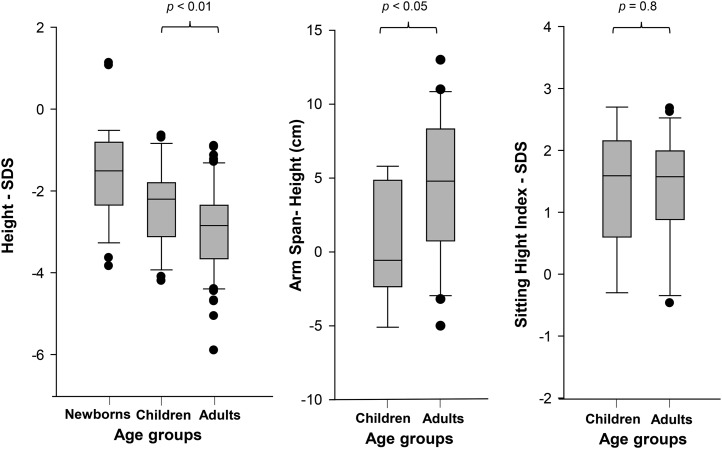
Figure 3.
Height SDS, SHI SDS, and arm span minus height in individuals with heterozygous ACAN mutations at different ages. (A) Height SDS at birth, during childhood, and in adulthood. (B) SHI SDS during childhood and in adults. (C) Arm span minus height during childhood and adulthood.
During childhood [age <10 years (n = 26), 14 females, 12 males], short stature was more pronounced, with heights ranging from low-normal to substantially short [median height (n = 25), −2.0 SDS; range, −4.0 to −0.6 SDS; Figs. 3 and 4; Supplemental Fig. 2 (59.7MB, pdf) ]. Children were also mostly proportionate, with a SHI SDS in the upper part of the normal range [6 of 9 children SHI within ± 2 SDS; median SHI (n = 9), 1.6 SDS; range, −0.3 to 2.7 SDS]. During childhood, the arm span was similar to the height [median arm span − height (n = 9), −0.6 cm; range, −5.1 to 5.8 cm].
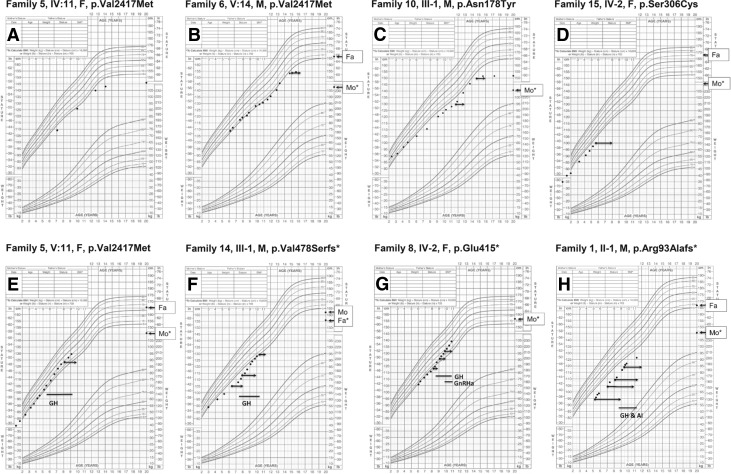
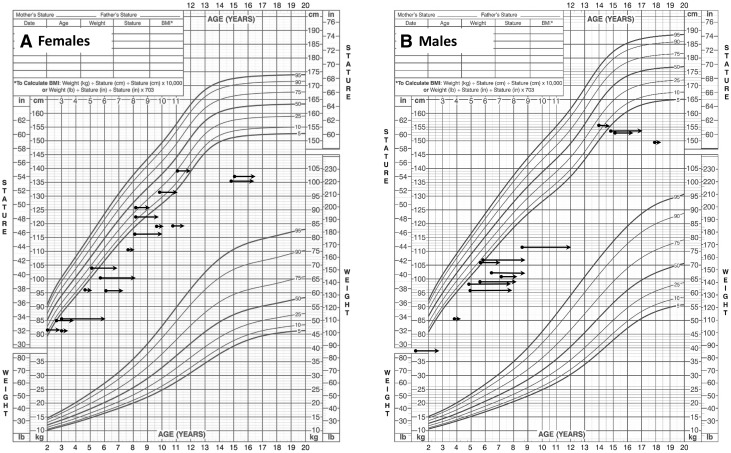
Figure 4.
Growth charts of individuals with heterozygous ACAN mutations. (A) Mother of proband in family 5 (IV:11). (B) ACAN mutation-positive child of family 6 (V:14). (C) Proband of family 10 (III:1). (D) Proband of family 15 (IV:2). (E) ACAN mutation-positive child of family 5 (V:11). (F) Proband of family 14 (III:1). (G) Proband of family 8 (IV:2). (H) Proband of family 1 (II:1). The left end of each arrow represents the subject’s chronological age (CA) and height and the right end, the BA. Lines indicate periods with GH or gonadotropin-releasing hormone analog (GnRHa) or GH and GnRHa or GH and aromatase inhibitor (AI) letrozole treatment. Asterisk indicates parent carrying the mutation. Fa, father’s height; Mo, mother’s height.
Of 31 children with heterozygous ACAN mutations, 20 presented with a BA that was >12 months advanced, and no child had delayed BA [BA − chronological age; median (n = 31), +1.3 years; range, +0.0 to +3.7 years; Fig. 5].
Figure 5.
Height and BA in girls (A) and boys (B) with heterozygous ACAN mutations. BA was assessed according to the method of Greulich and Pyle (12) by a single, blinded, expert reader. The left end of each arrow represents the subject’s chronological age (CA) and the right end represents the BA. Each subject is represented only once on the graph.
Children in 6 families (families 1, 4, 12, 15, 18, and 20) had markedly advanced skeletal maturation (BA, 3 to 4 years advanced); however, the children in the other 14 families had normal or modestly advanced BA (BA, 0 to 2 years advanced; Fig. 5). This relative advancement of BA appears to reflect decreased remaining growth potential as evidenced by the history of early growth cessation in subjects [median age of growth cessation (n = 23), 12 years; range, 9 to 16 years] and/or decreased pubertal growth spurts (Fig. 4). In this cohort, no child presented with delayed BA. This is very different from the cohorts of patients with idiopathic short stature in which average BA delays of ≥2 years have been reported (16, 17).
Consequently, most adult individuals had a more obvious phenotype with short stature [median height (n = 62), −2.9 SDS; range, −5.9 to −0.9 SDS; Figs. 3 and 4] and often with some disproportion. Males (n = 24) and females (n = 38) had a similar degree of growth failure (average height of females, 143.9 cm; −3.0 SDS; average height of males, 156 cm; −2.9 SDS). The average adult height was similar in families with missense and truncating variants (average height, −3.0 SDS; range, −2.4 to −4.5; vs −3.2 SDS; range, −1.0 to −4.3; P = 0.66, respectively). In addition, the variation of adult height in our cohort (adult height SDS, female, 7.0; male, 6.9) was not larger than that of the general population (18). Taken together, these findings suggest that different mutations have a similar effect on final height. In adult individuals, the SHI was commonly at the upper part of the normal range or slightly elevated [median SHI ratio (n = 22), +1.6 SDS; range, −0.5 to 2.7 SDS]. The growth of the upper extremities appeared to be less affected, with arm spans commonly greater than the height [median arm span − height (n = 22), 5.0 cm; range, −5.0 to 13.3 cm]. Most patients had a normal head circumference [median for females (n = 11), 54.5 cm; +0.2 SDS; range, 51.3 to 58.5 cm; median for males (n = 12), 56 cm; 1.0 SDS; range, 53.3 to 58.5 cm]. Two male patients and 1 female patient had a head circumference >+2 SDS.
Other skeletal features
In some families, the mutations were associated with mild midface hypoplasia and flat nasal bridge (8 of 20 families; Supplemental Fig. 3 (59.7MB, pdf) ), and young children in 2 families were noted to have frontal bossing (families 16 and 18).
The growth of the hands varied. Brachydactyly was reported in 5 of 20 families. In 4 of these families, the affected individuals also had notably short thumbs (Supplemental Fig. 3 (59.7MB, pdf) ). Complete skeletal surveys were performed on 8 individuals with no or only subtle signs of skeletal dysplasia noted (Supplemental Fig. 4 (59.7MB, pdf) ).
Early-onset OA and osteochondritis dissecans
Heterozygous ACAN mutations can also affect the articular cartilage (4, 19). In our cohort, early-onset OA was reported in 12 (families 3, 5 to 9, 11, 12, 14, 16, 19, 20) of 20 families, with knee pain the most commonly reported complaint (7 children and 34 adults), except for short stature and commonly started in late adolescence. In these families, severe OA was established during the second and fourth decade of life. Penetrance appeared to be complete but with a variable degree of severity among the families. The knees were the most commonly affected joints, but other joints were also affected (4, 13). The joint disease was associated with missense, truncating, and nonsense mutations located in various regions of the gene (Fig. 1). Thus, in this cohort of 20 families, no correlation was no evident between the presence or absence of joint disease and either the domain in which the mutation occurred or the type of mutation. In some families (families 3, 5, and 6), the ACAN mutations were associated with osteochondritis dissecans (OD) (4, 13, 20). In these families, the 2 ACAN mutations occurred in the CLD region.
Intervertebral disc disease
Aggrecan is a major proteoglycan of the cartilaginous intervertebral discs, and its degradation has been suggested to be an important pathogenic mechanism in intervertebral disc degenerative disease (21). Back pain was also a common complaint in affected adult individuals of 11 families (families 1, 5 to 9, 12, 15, 16, 19, and 20), who reported back problems with confirmed disc disease or symptoms suspicious for intervertebral disc disease (3 children and 14 adults). Symptoms of back problems typically started later than the joint disease, with symptoms beginning in the fourth and fifth decade of life and commonly progressing to severe disease with neurologic symptoms often requiring surgical intervention. However, 2 sisters from family 20 presented with symptoms in the first and second decade of life.
Treatment of short stature
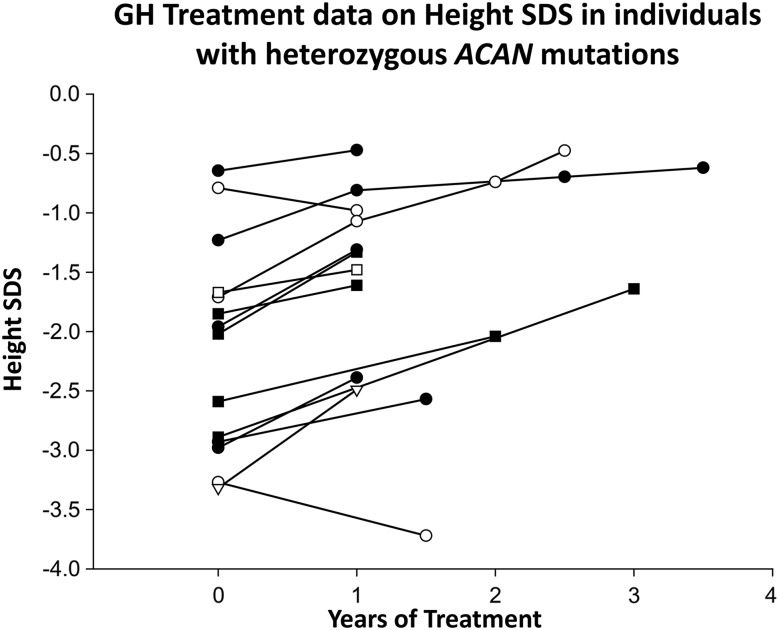
In total, 14 children from 8 families had received growth hormone (GH) treatment of short stature (8 females, 6 males) at 1 of 3 different doses: 30 µg/kg/d (4 individuals), 43 µg/kg/d (9 individuals), and 50 µg/kg/d (1 individual). In addition, 5 adult individuals had previously received GH treatment of short stature. The average height SDS of these 5 GH-treated adult individuals was −2.5 and that of untreated adult individuals (n = 65) was −3.0 SDS. Longitudinal growth data were available for the 14 children treated with GH for ≥12 months, including growth data after 2 (n = 3) and 3 (n = 3) years in some individuals. During GH treatment, the height SDS increased on average by +0.4, +0.7, and +1.0 SDS during the first year, the first 2 years, and the first 3 years of treatment, respectively (Fig. 6). In addition to GH treatment, 5 subjects had received treatment with gonadotropin-releasing hormone analogs (4 females, 1 male; families 3, 5, 8, and 20; Fig. 6) to delay BA progression and thereby increase adult height. BA radiographs were available before and after the beginning of treatment in 2 individuals. During treatment, BA progression was halted (0 years of progression/chronological year) during 9 and 22 months of gonadotropin-releasing hormone analog treatment, respectively. Thus, treatment appeared to halt BA progression. In addition, 1 male patient had received treatment with GH and an aromatase inhibitor (letrozole; family 1) for 11 months. During letrozole treatment, BA progression was halted (Fig. 6). Moreover, no adverse events or serious side effects from the treatments, including no new or worsened joint pain, were recorded for any of the patients.
Figure 6.
Change in height SDS of subjects during treatment of short stature. Longitudinal growth data were available for 14 individuals with ACAN mutations from different families who had received GH treatment of short stature (8 females and 6 males). Solid squares represent boys who had received GH only; solid circles, girls who had received GH only; open circles, girls who received GH and gonadotropin-releasing hormone agonist treatment; open triangles, 1 boy who had received GH and aromatase inhibitor (letrozole) therapy.
Discussion
We present the clinical characterization of a large international cohort of patients with 19 heterozygous ACAN mutations, including 103 mutation-positive individuals from 20 families (Supplemental Fig. 1 (59.7MB, pdf) ). Our study results greatly widen the genotypic and phenotypic spectrum of ACAN deficiency, demonstrating a lack of a simple genotype/phenotype correlation with regard to joint disease, and better defining the associated comorbidities and the natural history and response to growth-promoting therapies (2, 3).
The probands of all 20 families presented with autosomal dominant short stature and, in most cases, no or only subtle indications of chondrodysplasia. Affected individuals typically manifested mildly disproportionate short stature, often with advanced BA, a family history of early growth cessation, and/or a family history of early-onset OA (2, 3, 22–24).
In each family, a rare, nonsynonymous ACAN variant predicted to perturb protein function was identified by Sanger sequencing of ACAN, targeted next-generation sequencing, or exome sequencing with confirmation by Sanger sequencing. In each family, the rare variant cosegregated perfectly with the phenotype (Fig. 2; Supplemental Fig. 1 (59.7MB, pdf) ; Supplemental Table 2 (59.7MB, pdf) ). The variants were spread throughout the gene and included truncating, splice site, and missense variants, and included both missense and truncating variants in the G1, chondroitin sulfate attachment region, and G3 domains, and truncating variants in the G2 domain (Fig. 1) (6), thus greatly broadening the spectrum of the 7 previously published ACAN mutations (2, 3, 19, 25–27).
All the current and previously reported heterozygous aggrecan mutations cause adult short stature of similar severity (2, 3, 13, 19, 26). In addition, the variation in adult height in our cohort was similar to the adult height variation in the general population. Taken together, these findings suggest that different disease-causing heterozygous ACAN variants impair growth plate chondrogenesis similarly, which, in turn, suggests a common mechanism. Therefore, it is likely that the impairment of growth plate chondrogenesis is due to functional haploinsufficiency of aggrecan, rather than different mutation-specific mechanisms (25).
Before the present report, early-onset OA due to heterozygous ACAN mutations, including 1 truncating mutation in the chondroitin-sulfate domain and 2 missense mutations in the G3 lectin-binding domain, had been reported in 3 of 7 families (2, 4, 13, 19). In the present study, we report 12 families with 11 mutations associated with early-onset OA. Two of the 11 mutations associated with early-onset OA had been reported previously and 9 of the mutations have not been previously reported (2, 4). New mutations associated with early-onset OA include early truncating mutations in the G1 and G2 domains and missense mutations in the G1 and chondroitin-sulfate 1 domains (Fig. 1). Mutations not associated with early-onset OA also include early and late truncating mutations and missense mutations involving different regions of the protein. Therefore, no obvious genotype–phenotype correlation for early-onset OA could be detected in the present study, suggesting that additional genetic and environmental factors might ay modify the susceptibility to OA development caused by pathogenic gene variants in ACAN. Moreover, OA was common in adult individuals (53%) and children (21%) with heterozygous ACAN mutations and usually established well before 40 years of age. This is different from the general population, in which OA prevalence is very low, at approximately 0.04% in children (age <18 years), remains low until the mid-30s, and then increases rapidly, such that approximately 33% of the population aged >65 years are affected (28–31). Radiologic signs of OD have to date only been detected in individuals with missense mutations in the CLD. Whether OD also occurs in individuals with truncating mutations and missense mutations in other domains remains to be determined.
In our cohort, the affected individuals of several families also reported early-onset intervertebral disc disease that often caused neurologic symptoms and required surgical intervention. This finding is consistent with aggrecan being the major proteoglycan component of the intervertebral disc and loss of aggrecan being an important pathogenic mechanism for degenerative disc disease (21).
Several of the affected children were treated with GH for short stature. We also compared previously GH-treated and non–GH-treated adult individuals and calculated the change in height SDS during treatment. Both of these observations suggest a modest response to GH, with a magnitude similar to that seen with GH treatment of idiopathic short stature (32, 33). However, to rigorously assess the efficacy and safety of GHs for the treatment of children with short stature due to ACAN mutations, a randomized controlled trial would be required.
In summary, we report 20 families with heterozygous variants in ACAN associated with short stature, advanced BA, variable mild dysmorphic features, early-onset OA, and degenerative disc disease. The findings from the families indicate that heterozygous ACAN mutations sometimes present to the clinician as evident skeletal dysplasia or with early-onset OA but more often as autosomal dominant short stature with normal or advanced BA and early growth cessation despite normally timed puberty. Our findings greatly increase the number of reported families with ACAN mutations and suggest that heterozygous ACAN mutations often cause early-onset OA and intervertebral disc disease and that heterozygous ACAN mutations might be relatively common in families with autosomal dominant short stature with accelerated skeletal maturation and early growth cessation.
Acknowledgments
We thank Dr. Dana Zemkova for the anthropological assessment of the Czech patients.
Acknowledgments
The work of A.D. was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (Grant 1K23HD073351). The work of J.B. was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. The work of O.N. and A.G. was supported by the Swedish Research Council (Grants 521-2014-3063 and 2015-02227), the Swedish Governmental Agency for Innovation Systems (Vinnova) (Grant 2014-01438), the Marianne and Marcus Wallenberg Foundation (Grant 2014-0096), the Stockholm County Council, the Swedish Society of Medicine, Byggmästare Olle Engkvist’s Foundation, HKH Kronprinsessan Lovisas förening för barnasjukvård, Sällskapet Barnavård, Stiftelsen Frimurare Barnhuset i Stockholm, and Karolinska Institutet. A.A.L.J. was supported by Grant 2013/03236–5 from the São Paulo Research Foundation (FAPESP), Brazil. The work of K.E.H. and L.S. was supported by grants from the Spanish Ministry of Education and Science (Grants SAF2012-30871 and SAF2015-66831-R). The work of J.L., S.P., and L.E. was supported by the Czech Health Research Council (Grant AZV 16-31211A) and the Project for Conceptual Development of Research Organization (Grant 00064203/6001), Ministry of Health, Czech Republic.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACAN
- aggrecan
- BA
- bone age
- CLD
- C-type lectin-binding domain
- FOD
- familial osteochondritis dissecans
- GH
- growth hormone
- OA
- osteoarthritis
- OD
- osteochondritis dissecans
- SDS
- standard deviation score
- SHI
- sitting height index
References
- 1.Baron J, Sävendahl L, De Luca F, Dauber A, Phillip M, Wit JM, Nilsson O. Short and tall stature: a new paradigm emerges. Nat Rev Endocrinol. 2015;11(12):735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nilsson O, Guo MH, Dunbar N, Popovic J, Flynn D, Jacobsen C, Lui JC, Hirschhorn JN, Baron J, Dauber A. Short stature, accelerated bone maturation, and early growth cessation due to heterozygous aggrecan mutations. J Clin Endocrinol Metab. 2014;99(8):E1510–E1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quintos JB, Guo MH, Dauber A. Idiopathic short stature due to novel heterozygous mutation of the aggrecan gene. J Pediatr Endocrinol Metab. 2015;28(7-8):927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stattin EL, Wiklund F, Lindblom K, Onnerfjord P, Jonsson BA, Tegner Y, Sasaki T, Struglics A, Lohmander S, Dahl N, Heinegård D, Aspberg A. A missense mutation in the aggrecan C-type lectin domain disrupts extracellular matrix interactions and causes dominant familial osteochondritis dissecans. Am J Hum Genet. 2010;86(2):126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roughley PJ, Mort JS. The role of aggrecan in normal and osteoarthritic cartilage. J Exp Orthop. 2014;1(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspberg A. The different roles of aggrecan interaction domains. J Histochem Cytochem. 2012;60(12):987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDowell MA, Fryar CD, Ogden CL. Anthropometric reference data for children and adults: United States, 1988–1994. Vital and Health Statistics, Series 11. Bethesda, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2009;(249):1–68. [PubMed] [Google Scholar]
- 8.Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007–2010. Vital and Health Statistics, Series 11. Bethesda, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2012;(252):1–48. [PubMed] [Google Scholar]
- 9.Rollins JD, Collins JS, Holden KR. United States head circumference growth reference charts: birth to 21 years. J Pediatr. 2010;156(6):907–913, 913.e901–913.e902. [DOI] [PubMed] [Google Scholar]
- 10.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greulich WW, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. Stanford, CA: Stanford University Press; 1959. [Google Scholar]
- 13.Stattin EL, Tegner Y, Domellof M, Dahl N. Familial osteochondritis dissecans associated with early osteoarthritis and disproportionate short stature. Osteoarthritis Cartilage. 2008;16(8):890–896. [DOI] [PubMed] [Google Scholar]
- 14.Valhmu WB, Palmer GD, Rivers PA, Ebara S, Cheng JF, Fischer S, Ratcliffe A. Structure of the human aggrecan gene: exon-intron organization and association with the protein domains. Biochem J. 1995;309(Pt 2):535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowe BJ, Rekers-Mombarg LT, Robling K, Wolka AM, Cutler GB Jr, Wit JM; European Idiopathic Short Stature Group . Effect of growth hormone dose on bone maturation and puberty in children with idiopathic short stature. J Clin Endocrinol Metab. 2006;91(1):169–175. [DOI] [PubMed] [Google Scholar]
- 17.Wit JM, Rekers-Mombarg LT; Dutch Growth Hormone Advisory Group . Final height gain by GH therapy in children with idiopathic short stature is dose dependent. J Clin Endocrinol Metab. 2002;87(2):604–611. [DOI] [PubMed] [Google Scholar]
- 18.Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, Chu AY, Estrada K, Luan J, Kutalik Z, Amin N, Buchkovich ML, Croteau-Chonka DC, Day FR, Duan Y, Fall T, Fehrmann R, Ferreira T, Jackson AU, Karjalainen J, Lo KS, Locke AE, Mägi R, Mihailov E, Porcu E, Randall JC, Scherag A, Vinkhuyzen AA, Westra HJ, Winkler TW, Workalemahu T, Zhao JH, Absher D, Albrecht E, Anderson D, Baron J, Beekman M, Demirkan A, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Fraser RM, Goel A, Gong J, Justice AE, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Lui JC, Mangino M, Mateo Leach I, Medina-Gomez C, Nalls MA, Nyholt DR, Palmer CD, Pasko D, Pechlivanis S, Prokopenko I, Ried JS, Ripke S, Shungin D, Stancáková A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Afzal U, Arnlöv J, Arscott GM, Bandinelli S, Barrett A, Bellis C, Bennett AJ, Berne C, Blüher M, Bolton JL, Böttcher Y, Boyd HA, Bruinenberg M, Buckley BM, Buyske S, Caspersen IH, Chines PS, Clarke R, Claudi-Boehm S, Cooper M, Daw EW, De Jong PA, Deelen J, Delgado G, Denny JC, Dhonukshe-Rutten R, Dimitriou M, Doney AS, Dörr M, Eklund N, Eury E, Folkersen L, Garcia ME, Geller F, Giedraitis V, Go AS, Grallert H, Grammer TB, Gräßler J, Grönberg H, de Groot LC, Groves CJ, Haessler J, Hall P, Haller T, Hallmans G, Hannemann A, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hemani G, Henders AK, Hillege HL, Hlatky MA, Hoffmann W, Hoffmann P, Holmen O, Houwing-Duistermaat JJ, Illig T, Isaacs A, James AL, Jeff J, Johansen B, Johansson Å, Jolley J, Juliusdottir T, Junttila J, Kho AN, Kinnunen L, Klopp N, Kocher T, Kratzer W, Lichtner P, Lind L, Lindström J, Lobbens S, Lorentzon M, Lu Y, Lyssenko V, Magnusson PK, Mahajan A, Maillard M, McArdle WL, McKenzie CA, McLachlan S, McLaren PJ, Menni C, Merger S, Milani L, Moayyeri A, Monda KL, Morken MA, Müller G, Müller-Nurasyid M, Musk AW, Narisu N, Nauck M, Nolte IM, Nöthen MM, Oozageer L, Pilz S, Rayner NW, Renstrom F, Robertson NR, Rose LM, Roussel R, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Schunkert H, Scott RA, Sehmi J, Seufferlein T, Shi J, Silventoinen K, Smit JH, Smith AV, Smolonska J, Stanton AV, Stirrups K, Stott DJ, Stringham HM, Sundström J, Swertz MA, Syvänen AC, Tayo BO, Thorleifsson G, Tyrer JP, van Dijk S, van Schoor NM, van der Velde N, van Heemst D, van Oort FV, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Waldenberger M, Wennauer R, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, Arveiler D, Bakker SJ, Beilby J, Bergman RN, Bergmann S, Biffar R, Blangero J, Boomsma DI, Bornstein SR, Bovet P, Brambilla P, Brown MJ, Campbell H, Caulfield MJ, Chakravarti A, Collins R, Collins FS, Crawford DC, Cupples LA, Danesh J, de Faire U, den Ruijter HM, Erbel R, Erdmann J, Eriksson JG, Farrall M, Ferrannini E, Ferrières J, Ford I, Forouhi NG, Forrester T, Gansevoort RT, Gejman PV, Gieger C, Golay A, Gottesman O, Gudnason V, Gyllensten U, Haas DW, Hall AS, Harris TB, Hattersley AT, Heath AC, Hengstenberg C, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Hovingh GK, Humphries SE, Hunt SC, Hypponen E, Jacobs KB, Jarvelin MR, Jousilahti P, Jula AM, Kaprio J, Kastelein JJ, Kayser M, Kee F, Keinanen-Kiukaanniemi SM, Kiemeney LA, Kooner JS, Kooperberg C, Koskinen S, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Le Marchand L, Lehtimäki T, Lupoli S, Madden PA, Männistö S, Manunta P, Marette A, Matise TC, McKnight B, Meitinger T, Moll FL, Montgomery GW, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Ouwehand WH, Pasterkamp G, Peters A, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ritchie M, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schwarz PE, Sebert S, Sever P, Shuldiner AR, Sinisalo J, Steinthorsdottir V, Stolk RP, Tardif JC, Tönjes A, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PI, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hayes MG, Hui J, Hunter DJ, Hveem K, Jukema JW, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, März W, Melbye M, Moebus S, Munroe PB, Njølstad I, Oostra BA, Palmer CN, Pedersen NL, Perola M, Pérusse L, Peters U, Powell JE, Power C, Quertermous T, Rauramaa R, Reinmaa E, Ridker PM, Rivadeneira F, Rotter JI, Saaristo TE, Saleheen D, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Strauch K, Stumvoll M, Tuomilehto J, Uusitupa M, van der Harst P, Völzke H, Walker M, Wareham NJ, Watkins H, Wichmann HE, Wilson JF, Zanen P, Deloukas P, Heid IM, Lindgren CM, Mohlke KL, Speliotes EK, Thorsteinsdottir U, Barroso I, Fox CS, North KE, Strachan DP, Beckmann JS, Berndt SI, Boehnke M, Borecki IB, McCarthy MI, Metspalu A, Stefansson K, Uitterlinden AG, van Duijn CM, Franke L, Willer CJ, Price AL, Lettre G, Loos RJ, Weedon MN, Ingelsson E, O’Connell JR, Abecasis GR, Chasman DI, Goddard ME, Visscher PM, Hirschhorn JN, Frayling TM; Electronic Medical Records and Genomics (eMEMERGEGE) Consortium; MIGen Consortium; PAGEGE Consortium; LifeLines Cohort Study . Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46(11):1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleghorn L, Ramesar R, Beighton P, Wallis G. A mutation in the variable repeat region of the aggrecan gene (AGC1) causes a form of spondyloepiphyseal dysplasia associated with severe, premature osteoarthritis. Am J Hum Genet. 2005;77(3):484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edmonds EW, Polousky J. A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from König to the ROCK study group. Clin Orthop Relat Res. 2013;471(4):1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivan SS, Wachtel E, Roughley P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. Biochim Biophys Acta. 2014;1840(10):3181–3189. [DOI] [PubMed] [Google Scholar]
- 22.Domowicz MS, Cortes M, Henry JG, Schwartz NB. Aggrecan modulation of growth plate morphogenesis. Dev Biol. 2009;329(2):242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe H, Yamada Y. Chondrodysplasia of gene knockout mice for aggrecan and link protein. Glycoconj J. 2002;19(4-5):269–273. [DOI] [PubMed] [Google Scholar]
- 24.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. [DOI] [PubMed] [Google Scholar]
- 25.Gibson BG, Briggs MD. The aggrecanopathies: an evolving phenotypic spectrum of human genetic skeletal diseases. Orphanet J Rare Dis. 2016;11(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tompson SW, Merriman B, Funari VA, Fresquet M, Lachman RS, Rimoin DL, Nelson SF, Briggs MD, Cohn DH, Krakow D. A recessive skeletal dysplasia, SEMD aggrecan type, results from a missense mutation affecting the C-type lectin domain of aggrecan. Am J Hum Genet. 2009;84(1):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spranger JW, Brill P, Superti-Furga A, Unger S, Nishimura G Bone Dysplasias: An Atlas of Genetic Disorders of Skeletal Development. Oxford, UK: Oxford University Press; 2012. [Google Scholar]
- 28.Sacks JJ, Helmick CG, Luo YH, Ilowite NT, Bowyer S. Prevalence of and annual ambulatory health care visits for pediatric arthritis and other rheumatologic conditions in the United States in 2001-2004. Arthritis Rheum. 2007;57(8):1439–1445. [DOI] [PubMed] [Google Scholar]
- 29.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F; National Arthritis Data Workgroup . Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leschek EW, Rose SR, Yanovski JA, Troendle JF, Quigley CA, Chipman JJ, Crowe BJ, Ross JL, Cassorla FG, Blum WF, Cutler GB Jr, Baron J; National Institute of Child Health and Human Development-Eli Lilly & Co. Growth Hormone Collaborative Group . Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89(7):3140–3148. [DOI] [PubMed] [Google Scholar]
- 33.Albertsson-Wikland K, Aronson AS, Gustafsson J, Hagenäs L, Ivarsson SA, Jonsson B, Kriström B, Marcus C, Nilsson KO, Ritzén EM, Tuvemo T, Westphal O, Aman J. Dose-dependent effect of growth hormone on final height in children with short stature without growth hormone deficiency. J Clin Endocrinol Metab. 2008;93(11):4342–4350. [DOI] [PubMed] [Google Scholar]