Abstract
Context:
The RAF inhibitor vemurafenib has provided a major advance for the treatment of patients with BRAF-mutant metastatic melanoma. However, BRAF-mutant thyroid cancer is relatively resistant to vemurafenib, and the reason for this disparity remains unclear. Anticancer therapy–induced autophagy can trigger adaptive drug resistance in a variety of cancer types and treatments. To date, role of autophagy during BRAF inhibition in thyroid cancer remains unknown.
Objective:
In this study, we investigate if autophagy is activated in vemurafenib-treated BRAF-mutant thyroid cancer cells, and whether autophagy inhibition improves or impairs the treatment efficacy of vemurafenib.
Design:
Autophagy level was determined by western blot assay and transmission electron microscopy. The combined effects of autophagy inhibitor and vemurafenib were assessed in terms of cell viability in vitro and tumor growth rate in vivo. Whether the endoplasmic reticulum (ER) stress was in response to vemurafenib-induced autophagy was also analyzed.
Results:
Vemurafenib induced a high level of autophagy in BRAF-mutant thyroid cancer cells. Inhibition of autophagy by either a pharmacological inhibitor or interfering RNA knockdown of essential autophagy genes augmented vemurafenib-induced cell death. Vemurafenib-induced autophagy was independent of MAPK signaling pathway and was mediated through the ER stress response. Finally, administration of vemurafenib with the autophagy inhibitor hydroxychloroquine promoted more pronounced tumor suppression in vivo.
Conclusions:
Our data demonstrate that vemurafenib induces ER stress response–mediated autophagy in thyroid cancer and autophagy inhibition may be a beneficial strategy to sensitize BRAF-mutant thyroid cancer to vemurafenib.
Vemurafenib induces cytoprotective autophagy in BRAF-mutant thyroid cancer cells. Pharmacologic or genetic inhibition of autophagy augments the antitumor effect of vemurafenib in vitro and in vivo.
Thyroid cancer incidence has increased significantly in recent years, and it is currently the fifth most frequently diagnosed malignancy in the United States in women (1). Among different histologic variants of thyroid cancer, papillary thyroid carcinoma (PTC) is the most common type and usually has a favorable prognosis. However, about 5% of PTC patients develop aggressive growth, metastatic spread and loss of response to conventional therapy (radioactive iodine and thyrotropin suppressive therapy), and these tumors prove to be primarily responsible for deaths attributed to thyroid cancer (2, 3). The 10-year survival rate for these patients is only 10% and it has been fairly unchanged for many years because of limited progress in the treatment of this aggressive subset of tumors (4, 5). Anaplastic thyroid carcinoma (ATC) is another histologic type that is relatively uncommon but is an extremely aggressive and lethal cancer (6). Therefore, new treatments are critically needed for these patients with incurable thyroid cancer.
Within different types of thyroid cancer, the BRAFV600E mutation is found in PTC and ATC, with an average incidence of 45% and 25%, respectively (7). Recurrent or metastatic PTCs have a particularly high rate of BRAFV600E mutation (around 80%) (8, 9). BRAF is a key component of the RAS/RAF/MEK/MAPK/ERK signaling pathway (MAPK pathway) that transmits mitogenic signals from the cell membrane to the nucleus and promotes cell division and proliferation (10). The BRAFV600E mutation has been consistently identified to be associated with 1 or more conventional high-risk clinicopathologic characteristics in PTC and is confirmed to be an important independent prognostic factor that may influence therapy options (11). Vemurafenib, the selective RAF inhibitor that was approved by the U.S. Food and Drug Administration for patients with BRAFV600E metastatic melanoma, has been shown to increase disease free survival and overall survival in these treated patients (12). However, the majority of BRAF-mutant thyroid cancer cell lines have proven to be resistant to vemurafenib, and the reason for this disparity remains unclear. A feedback induced ligand-dependent activation of HER2/HER3 signaling was proposed to cause the decreased sensitivity of thyroid cancer cell lines to growth inhibition of vemurafenib (13). A recent clinical trial also showed that BRAFV600E anaplastic thyroid cancer had a lower response rate to vemurafenib than non–small cell lung cancer and Erdheim–Chester disease or Langerhans’ cell histiocytosis (14).
Macroautophagy (hereafter referred to as autophagy) is a highly conserved catabolic process in eukaryotic cells that degrades and recycles proteins and organelles to support metabolism and survival (15). It is typically upregulated when cells require intracellular nutrients and energy, such as during starvation. This process is supposed to be a survival strategy for cells. Accumulating evidence suggests that autophagy may also induce adaptive drug resistance in a variety of cancer types and treatments. Autophagy can facilitate removal of proteins or organelles that are damaged by drug treatment in cancer cells, and provide fuel for cancer cells from the degraded cellular components (16, 17). Metastatic melanomas with an elevated rate of basal autophagy have been reported to be more invasive and less responsive to cytotoxic chemotherapy and associated with shorter survival (18). Autophagy has also been implicated in thyroid cancer development and influences thyroid cancer treatment as well (19–22). However, in contrast to other tumors, enhanced autophagy levels following a number of antitumor treatment regimens in thyroid cancer may promote tumor cells to be more sensitive to the anticancer therapy (20–22). To date, role of autophagy during BRAF inhibition in thyroid cancer remains unknown. Here, we looked to determine if autophagy is activated in BRAF-mutant thyroid cancer cells during BRAF inhibition and whether autophagy inhibition improves or impairs the treatment efficacy of vemurafenib.
Materials and Methods
Reagents
Primary antibodies were used as follows: anti-LC3B (Sigma-Aldrich, St. Louis, MO), anti-GAPDH (Cell Signaling, Danvers, MA), antip62/SQSTM1 (Santa Cruz, Dallas, TX), antiphospho-ERK (E4, Santa Cruz), anti-ERK1/2 (Cell Signaling), anti-ATG 5 (D5F5U, Cell Signaling), anti–C/EBP homologous protein (CHOP)/GADD153 (B3, Santa Cruz), anti-Phospho-eukaryotic initiation factor 2-α (eIF2α; Ser51, Cell Signaling). The following reagents were used: PLX4032 (for in vitro, Selleck Chemicals, Houston, TX), PLX4720 (for in vivo, Selleck Chemicals), hydroxychloroquine (HCQ; Spectrum Chemical, New Brunswick, NJ), GSK2606414 (Selleck Chemicals), U0126 (Sigma-Aldrich).
Cell culture
Thyroid cancer cell lines BCPAP and 8505C were purchased from DSMZ (Braunschweig, Germany). Thyroid cancer cell line FRO was kindly provided by Dr. James A. Fagin (Memorial Sloan–Kettering Cancer Institute, New York, New York) and was authenticated using short tandem repeat- and single-nucleotide polymorphism array analysis (23).
Western blot analysis
Harvested cells were lysed by the RIPA Buffer Kit (Santa Cruz Biotechnology). Aliquots of the proteins were size fractionated by SDS-PAGE and then transferred to nitrocellulose membranes. Membranes were probed with primary antibodies and then with polyclonal HRP-conjugated secondary antibodies. Immunoreactive bands were visualized with ECL detection reagent (Pierce, Rockford, IL) in accordance with the manufacturer’s recommendations. The intensities of bands were quantified by densitometry using ImageJ software (National Institutes of Health).
GFP-LC3 cells stabilization and confocal microscopy
Cells stably expressing GFP-LC3 were generated as previously described (24). Briefly, Human LC3a was subcloned into pBabe/blast containing an N-terminal GFP tag. FRO cells were stably transduced using a Moloney murine leukemia retrovirus-based pBabe system with 293T cells used as the packaging cell line. FRO cells stably expressing GFP-LC3 were grown overnight on glass coverslips coated with a 0.1% gelatin solution. After drug treatment, coverslips were fixed with 3.7% paraformaldehyde (w/v) in 20 mM HEPES pH 7.5 for 20 minutes at room temperature. Fixed coverslips were rinsed in phosphate-buffered saline and mounted on microscopic glass slides with an antifade mounting media (ProLong, Invitrogen). After curing, the slides were examined using the 60x magnification objective on a Nikon confocal microscope.
Transmission electron microscopy
Treated cells were washed with serum-free medium and fixed with modified Karnovsky’s fixative (2.5% glutaraldehyde, 4% paraformaldehyde, and 0.02% picric acid in 0.1 M sodium cacodylate buffer, pH 7.3) over night. Cells were postfixed in 1% osmium tetroxide/1.5% potassium-ferricyanide, stained with 1.5% uranyl acetate, and dehydrated through a graded ethanol series. After embedding in Spurr’s resin, ultrathin sections (55-60 nm) were cut using a diamond knife (Diatome) on an ultramicrotome (Ultracut S; Leica, Austria). Sections were contrasted with lead citrate and viewed on a JEM-1400 electron microscope (JEOL, Tokyo, Japan) operated at 120 kV.
Cell viability assay
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Cells were seeded into 96-well plates (5,000/well) and treated with chemicals at different concentrations. After 72 hours’ incubation, absorbance of the solution was measured using a model iMark Micro Plate Reader (BioRad, USA) at 490 nm wavelength. Cell viability evaluations are presented as percentages relative to the control group [mean ± standard deviation (SD)] and IC 50 results are calculated by using nonlinear regression analysis (Prism 6.0, GraphPad Software Inc.).
Clonogenic assay
Cells were plated in a 6-well plate (1,000 cells/well) and treated with different reagent for 5-7 days. Fresh media without any drug was then added to the cells and cultured for another 3 days. Colonies were fixed with methanol and stained with 0.1% crystal violet. All the experiments were performed in triplicate and repeated at least 3 times.
RNA interference
ATG 5 small interfering RNA (siRNA-ATG5) and nontarget small interfering RNA (siRNA-NT) were purchased from Cell Signaling. Cells were transfected with siRNA-ATG5 or nontarget siRNA using HiPerFect (Qiagen, Hilden, Germany) according to manufacturer’s protocol. Twenty-four hours after transfection, cells were treated with either DMSO or vemurafenib. Seventy-two hours after the treatment, cell viability assay and Western blot analysis were performed.
Xenograft experiments
Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Weill Cornell Medical College. 5-7 weeks old male SCID mice (Charles River Laboratories, Wilmington, MA) were used to generate the xenograft tumor models. 5x106 BCPAP cells in Matrigel were injected subcutaneously. One week after implantation, when the average tumor volume reached approximately 350 mm3, mice were randomly assigned to different groups. PLX4720 was dissolved in DMSO (120 mg/mL) and then suspended in a 1% solution of carboxymethycellulose (Sigma, St. Louis). HCQ was dissolved in phosphate-buffered saline (120 mg/mL) and then also suspended in a 1% solution of carboxymethycellulose. Tumor size measurements and monitoring for signs of toxicity by body weight loss were performed at least twice a week. Tumor volume was calculated using the formula V = AB2/2, where A and B are the long and short diameter of the tumor, respectively. Three hours after the last treatment, animals were killed and transmission electron microscopy was performed to evaluate the autophagosome number.
Statistical analysis
Comparisons between 2 groups were performed by Student unpaired, 2-tailed t test. A P value less than 0.05 was considered significantly different.
Results
Vemurafenib induces autophagy in BRAF-mutant thyroid cancer cell lines
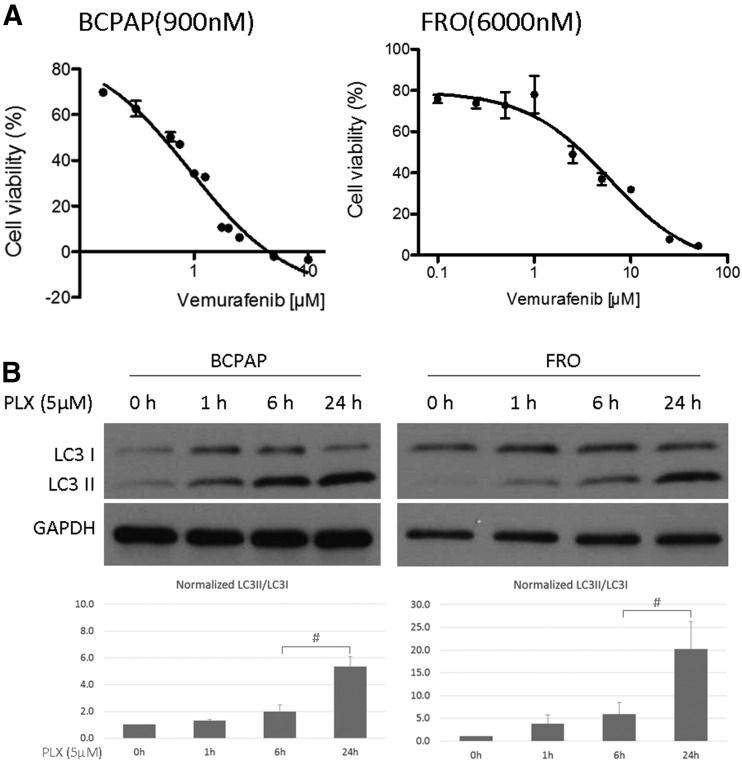
To evaluate the effect of vemurafenib treatment on autophagy in BRAF-mutant thyroid cancer cell lines, we first determined the sensitivity to vemurafenib (PLX4032) in the BRAF-mutant PTC cell line BCPAP and the ATC cell line FRO. Both cell lines are relatively resistant to vemurafenib with IC50 of 900 nM and 6000 nM, respectively [Fig. 1(A)], which is consistent with other reports (13). We then measured the expression level of LC3, a microtubule-associated protein that is a key marker of autophagy. During the progress of autophagy, the cytoplasmic form of LC3 (LC3I) is conjugated to phosphatidylethanolamine and targeted to autophagic membranes. Therefore, the ratio of LC3II (lipidated form of LC3) to LC3 I is used as a measure of autophagy in cells (25). When treated with vemurafenib, the ratio of LC3II/LC3I started to increase as early as 1 hour after treatment and reached significant change by 24 hours in both BCPAP and FRO cells [Fig. 1(B)], and in the third thyroid cancer cell line—8505C—as well (Supplemental Fig. 1 (1.2MB, tif) ). We also found a dose responsive increase in LC3II/LC3I in response to increasing doses of vemurafenib (Supplemental Fig. 2 (447.6KB, tif) ).
Figure 1.
Vemurafenib treatment in thyroid cancer cells. (A) Activity of vemurafenib detected using MTT assay in BCPAP and FRO cells. IC50 values are shown in brackets behind the name of each cell (nmol/L). Results shown are representative of at least 3 independent experiments. (B) Immunoblots and gel density quantifications against autophagy marker (LC3) in BCPAP and FRO cells. Cells were treated with 5.0 µM vemurafenib (PLX) for the indicated intervals. LC3 (I/II) and GAPDH levels were evaluated by immunoblot analysis. Intensity of LC3I and LC3II were determined by ImageJ densitometry analysis. Bar graphs shown represent normalized intensity levels of LC3II/LC3I relative to no treatment control (0 h). Error bars, SD from 3 independent replicates. #P < 0.05.
To determine whether the accumulation of LC3II induced by vemurafenib was due to enhanced autophagosome formation or inhibition of autophagosome degradation, BCPAP and FRO cells were treated with vehicle (DMSO) or vemurafenib with/without the presence of HCQ. HCQ passively diffuses into lysosomes to increase the lysosome PH, and ultimately inhibits autophagosome degradation by blocking fusion of the autophagosome with lysosomes (26). Firstly, vemurafenib treatment triggered accumulation of LC3II in both FRO and BCPAP cells [Fig. 2(A) and Supplemental Fig. 3 (408.5KB, tif) ]. When cells were cotreated with vemurafenib and HCQ, accumulation of LC3II was further enhanced compared with the vemurafenib-treated group. To further confirm this observation, the compartmentalization of endogenous LC3II in cells treated with vemurafenib was monitored by examining the GFP positive puncta in FRO cells stably expressing GFP-LC3 (FRO-GFP-LC3). In the DMSO group, smaller GFP-positive puncta were observed, which reflects the basal level of autophagy in FRO cells. In contrast, cells treated with vemurafenib produced larger puncta [Fig. 2(B)], indicating augmentation of autophagosome formation. Treatment with HCQ was associated with the development of numerous large green puncta due to blockade of autophagosome degradation. Combined treatment of vemurafenib and HCQ resulted in markedly increased number of large green puncta over vemurafenib treatment only, suggestive of a vemurafenib effect on autophagosome formation. These results indicate that the accumulation of LC3II induced by vemurafenib is a result of induction of autophagosome formation rather than inhibition of autophagosome degradation.
Figure 2.
Vemurafenib treatment increased autophagosome formation in thyroid cancer cells. (A) Representative western blot result of FRO cells treated with DMSO, 5 µM PLX, 10 µM HCQ, and a combination of PLX and HCQ. The histogram presents ratio of LC3II/LC3I in 4 different groups. Error bars, SD from 3 independent experiments. (B) Representative images of FRO-GFP-LC3 cells under the treatment with vehicle (DMSO) or vemurafenib (PLX) for 48 hours with/without the presence of HCQ. (C) Transmission electron microscopy images of FRO cells exposed to PLX or DMSO for 48 hours. Typical autophagic vacuoles (AV) with multivesicular and double-layer membrane were frequently observed in PLX treated group but not in DMSO group (as indicated by black arrows). Graph shows quantification of mean ±SD of number of AVs per cell. Magnification ×5000 to 50,000. #P < 0.05. N, nucleus.
Finally, transition electron microscopy was performed to evaluate formation of autophagic vacuoles (AVs) after vemurafenib treatment in FRO cells. Typical AVs with multivesicular and double layer membrane were frequently observed in vemurafenib-treated group (Supplemental Fig. 4 (7.8MB, tif) ). Abundant AVs with significantly higher autophagic index (the number of AV per cell) were found in vemurafenib treated FRO cells, as well as in combination group but not in untreated cells [Fig. 2(B) and Supplemental Fig. 5 (4.3MB, tif) ] Collectively, these results demonstrate that vemurafenib induces autophagy in thyroid cancer cells.
Autophagy inhibition sensitizes BRAF-mutant thyroid cancer cells to vemurafenib
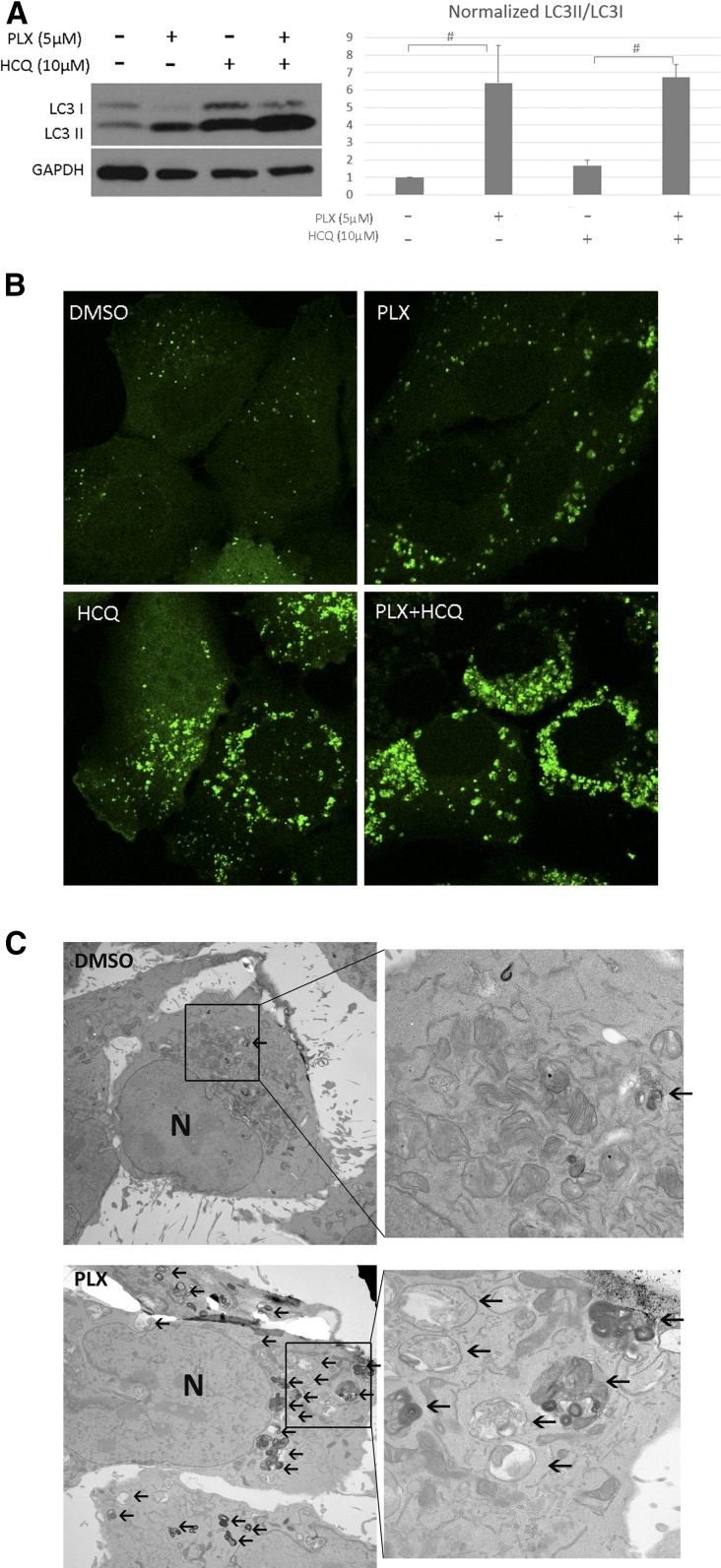
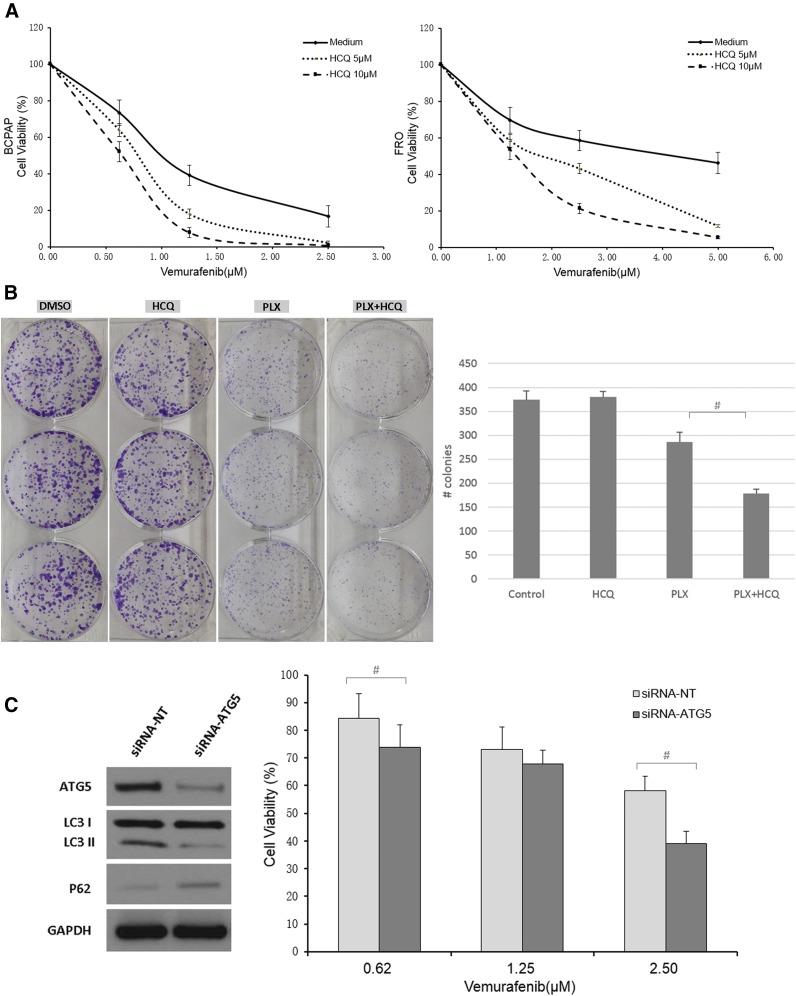
To determine whether autophagy inhibition would be an effective therapeutic intervention in BRAF-mutant thyroid cancer cells, we treated cells with HCQ, an agent that is already clinically available as an antimalarial reagent. To this end, BCPAP and FRO cells were cotreated with vemurafenib and HCQ. After 72 hours’ treatment, a dose dependent reduction of cell growth, measured by MTT assay, was observed in all vemurafenib-treated groups [Fig. 3(A)]. Moreover, combination with either 5 µM or 10 µM HCQ triggered much greater cell growth inhibition than vemurafenib alone, with the most potent effect seen in the 10 µM HCQ group. These concentrations of HCQ (5 and 10 µM) were found to have minimal toxicity to BCPAP and FRO cells when used as the sole treatment agent (Supplemental Fig. 6 (582.5KB, TIF) ). A clonogenic assay was performed to determine whether long-term autophagy inhibition augments vemurafenib-induced suppression of colony formation in BRAF-mutant thyroid cancer cells. To this end, we treated FRO cells with DMSO, HCQ, vemurafenib or a combination of vemurafenib and HCQ, and found the combination was the most effective way to reduce colony numbers, with significantly fewer colonies than in the vemurafenib only group [Fig. 3(B)]. We also treated BCPAP cells in the same way as that on the FRO cells and found they cannot form cell colony. Therefore, we stained the cells right after 4 days’ drug treatment and found very similar results as from FRO’s (Supplemental Fig. 7 (6.8MB, tif) ). As expected, HCQ had little effect on the clonogenicity of cells. Taken together, these results strongly suggest that the minimally toxic concentrations of the autophagy inhibitor (HCQ) markedly enhance the efficacy of vemurafenib in thyroid cancer cells.
Figure 3.
Autophagy inhibition augmented the antitumor effect of vemurafenib in thyroid cancer cells. (A) BCPAP and FRO cells were treated with increasing doses of vemurafenib in medium with or without HCQ for 72 hours and MTT assays were performed to assess cell proliferation. (B) Left panel, representative images of colonies stained with crystal violet in FRO cells treated with DMSO, 5 µM HCQ, 2 µM vemurafenib (PLX), and a combination of vemurafenib and HCQ (PLX + HCQ). The histogram in the right panel presents quantitative analysis of colony numbers in 4 different groups. Error bars, SD from 3 independent experiments. (C) Left panel, siRNA-ATG5 treatment of BCPAP cells showed effective knockdown of ATG and inhibited autophagy. Right panel showed that autophagy inhibition by siRNA-ATG5 sensitized BCPAP cells to vemurafenib treatment. Results shown are representative of 3 independent experiments. #P < 0.05.
To further explore whether the effects of HCQ were the result of autophagy inhibition and not another unknown effect of HCQ, we genetically inhibited autophagy. BCPAP cells were treated with siRNA targeting the essential autophagy related (ATG) protein ATG5. First, we examined the effect of ATG5 siRNA on BCPAP cells in normal physiological conditions. ATG5 siRNA robustly suppressed ATG5 expression and clearly inhibited autophagy as shown by decreased LC3II and accumulation of p62 [Fig. 3(C)]. The p62 protein is a ubiquitin-binding scaffold protein that binds directly to LC3 and is degraded during autophagy (27). We then combined ATG5 siRNA with different doses of vemurafenib. The ATG5 siRNA-vemurafenib combination effectively reduced cell viability as compared with the vemurafenib alone treatment group, with significant changes at 0.6 µM and 2.50 µM vemurafenib combinations [Fig. 3(C)]. Our findings indicate that vemurafenib-induced autophagy is cytoprotective in thyroid cancer cells and autophagy inhibition could sensitize them to vemurafenib.
Vemurafenib-induced autophagy is mediated by enhanced ER stress response
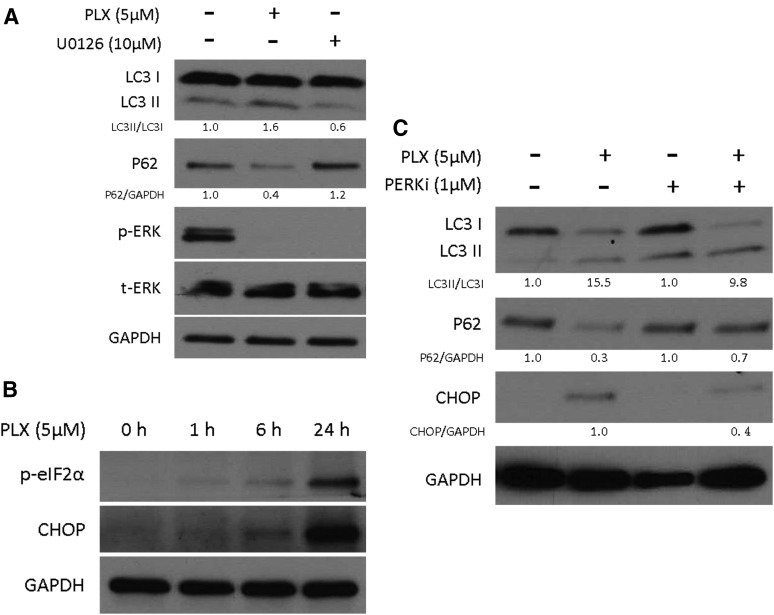
Vemurafenib is a specific inhibitor of BRAF V600E mutation, and therefore, it can suppress the MAPK signaling pathway in treated cells. The MAPK pathway is 1 of the signaling pathways that regulates autophagy in mammalian cells (28). To test the possibility that vemurafenib-induced autophagy is associated with the inactivation of MAPK signaling pathway, we compared the effect of vemurafenib with U0126, a specific inhibitor of MEK1 and MEK2. After 6 hours’ treatment, ERK phosphorylation was completely inhibited by both vemurafenib and U0126 [Fig. 4(A)]. However, U0126 triggered a decrease of LC3II and accumulation of p62, indicating that autophagy was inhibited rather than being activated (as with vemurafenib) during U0126-mediated MAPK signaling pathway inhibition. Therefore, vemurafenib-induced autophagy appears to be independent of the MAPK signaling pathway in thyroid cancer cells.
Figure 4.
Vemurafenib treatment induced ER stress response in thyroid cancer cells. (A) Vemurafenib (PLX) triggered autophagy independently of MAPK signaling pathway inactivation. BCPAP cells were treated with 5 μM PLX and 10 μM U0126 for 6 hours, after which protein lysates were prepared and subjected to protein gel blot analysis using the indicated antibodies. Relative quantity of LC3 II/LC3 I was calculated by ImageJ densitometric analysis and normalized to control (DMSO). (B) Vemurafenib treatment induced ER stress response in a time dependent manner. BCPAP cells were treated with 5 μM PLX for the indicated times. Whole-cell lysates were subjected to immunoblotting against the indicated antibodies. (C) PERK (PKR-like ER stress kinase) inhibitor (PERKi) partially reverted vemurafenib-induced autophagy. BCPAP cells were treated with 5 μM PLX and/or 1 μM PERKi for 24 hours. After that, protein lysates were prepared and subjected to immunoblotting against the indicated antibodies. Relative quantity of LC3II/LC3I was calculated by ImageJ densitometric analysis and normalized to control (DMSO / DMSO + PERKi). p-, phospho-; t-,total.
Another well-established signaling regulator of autophagy is the endoplasmic reticulum (ER) stress response. When unfolded/misfolded proteins exceed the capacity of ER-associated degradation, the ER stress response can be activated via stimulation of the ER chaperone GRP78 and 3 signaling pathways: PKR-like ER stress kinase (PERK)/eIF2α/CHOP, IRE-1/Xbp-1, and ATF6α. Then an alternate degradation system autophagy will be elicited (29, 30). Active PERK phosphorylates eIF2α and induces the expression of the transcription factors activating transcription factor 4 and CHOP (31). In BCPAP cells, vemurafenib induced eIF2α phosphorylation and CHOP expression in a time-dependent manner [Fig. 4(B)]. Phosphorylated eIF2α and CHOP increased as early as 1 hour after vemurafenib treatment. By 24 hours, when there was the strongest evidence of vemurafenib-induced autophagy, both phosphorylated eIF2α and CHOP increased dramatically, indicating that the ER stress response was highly activated.
To further determine whether this ER stress response activation is required for vemurafenib-induced autophagy in thyroid cancer, we inhibited the ER stress response by using a specific PERK inhibitor (GSK2606414). After 24 hours of incubation, the PERK inhibitor reduced the ratio of LC3II/LC3I and suppressed the digestion of p62 [Fig. 4(C)], indicating that vemurafenib-induced autophagy was reverted. Meanwhile, the increase in CHOP protein level was suppressed after combining the PERK inhibitor, supporting the fact that the ER stress response was partially inhibited by the PERK inhibitor. Thus, we conclude that the ER stress response plays a significant role in inducing autophagy during BRAF inhibition in thyroid cancer.
Autophagy inhibition enhances the antitumor effect of vemurafenib in vivo
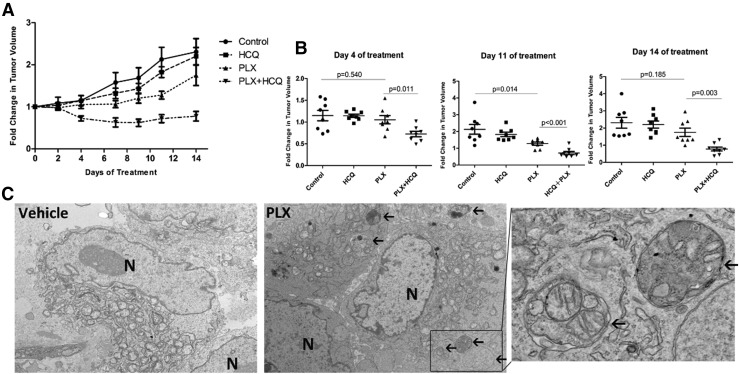
To further investigate the therapeutic benefit of inhibiting autophagy in combination with vemurafenib, SCID mice with established BCPAP xenograft tumors were divided into 4 groups: vehicle, HCQ alone, vemurafenib alone, and a combination of vemurafenib and HCQ. As shown in Fig. 5(A); the average tumor volume shown in Supplemental Fig. 8 (684.7KB, tif) ), HCQ alone had minimal effect on the tumor growth. Vemurafenib alone displayed moderate antitumor activity, with statistically significant suppression of tumor growth on the 11th day of treatment (P = 0.014). In contrast, mice treated with the combination of vemurafenib and HCQ showed significantly reduced tumor growth compared with monotherapy with vemurafenib as early as 4 days after initiation of the treatment (P = 0.011). This continued until at least 14 days of treatment (P = 0.003). Furthermore, no significant weight loss was observed in any treated groups, indicating the toxicity of the regimens was mild (Supplemental Fig. 9 (263.9KB, tif) ). Electron microscopy demonstrated accumulation of AVs in tumors treated with vemurafenib compared with vehicle treatment [Fig. 5(B)]. Taken together, these findings suggest that the autophagy inhibitor (HCQ) potently enhances antitumor activity of vemurafenib in thyroid cancer and is well tolerated in vivo.
Figure 5.
Combinatorial vemurafenib (PLX) and hydroxychloroquine (HCQ) treatment reduced tumor growth in xenograft models. (A) Tumor growth curves of BCPAP xenografts following initiation of treatment with vehicle (n = 8), HCQ (150 mg/kg per day, n = 8), PLX4720 (30 mg/kg per day, n = 8), or a combination of PLX4720 and HCQ (n = 8) by oral gavage once daily (6 days/week) for 2 weeks. Tumor volume was evaluated every other day. The relative tumor volumes were normalized to their original sizes and plotted as the fold change relative to day 0. (B) Statistical analysis of tumor volume among different groups at indicated time course of treatment. (C) Representative transition electron micrographs of BCPAP xenografts under the treatment with vehicle (DMSO) or vemurafenib (PLX). Black arrows denote AVs. Magnification ×5000 to 50,000. Error bars, standard error of the mean of tumor volumes in each group.
Discussion
Autophagy is thought to play a dual role in cancer development and progression (17). It can act as a tumor suppressor in tumorigenesis. The essential autophagy gene beclin1 is frequently deleted in human breast, ovarian and prostate cancer (32, 33). Introduction of beclin1 into breast cancer cells induced autophagy and inhibited tumorigenesis. Conversely, in advanced stages of tumor development, autophagy can sustain tumor metabolism and promote tumor survival via nutrient recycling (34). Determining the contextual role of autophagy in cancer is therefore important, and the role that autophagy plays in the cellular escape from cancer therapies is becoming a new focus of interest. The current study, to our best knowledge, is the first to explore the role of autophagy during BRAF inhibition in thyroid cancer. Here, we demonstrate that vemurafenib treatment induces cytoprotective autophagy in thyroid cancer cells. Pharmacologic or genetic inhibition of autophagy augments the antitumor effect of vemurafenib in vitro, which is further evidenced in vivo.
Previously, enhanced autophagy following a number of antitumor treatment regimens was reported to promote tumor cell death in thyroid cancer. Lin et al. (20) reported that doxorubicin and radiation induced autophagy in thyroid PTC and ATC cell lines, and inhibition of autophagy decreased the radio-sensitivity and chemo-sensitivity of these cells. They further found that combining therapy with an autophagy activator resulted in sensitization of thyroid cancer cells to both doxorubicin and radiation (21). Zhang et al. (22) recently showed that autophagy was induced in PTC cells treated with apigenin (a potential chemotherapeutic agent), and an autophagy inhibitor rescued the cells from apigenin-induced cell death. In contrast, here we have demonstrated that autophagy induced by vemurafenib was cytoprotective to thyroid cancer cells, and that pharmacologic or genetic inhibition of autophagy augmented the antitumor effects of vemurafenib. It has recently been shown, consistent with our findings, that the addition of an autophagy inhibitor helped to overcome BRAF-inhibitor resistance in melanoma (35).
HCQ is a potential autophagy inhibitor that has been safely used for decades in patients for malaria prophylaxis and for treatment of rheumatoid arthritis (36). Based on the reports that autophagy inhibition could augment the efficacy of a number of cancer therapies, clinical trials involving HCQ in combination with cytotoxic chemotherapy or targeted therapy for the treatment of different cancers, including breast, colon, and brain, are currently under way (37). Our work shows that cotreatment with vemurafenib and HCQ could effectively enhance the cell death induced by vemurafenib in thyroid cancer cells in vitro, as well as in vivo in a xenograft mouse model. Importantly, the dose of HCQ we used did not inhibit thyroid cancer cell growth in vitro alone, and cotreatment did not result in any additional toxicity in vivo. These data provide new opportunities for future clinical trials to explore this possible combination therapy in advanced thyroid cancer patients.
The activated MAPK pathway has been previously described to promote autophagy in leukemia and colon cancer cells (38, 39). However, in the current study, we found that vemurafenib-induced autophagy was independent of MAPK signaling pathway. The ER stress response has been previously shown to be involved in inducing autophagy during anticancer treatment in several cancer types (35, 40). Here, we have provided evidence that autophagy is also activated at least in part by the ER stress response in thyroid cancer. By the time there was clear evidence of vemurafenib-induced autophagy, the ER stress response was highly activated. Pharmacological inhibition of ER stress response reversed vemurafenib-induced autophagy. Taken together, our data identifies the ER stress response as having a significant role in inducing autophagy during BRAF inhibition in thyroid cancer. Mutant BRAF is described to bind to GRP78, the gatekeeper that controls the ER stress response, and BRAF inhibition can promote further binding of mutant BRAF and GRP78, suggesting a possible mechanism as to how vemurafenib activates the ER stress response (35).
In conclusion, our study demonstrates that autophagy induced upon vemurafenib treatment is an adaptive response in BRAF-mutant thyroid cancer cells, raising the exciting potential for targeting autophagy in future therapeutic strategies in BRAF-mutated advanced thyroid cancer patients.
Acknowledgments
The authors thank James A. Fagin (Memorial Sloan-Kettering Cancer Institute) for providing FRO cells. The authors also thank Leona Cohen-Gould and Juan Pablo Jimenez from CLC Imaging Core Facility of Weill Cornell Medical College for transition electron microscopy experiments.
Acknowledgments
This study is partially supported by the Dancer's Care Foundation, Goodwin Experimental Therapeutic Center fund, a Cycle for Survival fund, National Institutes of Health (No. R01CA166413 and R01GM113013), Grants from the National Natural Science Foundation of China (No. 81202141), scholarship from China Scholarship Council (No. 201308330175), and the Key Project of Scientific and Technological Innovation of Zhejiang Province (No. 2015C03G2010206).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATC
- anaplastic thyroid carcinoma
- AV
- autophagic vacuole
- CHOP
- C/EBP homologous protein
- ER
- endoplasmic reticulum
- HCQ
- hydroxychloroquine
- PERK
- PKR-like ER stress kinase
- PTC
- papillary thyroid carcinoma
- SD
- standard deviation
- siRNA
- small interfering RNA
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 2.Tuttle RM, Haddad RI, Ball DW, Byrd D, Dickson P, Duh QY, Ehya H, Haymart M, Hoh C, Hunt JP, Iagaru A, Kandeel F, Kopp P, Lamonica DM, Lydiatt WM, McCaffrey J, Moley JF, Parks L, Raeburn CD, Ridge JA, Ringel MD, Scheri RP, Shah JP, Sherman SI, Sturgeon C, Waguespack SG, Wang TN, Wirth LJ, Hoffmann KG, Hughes M Thyroid carcinoma, version 2.2014. J Natl Compr Canc Netw. 2014;12:1671–1680. [DOI] [PubMed] [Google Scholar]
- 3.Antonelli A, Fallahi P, Ferrari SM, Carpi A, Berti P, Materazzi G, Minuto M, Guastalli M, Miccoli P. Dedifferentiated thyroid cancer: a therapeutic challenge. Biomed Pharmacother. 2008;62(8):559–563. [DOI] [PubMed] [Google Scholar]
- 4.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–2899. [DOI] [PubMed] [Google Scholar]
- 5.Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, Pacini F. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014;2(5):356–358. [DOI] [PubMed] [Google Scholar]
- 6.Ain KB. Anaplastic thyroid carcinoma: a therapeutic challenge. Semin Surg Oncol. 1999;16(1):64–69. [DOI] [PubMed] [Google Scholar]
- 7.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–262. [DOI] [PubMed] [Google Scholar]
- 8.Lupi C, Giannini R, Ugolini C, Proietti A, Berti P, Minuto M, Materazzi G, Elisei R, Santoro M, Miccoli P, Basolo F. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92(11):4085–4090. [DOI] [PubMed] [Google Scholar]
- 9.Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A, Basolo F. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93(10):3943–3949. [DOI] [PubMed] [Google Scholar]
- 10.Mercer KE, Pritchard CA. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta. 2003;1653(1):25–40. [DOI] [PubMed] [Google Scholar]
- 11.Oler G, Cerutti JM. High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer. 2009;115(5):972–980. [DOI] [PubMed] [Google Scholar]
- 12.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, Hersey P, Kefford R, Lawrence D, Puzanov I, Lewis KD, Amaravadi RK, Chmielowski B, Lawrence HJ, Shyr Y, Ye F, Li J, Nolop KB, Lee RJ, Joe AK, Ribas A. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366(8):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, Ryder M, Ghossein RA, Rosen N, Fagin JA. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3(5):520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, Wolf J, Raje NS, Diamond EL, Hollebecque A, Gervais R, Elez-Fernandez ME, Italiano A, Hofheinz RD, Hidalgo M, Chan E, Schuler M, Lasserre SF, Makrutzki M, Sirzen F, Veronese ML, Tabernero J, Baselga J. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330(6009):1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazova R, Camp RL, Klump V, Siddiqui SF, Amaravadi RK, Pawelek JM. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin Cancer Res. 2012;18(2):370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5(9):726–734. [DOI] [PubMed] [Google Scholar]
- 18.Ma XH, Piao S, Wang D, McAfee QW, Nathanson KL, Lum JJ, Li LZ, Amaravadi RK. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin Cancer Res. 2011;17(10):3478–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plantinga TS, van de Vosse E, Huijbers A, Netea MG, Joosten LA, Smit JW, Netea-Maier RT. Role of genetic variants of autophagy genes in susceptibility for non-medullary thyroid cancer and patients outcome. PLoS One. 2014;9(4):e94086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CI, Whang EE, Abramson MA, Jiang X, Price BD, Donner DB, Moore FD Jr, Ruan DT. Autophagy: a new target for advanced papillary thyroid cancer therapy. Surgery. 2009;146(6):1208–1214. [DOI] [PubMed] [Google Scholar]
- 21.Lin CI, Whang EE, Donner DB, Du J, Lorch J, He F, Jiang X, Price BD, Moore FD Jr, Ruan DT. Autophagy induction with RAD001 enhances chemosensitivity and radiosensitivity through Met inhibition in papillary thyroid cancer. Mol Cancer Res. 2010;8(9):1217–1226. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Cheng X, Gao Y, Zheng J, Xu Q, Sun Y, Guan H, Yu H, Sun Z. Apigenin induces autophagic cell death in human papillary thyroid carcinoma BCPAP cells. Food Funct. 2015;6(11):3464–3472. [DOI] [PubMed] [Google Scholar]
- 23.Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland JA, Smallridge RC, Haugen BR. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93(11):4331–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganley IG, Wong PM, Gammoh N, Jiang X. Distinct autophagosomal-lysosomal fusion mechanism revealed by thapsigargin-induced autophagy arrest. Mol Cell. 2011;42(6):731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36(12):2503–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boya P, González-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25(3):1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rusten TE, Stenmark H. p62, an autophagy hero or culprit? Nat Cell Biol. 2010;12(3):207–209. [DOI] [PubMed] [Google Scholar]
- 28.Kondo Y, Kondo S. Autophagy and cancer therapy. Autophagy. 2006;2(2):85–90. [DOI] [PubMed] [Google Scholar]
- 29.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–891. [DOI] [PubMed] [Google Scholar]
- 31.Blais JD, Filipenko V, Bi M, Harding HP, Ron D, Koumenis C, Wouters BG, Bell JC. Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol. 2004;24(17):7469–7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–676. [DOI] [PubMed] [Google Scholar]
- 33.Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59(1):59–65. [DOI] [PubMed] [Google Scholar]
- 34.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12(6):401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma XH, Piao SF, Dey S, McAfee Q, Karakousis G, Villanueva J, Hart LS, Levi S, Hu J, Zhang G, Lazova R, Klump V, Pawelek JM, Xu X, Xu W, Schuchter LM, Davies MA, Herlyn M, Winkler J, Koumenis C, Amaravadi RK. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J Clin Invest. 2014;124(3):1406–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Neill PM, Bray PG, Hawley SR, Ward SA, Park BK. 4-Aminoquinolines—past, present, and future: a chemical perspective. Pharmacol Ther. 1998;77(1):29–58. [DOI] [PubMed] [Google Scholar]
- 37.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17(4):654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han W, Sun J, Feng L, Wang K, Li D, Pan Q, Chen Y, Jin W, Wang X, Pan H, Jin H. Autophagy inhibition enhances daunorubicin-induced apoptosis in K562 cells. PLoS One. 2011;6(12):e28491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Whiteman MW, Lian H, Wang G, Singh A, Huang D, Denmark T. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating beclin 1. J Biol Chem. 2009;284(32):21412–21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi YH, Ding ZB, Zhou J, Hui B, Shi GM, Ke AW, Wang XY, Dai Z, Peng YF, Gu CY, Qiu SJ, Fan J. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy. 2011;7(10):1159–1172. [DOI] [PubMed] [Google Scholar]