Abstract
Context:
Findings of studies of testosterone’s effects on muscle strength and physical function in older men have been inconsistent; its effects on muscle power and fatigability have not been studied.
Objective:
To determine the effects of testosterone administration for 3 years in older men on muscle strength, power, fatigability, and physical function.
Design, Setting, and Participants:
This was a double-blind, placebo-controlled, randomized trial of healthy men ≥60 years old with total testosterone levels of 100 to 400 ng/dL or free testosterone levels <50 pg/mL.
Interventions:
Random assignment to 7.5 g of 1% testosterone or placebo gel daily for 3 years.
Outcome Measures:
Loaded and unloaded stair-climbing power, muscle strength, power, and fatigability in leg press and chest press exercises, and lean mass at baseline, 6, 18, and 36 months.
Results:
The groups were similar at baseline. Testosterone administration for 3 years was associated with significantly greater performance in unloaded and loaded stair-climbing power than placebo (mean estimated between-group difference, 10.7 W [95% confidence interval (CI), −4.0 to 25.5], P = 0.026; and 22.4 W [95% CI, 4.6 to 40.3], P = 0.027), respectively. Changes in chest-press strength (estimated mean difference, 16.3 N; 95% CI, 5.5 to 27.1; P < 0.001) and power (mean difference 22.5 W; 95% CI, 7.5 to 37.5; P < 0.001), and leg-press power were significantly greater in men randomized to testosterone than in those randomized to placebo. Lean body mass significantly increased more in the testosterone group.
Conclusion:
Compared with placebo, testosterone replacement in older men for 3 years was associated with modest but significantly greater improvements in stair-climbing power, muscle mass, and power. Clinical meaningfulness of these treatment effects and their impact on disability in older adults with functional limitations remains to be studied.
Testosterone replacement in older men for 3 years was associated with modest but significantly greater improvements in muscle power and physical function compared with placebo.
Testosterone levels decline with advancing age (1, 2) and have been associated with the age-related changes in lean body mass, muscle strength, and self-reported and performance-based measures of physical function (3–6). Furthermore, testosterone supplementation has been shown to consistently increase whole-body and appendicular lean body mass (3, 7–13). These observations have stimulated enormous interest in investigating the anabolic applications of androgens to improve physical function and reduce the burden of disability in older adults. However, the effects of testosterone supplementation on muscle performance and physical function have been inconsistent in previous trials (3, 7–18). Many previous trials have been limited by their relatively short duration, small sample sizes, and the heterogeneity of testosterone doses, regimens, and on-treatment testosterone levels (7–18). Furthermore, the effects of testosterone on other important measures of muscle performance, including muscle power and fatigability, have not been well evaluated.
The Testosterone’s Effects on Atherosclerosis Progression in Aging Men (TEAAM) Trial was a randomized, placebo-controlled trial whose primary aim was to determine the effects of testosterone supplementation on atherosclerosis progression in older men with low or low-normal testosterone levels (19). The primary results of this trial have been published (19). A secondary aim of the TEAAM Trial was to determine the effects of raising testosterone levels into the midrange for healthy young men on physical function and muscle performance in older men with low or low-normal testosterone levels. In addition to muscle strength, we also determined the effects of testosterone on muscle power and fatigability, 2 additional important measures of muscle performance. Muscle power, the rate at which a muscle generates force, is more strongly associated with measures of physical function than muscle strength, and diminishes faster than strength with aging (20–23). Local muscle fatigability has been associated with one’s ability to delay the onset of muscle fatigue, especially when performing work at high workloads (24, 25) Testosterone’s effects on these measures have not been well studied in older men.
Several explanations have been offered to explain why previous testosterone trials have failed to demonstrate consistent improvements in measures of physical function despite significant gains in muscle mass, including suboptimal intervention duration, failure to raise testosterone levels into the mid-normal range, and low ceiling of the measures of physical function tests used in these trials. To overcome these limitations of previous trials, the participants of the TEAAM Trial received the intervention for 3 years. Testosterone levels were measured during intervention and the testosterone dose was adjusted to maintain serum testosterone levels in the testosterone arm between 500 and 900 ng/dL (17.3–31.2 nmol/L). Furthermore, we used the stair-climb test as a measure of physical function, because of its higher ceiling and stronger association with lower extremity strength than some other measures of physical function, such as gait speed (14). We also assessed performance in a loaded stair-climb test, in which participants carried a load while ascending stairs, thus raising the ceiling even higher than that of the unloaded stair-climbing test. We have previously shown the loaded stair-climb test to be a better discriminator of testosterone efficacy among young and older healthy men and older men with mobility limitations (26).
Methods
Study design
The TEAAM trial was a 3-site, randomized, placebo-controlled, parallel-group, double-blind trial designed to investigate long-term effects of testosterone supplementation on atherosclerosis progression in older men with low testosterone (19). This report describes the secondary results specific to muscle performance, physical function, and lean body mass in the TEAAM Trial. The participant sites included Charles Drew University of Medicine and Science, Los Angeles, California; Boston University Medical Center, Boston, Massachusetts; and the Kronos Longevity Research Institute, Phoenix, Arizona. The study protocol was approved by the Western Institutional Review Board, Puyallup, Washington; for Kronos Longevity Research Institute; and by the respective institutional review boards of the other institutions.
Participants
The details of inclusion and exclusion criteria have been reported (19). Briefly, participants were community-dwelling men age 60 years or older with low to low-normal testosterone defined as total serum testosterone levels between 100 and 400 ng/dL (3.5–13.9 nmol/L) or free testosterone level less than 50 pg/mL (174 pmol/L) obtained in a fasting, morning sample. We included men with low or low-normal testosterone levels (19) who were similar to a substantial fraction of middle-aged and older men receiving testosterone prescription (27).
None of the subjects were engaged in resistance exercise training before or during the 36 months of the study. Before participation in the study, all subjects provided written informed consent.
Randomization
The participants were stratified by age group and site, and randomly assigned to receive either 1% testosterone transdermal gel or placebo gel. The investigational drug pharmacist assigned the randomization number in a prespecified sequence generated by the biostatistician.
Intervention
Participants were randomly assigned to receive either 7.5 g of 1% testosterone gel (75 mg of testosterone) or placebo gel daily for 3 years. Two weeks after randomization, total testosterone levels were measured 2 to 12 hours after gel application. If the total testosterone concentration was lower than 500 ng/dL (17.3 nmol/L), the testosterone dose was increased to 10 g daily. The men with a total testosterone level higher than 900 ng/dL (31.2 nmol/L) had their testosterone dose reduced to 5 g. To maintain blinding, an unblinded study staff adjusted the dose in the placebo group simultaneously. Subsequently, testosterone levels were measured at 6, 18, and 36 months. Compliance with the treatment paradigm was assessed by counting the number of unused gel packs returned by the participants.
Outcomes
Muscle performance
Muscle strength, power, and fatigability were measured at baseline and 6, 18, and 36 months. The measurement procedures and equipment were standardized across sites. Some participants were not able to perform certain tests during some of the visits; therefore, the sample size differed for various tests.
Muscle strength
Maximal voluntary strength of the lower and upper extremities was assessed using the 1-repetition maximum (1-RM) method (28) for the seated leg-press and chest-press exercises (Keiser Sport, Fresno, CA). Subjects were positioned in the standardized seat position and foot placement that allowed 90° of knee flexion for the leg-press exercise. Seat height and handle position were standardized for the chest press. Subjects were familiarized with the exercises, practiced the technique, and completed a 5-minute warm-up. The 1-RM procedure has been previously described (8). Briefly, it consisted of a warmup set with 5 to 8 repetitions at a resistance set to about 50% of the participant’s estimated 1-RM and progressed with increasing loads interspersed with standardized rest periods until the subject was able to perform only 1 full range-of-motion repetition. The participants were tested twice on nonconsecutive days with the better of the 2 trials reported as the 1-RM.
Muscle power
Leg-press and chest-press power were measured using the same equipment and positioning used for the 1-RM assessments (8). The pneumatic resistance machines were instrumented with an electronics package (A420, Keiser Sport) that enabled the measurement of force and velocity and, hence, power. Subjects performed 5 repetitions with 30 seconds of rest between repetitions at 50%, 60%, and 70% of the baseline 1-RM for the leg press and 40%, 50%, and 60% of the chest-press 1-RM. Peak power for each load was analyzed as well as the highest peak power across all loads. The same absolute load was used at all test intervals.
Muscle fatigability
This test measured the fatigability of the muscle groups used to perform the leg-press and chest-press exercises (8). Subjects were instructed to perform as many full range-of-motion repetitions as possible at a fixed cadence of 4 seconds per repetition with a load equal to 80% or 70% of the baseline 1-RM for the leg press and chest press, respectively. The same absolute loads were used for all tests over the 3-year study.
Physical function
Stair-climb power
Each test site conducted 2 tests of stair climb power using an indoor 12-step staircase. One test consisted of ascending the 12 steps as rapidly as possible without running (unloaded stair climb), whereas the second test required subjects to carry a load equivalent to 20% of their baseline body weight evenly distributed in 2 canvas tote bags (loaded stair climb) (13, 14). Time to ascend the stairs was measured electronically with a digital clock and switch mats (Lafayette Instruments, Lafayette, IN) placed at the base of the steps and on the 12th step. The same timing equipment was used at all sites. Stair-climb power (measured in watts) was calculated from the product of the total rise of the 12 steps, body weight plus load carried, and acceleration of gravity, all divided by time. Subjects performed 2 trials of the unloaded stair climb with a 2-minute rest between trials, rested 10 minutes, and then completed 2 trials of the loaded stair climb with a 2-minute rest between trials. The same absolute load carried at baseline was used at the 6-, 18-, and 36-month assessments.
Lean body mass
Lean body mass was measured by dual energy x-ray absorptiometry, using the Hologic QDR4500 (Hologic Inc., Marlborough, MA) or the GE Lunar Prodigy (site 3 only; General Electric Co., Little Chalfont, United Kingdom) instruments. The GE Lunar Prodigy data were corrected using cross-validation measurements in 8 men aged 20 to 60 years who were tested on both instruments. All dual energy x-ray absorptiometry instruments were cross-validated using a whole-body phantom and calibrated daily following manufacturer’s recommendations.
Hormone assays
Total testosterone was measured at Quest Diagnostics (San Juan Capistrano, CA) using a Bayer Advia Centaur immunoassay (Siemens Medical Solutions, Malvern, PA) after extraction of serum with ethyl acetate and hexane, followed by celite chromatography; this assay, validated against liquid chromatography coupled to tandem mass spectrometry, has a sensitivity of 10 ng/dL (0.3 nmol/L); the intra- and interassay coefficients of variation are 11.8% and 17%, respectively (29). Free testosterone was calculated as previously described (30).
Statistical analysis
The primary analyses were performed on an intention-to-treat basis (i.e., all randomized subjects with a baseline and at least 1 postrandomization assessment were included in the analyses regardless of compliance). Baseline characteristics were summarized by treatment groups using means and standard deviations (SDs). Change from baseline in muscle performance, physical function, and body composition measures was analyzed using mixed-effects linear regression models with repeated measures allowing for within-subject correlation of outcomes over each outcome measurement during the entire postrandomization intervention period. Models were adjusted for baseline outcomes as well as age group (men 65–75 years old and men >75 years old), and contained a site factor as a random effect. The visit number (representing time in treatment), randomization assignment, and visit-by-randomization interaction were treated as fixed effects; to accommodate potential nonlinearities in change in outcomes, time was considered a discrete (i.e,. a class or factor) variable.
The primary hypothesis of difference between treatment effects was assessed using a combined test of main effect and visit-by-randomization interaction over time. Estimated mean changes and 95% confidence intervals (CIs), within and between arms, were calculated as an average of changes from baseline for t3 postrandomization visits. Additionally, a prespecified, per-protocol analysis was conducted on participants who completed 3 years of intervention and had both baseline and 36-month outcome assessment data.To determine whether baseline testosterone levels affected response to testosterone, a sensitivity analysis was performed on participants who had a baseline serum testosterone level ≤300 ng/dL (10.4 nmol/L) or >300 ng/dL. The association between the change in total serum testosterone and change in muscle performance, physical function, or body composition was evaluated by a linear mixed-effect regression model. Additionally, the association between total lean mass change and physical function measures was examined using mixed-model linear regression.
For limited hypothesis testing, the nominal type 1 error was set at α = 0.05. Primary analyses were prespecified, hence no adjustments were made for multiplicity. All analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC) and R software version 2.15.1 (https://www.r-project.org/).
Results
The flow of participants through the trial
Of the 306 men who were randomly assigned to the parent trial, 256 men had a baseline assessment (135 men randomly assigned to the testosterone arm and 121 men to the placebo arm) and at least 1 postrandomization assessment of physical function and muscle performance and constituted the intention-to-treat analytic sample. A total of 106 participants in the testosterone arm and 97 men in the placebo arm completed 3 years of the study and constituted the sample for the prespecified per-protocol sensitivity analyses of completers.
Baseline characteristics of the participants
The participants in this report averaged 67 years of age and had a mean body mass index of 30 kg/m2. The baseline age, body mass index, hormone levels, and measures of muscle performance, physical function, and lean body mass were comparable between groups (Table 1).
Table 1.
Baseline Characteristics of Participants by Treatment Arm
| Variables | Testosterone, Mean ± SD (n = 135)a | Placebo, Mean ± SD (n = 121)a |
|---|---|---|
| Age, y | 66.6 ± 5.4 | 68.0 ± 5.1 |
| Weight, kg | 91.7 ± 23.4 | 94.1 ± 31.2 |
| Body mass index, kg/m2 | 30.1 ± 7.7 | 30.8 ± 10.1 |
| Total testosterone, ng/dL | 306.7 ± 66.6 | 302.3 ± 67.0 |
| ≤200 | 186.0 ± 17.4 | 153.0 ± 44.3 |
| No. | 8 | 8 |
| 201–300 | 253.4 ± 29.3 | 250.4 ± 30.2 |
| No. | 52 | 42 |
| >300 | 356.6 ± 36.5 | 347.8 ± 31.8 |
| No. | 75 | 71 |
| Free testosterone, pg/mL | 63.2 ± 17.3 | 61.3 ± 18.2 |
| Hemoglobin, g/dL | 14.5 ± 1.24 | 14.5 ± 1.42 |
| Chest-press strength, no. | 517.0 ± 133 | 513.8 ± 105 |
| Leg-press strength, no. | 2270.4 ± 424 | 2233.7 ± 415 |
| Chest-press peak power,b W | 400.8 ± 109 | 401.2 ± 102 |
| Leg-press peak power,c W | 1286.4 ± 312 | 1318.1 ± 325 |
| Chest-press fatigability,d no. | 14.0 ± 4.1 | 14.3 ± 4.3 |
| Leg-press fatigability,d no. | 15.1 ± 6.4 | 15.7 ± 7.7 |
| Unloaded stair-climb power,e W | 536.3 ± 134 | 535.8 ± 125 |
| Loaded stair-climb power,e W | 581.2 ± 148 | 594.8 ± 150 |
| Lean body mass, kg | 56.2 ± 6.3 | 56.7 ± 6.1 |
Number of subjects with baseline and at least 1 postrandomization physical function test or body composition record.
Chest-press peak power is the highest peak power measured at 40%, 50%, and 60% of the chest press 1-RM.
Leg-press peak power is the highest peak power measured at 50%, 60%, and 70% of the leg press 1-RM.
Chest-press and leg-press fatigability values reflect the number of leg-press and chest-press repetitions to failure.
Stair-climb power (both unloaded and loaded) reflects peak power measured over 12 steps.
Hormone levels
The mean (±SD) total testosterone level in the analytical sample, derived from the mean of the 6-, 18-, and 36-month measurements, increased from 307 (±67) ng/dL [10.6 (±2.3) nmol/L] at baseline to 567 (±244) ng/dL [19.7 (±8.5) nmol/L] in testosterone-treated men. Free testosterone increased from 63 (±17) pg/dL [219 (±59) pmol/L] to 105 (±59) pg/dL [364 (±205) pmol/L]. Neither total nor free mean testosterone level changed in men receiving placebo (302 (±67) [10.5 (±2.3) nmol/L] to 331 (±103) ng/dL [11.5 (±3.6) nmol/L] and 61 (±18) to 51 (±20) pg/dL [177 (±69) pmol/L], respectively). These data are presented in Supplemental Fig. 1 (165.9KB, tif) .
Estimated mean changes from baseline
The estimated mean changes from baseline and their 95% CIs, calculated as an average of changes from baseline for 3 postrandomization visits, for the testosterone and placebo groups are summarized in Table 2. The estimated mean 3-year difference and 95% CIs between groups are also presented in Table 2. Estimated mean percent changes and 95% CIs between and within groups over 3 years are presented in Supplemental Table 1 (15.5KB, docx) .
Table 2.
Estimated Mean Changes and 95% CIs Between and Within Groups Over 3 Years for Physical Function, Muscle Performance, and Lean Body Mass
| Variables | Testosterone |
Placebo |
Estimated Mean Difference (95% CI) | Pb |
|---|---|---|---|---|
| Estimated Mean Change (95% CI)a | Estimated Mean Change (95% CI)a | |||
| Chest-press strength, no. | 4.2 (−3.3 to 11.6) | −12.1 (−20.0 to −4.3) | 16.3 (5.5–27.1) | <0.001 |
| Leg-press strength, no. | 14.1 (−22.1 to 50.3) | −17.7 (−56.4 to 21.0) | 31.8 (−21.3 to 84.8) | 0.280 |
| Chest-press peak power, W | 8.3 (−1.9 to 18.5) | −14.3 (−25.2 to -3.3) | 22.5 (7.5–37.5) | <0.001 |
| Leg-press peak power, W | 29.0 (−4.0 to 61.9) | −54.8 (−90.2 to −19.5) | 83.8 (35.4–132.2) | <0.001 |
| Chest-press fatigability, no. | 0.4 (−1.7 to 2.6) | −0.0 (−2.2 to 2.1) | 0.4 (−0.4 to 1.3) | 0.202 |
| Leg-press fatigability, no. | −0.4 (−3.4 to 2.3) | −0.6 (−3.7 to 2.4) | 0.3 (−1.2 to 1.8) | 0.911 |
| Unloaded stair-climb power, W | 0.4 (−24.8 to 25.6) | −10.3 (−35.9 to 15.2) | 10.7 (−4.0 to 25.5) | 0.026 |
| Loaded stair-climb power, W | 17.4 (5.3–29.5) | −5.1 (−18.2 to 8.1) | 22.4 (4.6–40.3) | 0.027 |
| Lean body mass, kg | 0.7 (0.2–1.2) | −0.2 (−0.8 to 0.3) | 0.9 (0.5–1.4) | <0.001 |
Estimated mean change and 95% CIs were determined over the entire 3-year study period.
P value extracted from mixed-model regression (combined test of main effect and visit-by-treatment interaction).
Changes in hemoglobin
Hemoglobin levels in our analytical sample were similar at baseline in both randomization groups: 14.54 ± 1.24 g/dL and 14.48 ± 1.42 g/dL for the on-treatment and placebo groups, respectively. In the intervention group, mean hemoglobin level changes, calculated as an average value across 3 postrandomization time points, increased to 15.33 ± 1.39 g/dL over the 3-year study period, but remained almost unchanged in the placebo group (14.40 ± 1.15 g/dL).
Measures of muscle performance
Chest-press strength
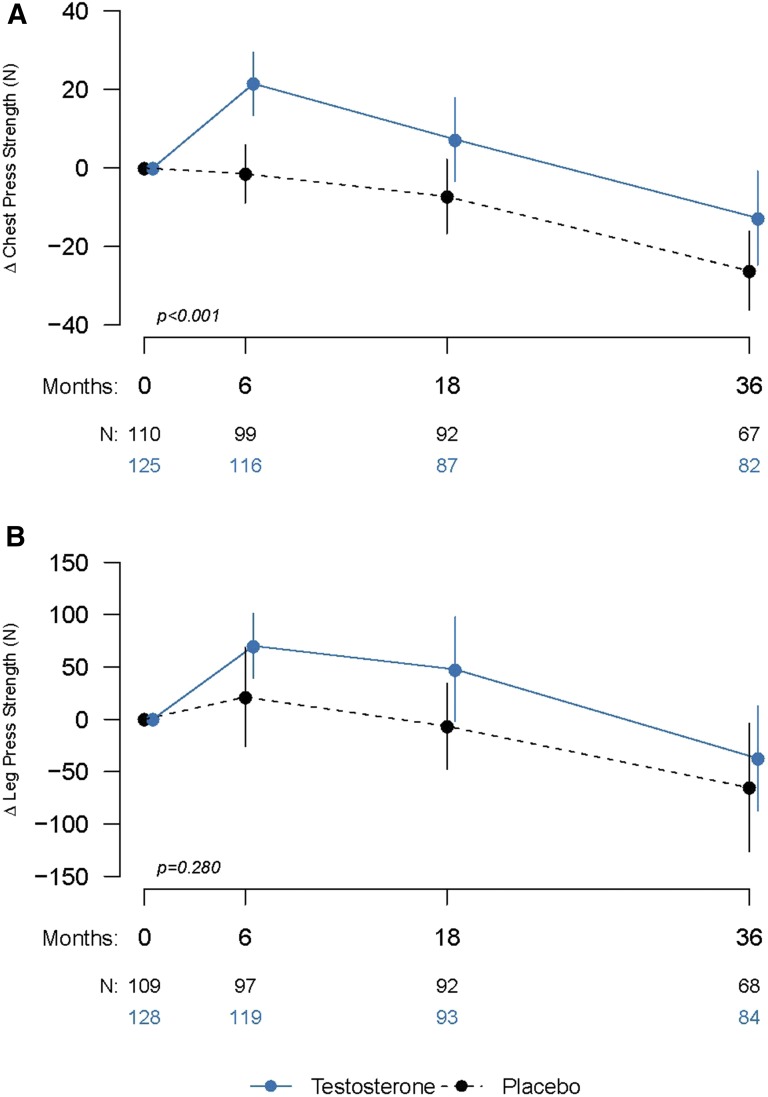
Chest-press strength increased significantly more in men randomly assigned to the testosterone arm than in those assigned to the placebo arm (Fig. 1). Changes from baseline in chest-press strength for both groups as well as the estimated mean difference between groups over the 3-year study are shown in Table 2; the difference between groups was significant (P < 0.001). Although the treatment effect seemed to wear off over time (Fig. 1), the statistical test of visit-by-treatment interaction (expressing change in the distance between the 2 plotted lines in the figures) was not significant. A strong and significant association was seen between the change in chest-press strength and change in total and free testosterone levels (P < 0.001 for each; Supplemental Figs. 2 (242.4KB, tif) and 3 (240.6KB, tif) ). The change in chest-press strength was significantly associated with the change in whole-body lean mass (P = 0.031) in the testosterone group.
Figure 1.
(A) Changes in strength in the chest press exercise. (B) Changes in strength in the leg press exercise. Results represent change in mean maximal voluntary strength from baseline for each visit for men randomly assigned to testosterone (solid line) or placebo arms (dashed line) and the number of subjects completing each visit. Error bars are 95% CIs. P values are provided from linear mixed-model regression controlling for baseline values and age category.
Leg-press strength
The change in leg-press strength over 3 years did not differ significantly between the 2 groups. The estimated mean (95% CI) difference between groups was 31. 8 N (−21.3 to 84.8; P = 0.280; Fig. 1; Table 2). Estimated mean changes from baseline for the testosterone and placebo groups are given in Table 2. The change in leg-press strength was associated significantly with the change in total (P = 0.024) and free testosterone levels (P = 0.031) over the 3 years (Supplemental Figs. 2 (242.4KB, tif) and 3 (242.4KB, tif) ). Leg-press strength in the testosterone group was not significantly associated with the change in lean body mass (P = 0.391), but was significantly associated with leg-press power and loaded and unloaded stair-climbing power (all, P < 0.001).
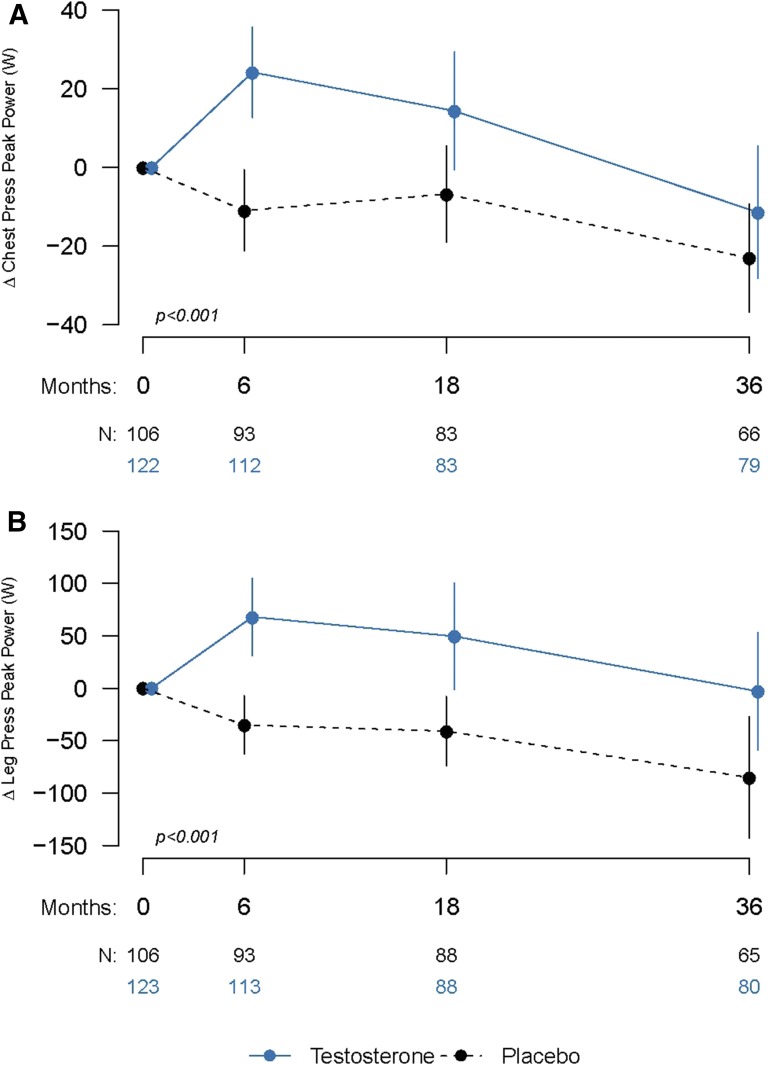
Muscle power
The changes in chest-press and leg-press peak power (Fig. 2) were significantly greater in the men randomly assigned to the testosterone group compared with those randomly assigned to the placebo group. The estimated mean changes from baseline for chest-press and leg-press peak power for the testosterone and placebo groups are summarized in Table 2. The estimated mean between-group differences in the change in peak chest-press and leg-press power were significant (P < 0.001 for both); the estimated mean differences (95% CI) for chest-press peak power and leg-press peak power were 22.5 W (7.5–37.5 W) and 83.8 W (35.4–132.2 W), respectively. Both chest-press and leg-press peak power (Supplemental Figs. 2 (242.4KB, tif) and 3 (240.6KB, tif) ) were significantly associated with changes in total (P = 0.015 and P = 0.017, respectively) and free testosterone (P = 0.016 and P = 0.026, respectively) levels. The change is leg-press peak power was not significantly associated with the change in whole-body lean mass (P = 0.058), but was significantly associated with loaded and unloaded stair-climbing power (P < 0.001 for both).
Figure 2.
(A) Changes for peak power in the chest-press exercise. (B) Changes for peak power in the leg-press exercise. Data indicate changes from baseline in mean peak power for testosterone (solid lines) and placebo (dashed lines) groups for each visit and the number of participants completing each visit (N). Error bars are 95% CIs. P values are provided from linear mixed-model regression controlling for baseline values and age category.
Fatigability
There was no statistically significant treatment effect on leg-press or chest-press fatigability (data not shown). The changes in muscle fatigability during the chest-press and leg-press exercises were not significantly associated with changes in total or free testosterone levels (P = 0.202 and P = 0.911, respectively; Supplemental Fig. 4 (144KB, tif) ).
Measures of physical function
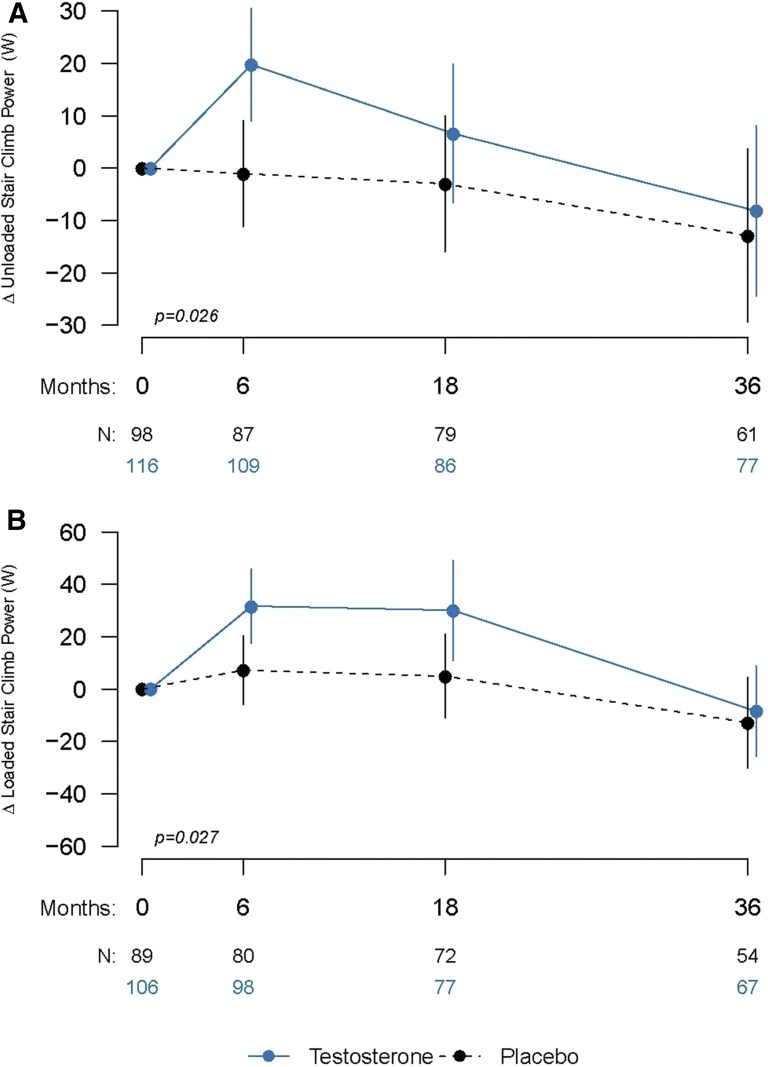
Stair-climb power
Testosterone supplementation for 3 years was associated with significantly greater improvements in the power generated during both the unloaded and loaded stair-climb tests than with placebo (Fig. 3). The estimated mean (95% CI) changes from baseline in unloaded and loaded stair-climb power for the testosterone and placebo groups are shown in Table 2. The estimated mean (95% CI) between-group differences in unloaded and loaded stair-climb power was 10.7 W (−4.0 to 25.5 W; P = 0.026) and 22.4 W (4.6–40.3 W; P = 0.027), respectively. The changes observed in both unloaded and loaded stair-climbing power were not significantly related to changes in total testosterone (P = 0.561 and P = 0.069, respectively). The visit-by-treatment interaction was not statistically significant although, numerically, the magnitude of the effect appeared to be waning with time.
Figure 3.
Mean changes from baseline at each visit for (A) unloaded and (B) loaded stair climb power. Solid lines represent data for the testosterone group; dashed lines indicate changes in the placebo group. The number of subjects completing each visit (N) is indicated. Error bars are 95% confidence intervals. P values are provided from linear mixed model regression controlling for baseline values and age category.
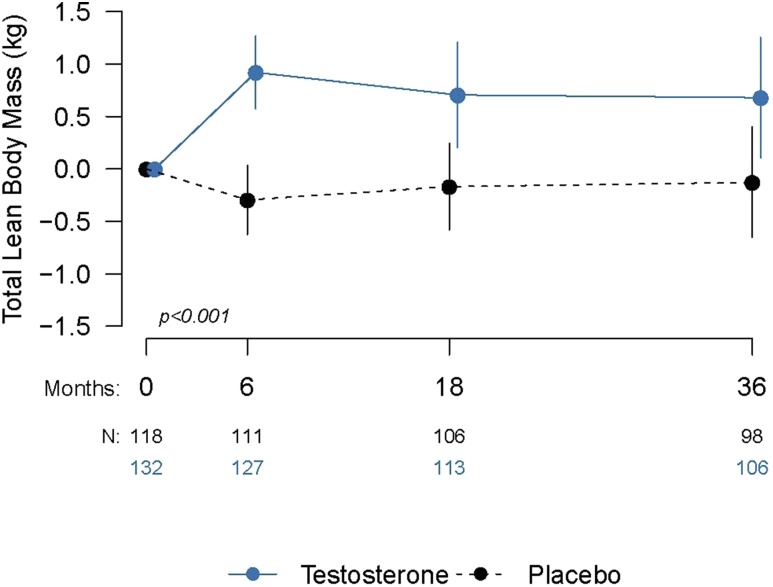
Lean body mass
Testosterone administration was associated with significantly greater increments in lean body mass over 3 years compared with placebo (Fig. 4). However, the estimated treatment effect was small (estimated mean difference [95% CI] between arms over 3 years, 0.9 kg [0.5–1.4 kg]; P < 0.001; Table 2). Among men assigned to the testosterone arm, the change in lean body mass was significantly associated with the change in total (P = 0.006) and free testosterone level (P = 0.002). As with muscle strength, muscle power, and stair-climbing power, the magnitude of the effect appeared wane over time despite a statistically nonsignificant visit-by-treatment interaction (Fig. 4).
Figure 4.
Mean changes in lean body mass from baseline at each visit and the number of subjects at each visit (N). Solid lines represent changes for the testosterone group, and dashed lines indicate changes in the placebo group. Error bars are 95% confidence intervals. P values are provided from linear mixed model regression controlling for baseline values and age category.
Sensitivity analysis
Prespecified per-protocol analyses of participants randomly assigned to the testosterone arm who completed 36 months of intervention yielded similar results (Supplemental Table 2 (17.5KB, docx) ). The treatment effects on muscle strength, power, fatigability, stair-climbing power, and lean body mass were qualitatively similar in men whose baseline testosterone level was less than 300 ng/dL (10.4 nmol/L) and those whose baseline testosterone level was more than 300 ng/dL (this analysis was not prespecified).
Discussion
Testosterone replacement for 3 years was associated with modest improvements in chest-press strength, muscle power, unloaded and loaded stair-climbing power, and lean body mass. Although these results are promising, the magnitude of change in the measures of muscle performance, physical function, and lean mass in this study was small and the clinical meaningfulness of these changes remains unclear. The potential impact of the improvements in these laboratory measures of muscle performance and physical function observed in the setting of a controlled trial on health outcomes and disability in older adults with functional limitations remains to be studied.
Unique to this 3-year study of testosterone administration in older men is the assessment of muscle power and fatigability. Aging is associated with a preferential loss of type II motor units (20, 31) and the consequent loss in the rate of force development (i.e., power). Because muscle power is more strongly associated with functional performance in activities such as stair climbing, walking, and rising from a chair (22), assessing the effectiveness of anabolic therapies on muscle power is important. Men in the testosterone-treated group significantly increased both lower and upper extremity power more than men receiving placebo. In addition, the changes in leg-press and chest-press power were significantly associated with changes in stair-climbing power, reinforcing the role of muscle power in performance of functional activities.
Muscle fatigability, assessed by the number of repetitions to failure at 80% of baseline chest-press and leg-press strength (1-RM) did not change in either group. Because local muscle fatigability is on a continuum with muscle strength (28), the small 1% to 2% changes observed in strength over the course of the study in men receiving testosterone may not have been adequate to reflect changes in fatigability. Furthermore, the principle of specificity suggests that improvement in fatigability is more likely to occur with resistance exercise performed systematically over time with high-volume, low-intensity training (28). This was not the case in the men participating in this study, because resistance exercise training was disallowed.
The changes in leg-press strength did not differ between the intervention groups even though upper extremity strength improved significantly more in the testosterone-treated men. These regional differences in the response of different muscle groups to androgens have been reported in other studies (32). Some long-term testosterone trials also found no improvement in lower extremity strength with testosterone replacement (9, 16). In general, trials that used larger doses of testosterone and achieved greater gains in lean body mass, and measured maximal voluntary strength with isoinertial exercise devices have tended to show significant improvements in lower extremity strength (3, 14). In contrast, those studies that have used relatively lower testosterone doses, achieved smaller gains in lean mass, and used isokinetic exercise have failed to do so. One possible contributor to the failure to observe significant improvements in leg-press strength in this study might be the relatively small 0.9-kg increase in total lean body mass seen at 6 months followed by its decline to 0.32 kg over the remaining 24 months. Indeed, in our previous dose-response studies, substantially larger gains in lean body mass with higher doses of testosterone than those used in this trial were associated with substantially greater increments in leg-press strength (3).
The intervention effect for all the outcomes appeared to wane over time, although the statistical tests for time-treatment interaction were not significant. The possible reasons for attenuation of the treatment effect over time could be decreased compliance over time or the age-related decline in function over time. Although compliance in both groups was greater than 90%, it is possible that over time, the subjects may have been less diligent in applying the gel. The age-related decline on physical function seems likely because the placebo-treated men also appeared to show a decline in muscle mass, power, and physical function over time. It is possible that testosterone plays a role in attenuating the age-related decline in these measures. Testosterone’s effect on attenuating the age-related decline in aerobic capacity has been reported (33).
The TEAAM Trial has several strengths and some limitations. The study had many attributes of a good trial design, including masked random allocation of participants to interventions, blinding, parallel groups, and prespecified intent-to-treat analytical strategy. The TEAAM Trial is among the largest and longest of testosterone trials. The testosterone dose raised and maintained serum testosterone concentrations in the mid-normal range for healthy young men. A battery of muscle performance measures, including muscle strength, muscle power and fatigability, enabled a more comprehensive assessment of muscle performance than has been performed in any other testosterone trial in older men, to our knowledge. We included stair climbing as a measure of physical function, which has a higher ceiling and is more strongly associated with lower extremity muscle strength than gait speed (22). The 3-year intervention duration was long enough to observe functional changes in both lower- and higher-ceiling (unloaded and loaded stair climbing) measures of physical function.
The trial also had some limitations. The participants included in the TEAAM Trial were healthy community-dwelling men without any functional limitations; the improvements in functionally limited older adults could be greater or smaller than those observed in healthy men. These participants had low-normal or slightly low testosterone levels, similar to a majority of middle-aged men receiving testosterone therapy in clinical practice. These men were not hypogonadal; therefore, these findings should not be extrapolated to hypogonadal men with known diseases of the testes, pituitary, or the hypothalamus. The drop-out rates were high but not dissimilar from other intervention trials of similar duration, and were substantially better than the treatment discontinuation rates observed in clinical practice.
The findings of the TEAAM Trial, when viewed together with the recent results of the Testosterone Trials (TTrials) (34), suggest that testosterone replacement in community-dwelling older adults with either no functional limitations or with mild to moderate mobility limitation is associated with only modest improvements in performance-based measures of physical function, such as 6-minute walking distance and stair-climbing power. These data contrast with published testosterone dose-response studies (3, 7) and empiric experience from athletes abusing large doses of androgens, which have shown substantially larger gains in skeletal muscle mass and maximal voluntary strength. Hence, their clinical meaningfulness of testosterone replacement in healthy older men with low or low-normal testosterone remains unclear. Further studies are needed to determine whether the observed gains in these laboratory measures of muscle mass, muscle performance, and physical function can be associated with patient-important improvements in distal health outcomes, such as ability to undertake activities of daily living, reduction in falls, or disability.
Resistance exercise training has been shown to enhance the anabolic effects of androgens on muscle mass, maximal voluntary strength, muscle power, and physical function (35–37). Similarly, athletes who use supraphysiologic doses of androgens to improve athletic performance often combine these drugs with task-specific training with the resultant performance enhancement (38). Therefore, it is possible that adjunctive functional exercise training may be needed to augment and translate the modest gains in skeletal muscle mass, muscle power, and laboratory measures of physical function into clinically meaningful improvements in health outcomes and disability. This hypothesis should be tested in adequately powered randomized trials. Furthermore, the TEAAM Trial was not powered to detect intervention effects on cardiovascular or prostate events; therefore, the long-term safety of testosterone therapy and the risk-benefit ratio cannot be ascertained from these data.
Acknowledgments
The authors thank the staff of the General Clinical Research Unit of Boston University’s Clinical and Translational Science Institute and the Clinical Research Center of Charles Drew University of Medicine and Science for their help with these studies, and the study participants for their commitment and generosity. The authors thank Dr Thomas Yoshikawa, David Geffen School of Medicine at University of California, Los Angeles (UCLA), California (Chair); Dr William French, Division of Cardiology, Harbor-UCLA Medical Center, Torrance, California; and Dr Nand Datta, Department of Urology, Charles Drew University, Los Angeles, California; of the Data Safety Monitoring Board.
Acknowledgments
This investigator-initiated study was funded by a grant from AbbVie and by a grant from the Aurora Foundation to Kronos Longevity Research Institute. Additional support was provided by the National Institute on Aging-funded Boston Claude D. Pepper Older Americans Independence Center (Grant 2 P30 AG031679).
Clinical Trials registry: ClinicalTrials.gov no. NCT00287586
Disclosure Summary: T.W.S. has received consulting fees from Regeneron Pharmaceuticals. S. Basaria has received research grants from AbbVie and consulting fees from Eli Lilly and Takeda. S. Bhasin has received research grants from AbbVie, Eli Lilly & Co, Regeneron, Transition Therapeutics, and Novartis; consulting fees from Sanofi, Novartis, Lilly, and AbbVie; has equity interest in FPT, LLC; has filed patent applications on a method to calculate free testosterone and for a selective anabolic therapy; and has served on the American Board of Internal Medicine Endocrinology Examination Writing Committee and the Endocrine Society Council. The remaining authors have nothing to disclose.
Footnotes
- 1-RM
- 1-repetition maximum
- CI
- confidence interval
- SD
- standard deviation
- TEAMM
- Testosterone’s Effects on Atherosclerosis Progression in Aging Men
- TTrials
- Testosterone Trials
References
- 1.Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D’Agostino R, Vasan RS. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96(8):2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu FCW, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Lean MEJ, Pendleton N, Punab M, Boonen S, Vanderschueren D, Labrie F, Huhtaniemi IT; EMAS Group . Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135. [DOI] [PubMed] [Google Scholar]
- 3.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90(2):678–688. [DOI] [PubMed] [Google Scholar]
- 4.Krasnoff JB, Basaria S, Pencina MJ, Jasuja GK, Vasan RS, Ulloor J, Zhang A, Coviello A, Kelly-Hayes M, D’Agostino RB, Wolf PA, Bhasin S, Murabito JM. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab. 2010;95(6):2790–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy TA, Blackman MR, Harman SM, Tobin JD, Schrager M, Metter EJ. Interrelationships of serum testosterone and free testosterone index with FFM and strength in aging men. Am J Physiol Endocrinol Metab. 2002;283(2):E284–E294. [DOI] [PubMed] [Google Scholar]
- 6.Orwoll E, Lambert LC, Marshall LM, Blank J, Barrett-Connor E, Cauley J, Ensrud K, Cummings SR; Osteoporotic Fractures in Men Study Group . Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med. 2006;166(19):2124–2131. [DOI] [PubMed] [Google Scholar]
- 7.Storer TW, Woodhouse L, Magliano L, Singh AB, Dzekov C, Dzekov J, Bhasin S. Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. J Am Geriatr Soc. 2008;56(11):1991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J, Casaburi R, Bhasin S. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88(4):1478–1485. [DOI] [PubMed] [Google Scholar]
- 9.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84(8):2647–2653. [DOI] [PubMed] [Google Scholar]
- 10.Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90(3):1502–1510. [DOI] [PubMed] [Google Scholar]
- 11.Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56(5):M266–M272. [DOI] [PubMed] [Google Scholar]
- 12.Blackman MR, Sorkin JD, Münzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O’Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288(18):2282–2292. [DOI] [PubMed] [Google Scholar]
- 13.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Travison TG, Basaria S, Storer TW, Jette AM, Miciek R, Farwell WR, Choong K, Lakshman K, Mazer NA, Coviello AD, Knapp PE, Ulloor J, Zhang A, Brooks B, Nguyen A-H, Eder R, LeBrasseur N, Elmi A, Appleman E, Hede-Brierley L, Bhasin G, Bhatia A, Lazzari A, Davis S, Ni P, Collins L, Bhasin S. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66(10):1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MDL, Adams JE, Oldham JA, Wu FCW. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95(2):639–650. [DOI] [PubMed] [Google Scholar]
- 16.Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ III, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647–1659. [DOI] [PubMed] [Google Scholar]
- 17.Emmelot-Vonk MH, Verhaar HJJ, Nakhai Pour HR, Aleman A, Lock TMTW, Bosch JLHR, Grobbee DE, van der Schouw YT. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299(1):39–52. [DOI] [PubMed] [Google Scholar]
- 18.Liverman CT, Blazer DG, eds. Testosterone and Aging: Clinical Research Directions. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 19.Basaria S, Harman SM, Travison TG, Hodis H, Tsitouras P, Budoff M, Pencina KM, Vita J, Dzekov C, Mazer NA, Coviello AD, Knapp PE, Hally K, Pinjic E, Yan M, Storer TW, Bhasin S. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314(6):570–581. [DOI] [PubMed] [Google Scholar]
- 20.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46(3):451–456. [DOI] [PubMed] [Google Scholar]
- 21.Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin. Sci. Lond. 1992;82(3):321–327. [DOI] [PubMed] [Google Scholar]
- 22.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40(1):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark DJ, Pojednic RM, Reid KF, Patten C, Pasha EP, Phillips EM, Fielding RA. Longitudinal decline of neuromuscular activation and power in healthy older adults. J Gerontol A Biol Sci Med Sci. 2013;68(11):1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Justice JN, Mani D, Pierpoint LA, Enoka RM. Fatigability of the dorsiflexors and associations among multiple domains of motor function in young and old adults. Exp Gerontol. 2014;55:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, Manini TM, Wohlgemuth SE, Leeuwenburgh C, Cummings SR, Newman AB, Ferrucci L, Toledo FGS, Shankland E, Conley KE, Goodpaster BH. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68(4):447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeBrasseur NK, Bhasin S, Miciek R, Storer TW. Tests of muscle strength and physical function: reliability and discrimination of performance in younger and older men and older men with mobility limitations. J Am Geriatr Soc. 2008;56(11):2118–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jasuja GK, Bhasin S, Reisman JI, Berlowitz DR, Rose AJ. Ascertainment of testosterone prescribing practices in the VA. Med Care. 2015;53(9):746–752. [DOI] [PubMed] [Google Scholar]
- 28.Baechle TR, Earle RW, Wathen D. Resistance training. In: National Strength & Conditioning Association. Essentials of Strength Training and Conditioning, 2nd ed. Champaign, IL: Human Kinetics;2000:407–409. [Google Scholar]
- 29.Salameh WA, Redor-Goldman MM, Clarke NJ, Reitz RE, Caulfield MP. Validation of a total testosterone assay using high-turbulence liquid chromatography tandem mass spectrometry: total and free testosterone reference ranges. Steroids. 2010;75(2):169–175. [DOI] [PubMed] [Google Scholar]
- 30.Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74(6):512–519. [DOI] [PubMed] [Google Scholar]
- 31.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):11–16. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder ET, Singh A, Bhasin S, Storer TW, Azen C, Davidson T, Martinez C, Sinha-Hikim I, Jaque SV, Terk M, Sattler FR. Effects of an oral androgen on muscle and metabolism in older, community-dwelling men. Am J Physiol Endocrinol Metab. 2003;284(1):E120–E128. [DOI] [PubMed] [Google Scholar]
- 33.Storer TW, Bhasin S, Travison TG, Pencina K, Miciek R, McKinnon J, Basaria S. Testosterone attenuates age-related fall in aerobic function in mobility limited older men with low testosterone. J Clin Endocrinol Metab. 2016;101(6):2562–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Cifelli D, Dougar D, Fluharty L, Resnick SM, Storer TW, Anton S, Basaria S, Diem SJ, Hou X, Mohler ER III, Parsons JK, Wenger NK, Zeldow B, Landis JR, Ellenberg SS; Testosterone Trials Investigators . Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coetsee C, Terblanche E. The time course of changes induced by resistance training and detraining on muscular and physical function in older adults. Eur Rev Aging Phys Act. 2015;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Labra C, Guimaraes-Pinheiro C, Maseda A, Lorenzo T, Millán-Calenti JC. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr. 2015;15:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu C-J, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;(3):CD002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffman JR, Kraemer WJ, Bhasin S, Storer T, Ratamess NA, Haff GG, Willoughby DS, Rogol AD. Position stand on androgen and human growth hormone use. J Strength Cond Res. 2009;23(5 Suppl):S1–S59. [DOI] [PubMed] [Google Scholar]