Abstract
Context:
Thyroiditis is one of the most common extrahepatic manifestations of hepatitis C virus (HCV) infection. By binding to surface cell receptor CD81, HCV envelope glycoprotein E2 mediates entry of HCV into cells. Studies have shown that different viral proteins may individually induce host responses to infection. We hypothesized that HCV E2 protein binding to CD81 expressed on thyroid cells activates a cascade of inflammatory responses that can trigger autoimmune thyroiditis in susceptible individuals.
Setting:
Human thyroid cell lines ML-1 and human thyrocytes in primary cell culture were treated with HCV recombinant E2 protein. The expression of major proinflammatory cytokines was measured at the messenger RNA and protein levels. Next-generation transcriptome analysis was used to identify early changes in gene expression in thyroid cells induced by E2.
Results:
HCV envelope protein E2 induced strong inflammatory responses in human thyrocytes, resulting in production of interleukin (IL)-8, IL-6, and tumor necrosis factor-α. Furthermore, the E2 protein induced production of several heat shock proteins including HSP60, HSP70p12A, and HSP10, in human primary thyrocytes. In thyroid cell line ML-1, RNA sequencing identified upregulation of molecules involved in innate immune pathways with high levels of proinflammatory cytokines and chemokines and increased expression of costimulatory molecules, specifically CD40, known to be a major thyroid autoimmunity gene.
Conclusion:
Our data support a key role for HCV envelope protein E2 in triggering thyroid autoimmunity through activation of cytokine pathways by bystander mechanisms.
The interaction between E2 and CD81 on human thyrocytes, independent of HCV entry, mediated activation of pathways known to be involved in immune response to viral infection and thyroid autoimmunity.
The causes of autoimmune thyroid diseases (AITDs) are not fully understood. There is, however, growing evidence that environmental factors such as viral infections may trigger the development of AITD, most likely in genetically predisposed individuals (1). Hepatitis C virus (HCV) infection is a prevalent disease worldwide (2). In addition to liver complications, extrahepatic manifestations are frequently observed in patients with chronic hepatitis C. These manifestations are mainly autoimmune and involve different organs. Studies report a high prevalence of thyroid peroxidase antibodies and hypothyroidism in hepatitis C patients compared with both healthy controls and hepatitis B patients [reviewed in (3)]. Indeed, thyroiditis is among the most common extrahepatic manifestations of HCV infection.
Viral infection may induce autoimmunity by various mechanisms: triggering changes in self-antigens, inducing local inflammation (bystander mechanisms), molecular mimicry, inducing heat shock protein (HSP), and activating expression of major histocompatibility complex proteins on nonimmune cells (4). Our group demonstrated that HCV can directly infect human thyroid cells (5). However, little is known about the immunological events following HCV infection of the thyroid. Such an infection may trigger a primary immune response with in situ production of cytokines, but dysregulation of the cytokine network during infection may initiate autoimmunity.
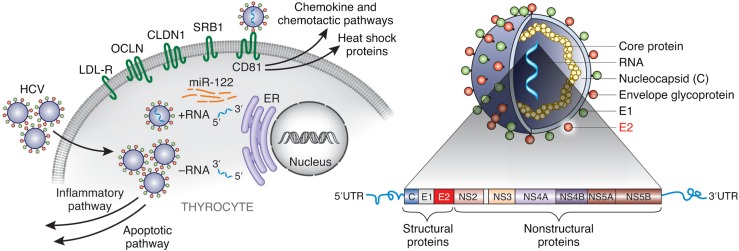
HCV contains a single positive-strand RNA coding for 10 different proteins: core protein, envelope proteins (E1 and E2), and 7 nonstructural proteins (Fig. 1). Studies have shown that different HCV proteins may individually interfere with cellular functions (6). E2, the main component of the viral envelope, is believed to be the primary mediator of viral attachment and entry into cells by binding to its receptor CD81 (7). Interestingly, it was reported that the interaction between E2 and CD81 increased T-cell proliferation (8) and inhibited cytotoxicity of natural killer cells (9). Therefore, E2 might have a central role in development of autoimmune phenomena seen in HCV-infected individuals.
Figure 1.
HCV entry is initiated by the interaction between E2 and CD81. By binding to the large extracellular loop of surface receptor CD81, HCV envelope protein E2 induces production of inflammatory signaling pathways and production of HSPs.
We hypothesized that HCV E2 protein binds to CD81 expressed on thyroid cells and activates a cascade of inflammatory responses that trigger autoimmunity. The aim of this study was to test this hypothesis and dissect the underlying mechanisms. Our data support the hypothesis that HCV may induce autoimmune thyroiditis by triggering cytokines and HSP production through binding to CD81.
Material and Methods
Cell cultures
Human thyroid cell line ML-1 (female origin; a gift from Dr. Schönberger, University of Regensburg, Germany) is derived from a differentiated follicular thyroid carcinoma (10). ML-1 cells express thyroglobulin (Tg) and represent a suitable model for biological studies of E2-thyrocyte interactions. ML-1 cells were maintained in Dulbecco’s Modified Eagle’s Medium (Thermo Fischer Scientific Inc., Waltham, MA), supplemented with 10% fetal calf serum, 1% penicillin (100 μg/mL), and streptomycin (100 μg/mL). ML-1 cells were authenticated by measurement of their key phenotypic marker, Tg by polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay.
Human hepatocyte cell line Huh7.5 (male origin) was kindly provided by Apath LLC (St. Louis, MO) and maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Huh7.5-JFH1, a subclone of Huh7.5, was authenticated by its ability to sustain and propagate HCV infection. Human hepatocellular carcinoma (HepG2; male origin) was obtained from ATCC (authenticated by ATCC) and was cultured in Eagle’s Minimum Essential Medium supplemented with 10% FBS and 1% penicillin-streptomycin. Cells were cultured at 37°C in 5% CO2, and medium was replaced every 48 hours until confluent.
Human thyroid primary cells
The project was approved as exempt by the Icahn School of Medicine Institutional Review Board. Human primary thyrocytes were prepared from fresh normal deidentified thyroid tissue adjacent to thyroid tumor collected during surgery (8 females, 2 males). Briefly, ∼0.3 to 1.2 g of tissue was washed in phosphate-buffered saline, minced on ice, and incubated with 200 U/mL collagenase solution for 45 minutes at 37°C on a shaker 3 times. Cells were harvested and cultured with medium E199/EBSS (Thermo Fischer Scientific) supplemented with 10% FBS and 1% penicillin-streptomycin. After overnight culture in 37°C humidified air at 5% CO2, cells were washed twice to remove mononuclear cells and kept in culture for 2 days. Each set of experiments was repeated in duplicate or triplicate.
Binding of anti-CD81 monoclonal antibody
Flow cytometry for CD81 was performed as previously described (11). ML-1 cells, cultured in 6-cm dishes, were harvested and washed with phosphate-buffered saline and resuspended in flow cytometry wash buffer supplemented with 0.02% sodium azide. Cells (5 × 105) were incubated for 30 minutes at 4°C with 10 μL of fluorescein isothiocyanate–conjugated mouse anti–CD81 monoclonal antibody (BD Biosciences Pharmingen, San Jose, CA; cat. No. 551108) or fluorescein isothiocyanate–conjugated nonspecific immunoglobulin G1 control antibody and washed twice. CD81 binding was quantified by flow cytometry (mean fluorescence intensity), using a Beckman Coulter Epics XL-MCL Flow Cytometer (Fullerton, CA).
Stimulation of cells with E2
To investigate the effect of E2, cells were incubated with 5 μg/mL of recombinant HCV E2 protein (ImmunoDx, LLC, Woburn, MA) for different periods of time as indicated in individual experiments. The choice of this concentration was based on our previous study showing that this concentration did not reduce the viability of thyroid cells (11). Cells (4 × 105 ML-1; 2 × 105 Huh7.5, HepG2, or human thyroid primary cells) were seeded into 6-well plates for 24 hours. Medium was then removed; cells were washed and incubated with 5 µg E2/mL culture medium.
Cytokine measurement
Supernatants of ML-1 cells in culture incubated with or without E2 were collected after 12, 24, and 48 hours. Supernatants of thyroid primary cells were collected after 12, 24, and 48 hours and 3, 5, and 7 days of incubation with or without E2. Interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, IL-1β, IL-12(p40), IL-17, and interferon (IFN)-γ were measured using Luminex multiplex assay (EMD; Millipore, Billerica, MA). IFN-α was measured using the VeriKine Human Interferon Alpha ELISA Kit (PBL Assay Science, Piscataway, NJ).
Tg measurement
To assess the function of ML-1 cells, Tg was measured in supernatant from ML-1 cells and thyroid primary cells in culture and cell lysate from ML-1, using Access DXI competitive-binding immunoenzymatic assay (Kit: Lot #434727; Beckman Coulter Inc., Jersey City, NJ).
RNA extraction
Total cellular RNA was extracted from cells using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol.
Quantitative real-time PCR (real-time qPCR)
Complementary DNA (cDNA) was synthesized by SuperScript III First-Strand Kit (Invitrogen) using 1 µg of RNA. Messenger RNA (mRNA) levels of IL-6, IL-8, and TNFα were measured by qPCR using commercially available TaqMan gene expression–specific primers (Applied Biosystems, Foster City, CA).
For analysis of microRNAs, total RNA was extracted from ML-1, primary thyroid cells, Huh7.5, and HepG2 in culture, and reverse transcription was carried on 1 µg total RNA using TaqMan microRNA (miRNA) reverse transcription kit (Applied Biosystems). Single-stranded cDNA was synthesized using commercially available specific 5× RT primers for the target miRNA miR-122 and endogenous control miR-16 (Applied Biosystems). Therefore, only the miRNA of interest was reverse transcribed into cDNA. The cDNA was amplified using Taqman Universal PCR Master Mix with uracil N-glycosylase and miRNA-specific Taqman probes hsa-miR-122-5p (UGGAGUGUGACAAUGGUGUUUG) and hsa-miR-16-5p (UAGCAGCACGUAAAUAUUGGCG). The results were quantified by 7300 Real-Time PCR Biosystem (Applied Biosystems). RNA from Huh cells highly expressing miR-122 (courtesy of Dr. Evans, MS, at Mount Sinai, NY) was used as positive control and RNA from HepG2 as negative control (12).
Transcriptome and pathway analyses
Extracted RNA (500 ng) from cells in culture (treated with E2 for 12 hours or untreated) was used for RNA sequencing (RNA-seq). RNA-seq and transcriptome analysis were performed as previously described (13). We performed pathway analysis to define functional networks and to identify genes that are upregulated or downregulated by E2. The Ingenuity Pathway Analysis (IPA; https://analysis.ingenuity.com/) was used to perform a core analysis on the data set gene files generated by RNA-seq. A cutoff of fold change >2 and P value <0.05 were used for including genes in the pathway analysis.
Statistics
Data are expressed as mean ± standard error of the mean. Mean values were tested for statistical significance using the Student t test. A significance level of 5% (P < 0.05) was applied when comparing treated cells with control cells. Statistical analyses were performed using GraphPad Prism 5 (San Diego, CA).
For RNA-seq, expressed transcripts were subjected to pathway analysis by Gene Ontology (GO) and IPA. Fisher’s exact test was used to calculate P value for the probability that a pathway was significantly enriched in input genes compared with the genome, and the pathways/networks were ranked by fold change expressed in log2 ratio and P values.
Results
CD81 is expressed on ML-1 thyroid cell line
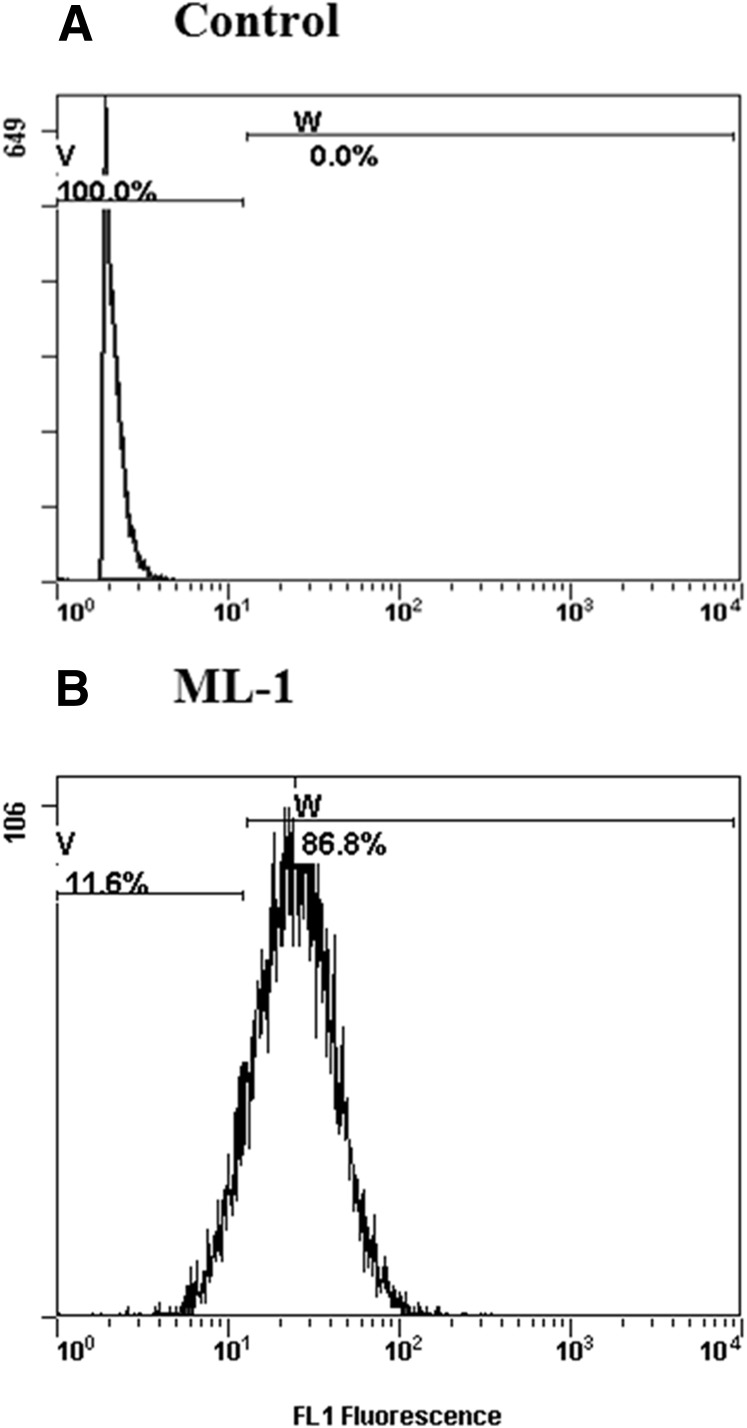
We first examined the expression of CD81 on thyroid cell line by flow cytometry. The analysis showed a robust expression of CD81 on ML-1 cells (Fig. 2), comparable with what we previously reported in human thyroid primary cells and in Huh cells (5, 11).
Figure 2.
Expression of human CD81 receptor on ML-1 cells. ML-1 cells were incubated with (A) immunoglobulin G1 isotope control or (B) human anti-CD81 antibody. Flow cytometry shows a robust expression of HCV surface receptor CD81 on ML-1 cells in culture.
E2 induces IL-8 and IL-6 production in ML-1 cells
Cytokine mRNA expression levels
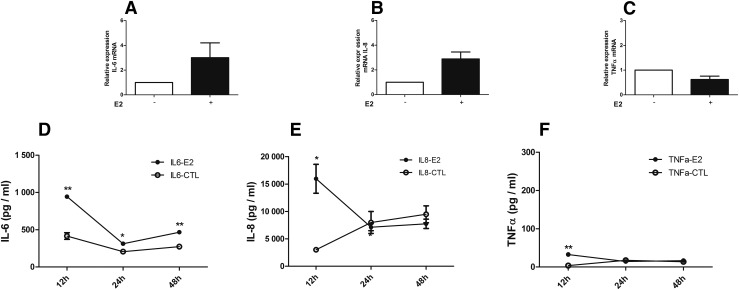
We examined the expression of IL-6, IL-8, and TNFα mRNA in ML-1 cells after 12 hours of incubation with recombinant E2 protein. These were selected because they showed increased protein levels (see later). The results showed a significant increase in the mRNA expression levels (corrected to glyceraldehyde 3-phosphate dehydrogenase) of IL-6 (∼threefold) and IL-8 (∼threefold) but not TNFα in E2-treated cells compared with untreated cells [Fig. 3(A–C)].
Figure 3.
Expression of cytokines in ML-1 cells in culture incubated with HCV envelope protein E2. (A–C) Incubation of ML-1 cells with recombinant HCV E2 protein (5 μg/mL) induced increased expression of (A) IL-6 and (B) IL-8 mRNA levels, but (C) TNFα mRNA levels were unchanged. Relative expression of mRNA was determined by real-time qPCR (corrected to glyceraldehyde 3-phosphate dehydrogenase): (A) IL-6, (B) IL-8, and (C) TNFα. Similarly, protein levels of (D) IL-6 and (E) IL-8 were increased, whereas (F) TNFα was only slightly increased at 12 hours. Concentration of cytokines (pg/mL) in supernatants of cultured cells: (D) IL-6, (E) IL-8, and (F) TNFα. The results are presented as mean ± standard error of the mean. *P < 0.05; **P < 0.01. CTL, control (untreated cells).
Cytokine protein expression levels
We tested the global cytokine responses of ML-1 cells upon exposure to E2; protein levels of 8 major cytokines/chemokines implicated in thyroid autoimmunity were measured in the supernatant of cells in culture. We incubated cells with E2 up to 48 hours as ML1 cells proliferate rapidly and medium needs to be changed every 48 hours. A significant increase in concentration of IL-6 was observed in supernatants of ML-1 cells treated with E2 for 12, 24, and 48 hours compared with untreated cells [Fig. 3(D)], consistent with the increases we observed in mRNA levels of this cytokine. E2 also induced a significant increase in the concentrations of IL-8 and TNFα at 12 hours [Fig. 3(E) and 3(F)]. Of note, the fold change in TNFα at 12 hours was ∼8, IL-8 was ∼4, and IL-6 was ∼2 (Fig. 3). The levels of IL-1β, IL-12(p40), IL-17, and IFN-γ were all lower than the detection limit (3.2 pmol/L) in treated and untreated cells. In all tested samples, the levels of IFN-α were lower than the detection limit of the kit we used (12.5 pg/mL).
E2 induces IL-6, IL-8, and TNFα expression in human thyroid cells in primary culture
Cytokine mRNA expression levels
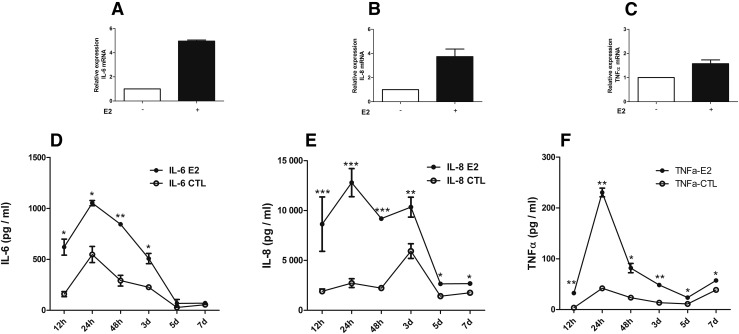
At 12 hours, a robust increase in IL-6 (approximately fivefold) and IL-8 (approximately fourfold) mRNA expression was observed [Fig. 4(A) and 4(B)]. The relative expression of TNFα mRNA was increased by approximately twofold [Fig. 4(C)]. Thus, the cytokine response to E2 protein was stronger in thyrocytes in primary culture than in ML-1, and they also showed increase in TNFα.
Figure 4.
Expression of cytokines in human thyroid primary cells in culture incubated with recombinant HCV envelope protein E2. Recombinant HCV E2 protein at a concentration of 5 µg/mL induced an increased expression of IL-6, IL-8, and TNFα both at the mRNA and at the protein levels. Time course up to 1 week shows increased protein secretion in supernatants of human primary thyrocytes. (A–C) Relative expression of mRNA by real-time qPCR (corrected to glyceraldehyde 3-phosphate dehydrogenase): (A) IL-6, (B) IL-8, and (C) TNFα. (D–F) Concentration of cytokines (pg/mL) in supernatant: (D) IL-6, (E) IL-8, and (F) TNFα. Results are presented as mean ± standard error of the mean. *P < 0.05; **P < 0.01; ***P < 0.001.
Cytokine protein expression levels
In human thyroid primary cells, the protein levels of cytokines showed a similar response to that of mRNA expression. A time course experiment showed a strong response with production of IL-6, IL-8, and TNFα in cells incubated with E2 protein compared with cells not exposed to E2 [Fig. 4(D–F)], increasing rapidly and remaining significantly higher for up to 7 days. The highest fold changes for IL-6 and TNFα were observed at 12 hours (∼3.0 and 8.0, respectively) and for IL-8 at 24 hours (∼4.0).
Similar to what we observed in ML-1, the levels of IL-1β, IL-12(p40), IL-17, and IFN-γ were lower than the detection limit. The levels of IFN-α were also undetectable, consistent with previous data showing that E2 protein inhibited production of IFNα by plasmacytoid dendritic cells, the main source for IFN-α (14). Please note that in contrast to thyroid tissue, it is likely that the number of immune cells was very low in thyroid cell cultures.
Primary thyroid cells do not proliferate and require time to acclimatize and reach confluence; therefore, primary thyrocytes were cultured and stimulated with E2 for up to 7 days. Long duration of cell culture, however, could result in growth of fibroblasts. Therefore, we mainly refer to the results up to 48 hours.
Effects of E2 on Tg levels
To confirm that cells used in this experiment consisted of well-differentiated thyroid cells, we measured Tg levels in cell supernatants (ML-1 and thyroid primary cells) and cell lysate (ML-1). After 48 hours of culture, ML-1 cells showed high levels of Tg in cell lysate but lower levels of secreted Tg (737 and 18.7 ng/mL, respectively). Supernatants from thyroid primary cells showed high levels of secreted Tg with and without E2 protein (122 and 108 ng/mL, respectively, at 24 hours). There was no significant difference in secreted Tg levels after incubation with E2 up to 7 days.
Transcriptome analysis
Pathway analysis—networks
RNA-seq identified 1677 upregulated and 2921 downregulated genes in ML-1 cells and 652 upregulated and 1157 downregulated genes in human thyroid primary cells after 12 hours of incubation with HCV E2 protein using a cutoff of fold change >2 and P < 0.05. To provide insights into the mechanisms underlying the induction of autoimmunity in the thyroid by HCV and to define molecular networks induced by E2, pathway analysis was performed.
E2 activates IL-8 and innate immune response pathways in ML-1 cells
GO analysis identified several pathways that were upregulated by E2: cell cycle process (P = 9 × 10−4), response to oxidative stress (P = 3.6 × 10−4), regulation of apoptosis (P = 1.5 × 10−4), and regulation of T-cell proliferation and migration (P = 2.0 × 10−3) (Supplemental Table 1 (43.5KB, xls) ).
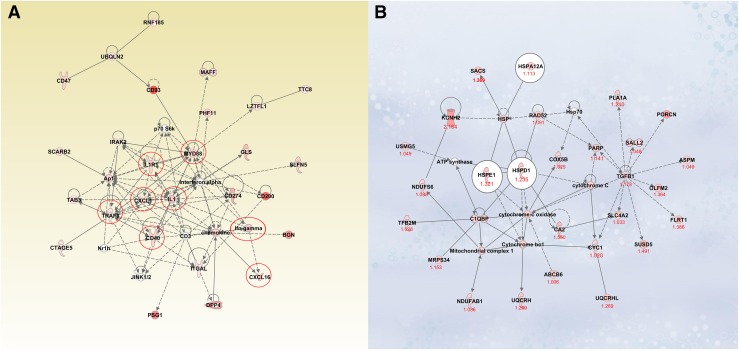
Interestingly, E2 upregulated several HCV surface receptors on thyroid cells: CD81 (log2 ratio, 1.4), Ocludin (log2 ratio, 3.9), Claudin (log2 ratio, 7.0), and LDL-R (log2 ratio, 1.4). Several cytoplasmic and nuclear molecules involved in the IL-8 signaling pathway were upregulated by E2 (e.g., PI3K, AKT) (Supplemental Fig. 1 (19.4MB, tif) ). E2 induced increased expression of transforming growth factor (TGF)β-1, a gene that was shown to be involved in HCV infection (15). IPA also demonstrated E2 induction of immune response genes, including CD40 [Fig. 5(A)] and other molecules participating in the nuclear factor κB (NF-κB) pathway such as PI3K, TRAF3, TRAF5, TRAF6, and Myd88. Furthermore, E2 induced upregulation of genes involved in inflammatory responses to infection, including IFN regulatory factors (IRF1, IFIT3 and IFIT5, IFIH1) and Mx1.
Figure 5.
(A) An example of innate immune response induced by E2 in ML-1 cells. Recombinant HCV E2 protein increased the expression of costimulatory molecules such as CD40 and the activation of other molecules involved in the NF-κB signaling pathways in the human thyroid cell line ML-1. (B) HSP network induced by HCV E2 protein in human thyroid primary cells. In human thyroid primary cells, the expression of several HSPs was increased by HCV envelope glycoprotein E2: HSP60 (HSPD1), HSP10 (HSPE1), and HSP12A (heat shock 70kDa protein12a). Ingenuity pathway analysis detected several molecules involved in this network. The numbers indicate the log2 ratio for each gene.
Furthermore, 5 gene signatures (SOCS3, CC2, GADD45A, KLF6, and CD74) that were reported to be expressed in thyroid tissues of patient with Graves disease (16) were also over-expressed in ML-1 cells treated with HCV E2 protein.
E2 induced activation of IL-8 pathway and production of HSPs in human thyroid primary cells
GO analysis showed that the main pathways induced by E2 in primary thyrocytes in culture were cell cycle process (P = 6.77 × 10−8), regulation of protein ubiquitination (P = 3.3 × 10−4), regulation of chemotaxis (P = 4 × 10−3), regulation of apoptosis (P = 6.3 × 10−3), and response to endogenous stimuli (P = 0.013) (Supplemental Table 2 (26KB, xls) ).
In addition, IPA detected an E2-mediated upregulation of several cytoplasmic molecules involved in the IL-8 signaling pathways (e.g., PI3K, PKC, and JNK) (Supplemental Fig. 1 (19.4MB, tif) ). IL-8 was previously associated with HCV infection (17), and HCV E2 protein triggered IL-8 secretion in hepatocytes and thyrocytes (11, 18). Similar to our observation in ML-1 cells, E2 upregulated the expression of TGFβ-1 in thyroid cells.
Interestingly, several HSPs, including HSP60 (HSPD1), HSPA12A (heat shock 70kDa protein 12A), and HSPE1 (HS 10kDa protein), were upregulated by E2 in primary thyrocytes. IPA analysis identified additional genes that are involved in the HSP network [Fig. 5(B)].
miR-122
miRNAs are small noncoding RNA sequences that play an important role in regulating gene expression in cells. Recent data showed that miR-122 enhances HCV viral replication in hepatocytes (19). The specific tropism of HCV for hepatocytes has partly been attributed to its interaction with miR-122, which is highly expressed in hepatocytes.
Therefore, we tested the expression of miR-122 in ML-1 thyroid cell line and thyroid primary cells. Our results showed that miR-122 is expressed in thyroid cells (cycle threshold ∼29), but its expression is not affected by E2 protein (relative expression corrected to mir16 ∼1.2 to 1.5). It remains to be determined if HCV infection induces overexpression of miR-122.
Discussion
Thyroiditis is one of the major extrahepatic manifestations in patients with chronic HCV infection (20), and we have previously shown that HCV can infect thyroid cells (5); this suggests that autoimmune thyroiditis may be triggered by direct viral infection of thyroid cells. However, it is possible that HCV can trigger autoimmune thyroiditis even without infecting thyroid cells directly. Our current study, showing that HCV E2 glycoprotein triggers strong cytokine and inflammatory responses by binding to its receptor CD81 on thyroid cells, supports this hypothesis (Fig. 1). Transcriptome analysis revealed HCV E2 mediated upregulation of several factors involved in both innate and adaptive immune responses.
HCV entry into cells, the first step in the cycle of infection, is initiated by the interaction between E2, the main HCV envelope protein, and the large extracellular loop of CD81 (21). CD81 is highly expressed on ML-1 (Fig. 2), as well as on human primary thyrocytes (11). In vitro studies have demonstrated that E2 protein is necessary not only for the entry of HCV into cells but also for viral replication and apoptosis of hepatocytes (22–24) and that anti–CD81mAb can efficiently block HCV dissemination in cells (25).
Although E2 induced increased cell death in hepatocytes, it did not inhibit cell growth in thyrocytes (11). Indeed, the interaction between E2 and CD81 increases T-cell proliferation (8) and inhibits cytotoxicity of natural killer cells (9). In human thyroid cells, E2 protein induces a cascade of signals altering the expression of several molecules implicated in immune response. We previously showed that E2 can bind to human thyroid cells and stimulate IL-8 production (12). In the current study, we expanded these studies to examine the global cellular and inflammatory effects of E2 in human thyroid cells. Our results confirmed the effect of E2 in inducing IL-8 production and in addition showed a robust induction of 2 other major inflammatory cytokines, TNFα and IL-6. TNFα is a cytokine implicated in autoimmune responses and in apoptosis (26). Binding to its receptor, TNFα induces production of other inflammatory cytokines through activation of the NF-κB signaling pathway (26); treating human thyroid primary cells with TNFα induced production of IL-8 and IL-10 (27). In a mouse model for granulomatous experimental thyroiditis, mice treated with anti–TNFα monoclonal antibody showed less fibrosis and were able to clear thyroid lesions earlier (28). Interestingly, the initial induction of TNFα by HCV in hepatocytes could be blocked by antibody against E2 (29). Anti–TNFα agents may, therefore, have a potential therapeutic effect in preventing the extrahepatic manifestations of HCV infection.
Studies have shown higher levels of peripheral blood Th17 cells and increased serum IL-6 in patients with Hashimoto thyroiditis (HT) (30). Th17 cells have been implicated in autoimmune thyroiditis; in fact, Th17 cell differentiation is triggered by a combination of IL-6 and TGFβ through the STAT3 pathway (31). IL-6 was upregulated by E2, suggesting that E2 protein could potentially contribute to Th17 cell differentiation in the setting of thyroidal inflammation. Further support for the role of IL-6 and TNFα in AITD comes from genetic studies showing that single nucleotide polymorphisms in IL-6 and TNFα were associated with AITD (32).
IL-8 mediates the activation and migration of lymphocytes into tissues (33) and is thought to be important in the development of autoimmune reactions. E2 induced expression of IL-8 in human thyroid cells, consistent with previously reported results (5, 11), and upregulation of several cytoplasmic and nuclear factors implicated in the IL-8 pathway in both ML-1 and primary thyroid cells. Similar results showing induction of IL-8 secretion by E2 were reported in other cells (34). It is likely that production of IL-8 is one of the first steps in the response to infection and that IL-8 secretion may participate in recruiting T cells to the thyroid and triggering autoimmune thyroiditis. However, TNFα induced increased secretion of IL-8 in primary thyroid cells and thyroid tumor cell lines (BCPAP and TPC-1) (35). Therefore, we cannot exclude the possibility that increased IL-8 is partly due to in situ production of TNFα in thyroid cells induced by E2. Interestingly, higher serum levels of IL-8 and TNFα were reported in HCV patients compared with controls, and IL-8 levels correlated with the degree of liver fibrosis (36). The levels of basal IL-8 were higher in ML-1 cells than in primary thyroid cells, consistent with previously reported results in other thyroid cancer cell lines (35). However, IL-8 response to E2 protein was similar in ML-1 cells and primary thyrocytes in culture at 12 hours.
There is also growing evidence that IL-8 is a tumor-promoting chemokine and may be a marker of aggressiveness in thyroid cancer (37), which may lead to development of new therapeutic strategies (35). Therefore, our data may suggest that HCV infection can worsen thyroid cancer prognosis.
RNA-seq detected upregulation of inflammatory and apoptotic pathways in thyroid cells exposed to E2. However, ML-1 is a cell line; therefore, responses to stimuli may differ from thyrocytes isolated from normal thyroid tissue. Indeed, RNA-seq detected upregulation of more genes in ML-1 than in primary thyroid cells. E2 induced overexpression of all HCV surface receptors in ML-1 cells.
Of special interest, the expression of HSPs such as HSP60 was increased mainly by E2 in primary thyrocytes. HSPs are present in cells under normal conditions but are upregulated in response to stress, such as infection. HSP60 has been shown to be involved in the development of HT; HSP60 levels were increased in the blood of HT patients (38), and its expression was increased in thyrocytes collected from thyroid tissues of patients with HT compared with controls (38, 39). Intriguingly, HSP60 was also overexpressed in orbital fat tissue in patients with Graves ophthalmopathy (40). Although the mechanisms by which HSP upregulation (e.g., during infection) contributes to the development of AITD are not known, one possibility is sequence homology with thyroid autoantigens, suggesting that upregulation of HSP60 may trigger thyroid autoimmunity by molecular mimicry (38).
Our transcriptome analysis in thyroid cells exposed to E2 also revealed upregulation of several factors involved in both innate and adaptive immune responses. Under physiological conditions, activation of innate immune responses is initiated by toll-like receptor (TLR) recognition of a virus, triggering recruitment of the adaptor protein MYD88 to mobilize the signaling factors, leading to activation of NF-κB (41). E2 increased expression of several of these signaling factors (MYD88, IRAK, TOLLIP, and TRAF6). Furthermore, E2 induced overexpression of CD40 in ML-1 cells. CD40 plays a crucial role in adaptive immunity and has been implicated in development of autoimmune thyroid diseases (42). There is also crosstalk between TLRs and CD40, and it was reported that TLR4 mediates HSP60 signaling (43).
In conclusion, our study demonstrated that the interaction between E2 and CD81 on human thyroid cells, independent of HCV entry into thyrocytes, mediated a cascade of intracellular signals, leading to strong cytokine and chemokine responses and activation of pathways known to be involved in immune responses to viral infection as well as pathways shown to participate in thyroid autoimmunity. Taken together, these data support a role for HCV and potentially other viral infections in triggering autoimmune thyroiditis through bystander mechanisms.
Acknowledgments
We thank Dr. Nikolina Babic for her assistance in Tg measurements and Dr. Angela Lombardi for her valuable comments.
Acknowledgments
This work was supported in part by Grants DK61659, DK067555, and DK073681 from the National Institute of Diabetes and Digestive and Kidney Diseases (to Y.T.). In addition, this material is based on work supported in part by VA Merit Award 1I01BX002031 from the US Department of Veterans Affairs Biomedical Laboratory Research and Development Service (to Y.T.). Further support was given by a grant from the South Eastern Norwegian Health Authority (HSØ) (to S.S.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AITD
- autoimmune thyroid disease
- FBS
- fetal bovine serum
- GO
- Gene Ontology
- HCV
- hepatitis C virus
- HepG2
- human hepatocellular carcinoma
- HSP
- heat shock protein
- HT
- Hashimoto thyroiditis
- IL
- interleukin
- IFN
- interferon
- IPA
- Ingenuity Pathway Analysis
- mRNA
- messenger RNA
- miRNA
- microRNA
- NF-κB
- nuclear factor κB
- PCR
- polymerase chain reaction
- RNA-seq
- RNA sequencing
- Tg
- thyroglobulin
- TGF
- transforming growth factor
- TNF
- tumor necrosis factor
- TLR
- toll-like receptor
References
- 1.Menconi F, Hasham A, Tomer Y. Environmental triggers of thyroiditis: hepatitis C and interferon-α. J Endocrinol Invest. 2011;34(1):78–84. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Hepatitis C. Available at: http://www.who.int/mediacentre/factsheets/fs164/en/. 2016.
- 3.Antonelli A, Ferri C, Pampana A, Fallahi P, Nesti C, Pasquini M, Marchi S, Ferrannini E. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117(1):10–13. [DOI] [PubMed] [Google Scholar]
- 4.Tomer Y. Hepatitis C and interferon induced thyroiditis. J Autoimmun. 2010;34(3):J322–J326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackard JT, Kong L, Huber AK, Tomer Y. Hepatitis C virus infection of a thyroid cell line: implications for pathogenesis of hepatitis C virus and thyroiditis. Thyroid. 2013;23(7):863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavio N, Lai MM. The hepatitis C virus persistence: how to evade the immune system? J Biosci. 2003;28(3):287–304. [DOI] [PubMed] [Google Scholar]
- 7.Voisset C, Dubuisson J. Functional hepatitis C virus envelope glycoproteins. Biol Cell. 2004;96(6):413–420. [DOI] [PubMed] [Google Scholar]
- 8.Wack A, Soldaini E, Tseng C, Nuti S, Klimpel G, Abrignani S. Binding of the hepatitis C virus envelope protein E2 to CD81 provides a co-stimulatory signal for human T cells. Eur J Immunol. 2001;31(1):166–175. [DOI] [PubMed] [Google Scholar]
- 9.Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schönberger J, Bauer J, Spruss T, Weber G, Chahoud I, Eilles C, Grimm D. Establishment and characterization of the follicular thyroid carcinoma cell line ML-1. J Mol Med (Berl). 2000;78(2):102–110. [DOI] [PubMed] [Google Scholar]
- 11.Akeno N, Blackard JT, Tomer Y. HCV E2 protein binds directly to thyroid cells and induces IL-8 production: a new mechanism for HCV induced thyroid autoimmunity. J Autoimmun. 2008;31(4):339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Wu S, Tong L, Luan T, Lin L, Lu S, Zhao W, Ma Q, Liu H, Zhong Z. miR-122 affects the viability and apoptosis of hepatocellular carcinoma cells. Scand J Gastroenterol. 2009;44(11):1332–1339. [DOI] [PubMed] [Google Scholar]
- 13.Akeno N, Smith EP, Stefan M, Huber AK, Zhang W, Keddache M, Tomer Y. IFN-α mediates the development of autoimmunity both by direct tissue toxicity and through immune cell recruitment mechanisms. J Immunol. 2011;186(8):4693–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florentin J, Aouar B, Dental C, Thumann C, Firaguay G, Gondois-Rey F, Soumelis V, Baumert TF, Nunès JA, Olive D, Hirsch I, Stranska R. HCV glycoprotein E2 is a novel BDCA-2 ligand and acts as an inhibitor of IFN production by plasmacytoid dendritic cells. Blood. 2012;120(23):4544–4551. [DOI] [PubMed] [Google Scholar]
- 15.Jee MH, Hong KY, Park JH, Lee JS, Kim HS, Lee SH, Jang SK. New mechanism of hepatic fibrogenesis: hepatitis C virus infection induces transforming growth factor β1 production through glucose-regulated protein 94. J Virol. 2015;90(6):3044–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin X, Sachidanandam R, Morshed S, Latif R, Shi R, Davies TF. mRNA-seq reveals novel molecular mechanisms and a robust fingerprint in Graves’ disease. J Clin Endocrinol Metab. 2014;99(10):E2076–E2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polyak SJ, Khabar KS, Rezeiq M, Gretch DR. Elevated levels of interleukin-8 in serum are associated with hepatitis C virus infection and resistance to interferon therapy. J Virol. 2001;75(13):6209–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balasubramanian A, Ganju RK, Groopman JE. Hepatitis C virus and HIV envelope proteins collaboratively mediate interleukin-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem. 2003;278(37):35755–35766. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Masaki T, Yamane D, McGivern DR, Lemon SM. Competing and noncompeting activities of miR-122 and the 5′ exonuclease Xrn1 in regulation of hepatitis C virus replication. Proc Natl Acad Sci USA. 2013;110(5):1881–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fallahi P, Ferrari SM, Politti U, Giuggioli D, Ferri C, Antonelli A. Autoimmune and neoplastic thyroid diseases associated with hepatitis C chronic infection. Int J Endocrinol. 2014;2014:935131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282(5390):938–941. [DOI] [PubMed] [Google Scholar]
- 22.Qin ZL, Ju HP, Wang WB, Ren H, Guan M, Zhao P, Qi ZT. The Arg719 residue at the C-terminal end of the stem region in hepatitis C virus JFH-1 E2 glycoprotein promotes viral infection. Virus Res. 2013;172(1–2):1–8. [DOI] [PubMed] [Google Scholar]
- 23.Chiou HL, Hsieh YS, Hsieh MR, Chen TY. HCV E2 may induce apoptosis of Huh-7 cells via a mitochondrial-related caspase pathway. Biochem Biophys Res Commun. 2006;345(1):453–458. [DOI] [PubMed] [Google Scholar]
- 24.Zhu LX, Liu J, Xie YH, Kong YY, Ye Y, Wang CL, Li GD, Wang Y. Expression of hepatitis C virus envelope protein 2 induces apoptosis in cultured mammalian cells. World J Gastroenterol. 2004;10(20):2972–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fofana I, Xiao F, Thumann C, Turek M, Zona L, Tawar RG, Grunert F, Thompson J, Zeisel MB, Baumert TF. A novel monoclonal anti-CD81 antibody produced by genetic immunization efficiently inhibits hepatitis C virus cell-cell transmission. PLoS One. 2013;8(5):e64221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3(9):745–756. [DOI] [PubMed] [Google Scholar]
- 27.Rotondi M, Coperchini F, Pignatti P, Sideri R, Groppelli G, Leporati P, La Manna L, Magri F, Mariotti S, Chiovato L. Interferon-γ and tumor necrosis factor-α sustain secretion of specific CXC chemokines in human thyrocytes: a first step toward a differentiation between autoimmune and tumor-related inflammation? J Clin Endocrinol Metab. 2013;98(1):308–313. [DOI] [PubMed] [Google Scholar]
- 28.Chen K, Wei Y, Sharp GC, Braley-Mullen H. Decreasing TNF-alpha results in less fibrosis and earlier resolution of granulomatous experimental autoimmune thyroiditis. J Leukoc Biol. 2007;81(1):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Tian Y, Chan ST, Kim JY, Cho C, Ou JH. TNF-α induced by hepatitis C virus via TLR7 and TLR8 in hepatocytes supports interferon signaling via an autocrine mechanism. PLoS Pathog. 2015;11(5):e1004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Figueroa-Vega N, Alfonso-Pérez M, Benedicto I, Sánchez-Madrid F, González-Amaro R, Marazuela M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2010;95(2):953–962. [DOI] [PubMed] [Google Scholar]
- 31.Ye J, Livergood RS, Peng G. The role and regulation of human Th17 cells in tumor immunity. Am J Pathol. 2013;182(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durães C, Moreira CS, Alvelos I, Mendes A, Santos LR, Machado JC, Melo M, Esteves C, Neves C, Sobrinho-Simões M, Soares P. Polymorphisms in the TNFA and IL6 genes represent risk factors for autoimmune thyroid disease. PLoS One. 2014;9(8):e105492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243(4897):1464–1466. [DOI] [PubMed] [Google Scholar]
- 34.Urbaczek AC, Ribeiro LC, Ximenes VF, Afonso A, Nogueira CT, Generoso WC, Alberice JV, Rudnicki M, Ferrer R, Fonseca LM, Costa PI. Inflammatory response of endothelial cells to hepatitis C virus recombinant envelope glycoprotein 2 protein exposure. Mem Inst Oswaldo Cruz. 2014;109(6):748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coperchini F, Pignatti P, Leporati P, Carbone A, Croce L, Magri F, Chiovato L, Rotondi M. Normal human thyroid cells, BCPAP, and TPC-1 thyroid tumor cell lines display different profile in both basal and TNF-α-induced CXCL8 secretion. Endocrine. 2016;54(1):123–128. [DOI] [PubMed] [Google Scholar]
- 36.Kaplanski G, Farnarier C, Payan MJ, Bongrand P, Durand JM. Increased levels of soluble adhesion molecules in the serum of patients with hepatitis C: correlation with cytokine concentrations and liver inflammation and fibrosis. Dig Dis Sci. 1997;42(11):2277–2284. [DOI] [PubMed] [Google Scholar]
- 37.Rotondi M, Coperchini F, Chiovato L. CXCL8 in thyroid disease: from basic notions to potential applications in clinical practice. Cytokine Growth Factor Rev. 2013;24(6):539–546. [DOI] [PubMed] [Google Scholar]
- 38.Marino Gammazza A, Rizzo M, Citarrella R, Rappa F, Campanella C, Bucchieri F, Patti A, Nikolic D, Cabibi D, Amico G, Conaldi PG, San Biagio PL, Montalto G, Farina F, Zummo G, Conway de Macario E, Macario AJ, Cappello F. Elevated blood Hsp60, its structural similarities and cross-reactivity with thyroid molecules, and its presence on the plasma membrane of oncocytes point to the chaperonin as an immunopathogenic factor in Hashimoto’s thyroiditis. Cell Stress Chaperones. 2014;19(3):343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotani T, Aratake Y, Hirai K, Hirai I, Ohtaki S. High expression of heat shock protein 60 in follicular cells of Hashimoto’s thyroiditis. Autoimmunity. 1996;25(1):1–8. [DOI] [PubMed] [Google Scholar]
- 40.Cheng KC, Huang HH, Hung CT, Chen CC, Wu WC, Suen JL, Chen KJ, Wu YJ, Chang CH. Proteomic analysis of the differences in orbital protein expression in thyroid orbitopathy. Graefes Arch Clin Exp Ophthalmol. 2013;251(12):2777–2787. [DOI] [PubMed] [Google Scholar]
- 41.Muralidharan S, Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol. 2013;94(6):1167–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HJ, Li CW, Hammerstad SS, Stefan M, Tomer Y. Immunogenetics of autoimmune thyroid diseases: a comprehensive review. J Autoimmun. 2015;64:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohashi K, Burkart V, Flohé S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164(2):558–561. [DOI] [PubMed] [Google Scholar]