Abstract
Identifying novel treatments that facilitate extinction learning could enhance cue-exposure therapy and reduce high relapse rates in alcoholics. Activation of mGlu5 receptors in the infralimbic prefrontal cortex (IL-PFC) facilitates learning during extinction of cue-conditioned alcohol-seeking behavior. Small-conductance calcium-activated potassium (KCa2) channels have also been implicated in extinction learning of fear memories, and mGlu5 receptor activation can reduce KCa2 channel function. Using a combination of electrophysiological, pharmacological, and behavioral approaches, this study examined KCa2 channels as a novel target to facilitate extinction of alcohol-seeking behavior in rats. This study also explored related neuronal and synaptic mechanisms within the IL-PFC that underlie mGlu5-dependent enhancement of extinction learning. Using whole-cell patch-clamp electrophysiology, activation of mGlu5 in ex vivo slices significantly reduced KCa2 channel currents in layer V IL-PFC pyramidal neurons, confirming functional downregulation of KCa2 channel activity by mGlu5 receptors. Additionally, positive modulation of KCa2 channels prevented mGlu5 receptor-dependent facilitation of long-term potentiation in the IL-PFC. Systemic and intra-IL-PFC treatment with apamin (KCa2 channel allosteric inhibitor) significantly enhanced extinction of alcohol-seeking behavior across multiple extinction sessions, an effect that persisted for 3 weeks, but was not observed after apamin microinfusions into the prelimbic PFC. Positive modulation of IL-PFC KCa2 channels significantly attenuated mGlu5-dependent facilitation of alcohol cue-conditioned extinction learning. These data suggest that mGlu5-dependent facilitation of extinction learning and synaptic plasticity in the IL-PFC involves functional inhibition of KCa2 channels. Moreover, these findings demonstrate that KCa2 channels are a novel target to facilitate long-lasting extinction of alcohol-seeking behavior.
SIGNIFICANCE STATEMENT Alcohol use disorder is a chronic relapsing disorder that is associated with compulsive alcohol-seeking behavior. One of the main causes of alcohol relapse is the craving caused by environmental cues that are associated with alcohol. These cues are formed by normal learning and memory principles, and the understanding of the brain mechanisms that help form these associations can lead to the development of drugs and/or behavior therapies that reduce the impact that these cues have on relapse in alcoholics.
Keywords: addiction, alcohol, cues, extinction, infralimbic cortex, mGlu5, SK channels
Introduction
Relapse to alcohol is influenced by learning processes in which alcohol availability becomes associated with environmental cues. Exposure to these cues in alcoholics evokes maladaptive conditioned responses that manifest as strong cravings and alcohol seeking observed in humans (Papachristou et al., 2012; Naqvi et al., 2015) and rodents (Bienkowski et al., 2000; Sinclair et al., 2012; Cannady et al., 2013). Cue-exposure therapy is a procedure proposed to reduce the effect of cues to elicit alcohol seeking through repeated noncontingent cue exposure. However, meta-analyses of cue-exposure therapy for alcohol-seeking revealed low efficacy (Childress et al., 1993; Conklin and Tiffany, 2002). Recent evidence suggests that cue-exposure therapy is enhanced when combined with acute pharmacotherapeutic treatment. Indeed, cognitive-enhancing agents facilitate inhibitory learning and reduce cue-induced craving and alcohol-seeking in humans (MacKillop et al., 2015) and rodents (Vengeliene et al., 2008; Nic Dhonnchadha and Kantak, 2011; Gass et al., 2014a). However, the cellular and molecular mechanisms that underlie extinction learning within the context of addiction have not been fully determined. Understanding of the neuronal substrates that regulate extinction/inhibitory learning is critical for the development of treatments that can enhance cue-exposure therapy and reduce relapse risk.
Small-conductance calcium-activated potassium (KCa2) channels are expressed throughout the brain (Stocker and Pedarzani, 2000) and have established roles in regulating neuronal intrinsic excitability, synaptic plasticity, and integration of dendritic signaling (Stocker et al., 1999; Blank et al., 2003; Cai et al., 2004; Villalobos et al., 2004; Fakler and Adelman, 2008; Adelman et al., 2012). Cortical and hippocampal KCa2 channels also mediate learning and memory, as blocking or enhancing KCa2 channel activity (via systemic or localized pharmacological manipulation) bidirectionally modulates performance in cognitive and associative learning tasks (Stackman et al., 2002; Deschaux and Bizot, 2005; Hammond et al., 2006; Brosh et al., 2007). Pharmacological inhibition of KCa2 channels with apamin in the infralimbic prefrontal cortex (IL-PFC) facilitates extinction of cue-conditioned fear, whereas stimulating these channels with 1-ethyl-2-benzimidazolinone (1-EBIO) has the opposite effect (Criado-Marrero et al., 2014). This finding is intriguing given the significant overlap between the neurocircuitry involved in extinction of conditioned fear and cue-induced drug seeking (Peters et al., 2009; Gass and Chandler, 2013). Together, these findings suggest that IL-PFC KCa2 channels may similarly regulate extinction of alcohol-seeking behavior.
Interestingly, there is a functional relationship between KCa2 channels and the group I metabotropic glutamate receptor subtype 5 (mGlu5). Activation of mGlu5 enhances intrinsic neuronal excitability that appears to be attributable to reduced KCa2 channel activity (Mannaioni et al., 2001; Sourdet et al., 2003). More recent evidence demonstrates that mGlu5 and KCa2 channels can coassemble to regulate neuronal activity (García-Negredoet al., 2014), and additional evidence suggests that the functional coupling may underlie neuroadaptations induced by fear extinction (Sepulveda-Orengo et al., 2013). mGlu5 has been proposed as a promising molecular target for the facilitation of extinction learning based on preclinical evidence. Our previous findings have demonstrated that positive allosteric modulation of mGlu5 in the IL-PFC by 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) facilitates extinction of cue-conditioned alcohol seeking via potentiation of glutamatergic synaptic plasticity (Gass et al., 2014a). Similar findings have been observed on extinction of cocaine-seeking behavior after mGlu5 activation (Gass and Olive, 2009; Cleva et al., 2011). Although there is an established role for mGlu5 and KCa2 channels to coregulate neuronal activity and fear extinction, no studies have examined how alcohol-seeking behavior may be regulated by this functional relationship.
Accordingly, we hypothesized that KCa2 channel inhibition would facilitate extinction of cue-conditioned alcohol-seeking behavior. Given the relationship of KCa2 channels and mGlu5 receptors and their overlapping roles in mediating extinction behavior and synaptic plasticity within the IL-PFC, we hypothesized that mGlu5-dependent facilitation of extinction learning is functionally regulated via control of KCa2 channel activity. Electrophysiological procedures and behavioral pharmacology were used to examine the role of KCa2 channels in modulating extinction of cue-conditioned alcohol-seeking behavior in rats. We also sought to determine whether IL-PFC KCa2 channels could functionally regulate mGlu5-mediated facilitation of synaptic plasticity and extinction learning.
Materials and Methods
Animals
All procedures were conducted in male Wistar rats weighing 250–275 g on arrival (Harlan). Rats were housed individually in standard polycarbonate cages where food and water were continuously available except during behavioral testing. Experiments were performed during the dark portion of a reverse 12 h light cycle (lights off at 9:00 A.M.). All procedures were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina and were performed within the guidelines set forth by the National Research Council's Guideline for the Care and Use of Mammals in Neuroscience and Behavioral Research (2003).
Drugs
CDPPB was custom synthesized by Chemir Analytical Services, purified to >95% purity by liquid chromatography–mass spectrometry, and suspended in 10% (v/v) Tween 80 (Sigma-Aldrich). 3-((2-Methyl-4-thiazolyl)ethynyl) pyridine (MTEP) hydrochloride was purchased from Abcam and dissolved in sterile artificial CSF (ACSF) at a concentration of 5 μg/μl. Apamin (Sigma-Aldrich) and (RS)-2-chloro-5-hydroxyphenylglycine sodium salt (CHPG; Tocris) were dissolved in either ACSF for electrophysiological recordings or 0.9% sterile saline for behavioral studies. 1-EBIO (Tocris) was dissolved in 0.1% DMSO.
Whole-cell patch-clamp recordings
Whole-cell patch-clamp recordings were recorded as described previously (Mulholland et al., 2011; Padula et al., 2015). Wistar rats (P60–P75) were deeply anesthetized with isoflurane gas and decapitated. Rat brains were rapidly removed and placed in an ice-cold dissection solution consisting of (in mm) 200 sucrose, 1.9 KCl, 1.2 NaH2PO4, 6.0 MgCl2, 0.5 CaCl2, 0.4 ascorbate, 10 glucose, and 25 NaHCO3. Brains were then sectioned into coronal slices (200 μm) containing the IL-PFC. Slices were transferred to an incubation chamber filled with ACSF solution containing (in mm) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1.3 MgCl2, 2.0 CaCl2, 0.4 ascorbate, 10 glucose, and 25 NaHCO3 at 34°C for 30 min and then at room temperature for at least 30 min before recording at 25°C. The pH of all solutions listed above was adjusted to 7.4, and osmolarity was measured to be ∼300 mOsm. All external buffers were continuously aerated with 5% carbon dioxide/95% oxygen.
KCa2-mediated afterhyperpolarization currents (IAHP) were recorded in layer V IL-PFC pyramidal neurons using borosilicate glass electrodes (2–3 MΩ) filled with an internal solution (pH 7.3 using KOH; osmolarity, ∼290 mOsm) containing (in mm) 140 K-methylsulfate, 8.0 NaCl, 1.0 MgCl2, 0.05 EGTA, 10 HEPES, and 5 Mg2ATP. To activate KCa2-mediated IAHP, layer V IL-PFC pyramidal neurons were held at −70 mV in voltage-clamp mode, and 40 mV depolarizing voltage steps (400 ms duration) were applied with 2 s between steps. The protocol was repeated (three times) before and after a 10 min bath application of drugs. Peak amplitude (in picoamperes) of the KCa2-mediated IAHP (up to 100 ms after each hyperpolarization) was measured, and each triplicate was averaged before and after drug treatment. Recordings were acquired with a Multiclamp 700B amplifier (Molecular Devices) and analyzed by computer using AxographX software (Axograph). Currents were filtered at 4 kHz and acquired at 10 kHz using an ITC-18 interface.
Examination of systemic mGlu5 receptor activation on IL-PFC IAHP ex vivo
Naive Wistar rats were given subcutaneous injections of 30 mg/kg CDPPB or vehicle (10% Tween 80 v/v) 1 h before brain extraction for whole-cell patch-clamp recordings in the IL-PFC. IAHP currents were evoked as described above.
Extracellular field potential recordings
For field potential recordings, acute coronal slices containing the IL-PFC were prepared exactly as previously described above (Padula et al., 2015), with the exception that slices were 350 μm in thickness and ACSF contained a CaCl2 concentration of 2.5 mm. ACSF-filled theta-glass stimulating pipettes were placed in layer II/III, and ACSF-filled recording pipettes (0.3–2 MΩ) were placed in layer V of the IL-PFC. Field EPSPs (fEPSPs) were recorded at 30°C. Input/output curves were generated to determine the maximum stimulation intensity for each recording, and stimulation was adjusted to 50–60% maximum intensity for the duration of each recording. During the baseline period, slices were stimulated at a rate of 0.02 Hz. All drugs were applied for 10 min before high-frequency stimulation (HFS) consisting of five trains (50 Hz stimulation, 2 s duration) with a 30 s intertrain interval (Moussawi et al., 2009). After HFS, fEPSPs were evoked once per minute (0.02 Hz). Synaptic strength/potentiation was measured as the change in the initial onset of the fEPSP (slope) after HFS, relative to the normalized baseline slope, and expressed as a percentage of baseline. Long-term potentiation (LTP) recordings were amplified (gain of 10–50), filtered (0.2–1.2 kHz), and digitized at 10 kHz using an ITC-18 interface.
Self-administration apparatus
Alcohol self-administration, extinction, and reinstatement test sessions were conducted in Plexiglas chambers (32 cm wide × 25 cm deep × 11 cm high; model ENV-008, Med Associates) located in melamine sound-attenuating cubicles. Each cubicle was equipped with an exhaust fan to provide air circulation and mask external noise. Mounted on one wall of the self-administration chamber were two response levers that flanked a liquid receptacle connected to a single-speed syringe infusion pump with polyethylene tubing. Responses on one lever, designated the active lever, resulted in delivery of the liquid reinforcer (see below), whereas responses on the other (designated inactive) lever had no programmed consequences. Located above the active lever was a 2.5-cm-diameter stimulus light, which was illuminated for 1.5 s during each reinforcer delivery. Located atop the chambers was a house light to provide general illumination and a Sonalert speaker that emitted a tone (2900 Hz, ∼65 dB) for 1.5 s during each reinforcer delivery. Chambers were interfaced to a PC that controlled experimental sessions and recorded data using commercially available software (MED-PC IV, MED Associates). Of the 42 rats trained to self-administer alcohol in an operant task and then subsequently exposed to extinction training, 1 was removed from the study before the commencement of extinction training because of failure to successfully acquire alcohol self-administration.
Self-administration training
The two-bottle choice (ethanol vs water) procedure was conducted to initiate drinking as described previously (Simms et al., 2008; McGuier et al., 2015). Rats were given 24 h intermittent access to alcohol (20%, v/v) on Monday, Wednesday, and Friday for 1 week. The day after the last two-bottle choice session, the rats were placed in operant chambers and trained to self-administer a 20% alcohol solution on an FR1 schedule of reinforcement during daily sessions (30 min) as described previously (Gass et al., 2014a,b). Active lever presses activated a syringe pump to deliver ∼45 μl of alcohol over a 1.5 s period. During reinforcer delivery, the stimulus light above the active lever was illuminated and the tone was presented. After each reinforcer delivery, a 4 s timeout period was initiated during which additional active lever presses were recorded but had no programmed consequences. After stable responding for 20% alcohol (∼10–12 sessions) was reached, alcohol concentration was reduced to 10% to maximize the number of lever responses during subsequent operant sessions (12–16 sessions).
Extinction procedures
Extinction procedures and treatment with the mGlu5-positive allosteric modulator CDPPB, the SK channel activator 1-EBIO, and/or the KCa2 channel blocker apamin commenced after maintenance criteria for the alcohol solution was reached. Maintenance criteria included the following: less than 20% variation in the number of active lever presses across three consecutive sessions, a minimum of 30 reinforcers per session, and at least 12 sessions with 10% alcohol as the reinforcer. Extinction training was conducted in 30 min daily sessions in the presence of alcohol-associated cues (e.g., presentation of the light/tone stimulus), since such procedures produce drug-seeking behavior that is more resistant to extinction than that observed in the absence of drug-associated cues (Ranaldi and Roberts, 1996; Feltenstein and See, 2006). No alcohol was given during extinction sessions, and presses on the inactive lever during extinction were recorded but produced no programmed consequences.
For groups exposed to extinction training, rats were administered vehicle (saline or 10% Tween 80 v/v), apamin (0.2 mg/kg, i.p., in saline; 5 min pretreatment), or CDPPB (30 mg/kg, s.c., in 10% Tween 80 v/v; 20 min pretreatment) before each extinction training session according to their group assignment and returned to their home cages. The dose of apamin was chosen based on effectiveness in previously published behavioral studies in rats (Deschaux et al., 1997; Deschaux and Bizot, 2005). The dose of CDPPB was based on our previous studies that showed facilitation of extinction learning (Gass and Olive, 2009; Cleva et al., 2011). Rats were placed in the self-administration apparatus after treatment, and lever-pressing behavior was recorded. Extinction criteria were met when the number of active lever presses was <20% (for 2 consecutive days) of those observed on the average of the last 2 d of active alcohol self-administration.
Spontaneous recovery procedure
After each rat treated with systemic apamin (intraperitoneally) reached extinction criteria, it was returned to its home cage for 3 weeks before testing in a single spontaneous recovery session (30 min) using previously published procedures (Rodd-Henricks et al., 2002; Dhaher et al., 2010; Hauser et al., 2015). During the spontaneous recovery test, rats were placed in the operant chamber with levers extended, the house/well light illuminated, and the house fan started. All conditions remained the same as extinction sessions (i.e., active lever presses resulted in a 1.5 s presentation of the light–tone stimulus that was previously paired with alcohol delivery, but no alcohol was delivered). Active and inactive lever presses were recorded.
Locomotor activity procedure
A separate cohort of rats with a history of alcohol self-administration was treated with apamin to determine any effects on locomotor activity in an open field using previously described methods (McGuier et al., 2015). Rats were habituated to a 57 × 58 × 63 cm opaque acrylic box for 1 h per day for 2 consecutive days. On the third day, rats were treated with 0.9% sterile saline or 0.2 mg/kg apamin (intraperitoneally) 15 min before a 30 min locomotor session. Activity was digitally recorded with an overhead video camera under red light. Total movement was automatically scored using EthoVision XT software (Noldus Information Technology). Locomotor data were binned into 5 min periods.
Stereotaxic surgery and microinjection procedures
Rats were assigned to microinjection experiments to examine whether inhibition of KCa2 channels within the IL-PFC (n = 52) or prelimbic prefrontal cortex (PrL-PFC; n = 12) would facilitate extinction of alcohol-seeking behavior. A separate group of rats (n = 25) was tested to determine whether administering the KCa2 channel activator (1-EBIO) directly in the IL-PFC would prevent the facilitating effects of systemic CDPPB on the extinction of alcohol-seeking behavior.
Microinjection cannula implantation
Cannulae implantation and microinjections were done as described previously (Gass et al., 2014a). Rats were anesthetized with isoflurane vaporized in medical-grade breathing air at a flow rate of 0.4 L/min and placed in a stereotaxic instrument (Kopf Instruments). Bilateral microinjection guide cannulae (26 ga outer diameter, Plastics One) were aimed to terminate 1 mm dorsal to the IL-PFC. The stereotaxic coordinates used for the IL-PFC were (in millimeters from bregma and skull surface) anteroposterior +3.24, mediolateral ±0.6, and dorsoventral −3.8, and coordinates for the PrL-PFC were anteroposterior +3.24, mediolateral ±0.6, and dorsoventral −2.2 (Paxinos and Watson, 2005). Microinjection cannulae were secured to the skull with stainless steel screws and dental cement. Removable obturators (33 ga outer diameter) were inserted in the full length of the guide cannulae to limit obstruction by tissue and contamination by external debris. The wound was treated with topical 2% xylocaine and 2% triple antibiotic ointments and sutured closed using 3-0 Vicryl sutures. After surgery, all rats were given carprofen (2.5 mg/kg, s.c., daily for 5 d) for postoperative pain management. After recovery from surgery, rats were trained to self-administer alcohol following our standard methods.
Microinjection procedure
Rats were assigned to treatment groups: intra-IL-PFC apamin [0, 0.1, 1, and 10 μm; based on the study by Criado-Marrero et al. (2014)], intra-PrL-PFC apamin (0 and 10 μm), or systemic vehicle (subcutaneously)/vehicle, CDPPB (30 mg/kg, s.c.)/vehicle, vehicle/1-EBIO (9.5 μg/μl), and CDPPB (30 mg/kg, s.c.)/1-EBIO (9.5 μg/μl); n = 6–7 rats per treatment group (EBIO dose based on Hopf et al., 2010). Importantly, CDPPB was not used for microinjection studies because of poor solubility. Thus, 1-EBIO was selected for localized microinjections to block systemic actions of CDPPB via positive modulation of IL-PFC KCa2 channel activity. This dose of CDPPB (30 mg/kg) was chosen based on previous studies showing effective enhancement of extinction learning (Gass et al., 2014a) and lack of effects on locomotor behavior (Vales et al., 2010; Cleva et al., 2011; Kufahl et al., 2013; Besheer et al., 2014). Immediately after the microinjection, each rat received a systemic injection of CDPPB (or vehicle, subcutaneously). For apamin microinjection experiments, apamin was microinjected bilaterally 5 min before extinction sessions. Bilateral microinjections were performed 25 min before each systemic CDPPB/IL-PFC EBIO extinction session. Rats were lightly restrained, and obturators were removed. Sterile 33 ga microinjection needles (Plastics One) were connected via microbore tubing to two 10 μl syringes (Hamilton). Syringes were mounted on a microinfusion pump (Harvard Apparatus) set to deliver fluids at a flow rate of 0.5 μl/min. Microinjection needles were inserted bilaterally to a depth 1 mm beyond the ventral tip of the guide cannula. Drug solutions were infused in a volume of 0.5 μl per side over a 1 min period. Microinjection needles were left in place for an additional 60 s period to allow drug diffusion. Next, injectors were removed and obturators were replaced.
Histological verification of microinjection sites
Verification of cannula placement was determined using previously published methods (Paxinos and Watson, 2005; Gass et al., 2014a). After behavioral procedures, rats were anesthetized with isoflurane and killed by decapitation. Brains were then removed, immersed in 10% (v/v) formalin for at least 1 week at 4°C, and immersed in a 30% (w/v) sucrose solution for at least 72 h at 4°C, followed by immersion in 15% (w/v) sucrose for at least 72 h at 4°C. Brains were then cut into 40 mm coronal sections on a cryostat (Leica CM1900, Leica Microsystems), mounted onto microscope slides, and stained with cresyl violet for histological verification of cannula placement under light microscopy.
Statistical analyses
Electrophysiology experiments.
Averaged triplicate group mean peak IAHP values (in picoamperes) elicited by depolarizing voltage steps during baseline and after bath application of drugs were analyzed by paired t tests or two-way ANOVAs where appropriate. LTP determined by percentage changes in normalized fEPSP slope after HFS was analyzed using a mixed model procedure (SAS) with treatment and time (20–30 and 50–60 min) as between- and within-subject factors, respectively.
Extinction learning experiments.
For experiments involving analysis of extinction behavior, lever presses on the last 2 d of active self-administration of 10% alcohol (i.e., maintenance) were averaged and compared with each day of extinction training using a mixed two-way repeated-measures ANOVA with treatment (vehicle/apamin) as the between-subjects factor and experimental day (extinction session) as the within-subjects factor. An independent-samples t test or ANOVA was used to analyze the number of sessions required to reach extinction criteria.
Spontaneous recovery of alcohol-seeking behavior.
For the analysis of spontaneous recovery, the number of lever presses recorded during alcohol self-administration, the last day of the extinction training, and during the spontaneous test were compared using a mixed two-way ANOVA with treatment (control/apamin) as the between-subjects factor and experimental phase (self-administration, extinction, spontaneous recovery) as the within-subjects factor. Data from this study were analyzed based on the stage of the experiment using SPSS software (version 22.0, SPSS) and Prism (version 6, GraphPad Software).
Results
Examination of functional coupling of IL-PFC KCa2 channels and mGlu5 receptors
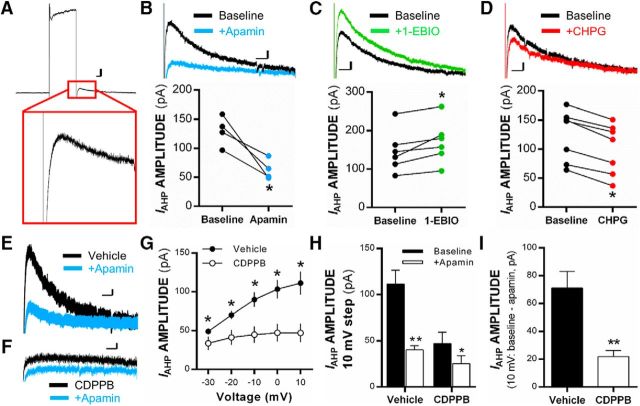
Previous studies have shown that mGlu5 receptor activation alters KCa2 channel activity in brain regions such as the hippocampus and somatosensory cortex (Mannaioni et al., 2001; Sourdet et al., 2003). We further explored this functional coupling between KCa2 channels and mGlu5 receptors in the IL-PFC. In voltage clamp, 400 ms depolarizing voltage steps were applied to layer V IL-PFC pyramidal neurons to isolate KCa2 channel-mediated IAHP (Fig. 1A). As expected, bath application of 100 nm apamin (KCa2 channel inhibitor) blocked the peak IAHP in IL-PFC pyramidal neurons relative to baseline (paired t(3) = 4.24, p = 0.02, n = 4 neurons/4 rats; Fig. 1B), whereas bath application of 400 μm 1-EBIO (KCa2 channel-positive modulator) significantly increased IAHP in IL-PFC pyramidal neurons relative to baseline (paired t(5) = 3.40, p < 0.02; n = 6 neurons/4 rats; Fig. 1C). Bath application of the mGlu5 receptor agonist CHPG significantly reduced IAHP relative to baseline (t(6) = 10.1, p < 0.01; n = 7 neurons/3 rats; Fig. 1D), demonstrating a functional reduction in KCa2 channel activity via mGlu5 activation in IL-PFC projection neurons.
Figure 1.
Localized and systemic mGlu5 receptor activation reduces IAHP in pyramidal neurons of the IL-PFC. A, Representative traces showing peak KCa2-mediated IAHP (outward potassium current) after being evoked by 400 ms depolarizing voltage steps in voltage clamp. B–D, Individual triplicate mean values and representative traces for KCa2-mediated IAHP after a voltage step from −70 to +10 mV in the presence of ACSF (baseline) or after bath application of 100 nm of apamin (B), 400 μm of 1-EBIO (C), or 100 μm CHPG (D; n = 4–7 neurons) in individual layer V pyramidal neurons of the IL-PFC. E, F, Representative traces of evoked IAHP from rats treated systemically with vehicle (E) or CDPPB (F) before and after bath application of 100 nm apamin. G, Mean values of peak IAHP current amplitude (in picoamperes) evoked in pyramidal neurons during the afterhyperpolarization phase of voltage steps after systemic treatment with 30 mg/kg CDPPB (subcutaneously) or vehicle (n = 4–6 neurons per treatment). H, Mean values of peak IAHP current amplitude (in picoamperes) evoked at the 10 mV voltage step before and after 100 nm apamin bath application in neurons from systemic CDPPB- or vehicle-treated rats. I, Mean values for the apamin-sensitive portion of IAHP amplitude (in picoamperes) at the 10 mV voltage step in neurons of rats treated with CDPPB or vehicle. Calibration: 20 pA, 100 ms. Values are expressed as group triplicate means ± SEM. *Significance was determined at p < 0.05, paired t test or two-way ANOVA.
To further examine the functional coupling of mGlu5 receptors and KCa2 channels, naive rats were given injections of vehicle or the mGlu5-positive modulator CDPPB (30 mg/kg, s.c.), and ex vivo slices containing the IL-PFC were prepared 60 min later for examination of IAHP (Fig. 1E,F). Systemic activation of mGlu5 with CDPPB resulted in a significant main effect of treatment (F(1,40) = 37.38, p < 0.001). Post hoc analysis indicated that systemic CDPPB treatment (n = 6 neurons/3 rats) significantly reduced KCa2-mediated IAHP amplitude at each voltage step relative to vehicle control cells (n = 4 neurons/3 rats, p < 0.001; Fig. 1G). An examination of KCa2-mediated IAHP (at 10 mV) before and after 100 nm apamin in IL-PFC pyramidal neurons from rats treated with systemic vehicle or CDPPB revealed a significant interaction of apamin × CDPPB treatments (F(1,8) = 20.20, p = 0.002). Post hoc analysis showed that neurons from rats treated with systemic CDPPB had significantly reduced IAHP currents and displayed reduced sensitivity to bath application of apamin compared with neurons from vehicle-treated rats (p = 0.006; Fig. 1H). Additional analysis of IAHP amplitude at the 10 mV step after systemic CDPPB (vs vehicle) demonstrated that the apamin-sensitive component of the IAHP (subtracted from baseline) was reduced in pyramidal neurons from rats treated with CDPPB (t(8) = 4.49, p = 0.002; Fig. 1I). These data confirm our ex vivo findings by showing that systemic activation of mGlu5 receptors reduces KCa2 channel activity within layer V IL-PFC pyramidal neurons.
Next, we determined whether KCa2 channels functionally regulate mGlu5-mediated synaptic plasticity of inputs onto IL-PFC neurons. Analysis revealed a main effect of treatment on a normalized fEPSP slope (F(4,48) = 3.84, p = 0.009; Fig. 2B). Post hoc analysis revealed that treatment with CHPG significantly increased normalized fEPSP slope relative to control treatment (p = 0.008; Fig. 2C), an effect that was blocked by coapplication of the selective mGlu5 antagonist MTEP (p = 0.03 relative to CHPG treatment; Fig. 2C). Interestingly, coapplication of the KCa2-positive modulator 1-EBIO prevented CHPG potentiation of LTP in IL-PFC slices (p = 0.004) (Fig. 2C). Importantly, the normalized fEPSP slope was not significantly reduced in slices treated with 1-EBIO alone (p = 0.32 vs controls; Fig. 2C), demonstrating that reversal of CHPG-induced enhancement of synaptic plasticity by combined treatment with 1-EBIO was not the result of an inability to induce LTP after positive modulation of KCa2 channels.
Figure 2.
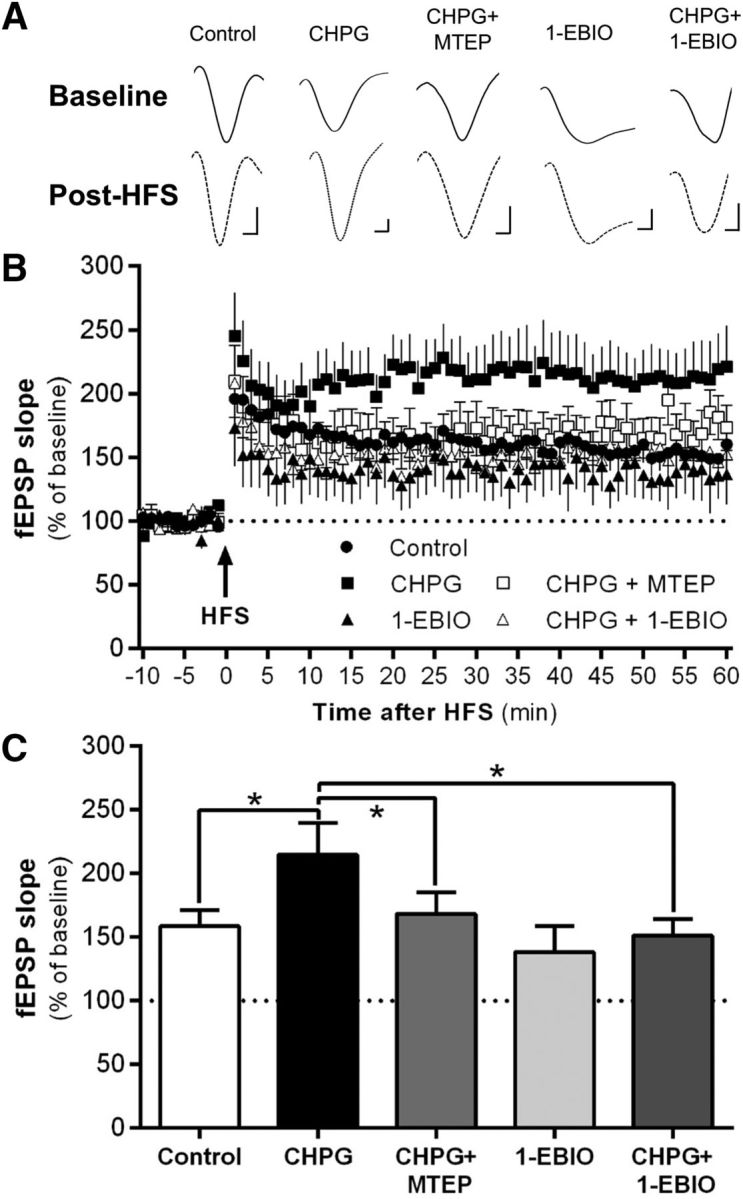
mGlu5-dependent enhancement of synaptic plasticity is regulated by KCa2 channels within the IL-PFC. A, Representative traces of normalized fEPSPs after bath application of ACSF (control), 100 μm CHPG, 100 μm CHPG plus 10 μm MTEP, 400 μm 1-EBIO, or 1 mm CHPG plus 400 μm 1-EBIO. Slices were stimulated in layer II/III and recorded once per minute in layer V of the IL-PFC. Calibration: 100 μV, 1 ms. B, Mean normalized fEPSP slope values before bath application of ACSF, CHPG, CHPG plus MTEP, CHPG plus 1-EBIO, and 1-EBIO after 50 Hz HFS. C, Collapsed binned analysis of the post-HFS normalized fEPSP slope values at 20–30 and 50–60 min time intervals (n = 10–12 slices per treatment group). Values are expressed as group means ± SEM. *Significance was determined at p < 0.05, mixed model procedure.
Examination of KCa2 channels in regulating extinction of alcohol-seeking behavior
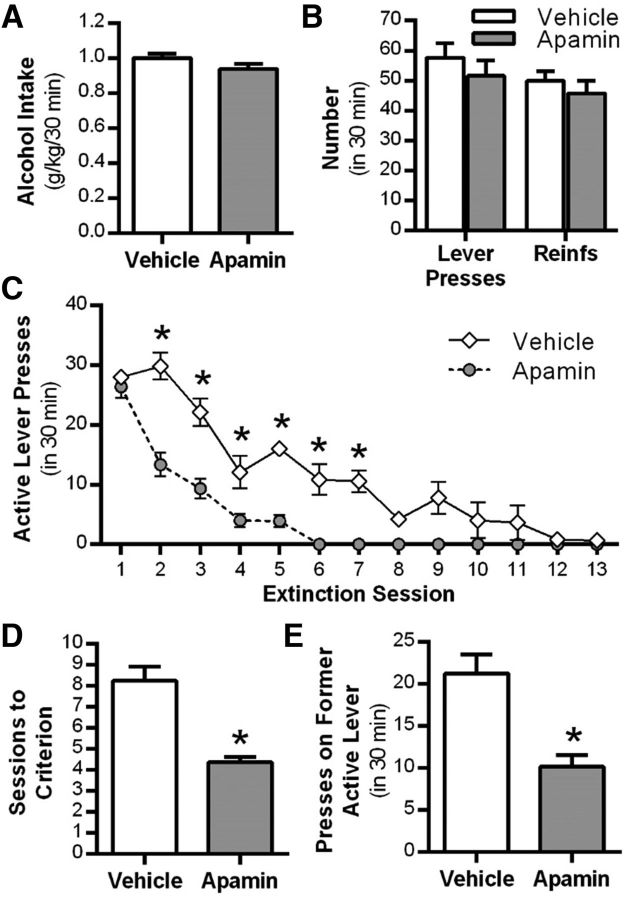
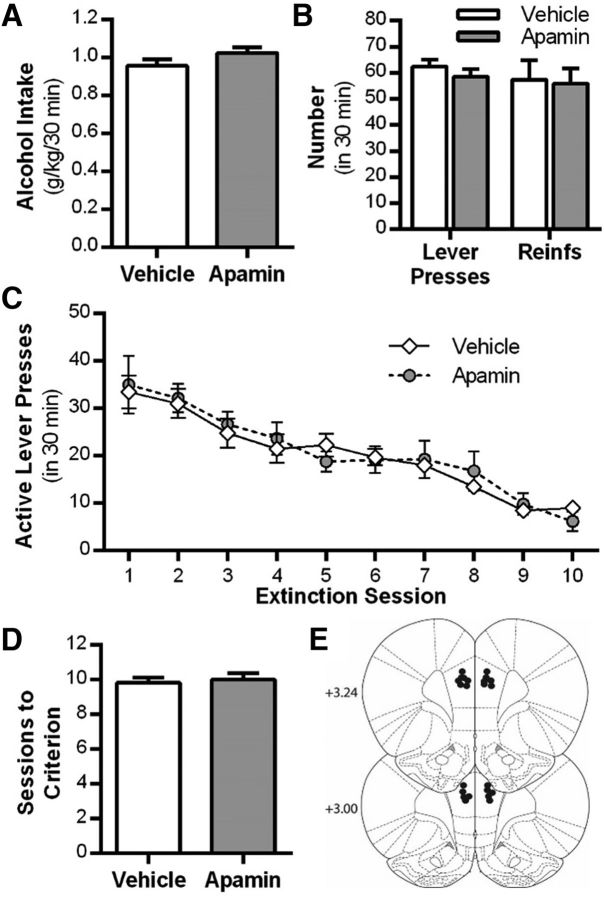
Given the significant role of KCa2 channels in modulating learning and memory (Stackman et al., 2002; Deschaux and Bizot, 2005; Hammond et al., 2006; Brosh et al., 2007) and regulation of cue-conditioned fear extinction (Criado-Marrero et al., 2014), we examined the effect of systemic administration of the KCa2 channel inhibitor apamin on the extinction of alcohol-seeking behavior in rats. As shown in Figure 3A, no differences were observed in the amount of alcohol consumed (t(10) = 1.6, p = 0.13). Also, there were no differences in the number of lever presses or reinforcements between groups during the final alcohol self-administration sessions before extinction (F(1,42) = 0.1, p = 0.835, n = 12 control, n = 11 apamin; Fig. 3B). Apamin administered before each daily extinction session resulted in a significant treatment × session interaction (F(12,252) = 11.5, p = 0.001, n = 11–12 per group). Post hoc analyses revealed that rats treated with apamin had significantly fewer responses on the previously active lever compared with vehicle-treated rats during extinction sessions 2–7 (*p < 0.05; Fig. 3C). Rats treated with apamin also required significantly fewer extinction sessions to reach extinction criteria compared with vehicle-treated rats (t(21) = 5.1, p = 0.001; Fig. 3D). To determine the persistence of the enhanced extinction learning produced by apamin, we assessed spontaneous recovery of alcohol-seeking behavior after 3 weeks in the home cage. Rats previously treated with apamin during extinction training showed a significant reduction in lever presses on the previously alcohol-reinforced lever (t(21) = 4.1, p = 0.001; Fig. 3E) compared with vehicle-treated controls.
Figure 3.
Systemic KCa2 channel inhibition facilitates extinction of alcohol-seeking behavior. A, Alcohol consumption during the maintenance phase of self-administration procedure. B, Mean number of lever presses and reinforcers after a history of operant alcohol self-administration. C, Mean active lever presses during extinction learning sessions after daily treatment with vehicle or the KCa2 channel inhibitor apamin. During extinction learning, lever pressing resulted in the presentation of alcohol cues, but no alcohol was delivered. D, Mean number of sessions to reach extinction criteria after vehicle or apamin treatment. E, Mean number of previously active lever presses after a single pavlovian spontaneous recovery test session conducted 3 weeks after extinction training and drug treatment (n = 12 vehicle group vs n = 11 apamin treatment group). Values are expressed as group means ± SEM. *Significance was determined at p < 0.05, t test or two-way ANOVA.
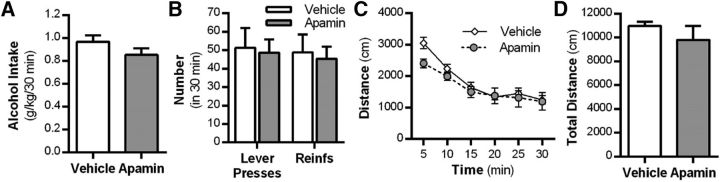
A potential concern with the apamin treatment was the possibility that deficits in motor behavior would be empirically perceived as an enhancement of extinction learning. Therefore, in a separate set of rats with a history of alcohol self-administration, we monitored locomotor activity in response to apamin (0.2 mg/kg, i.p.) administration. No differences were observed in alcohol intake (t(10) = 1.6, p = 0.13; Fig. 4A) or the number of lever presses or number of reinforcements between groups (F(1,42) = 0.698, p = 0.044; n = 5 control, n = 6 apamin; Fig. 4B) during the final alcohol self-administration sessions before locomotor testing. Apamin did not alter binned activity (F(5,45) = 1.698, p = 0.155) or overall total distance traveled (t(9) = 0.88, p = 0.401) in rats previously trained to self-administer alcohol (Fig. 4C,D).
Figure 4.
KCa2 channel inhibition does not affect locomotor behavior in rats with a history of alcohol self-administration. A, Alcohol intake during voluntary alcohol self-administration. B, Mean number of lever presses and reinforcers (Reinfs) after a history of operant alcohol self-administration. C, Cumulative distance traveled after treatment with vehicle or the KCa2 channel inhibitor apamin in an open-field test of locomotor activity. D, Total distance traveled after vehicle or apamin treatment (n = 5 vehicle group vs n = 6 apamin treatment group). Values are expressed as group means ± SEM.
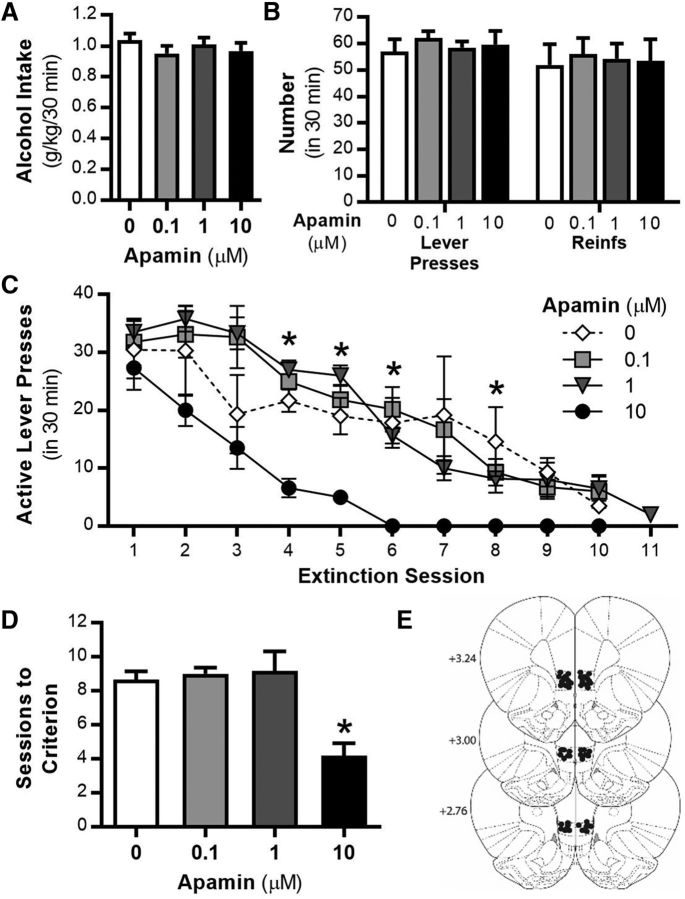
Given that the IL-PFC is a critical site that regulates inhibitory control of behavioral responding (Peters et al., 2009; Gass and Chandler, 2013; Barker et al., 2014) and the current results showing that systemic KCa2 inhibition facilitates extinction learning (Fig. 3), we investigated the effects of daily intra-IL-PFC KCa2 apamin pretreatment on extinction of alcohol-seeking behavior. No significant differences were observed in the amount of alcohol consumed (F(3,23) = 0.6, p = 0.595; Fig. 5A) or in the number of lever presses or reinforcements between groups during the final alcohol self-administration sessions before extinction (F(3,40) = 0.014, p = 0.998; n = 6 per group; Fig. 5B). Intra-IL-PFC apamin (10 μm) administered before each daily extinction session resulted in a significant treatment × session interaction (F(27,180) = 2.6, p = 0.007; Fig. 5C). Post hoc analyses revealed that rats given microinjections of 10 μm apamin had significantly fewer responses on the previously active lever compared with vehicle-treated rats on multiple extinction sessions (*p < 0.05; Fig. 5C). Rats given microinjections of 10 μm apamin also required significantly fewer extinction sessions to reach extinction criteria compared with vehicle-treated rats (F(3,20) = 32.9, p < 0.0001; Fig. 5D). These data demonstrate that KCa2 channel inhibition within the IL-PFC is required for the facilitation of alcohol cue-associated extinction learning.
Figure 5.
Inhibition of KCa2 channels in the IL-PFC facilitates extinction of alcohol-seeking behavior. A, Alcohol consumption during operant alcohol self-administration sessions. B, Mean number of lever presses and reinforcers (Reinfs) after a history of operant alcohol self-administration. C, Mean active lever presses during extinction learning sessions after daily IL-PFC microinjections of the KCa2 channel inhibitor apamin (0, 0.1, 1, and 10 μm). During extinction learning, lever pressing resulted in the presentation of alcohol cues, but no alcohol was delivered. D, Mean number of sessions to reach extinction criteria after vehicle or apamin microinjections. E, Diagram of coronal rat brain sections showing the location of microinjector tips (dark circles) in the IL-PFC. Illustrations were adapted from the atlas of Paxinos and Watson (2005). Numbers along the left side of each section represent the distance (in millimeters of that section from bregma; n = 6 per treatment group). Values are expressed as group means ± SEM. *Significance was determined at p < 0.05, t test or two-way ANOVA.
The PrL-PFC is a key region within the circuitry that processes and initiates cue-mediated reward seeking (Peters et al., 2008; Gourley and Taylor, 2016). Given the current findings demonstrating that IL-PFC KCa2 channel inhibition facilitates extinction learning (Fig. 5), we tested for region specificity by evaluating the effects of daily intra-PrL-PFC apamin pretreatment on extinction of alcohol-seeking behavior. No significant differences were observed in the amount of alcohol consumed (t(10) = 1.5, p = 0.171; Fig. 6A) or the number of lever presses or reinforcements between groups during the final alcohol self-administration sessions before extinction (F(1,20) = 0.05, p = .814; n = 6 per group; Fig. 6B). Intra-PrL-PFC apamin (10 μm) administered before each daily extinction session did not significantly alter lever responses on the previously active lever (F(9,90) = 0.3, p = 0.940; Fig. 6C). Furthermore, there was no effect on sessions to reach extinction criterion (t(10) = 0.3, p = 0.734; Fig. 6D). These data demonstrate that KCa2 channel inhibition within the IL-PFC, but not the PrL-PFC, is specifically required for the facilitation of alcohol cue-associated extinction learning.
Figure 6.
Inhibition of KCa2 channels in the PrL-PFC does not alter extinction of alcohol-seeking behavior. A, Alcohol consumption during the maintenance phase of the self-administration procedure. B, Mean number of lever presses and reinforcers (Reinfs) after a history of operant alcohol self-administration. C, Mean active lever presses during extinction learning sessions after daily PrL-PFC microinjections of the KCa2 channel inhibitor apamin (0 and 10 μm). D, Mean number of sessions to reach extinction criteria after vehicle or apamin microinjections. E, Diagram of coronal rat brain sections showing the location of microinjector tips (dark circles) in the PrL-PFC. Illustrations were adapted from the atlas of Paxinos and Watson (2005). Numbers along the left side of each section represent the distance (in millimeters of that section from bregma; n = 6 per treatment group). Values are expressed as group means ± SEM.
KCa2 channel–mGlu5 receptor interactions and extinction of alcohol-seeking behavior
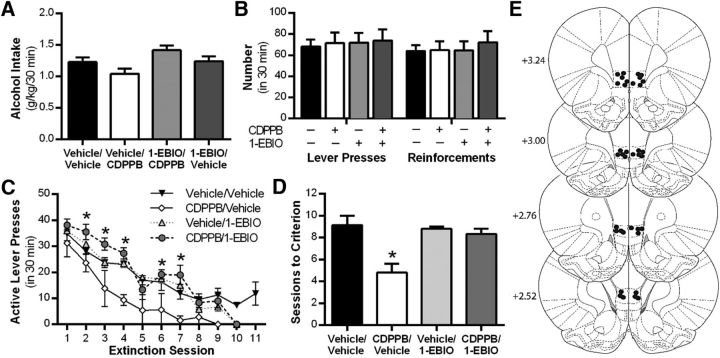
Our current data indicate that mGlu5 activation functionally reduces IL-PFC KCa2 channel activity (Fig. 1) and that this functional reduction in KCa2 activity is a requisite for mGlu5-dependent enhancement of LTP ex vivo (Fig. 2). Therefore, we examined the role of IL-PFC KCa2 channels in regulating mGlu5-dependent extinction of alcohol-seeking behavior by microinjecting 1-EBIO into the IL-PFC in rats treated with systemic CDPPB. No significant differences were observed in alcohol intake (F(3,23) = 1.2, p = 0.31; Fig. 7A) or the number of lever presses and reinforcements between groups during the final alcohol self-administration sessions before extinction (F(3,42) = 0.04, p = 0.988; Fig. 7B). An analysis of combined systemic and intra-IL-PFC drug treatment on active lever presses during extinction sessions resulted in a significant treatment × session interaction (F(27,171) = 2.8, p = 0.001; Fig. 7C). Post hoc analysis revealed that the systemic CDPPB treatment group responded significantly fewer times on the active lever (relative to vehicle/vehicle treatment) during extinction sessions 2, 3, 4, 6, and 7 (*p < 0.05), and this effect was blocked IL-PFC 1-EBIO microinfusions (Fig. 7C). Moreover, systemic CDPPB/vehicle produced a significant decrease in the number of sessions to reach extinction criteria relative to control treatment (F(3,19) = 7.8, p = 0.001; Fig. 7D), an effect reversed by KCa2 channel activation with intra-IL-PFC 1-EBIO. These data indicate that KCa2 channel inhibition in the IL-PFC is required for mGlu5-mediated facilitation of alcohol cue-associated extinction learning.
Figure 7.
Activation of KCa2 channels in the IL-PFC blocks mGlu5 receptor-mediated facilitation of alcohol-seeking behavior. A, Alcohol consumption during operant alcohol self-administration sessions. B, Mean number of lever presses and reinforcers after a history of operant alcohol self-administration. C, Mean number of active lever presses during extinction learning sessions after systemic treatment with vehicle or CDPPB (mGlu5-positive modulator) or in combination with local infusion of vehicle or 1-EBIO into the IL-PFC. D, Number of sessions to reach extinction criteria after systemic treatment with 1-EBIO or CDPPPB combined with local infusion of vehicle or 1-EBIO into the IL-PFC. E, Diagram of coronal rat brain sections showing the location of microinjector tips (dark circles) in the IL-PFC. Illustrations were adapted from the atlas of Paxinos and Watson (2005). Values are expressed as group means ± SEM (n = 7 per group). *Significance was determined at p < 0.05, one- or two-way ANOVA.
Discussion
The major findings from these studies demonstrate that both systemic and intra-IL-PFC inhibition of KCa2 channels facilitates extinction of cue-associated alcohol-seeking behavior and reveal a novel role for these channels in alcohol cue-related inhibitory learning. KCa2 inhibition in the PrL-PFC did not significantly alter extinction learning, revealing a subregion-specific role for IL-PFC KCa2 channels to regulate extinction of alcohol seeking. We also demonstrate that KCa2 inhibition is required for mGlu5-dependent facilitation of extinction. Furthermore, results of the current study show that KCa2 channels functionally couple with mGlu5 receptors in the IL-PFC to regulate KCa2 channel currents in layer V pyramidal neurons and to regulate mGlu5-dependent enhancement of LTP. Together, these data provide strong preclinical evidence that facilitation of extinction of alcohol-seeking behavior involves functional inhibition of KCa2 channels, through either direct or indirect activation of mGlu5 receptors. In addition, these data implicate the IL-PFC as a critical site of action in KCa2-mediated facilitation of extinction learning.
KCa2 channel inhibition and facilitated extinction learning
An intriguing finding in this study was the long-lasting (3 weeks) attenuation of alcohol seeking in rats previously treated with systemic apamin during extinction training. This effect was not attributable to reductions in locomotor activity as apamin treatment did not significantly alter spontaneous locomotor behavior in the open field. New memory formation is thought to be the underlying mechanism that mediates extinction learning. During extinction training, newly formed memories compete with associations formed during previous long-term repeated pairings of alcohol, drugs, or fearful stimuli with environmental stimuli (Bouton, 2000; Rescorla, 2004; Hutton-Bedbrook and McNally, 2013). The current findings showing that systemic KCa2 channel inhibition facilitated extinction learning are congruent with several studies demonstrating that KCa2 channels play a critical role in the formation of new memories (Stackman et al., 2002; Deschaux and Bizot, 2005; Hammond et al., 2006; Brosh et al., 2007). We hypothesize that KCa2 inhibition likely enhances the ability of the new memory to compete with older cue-associated memories that previously motivated alcohol-seeking behavior. The mechanism underlying KCa2 channel-dependent enhancement of learning and memory is attributed, at least in part, to increased neuronal intrinsic excitability after reductions in KCa2 channel function as evidenced by increased neuronal spiking after pharmacological block of KCa2 channels (Bond et al., 2004; Criado-Marrero et al., 2014). Thus, these persistent effects of KCa2 inhibition on alcohol seeking provide strong evidence that KCa2 channels are an ideal target for pharmacological enhancement of inhibitory learning.
The results of the present study also show that reduced KCa2 channel activity within the IL-PFC is selective in controlling extinction of alcohol-seeking behavior. Traditionally, the IL-PFC has been shown to play a more critical role in inhibiting, as opposed to the PrL-PFC, which initiates drug/reward-seeking behavior (Peters et al., 2008; Gass and Chandler, 2013; Barker et al., 2014) but see (Moorman and Aston-Jones, 2015). The current data provide the first preclinical evidence that inhibition of KCa2 channels in the IL-PFC, but not the PrL-PFC, facilitates extinction of alcohol-seeking behavior. These data are also congruent with previous work showing facilitation of fear extinction after IL-PFC microinjection with apamin (Criado-Marrero et al., 2014). Whereas apamin was thought to be highly selective for KCa2 channels, recent evidence has shown that apamin blocks KV1.3 channels (Voos et al., 2017). It is possible that apamin enhances extinction learning by reducing KV1.3 channel activity. This argument is weakened, however, because of our results demonstrating that 1-EBIO blocks CDPPB's effects on LTP and extinction learning. To our knowledge, 1-EBIO does not affect KV1.3 channel activity, suggesting that the ability of apamin to facilitate extinction learning is mediated by inhibition of KCa2, but not KV1.3, channel activity. Thus, reductions in KCa2 channel activity in the IL-PFC may functionally regulate inhibitory learning processes in multiple behavioral and preclinical models of extinction therapy.
Behavioral and physiological implications of functional coupling between mGlu5 receptors and KCa2 channels
Given the established role of IL-PFC KCa2 channels and mGlu5 receptors in modulating extinction of cue-conditioned fear (Sepulveda-Orengo et al., 2013; Criado-Marrero et al., 2014), we examined IL-PFC KCa2 channel regulation of mGlu5-dependent extinction of cue-conditioned alcohol-seeking behavior. Site-specific activation of IL-PFC KCa2 channels attenuated mGlu5-dependent enhancement of extinction learning. These data also extend previous findings showing that mGlu5 activation facilitates extinction learning (Gass et al., 2014a) by elucidating a requisite for reduced activity of IL-PFC KCa2 channels to enhance extinction of alcohol-seeking behavior after positive modulation of mGlu5 receptors. It is important to note that other forms of extinction learning, such as context-dependent extinction, are also regulated by mGlu5 receptors. mGlu5 receptor activity is critical for extinction of context-dependent fear memories (Riedel et al., 2000) and drug-seeking behavior (Gass and Olive, 2009; Chesworth et al., 2013; Kim et al., 2015). It is not fully clear, however, whether functional coupling of mGlu5 receptors and KCa2 channels plays a specific role in context-dependent extinction learning, and future studies are needed to determine this relationship.
The aforementioned behavioral data are in parallel with the electrophysiological findings that demonstrate functional coupling of mGlu5 receptors and KCa2 channels within the IL-PFC. Indeed, activation of mGlu5 receptors by local bath application and systemic treatment reduced KCa2-mediated IAHP in deep-layer IL-PFC pyramidal neurons. This finding is consistent with previous work showing that group I activation reduces KCa2 channel-mediated currents (Fontanez-Nuin et al., 2011; García-Negredoet al., 2014; Tigaret et al., 2016). Although the mechanism is unknown, this effect may involve removal of KCa2 channels from the plasma membrane after cyclic AMP-dependent protein kinase A (PKA) phosphorylation of KCa2 channels (Ren et al., 2006; Lin et al., 2008). Indeed, several studies have shown that positive modulation of mGlu5 receptors increases PKA activation (Lanté et al., 2006; Dell'anno et al., 2013). Other evidence demonstrates that Gq-mediated hydrolyzes of PIP2 (phosphatidylinositol bisphosphate) regulates calcium sensitivity of KCa2 channels via uncoupling of calmodulin binding (Zhang et al., 2014) and suggests that mGlu5 activation may be reducing KCa2 channel sensitivity to intracellular calcium levels. Thus, inhibitory learning may be enhanced by IL-PFC KCa2 channel endocytosis or reduced calcium sensitivity after mGlu5 activation. Regardless of the intracellular signaling mechanism, these data support the hypothesis that the reduction in KCa2 channel-mediated IAHP in the IL-PFC by activation of mGlu5 receptors is a key mechanism that enhances inhibitory learning.
Our previous work, as well as the work from other groups, has shown that facilitation of extinction learning has been associated with enhanced plasticity in the medial PFC (Herry and Garcia, 2002; Gass et al., 2014a; Zhong et al., 2015). In the present study, IL-PFC LTP was significantly and robustly enhanced after mGlu5 activation. This effect of enhanced LTP is similar to what others have observed after mGlu5 activation in the hippocampus (O'Leary and O'Connor, 1998; Ayala et al., 2009). Importantly, mGlu5-dependent facilitation of LTP was reversed by KCa2 channel activation, demonstrating that KCa2 channel inhibition is required for mGlu5-dependent enhancement of synaptic plasticity. A recent study demonstrated that activation of hippocampal mGlu1, but not mGlu5, enhances spike-timing-dependent synaptic plasticity via inhibition of KCa2 channels (Tigaret et al., 2016). Although these findings appear at odds with our results, they may be explained by different plasticity induction protocols and differing mGlu1 expression within the PFC relative to mGlu5 (Ferraguti and Shigemoto, 2006). Another study reported that KCa2 overexpression inhibits LTP induction in the hippocampus (Hammond et al., 2006). However, the concentration of 1-EBIO used in the present study did not significantly inhibit induction of IL-PFC synaptic plasticity, suggesting that overexpression of KCa2 channels may result in much stronger regulation of synaptic plasticity compared with KCa2 expression in intact neurons. Together, the current findings indicate a critical role for KCa2 channels in modulating mGlu5-dependent synaptic plasticity in the IL-PFC.
In conclusion, the findings of the present study show that apamin treatment during extinction learning facilitated a persistent reduction in alcohol-seeking behaviors and provide strong evidence that KCa2 channels are a novel therapeutic target for the enhancement of cue-exposure treatment in alcoholics. Furthermore, the functional coupling between KCa2 channels and mGlu5 receptors is a critical regulator of behavioral and synaptic plasticity within an important extinction-related brain region, the IL-PFC. Finally, these data add to the growing body of literature demonstrating the beneficial role of cognitive enhancement in facilitating extinction cue-exposure therapy for the treatment of alcohol use disorder.
Footnotes
This work was supported by NIH Grants AA020930 (P.J.M.), AA020537 (J.T.G.), AA024526 (J.T.G.), AA007474 (R.C.; MUSC T-32), AA009986 (J.J.W.), and AA010761 (P50 RC3) and by INIA Stress Consortium Grant AA013641.
The authors declare no competing financial interests.
References
- Adelman JP, Maylie J, Sah P (2012) Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol 74:245–269. 10.1146/annurev-physiol-020911-153336 [DOI] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ (2009) mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology 34:2057–2071. 10.1038/npp.2009.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR, Chandler LJ (2014) A unifying model of the role of the infralimbic cortex in extinction and habits. Learn Mem 21:441–448. 10.1101/lm.035501.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Jaramillo AA, Frisbee S, Cannady R (2014) Stress hormone exposure reduces mGluR5 expression in the nucleus accumbens: functional implications for interoceptive sensitivity to alcohol. Neuropsychopharmacology 39:2376–2386. 10.1038/npp.2014.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Koros E, Kostowski W, Bogucka-Bonikowska A (2000) Reinstatement of ethanol seeking in rats: behavioral analysis. Pharmacol Biochem Behav 66:123–128. 10.1016/S0091-3057(00)00194-5 [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Kye MJ, Radulovic J, Spiess J (2003) Small-conductance, Ca2+-activated K+ channel SK3 generates age-related memory and LTP deficits. Nat Neurosci 6:911–912. 10.1038/nn1101 [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP (2004) Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci 24:5301–5306. 10.1523/JNEUROSCI.0182-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. (2000) A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Health Psychol 19:57–63. 10.1037/0278-6133.19.Suppl1.57 [DOI] [PubMed] [Google Scholar]
- Brosh I, Rosenblum K, Barkai E (2007) Learning-induced modulation of SK channels-mediated effect on synaptic transmission. Eur J Neurosci 26:3253–3260. 10.1111/j.1460-9568.2007.05936.x [DOI] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM (2004) Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44:351–364. 10.1016/j.neuron.2004.09.026 [DOI] [PubMed] [Google Scholar]
- Cannady R, Fisher KR, Durant B, Besheer J, Hodge CW (2013) Enhanced AMPA receptor activity increases operant alcohol self-administration and cue-induced reinstatement. Addict Biol 18:54–65. 10.1111/adb.12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesworth R, Brown RM, Kim JH, Lawrence AJ (2013) The metabotropic glutamate 5 receptor modulates extinction and reinstatement of methamphetamine-seeking in mice. PLoS One 8:e68371. 10.1371/journal.pone.0068371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP (1993) Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 137:73–95. [PubMed] [Google Scholar]
- Cleva RM, Hicks MP, Gass JT, Wischerath KC, Plasters ET, Widholm JJ, Olive MF (2011) mGluR5 positive allosteric modulation enhances extinction learning following cocaine self-administration. Behav Neurosci 125:10–19. 10.1037/a0022339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST (2002) Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97:155–167. 10.1046/j.1360-0443.2002.00014.x [DOI] [PubMed] [Google Scholar]
- Criado-Marrero M, Santini E, Porter JT (2014) Modulating fear extinction memory by manipulating SK potassium channels in the infralimbic cortex. Front Behav Neurosci 8:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'anno MT, Pallottino S, Fisone G (2013) mGlu5R promotes glutamate AMPA receptor phosphorylation via activation of PKA/DARPP-32 signaling in striatopallidal medium spiny neurons. Neuropharmacology 66:179–186. 10.1016/j.neuropharm.2012.03.025 [DOI] [PubMed] [Google Scholar]
- Deschaux O, Bizot JC (2005) Apamin produces selective improvements of learning in rats. Neurosci Lett 386:5–8. 10.1016/j.neulet.2005.05.050 [DOI] [PubMed] [Google Scholar]
- Deschaux O, Bizot JC, Goyffon M (1997) Apamin improves learning in an object recognition task in rats. Neurosci Lett 222:159–162. 10.1016/S0304-3940(97)13367-5 [DOI] [PubMed] [Google Scholar]
- Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, Rodd ZA (2010) The orexin-1 receptor antagonist SB-334867 reduces alcohol relapse drinking, but not alcohol-seeking, in alcohol-preferring (P) rats. J Addict Med 4:153–159. 10.1097/ADM.0b013e3181bd893f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B, Adelman JP (2008) Control of K(Ca) channels by calcium nano/microdomains. Neuron 59:873–881. 10.1016/j.neuron.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE (2006) Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res 174:1–8. 10.1016/j.bbr.2006.06.039 [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R (2006) Metabotropic glutamate receptors. Cell Tissue Res 326:483–504. 10.1007/s00441-006-0266-5 [DOI] [PubMed] [Google Scholar]
- Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT (2011) Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex 21:727–735. 10.1093/cercor/bhq147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Negredo G, Soto D, Llorente J, Morató X, Galenkamp KM, Gómez-Soler M, Fernández-Dueñas V, Watanabe M, Adelman JP, Shigemoto R, Fukazawa Y, Luján R, Ciruela F (2014) Coassembly and coupling of SK2 channels and mGlu5 receptors. J Neurosci 34:14793–14802. 10.1523/JNEUROSCI.2038-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Chandler LJ (2013) The plasticity of extinction: contribution of the prefrontal cortex in treating addiction through inhibitory learning. Front Psychiatry 4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF (2009) Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry 65:717–720. 10.1016/j.biopsych.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Trantham-Davidson H, Kassab AS, Glen WB Jr, Olive MF, Chandler LJ (2014a) Enhancement of extinction learning attenuates ethanol-seeking behavior and alters plasticity in the prefrontal cortex. J Neurosci 34:7562–7574. 10.1523/JNEUROSCI.5616-12.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Glen WB Jr, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ (2014b) Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology 39:2570–2583. 10.1038/npp.2014.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Taylor JR (2016) Going and stopping: dichotomies in behavioral control by the prefrontal cortex. Nat Neurosci 19:656–664. 10.1038/nn.4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW (2006) Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci 26:1844–1853. 10.1523/JNEUROSCI.4106-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Deehan GA Jr, Dhaher R, Knight CP, Wilden JA, McBride WJ, Rodd ZA (2015) D1 receptors in the nucleus accumbens-shell, but not the core, are involved in mediating ethanol-seeking behavior of alcohol-preferring (P) rats. Neuroscience 295:243–251. 10.1016/j.neuroscience.2015.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Garcia R (2002) Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci 22:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Bowers MS, Chang SJ, Chen BT, Martin M, Seif T, Cho SL, Tye K, Bonci A (2010) Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron 65:682–694. 10.1016/j.neuron.2010.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton-Bedbrook K, McNally GP (2013) The promises and pitfalls of retrieval-extinction procedures in preventing relapse to drug seeking. Front Psychiatry 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Perry C, Luikinga S, Zbukvic I, Brown RM, Lawrence AJ (2015) Extinction of a cocaine-taking context that protects against drug-primed reinstatement is dependent on the metabotropic glutamate 5 receptor. Addict Biol 20:482–489. 10.1111/adb.12142 [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Nemirovsky NE, Watterson LR, Zautra N, Olive MF (2013) Positive or negative allosteric modulation of metabotropic glutamate receptor 5 (mGluR5) does not alter expression of behavioral sensitization to methamphetamine. F1000Res 2:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanté F, de JésusFerreira MC, Guiramand J, Récasens M, Vignes M (2006) Low-frequency stimulation induces a new form of LTP, metabotropic glutamate (mGlu5) receptor- and PKA-dependent, in the CA1 area of the rat hippocampus. Hippocampus 16:345–360. 10.1002/hipo.20146 [DOI] [PubMed] [Google Scholar]
- Lin MT, Luján R, Watanabe M, Adelman JP, Maylie J (2008) SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci 11:170–177. 10.1038/nn2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Few LR, Stojek MK, Murphy CM, Malutinok SF, Johnson FT, Hofmann SG, McGeary JE, Swift RM, Monti PM (2015) D-cycloserine to enhance extinction of cue-elicited craving for alcohol: a translational approach. Transl Psychiatry 5:e544. 10.1038/tp.2015.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ (2001) Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci 21:5925–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuier NS, Griffin WC 3rd, Gass JT, Padula AE, Chesler EJ, Mulholland PJ (2015) Kv7 channels in the nucleus accumbens are altered by chronic drinking and are targets for reducing alcohol consumption. Addict Biol 21:1097–1112. 10.1111/adb.12279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G (2015) Prefrontal neurons encode context-based response execution and inhibition in reward seeking and extinction. Proc Natl Acad Sci U S A 112:9472–9477. 10.1073/pnas.1507611112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW (2009) N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12:182–189. 10.1038/nn.2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Becker HC, Woodward JJ, Chandler LJ (2011) Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses. Biol Psychiatry 69:625–632. 10.1016/j.biopsych.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Ochsner KN, Kober H, Kuerbis A, Feng T, Wall M, Morgenstern J (2015) Cognitive regulation of craving in alcohol-dependent and social drinkers. Alcohol Clin Exp Res 39:343–349. 10.1111/acer.12637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BÁ, Kantak KM (2011) Cognitive enhancers for facilitating drug cue extinction: insights from animal models. Pharmacol Biochem Behav 99:229–244. 10.1016/j.pbb.2011.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DM, O'Connor JJ (1998) Priming of long-term potentiation by prior activation of group I and II metabotropic glutamate receptors in the rat dentate gyrus in vitro. Brain Res 809:91–96. 10.1016/S0006-8993(98)00897-X [DOI] [PubMed] [Google Scholar]
- Padula AE, Griffin WC 3rd, Lopez MF, Nimitvilai S, Cannady R, McGuier NS, Chesler EJ, Miles MF, Williams RW, Randall PK, Woodward JJ, Becker HC, Mulholland PJ (2015) KCNN genes that encode small-conductance Ca2+-activated K+ channels influence alcohol and drug addiction. Neuropsychopharmacology 40:1928–1939. 10.1038/npp.2015.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristou H, Nederkoorn C, Havermans R, van der Horst M, Jansen A (2012) Can't stop the craving: the effect of impulsivity on cue-elicited craving for alcohol in heavy and light social drinkers. Psychopharmacology (Berl) 219:511–518. 10.1007/s00213-011-2240-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates, Ed 5 San Diego: Academic. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28:6046–6053. 10.1523/JNEUROSCI.1045-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ (2009) Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16:279–288. 10.1101/lm.1041309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi R, Roberts DC (1996) Initiation, maintenance and extinction of cocaine self-administration with and without conditioned reward. Psychopharmacology (Berl) 128:89–96. 10.1007/s002130050114 [DOI] [PubMed] [Google Scholar]
- Ren Y, Barnwell LF, Alexander JC, Lubin FD, Adelman JP, Pfaffinger PJ, Schrader LA, Anderson AE (2006) Regulation of surface localization of the small conductance Ca2+-activated potassium channel, Sk2, through direct phosphorylation by cAMP-dependent protein kinase. J Biol Chem 281:11769–11779. 10.1074/jbc.M513125200 [DOI] [PubMed] [Google Scholar]
- Rescorla RA. (2004) Spontaneous recovery. Learn Mem 11:501–509. 10.1101/lm.77504 [DOI] [PubMed] [Google Scholar]
- Riedel G, Casabona G, Platt B, Macphail EM, Nicoletti F (2000) Fear conditioning-induced time- and subregion-specific increase in expression of mGlu5 receptor protein in rat hippocampus. Neuropharmacology 39:1943–1951. 10.1016/S0028-3908(00)00037-X [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK (2002) Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res 26:1642–1652. 10.1111/j.1530-0277.2002.tb02466.x [DOI] [PubMed] [Google Scholar]
- Sepulveda-Orengo MT, Lopez AV, Soler-Cedeño O, Porter JT (2013) Fear extinction induces mGluR5-mediated synaptic and intrinsic plasticity in infralimbic neurons. J Neurosci 33:7184–7193. 10.1523/JNEUROSCI.5198-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–1823. 10.1111/j.1530-0277.2008.00753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, Gass JT (2012) mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol Biochem Behav 101:329–335. 10.1016/j.pbb.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourdet V, Russier M, Daoudal G, Ankri N, Debanne D (2003) Long-term enhancement of neuronal excitability and temporal fidelity mediated by metabotropic glutamate receptor subtype 5. J Neurosci 23:10238–10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T (2002) Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci 22:10163–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P (2000) Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci 15:476–493. 10.1006/mcne.2000.0842 [DOI] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P (1999) An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A 96:4662–4667. 10.1073/pnas.96.8.4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigaret CM, Olivo V, Sadowski JH, Ashby MC, Mellor JR (2016) Coordinated activation of distinct Ca(2+) sources and metabotropic glutamate receptors encodes Hebbian synaptic plasticity. Nat Commun 7:10289. 10.1038/ncomms10289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vales K, Svoboda J, Benkovicova K, Bubenikova-Valesova V, Stuchlik A (2010) The difference in effect of mGlu2/3 and mGlu5 receptor agonists on cognitive impairment induced by MK-801. Eur J Pharmacol 639:91–98. 10.1016/j.ejphar.2009.11.067 [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Kiefer F, Spanagel R (2008) D-cycloserine facilitates extinction of conditioned alcohol-seeking behaviour in rats. Alcohol Alcohol 43:626–629. 10.1093/alcalc/agn067 [DOI] [PubMed] [Google Scholar]
- Villalobos C, Shakkottai VG, Chandy KG, Michelhaugh SK, Andrade R (2004) SKCa channels mediate the medium but not the slow calcium-activated afterhyperpolarization in cortical neurons. J Neurosci 24:3537–3542. 10.1523/JNEUROSCI.0380-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos P, Yazar M, Lautenschlager R, Rauh O, Moroni A, Thiel G (2017) The small neurotoxin apamin blocks not only small conductance Ca2+ activated K+ channels (SK type) but also the voltage dependent Kv1.3 channel. Eur Biophys J. Advance online publication. Retrieved January 20, 2017. doi: 10.1007/s00249-016-1196-0 [DOI] [PubMed] [Google Scholar]
- Zhang M, Meng XY, Cui M, Pascal JM, Logothetis DE, Zhang JF (2014) Selective phosphorylation modulates the PIP2 sensitivity of the CaM-SK channel complex. Nat Chem Biol 10:753–759. 10.1038/nchembio.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Brown J, Kramer A, Kaleka K, Petersen A, Krueger JN, Florence M, Muelbl MJ, Battle M, Murphy GG, Olsen CM, Gerges NZ (2015) Increased prefrontal cortex neurogranin enhances plasticity and extinction learning. J Neurosci 35:7503–7508. 10.1523/JNEUROSCI.0274-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]