Abstract
A growing body of evidence indicates that repeated exposure to cocaine leads to profound changes in glutamate transmission in limbic nuclei, particularly the nucleus accumbens. This review focuses on preclinical studies of cocaine-induced behavioral plasticity, including behavioral sensitization, self-administration, and the reinstatement of cocaine seeking. Behavioral, pharmacological, neurochemical, electrophysiological, biochemical, and molecular biological changes associated with cocaine-induced plasticity in glutamate systems are reviewed. The ultimate goal of these lines of research is to identify novel targets for the development of therapies for cocaine craving and addiction. Therefore, we also outline the progress and prospects of glutamate modulators for the treatment of cocaine addiction.
Keywords: nucleus accumbens, reinstatement, relapse, synaptic plasticity, pharmacotherapy, receptor
Introduction
Cocaine abuse remains a major public health problem in the United States.1 According to the National Survey on Drug Use and Health, it has been estimated that 34.15 million Americans ages 12 and older have used cocaine once in their lifetime and 2.1 million are current users of cocaine.1 One hallmark of cocaine addiction and the paramount issue in its treatment is the high rate of relapse to drug taking after detoxification.2,3 Despite decades of focused preclinical and clinical studies that have advanced our understanding of the anatomical and neurochemical bases of drug addiction, a safe and efficacious pharmacotherapy for cocaine craving remains to be discovered.
Cocaine craving and relapse of cocaine-taking behavior in abstinent human addicts are precipitated by three major stimuli: a stressful life event, an environmental stimulus previously associated or paired with the drug-taking event, or reexposure to the previously self-administered drug itself.4–8 Relapse to drug taking/seeking in humans is typically modeled in laboratory animals, including rodents and nonhuman primates, as follows: after a period of drug self-administration and the subsequent extinction of the drug-reinforced behavior, the ability of stress exposure, drug-associated stimuli, or reexposure to the drug itself to reinstate drug-seeking behavior is assessed.9 For example, after extinction of cocaine self-administration, systemic or intravenous administration of relatively low doses of cocaine reinstate operant responding in the absence of drug reinforcement in both nonhuman primates and rodents.5,10–13 Although the reinstatement model has good face validity in that similar stimuli reinstate cocaine seeking in animals that precipitate relapse in humans, the predictive validity of the reinstatement paradigm remains to be determined mainly because a successful pharmacotherapy for cocaine addiction has not been identified.14 As the most commonly used animal model of relapse, the reinstatement model has proven invaluable for elucidating the cellular and molecular mechanisms as well as the neural circuitry underlying cocaine-seeking behavior.
The reinforcing and rewarding effects of cocaine are mediated, in part, through the mesocorticolimbic dopamine system.15 However, a growing body of evidence has emerged indicating that cocaine indirectly influences glutamate transmission in the limbic system, producing persistent changes in neuronal function that alter the behavioral effects of cocaine. Recent studies have suggested that drug addiction is a disorder in which long-term neural adaptations in dopamine and glutamate systems result from, and contribute to, drug-associated learning.16 Furthermore, many similarities have been observed between the cellular and molecular mechanisms underlying addiction and neuronal plasticity associated with learning and memory.17,18 Thus, drugs of abuse, including cocaine, induce synaptic modifications in motivational networks through coordinated signaling of dopamine and glutamate systems that in turn lead to maladaptive behaviors, including cocaine craving and relapse.19–23 Therefore, studies further defining the cellular and molecular mechanisms underlying cocaine-induced plasticity in neuronal circuits that mediate drug-seeking behavior are essential to identify novel drug targets for cocaine craving and addiction.
Traditionally, research into the neurobiology of cocaine addiction has focused almost exclusively on the mesocorticolimbic dopamine system.15,24–26 However, a substantial body of literature has emerged supporting a role for glutamate in drug-associated learning and other adaptive processes that mediate addictive behaviors in laboratory animals, including the reinstatement of cocaine-seeking behavior.27–32 Here, we focus on the mechanisms underlying cocaine-induced behavioral and neuronal plasticity, with particular emphasis on the role of glutamate transmission in the nucleus accumbens, the primary input nucleus of the limbic portion of the basal ganglia.33 Recent studies describing cocaine-induced synaptic plasticity in the nucleus accumbens are presented along with the molecular mechanisms regulating glutamate receptor–mediated signaling and localization/expression of glutamate receptor subunits. Furthermore, the effect of cocaine-induced neuroplasticity in excitatory synapses within the accumbens is discussed with relation to preclinical cocaine self-administration studies, including the reinstatement paradigm, although important evidence from other models (such as behavioral sensitization) is presented as well. Finally, a summary of findings from clinical studies examining the efficacy of glutamate-modulating drugs for cocaine relapse is discussed. A more complete understanding of how cocaine-induced synaptic plasticity in the mesocorticolimbic dopamine system alters neuronal ensembles to produce reinstatement of drug-seeking behavior could lead to the development of novel, targeted pharmacotherapies for cocaine addiction and relapse.
Neuronal circuitry mediating reinstatement of cocaine seeking
Dopaminergic modulation of the limbic system
Drugs of abuse produce their reinforcing effects through actions in the limbic component of the basal ganglia, a circuit of nuclei that is responsible for the influence of motivational, emotional, contextual, and affective information on behavior (Fig. 1). Cocaine is a crystalline tropane alkaloid that binds to dopamine, norepinephrine, and serotonin transporters, thereby blocking reuptake of biogenic amines in the brain.34 Despite this binding profile, a growing literature indicates that dopamine is the biogenic amine primarily involved in cocaine reinforcement and the reinstatement of cocaine seeking.15,35
Figure 1.
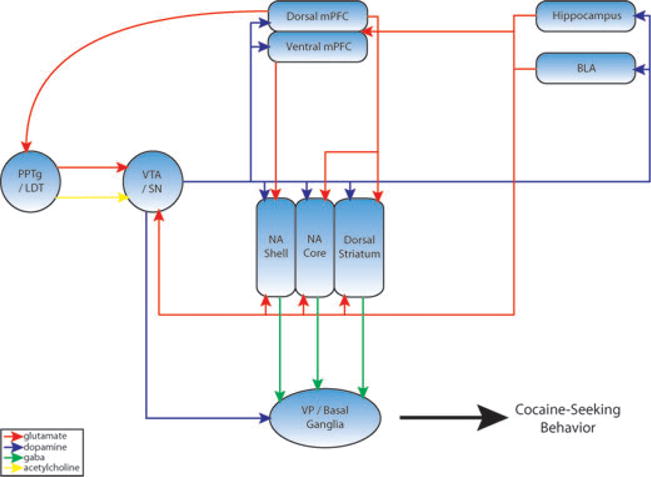
Proposed neuronal circuitry mediating cocaine priming–induced reinstatement of drug-seeking behavior. The medial prefrontal cortex (mPFC) sends segregated glutamatergic afferents to the nucleus accumbens (NA). These include excitatory projections from the dorsal mPFC (anterior cingulated cortex and dorsal prelimbic cortex) and ventral mPFC (ventral prelimbic cortex and infralimbic cortex) to the NA core and shell, respectively. The core and shell subregions of the accumbens also receive excitatory glutamatergic projections from both cortical (hippocampus) and subcortical (basolateral amygdala [BLA]) nuclei. Dopaminergic projections from the ventral tegmental area (VTA) and substantia nigra (SN) modulate the flow of emotional, declarative, and procedural memories through circuits centered on the NA, mPFC, BLA, and hippocampus. The activity of VTA and SN dopamine cells is regulated by excitatory glutamatergic projections from the pedunculopontine tegmental nucleus (PPTg)/laterodorsal tegmental nucleus (LDT), mPFC, hippocampus, and BLA, as well as inhibitory GABAergic/peptidergic projections from the NA and ventral pallidum (VP). Excitatory cholinergic afferents from the PPTg/LDT also synapse on midbrain dopamine neurons. The NA functions to translate the rewarding/reinforcing effects of drugs of abuse into drug-seeking behavior by processing, consolidating, and integrating information from limbic nuclei with motor functions of basal ganglia structures including the VP, thalamus, and motor cortex. (In color in Annals online.)
Limbic nuclei, including the amygdala, hippocampus, and medial prefrontal cortex (mPFC), send major glutamatergic projections to the nucleus accumbens, which is subdivided into the shell and core subregions.36–38 The nucleus accumbens sends segregated efferent GABAergic projections to the ventral pallidum and ventral tegmental area/substantia nigra.39–43 Both the ventral pallidum and ventral tegmental area, in turn, send GABAergic efferent projections to the medial dorsal thalamus.44,45 Glutamatergic projections from the medial dorsal thalamus to the mPFC close this limbic circuit.46–51 Dopaminergic neurons in the ventral tegmental area innervate the nucleus accumbens, amygdala, hippocampus, mPFC, and ventral pallidum, and changes in dopaminergic transmission play a critical role in modulating the flow of information through the limbic circuit comprising these interconnected nuclei.48,52–57
Nucleus accumbens: core and shell subregions
The nucleus accumbens can be divided according to histochemical criteria into two functionally discrete subregions, known as the core and shell.43,58–63 Growing evidence suggests that heterogeneity in ventral striatal subregions imparts distinct functional differences to the nucleus accumbens. For example, the nucleus accumbens shell, which is classified as a part of the limbic system, is implicated in the primary rewarding effects of drugs of abuse64–70 as well as regulating instrumental responding in the presence of motivationally relevant stimuli.71–75 Alternatively, the nucleus accumbens core, which is considered part of the basal ganglia, mediates the incentive value of reward-conditioned stimuli and contributes to drug-associated, cue-induced cocaine seeking.76–80 Neuronal circuits centered on the nucleus accumbens shell, nucleus accumbens core, and neostriatum are interconnected and can process information via parallel as well as integrated feedforward connections.81–83 Moreover, information can flow through the striatal complex hierarchically from the shell to the core and to the neostriatum.36,50,81 Thus, the nucleus accumbens serves as a functional interface between limbic and motor systems processing affective and motivational information from the limbic system and integrating it with the basal ganglia.84
Nucleus accumbens: dopamine and cocaine reinstatement
An extensive literature indicates that increased dopamine transmission through D1- and D2-like dopamine receptors plays a critical role in priming-induced reinstatement of cocaine seeking.35,85,86 For example, D2-like dopamine receptor agonists administered systemically or into the nucleus accumbens shell reinstate cocaine seeking.11,12,87–94 Consistent with these results, systemic or intra-accumbal shell administration of a D2-like dopamine receptor antagonist attenuates cocaine priming–induced reinstatement of drug-seeking behavior.12,90,91,95–98 In contrast, the precise contribution of D1-like dopamine receptors to reinstatement behavior is not clear. On their own, systemically administered D1-like dopamine receptor agonists do not reinstate cocaine-seeking behavior.11,12,88,90,99 However, systemically administered D1-like dopamine receptor agonists and antagonists both attenuate drug-seeking behavior induced by a priming injection of cocaine.11,12,90,100–102 When administered into the nucleus accumbens shell, D1-like dopamine receptor agonists reinstate drug-seeking behavior.23,91,93,94 Consistent with these results, intra-accumbal shell administration of a D1-like dopamine receptor antagonist attenuates drug seeking induced by a priming injection of cocaine.13,91 Taken together, these results indicate that D1- and D2-like dopamine receptors play critical roles in cocaine reinstatement and that D1-like dopamine receptors expressed in nuclei other than the nucleus accumbens shell may have different roles in drug-seeking behavior.
Converging neurotransmitter systems: corticostriatal glutamate afferents and mesostriatal dopamine afferents
There is considerable evidence that activation of the dopaminergic pathway from the ventral tegmental area to the mPFC contributes significantly to the reinstatement of cocaine seeking.103–107 Increases in extracellular dopamine levels in the mPFC appear to promote cocaine seeking by stimulating glutamatergic pyramidal neurons that project from the mPFC to the nucleus accumbens.105,108,109 Interestingly, there are two largely segregated glutamatergic afferents to the nucleus accumbens arising from the mPFC. Thus, the dorsal subregion of the mPFC (anterior cingulate cortex and dorsal prelimbic cortex) projects mainly to the accumbens core, whereas the ventral subregion of the mPFC (ventral prelimbic cortex and infralimbic cortex) sends glutamatergic projections to the accumbens shell.110–114
Within the nucleus accumbens and neostriatum, glutamatergic and dopaminergic afferent projections converge on the same spines of medium spiny GABAergic projection neurons.56,115–118 This convergence of glutamate and dopamine neurotransmitter systems at synaptic and extrasynaptic sites within the nucleus accumbens facilitates a unique synaptic triad whereby dopamine modulates excitatory input to the accumbens from the mPFC, hippocampus, and amygdala.119 Recent studies indicate that D2 dopamine receptors located on glutamatergic terminals regulate glutamate release in the striatum, and thus reinforce specific corticostriatal synapses by filtering less salient synaptic connections120–123 (however, see Ref. 124). Also, stimulation of the ventral tegmental area leads to a D2-like dopamine receptor–mediated attenuation of the response of nucleus accumbens neurons to limbic input from the mPFC.125 Taken together, these results indicate that excitatory input from cortical and sub-cortical structures to the nucleus accumbens is filtered and integrated by dopamine-mediated mechanisms, thereby shaping information output to the basal ganglia.
Glutamatergic neurotransmission
Glutamate is the major excitatory amino acid neurotransmitter in the central nervous system. Approximately 80–90% of synapses in the brain are glutamatergic, and it has been estimated that up to 90% of neurons in the brain use glutamate as a neurotransmitter.126 In the brain, glutamate is synthesized in presynaptic nerve terminals from glucose via the Krebs cycle and from glutamine that is synthesized in glial cells, released into the extracellular fluid, and transported into nerve terminals where it is converted into glutamate by the mitochondrial enzyme glutaminase. Within the nerve terminal, glutamate is loaded into synaptic vesicles by vesicular glutamate transporters (VGLUTs), multimeric protein complexes that function as proton–glutamate antiporters.127 To date, three different VGLUTs have been cloned (VGLUT1–3). Whereas VGLUT1 and 2 are expressed in functionally distinct populations of glutamatergic neurons, VGLUT3 is localized in serotonin and possibly dopamine neurons, cholinergic interneurons in the striatum, and GABAergic interneurons of the hippocampus and cortex, which suggests a novel role for glutamate.128–130 Upon depolarization of the presynaptic terminal, glutamate is released into the synaptic cleft, where it passively diffuses and binds to presynaptic, postsynaptic, and perisynaptic glutamate receptors. There are two main families of glutamate receptors: ligand-gated ionotropic glutamate receptors that mediate fast excitatory neurotransmission and metabotropic glutamate receptors that modulate pre- and postsynaptic responses through G protein activation of second-messenger systems.131 Glutamate signaling is terminated by a family of high-affinity, Na+-dependent excitatory amino acid transporters (EAATs 1–5) that have distinct anatomical and cellular distributions as well as unique pharmacological profiles.132 For example, EAAT1 and 2 are expressed on glial cells and neuronal EAATs (2–5) have specialized roles at presynaptic terminals (EAAT2 and 5) and postsynaptic membranes (EAAT3 and 4).132 In contrast to EAAT1 and 2, the Na+-independent cystine–glutamate antiporter maintains basal levels of extrasynaptic glutamate by exchanging extracellular cystine for intracellular glutamate in glial cells.133
Nucleus accumbens glutamate and cocaine reinstatement
A growing body of literature indicates that cocaine indirectly influences glutamate transmission in the limbic system, including the nucleus accumbens, producing persistent changes in neuronal function that alter the behavioral effects of cocaine.30,31,134,135 Thus, maladaptive forms of neuroplasticity in the nucleus accumbens contribute to cocaine-seeking behavior, and reversing these cocaine-induced neuroadaptations in glutamatergic transmission may prevent relapse of cocaine taking.
Basal levels of extracellular glutamate are decreased in the nucleus accumbens during withdrawal from repeated cocaine exposure
As a dopamine, serotonin, and norepinephrine reuptake inhibitor, cocaine does not act directly on glutamatergic neurons.34 Acute systemic administration of cocaine has little or no effect on extracellular glutamate levels in the nucleus accumbens136 (however see Refs. 137 and 138). In contrast, withdrawal from repeated exposure to cocaine reduces basal extracellular glutamate levels in the nucleus accumbens.35,136,139 It was subsequently shown that the decrease in basal accumbal glutamate during withdrawal from chronic cocaine exposure resulted from decreased activity of the cystine–glutamate antiporter.139,140 Consistent with these results, normalization of extracellular accumbal glutamate levels in animals with a history of cocaine self-administration with N-acetylcysteine (NAC), a cysteine prodrug that increases activity of the cystine–glutamate antiporter, prevented reinstatement of drug seeking induced by a priming injection of cocaine.140 Taken together, these results indicate that repeated cocaine treatment promotes neuroadaptations in glutamatergic transmission in the nucleus accumbens that influence the persistence of craving and drug-seeking behavior.
Glutamate release in the nucleus accumbens of cocaine-experienced rats promotes reinstatement of cocaine seeking
Multiple studies indicate that a cocaine challenge injection administered to rats pretreated with repeated cocaine injections results in increased glutamate release in the nucleus accumbens core.108,136,141–144 During withdrawal from repeated cocaine exposure, a challenge injection of cocaine also decreased presynaptic glutamate immunoreactivity in the accumbens core, but not the accumbens shell, suggesting that different neuroadaptations occur in these brain regions after repeated cocaine exposure.145–147 Similarly, cocaine priming–induced reinstatement of drug seeking was associated with increased glutamate release in the core of the nucleus accumbens, an effect that was attenuated by pharmacological inactivation of the mPFC.108 Consistent with these results, administration of an α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor antagonist into the nucleus accumbens blocked the reinstatement of cocaine seeking induced by administration of cocaine directly into the mPFC.105 Infusion of an AMPA receptor antagonist directly into the nucleus accumbens core or γ-aminobutyric acid (GABA) receptor agonists directly into the dorsal mPFC also alters GABA levels in the ventral pallidum after a challenge injection of cocaine in animals withdrawn from chronic cocaine exposure.148 These findings, collectively, suggest that activation of the glutamatergic projection from the mPFC to the nucleus accumbens promotes cocaine seeking, a finding supported by brain imaging studies of human cocaine addicts, which demonstrate that cocaine craving is associated with metabolic activation of the mPFC.149,150 These findings also demonstrate that stimulation of AMPA glutamate receptors in the nucleus accumbens plays a critical role in cocaine seeking.
Glutamate receptors and cocaine self-administration/seeking
The ionotropic family of glutamate receptors consists of three subfamilies of tetrameric receptors named for the agonists that bind to them: N-methyl-D-aspartate (NMDA) receptors, AMPA receptors, and kainate receptors (Fig. 2). Agonist binding induces a conformation change in NMDA, AMPA, and kainate receptors that increases the probability of channel opening. Different subunit compositions of ionotropic glutamate receptors produce functionally diverse NMDA, AMPA, and kainate receptors that are expressed differently throughout the brain.151
Figure 2.
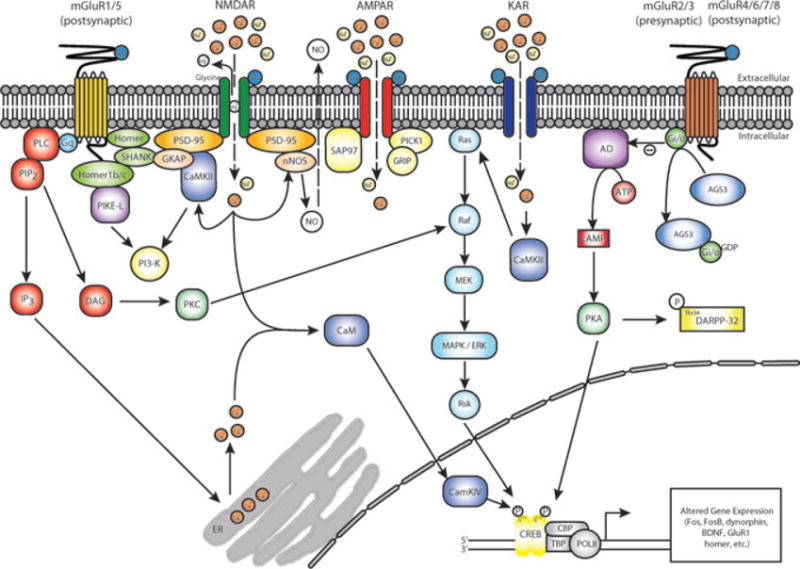
Glutamate receptor–mediated signaling. Glutamate released into the synaptic cleft binds to and activates ionotropic glutamate receptors (NMDA, AMPA, and kainate [KA] receptors) on postsynaptic membranes. Extracellular glutamate also binds to and activates perisynaptic metabotropic glutamate receptors located on presynaptic (mGluR2/3 autoreceptors) or postsynaptic (mGluR1/5s heteroreceptors) membranes. Influx of Na+, Ca2+, and K+ ions through activated AMPA/KA receptors depolarizes a neuron and subsequently relieves the Mg2+ block from voltage-sensitive NMDA receptors and activates voltage-gated Ca2+ channels (not shown). In addition to propagating action potentials, influx of cations through ionotropic glutamate receptors activates several intracellular signaling pathways including, but not limited to, Ras, CaMKII, and protein kinase A (PKA). Group I (mGluR1/5) and group II (mGluR2/3) metabotropic glutamate receptors are coupled via Gq and Gi/o, respectively, to intracellular enzymes. Stimulation of mGluR1/5s activates phospholipase C (PLC), which catalyzes the production of inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG) from phosphatidylinositol-4,5-bisphosphate (PIPs). The resulting increase in cytoplasmic IP3 triggers release of Ca2+ from intracellular stores, including the endoplasmic reticulum (ER). Stimulation of mGluR2/3s inhibits adenylyl cyclase (AC) activity, thus decreasing intracellular levels of cAMP and PKA. The cytoplasmic proteins PSD-95, glutamate receptor–interacting protein (GRIP), and Homer anchor glutamate receptors to the PSD complex. For example, Shank–Homer interactions link mGluR1/5s to NMDA receptors through PSD-95 and guanylate kinase–associated protein (GKAP). GluR1-containing AMPA receptors may be linked to mGluR1/5s through interactions between Homer and phosphoinositide 3 kinase (PI3-K) enhancer (PIKE-L). Metabotropic glutamate receptor–mediated signaling is influenced by regulators of G protein signaling (RGS), including activator of G protein signaling 3 (AGS3). AGS3 binds to and stabilizes the inactive guanosine diphosphate (GDP)–bound Gi conformation, preventing GDP release and thereby inhibiting Gi-mediated signaling. PKA phosphorylates dopamine- and cyclic AMP–regulated phosphoprotein (DARPP-32) at Thr34, which enhances extracellular signal–regulated kinase (ERK) signaling by inhibiting phosphatase activity. Activation of ionotropic and metabotropic glutamate receptors ultimately leads to phosphorylation of transcription factors, including cAMP response element–binding protein (CREB) at Ser133, changes in gene expression, and persistent changes in synaptic plasticity. See text for more detail on how repeated cocaine administration influences glutamate receptor–mediated signaling in the nucleus accumbens. (In color in Annals online.)
Ionotropic glutamate receptors: NMDA receptors
NMDA receptors are a heteromeric ligand-gated ion channels that are composed of three different subunits (NMDAR1–3) and are permeable to Ca2+, K+, and Na+. There are multiple subtypes of NMDA receptors that differ in their subunit composition and in their biophysical and pharmacological properties. Eight different splice variants of the NR1 subunit originate from a single gene. In contrast, six separate genes encode four different NR2 subunits (NR2A–D) and two genes encode two NR3 subunits (NR3A and B).151 Although the exact stoichiometry of NMDA receptors in vivo is unclear, at least one NR1 subunit and one NR2 subunit are required for functional NMDA receptors in vitro.152 The NMDA receptor is unique among all other neurotransmitter receptors in its requirement for the simultaneous binding of two different agonists (coagonists) for activation, glutamate and glycine or D-serine.153 At least one NR1 subunit and one NR2 subunit are required for functional NMDA receptors because glutamate and glycine bind to these respective subunits. In addition to binding coagonists, NMDA receptor activation requires membrane depolarization via AMPA receptor activation in order to remove the voltage-dependent block provided by Mg2+ at resting state.154 Furthermore, distinct recognition sites for endogenous and exogenous ligands regulate NMDA receptor function.151,152 NMDA receptors are expressed in different cell types, including microglia, astrocytes, oligodendrocytes, and neurons152,155–157 as well as on presynaptic and postsynaptic membranes in the nucleus accumbens.158,159 An emerging literature indicates that nucleus accumbens NMDA receptors play a role in the drug-induced neural plasticity underlying maladaptive behaviors, including addiction.17,160–163
Effect of repeated cocaine exposure on NMDA receptor expression in the nucleus accumbens
Studies examining NMDA receptor subunit mRNA and protein expression in the nucleus accumbens of animals receiving noncontingent injections of cocaine and in animals self-administering intravenous injections of cocaine report inconsistent findings.30 For example, some reports have demonstrated no change in the expression of NR1 and NR2A/B receptor subunits in the nucleus accumbens after 24 h of withdrawal from repeated cocaine exposure,164,165 whereas others have reported decreased expression of accumbal NR1 subunits at similar time points.166,167 In contrast, NR1 receptor subunit expression is increased in the nucleus accumbens after longer periods of withdrawal in animals that developed behavioral sensitization.164,166,168 Moreover, nucleus accumbens NR1 subunit expression is increased after protracted withdrawal periods in animals that previously self-administered cocaine.160,169 Behavioral sensitization to the locomotor-activating effects of cocaine is also associated with increased expression of NR2B in the nucleus accumbens shell after 24 h, but not 1 week, of withdrawal from cocaine, suggesting that changes in glutamate receptor subunit expression are time dependent.170,171 However, in rats that undergo withdrawal from cocaine, NR1 receptor subunit expression is decreased in the accumbens shell.172 Postmortem studies of human cocaine over-dose victims reveals increased expression of NR1.173 Collectively, these studies indicate that after a period of forced abstinence from cocaine administration there is increased expression of NMDA receptor subunits in the nucleus accumbens.
Nucleus accumbens NMDA receptors and cocaine reinforcement
NMDA receptors have been demonstrated to contribute to the reinforcing effects of cocaine. Systemic administration of noncompetitive NMDA receptor antagonists blocks the acquisition of cocaine self-administration behavior174 and attenuates responding for cocaine on a fixed-ratio (FR) schedule of reinforcement.175–178 Although these results indicate that blocking NMDA receptor–mediated signaling enhances the reinforcing efficacy of cocaine, MK-801 administration dose-dependently modulates breakpoint ratios (i.e., low doses potentiate and high doses attenuate the rewarding effects of cocaine) in rodents responding for cocaine under a progressive-ratio schedule of reinforcement.179 Interestingly, pharmacological antagonists of the NMDA receptor may have different effects on cocaine self-administration behavior owing to unique binding sites and modulatory kinetics.177 When administered directly into the nucleus accumbens, NMDA receptor antagonists attenuate responding for cocaine under a second-order schedule of reinforcement.180–182 However, NMDA receptor antagonists have reinforcing properties and thus abuse liability, because rats will self-administer pharmacologically diverse NMDA receptor antagonists directly into the nucleus accumbens shell.64
Nucleus accumbens NMDA receptors and the reinstatement of cocaine seeking
There also is evidence that accumbal NMDA receptors play a role in the reinstatement of cocaine seeking. Administration of a NMDA receptor agonist directly into the nucleus accumbens reinstated cocaine seeking in rats.143,144 However, intra-accumbal administration of a NMDA receptor agonist increased responding on an inactive lever, a nonspecific operant response not associated with drug infusion, suggesting that increased responding on the active lever may be due to a generalized increase in locomotor activity.143 In a subsequent study, it was found that systemic administration of a relatively low dose of an NMDA receptor antagonist neither prevented cocaine-primed reinstatement nor induced reinstatement when administered alone.144 In contrast, other studies have shown that systemic,183 intra-accumbal shell,105,161 or intra-accumbal core161 administration of an NMDA receptor antagonist reinstates cocaine-seeking behavior. Taken together, these results suggest that NMDA receptors in the nucleus accumbens modulate the reinstatement of cocaine seeking.
The extant literature examining the role of accumbal NMDA receptors in cocaine reinstatement does not provide an obvious interpretation of why NMDA receptor agonists and antagonists produce similar behaviors when administered directly into the nucleus accumbens.35,144 However, there are several plausible mechanisms to explain this discrepancy. For example, cocaine administration increases extracellular levels of acetylcholine in the cortex.184 Similarly, NMDA and a competitive NMDA receptor antagonist both increase extracellular acetylcholine levels in the mPFC when administered directly into the nucleus accumbens.185–187 Thus, one potential explanation for the similar behavioral effects of NMDA receptor agonists and antagonists involves acetylcholine release in the mPFC. It is also possible that the behavioral effects of NMDA receptor antagonists are mediated by presynaptic NMDA receptors in the accumbens. Although NMDA receptors are expressed predominantly on postsynaptic membranes, there is some evidence that these receptors are also expressed on corticostriatal terminals.158 Furthermore, systemic administration of an NMDA receptor antagonist increases extracellular glutamate levels in the nucleus accumbens.188 Therefore, it is possible that NMDA receptor antagonists administered into the nucleus accumbens promote cocaine seeking by blocking presynaptic NMDA receptors on glutamatergic afferents, thereby increasing extracellular glutamate levels188 and indirectly activating postsynaptic AMPA receptors to reinstate cocaine-seeking behavior.105,143,144 This hypothesis has not yet been examined experimentally. Finally, extracellular dopamine levels are increased in the nucleus accumbens after systemic, intra-accumbal, or intra-mPFC administration of NMDA receptor antagonists.188–191 Several studies have clearly demonstrated that increased dopaminergic transmission in the shell and medial core sub-regions of the accumbens reinstates cocaine seeking.13,91,93,94,97 On the basis of these studies, it is possible the NMDA receptor antagonists promote the reinstatement of cocaine seeking, in part, by increasing extracellular dopamine levels in the nucleus accumbens. Taken together, these results reveal that the functional role of nucleus accumbens NMDA receptors in the reinstatement of cocaine seeking is complex, and further studies are needed to determine the precise mechanism(s) by which NMDA receptor agonists and antagonists promote cocaine-seeking behavior.
Ionotropic glutamate receptors: AMPA and kainate receptors
Similar to NMDA receptors, AMPA receptors are heterotetrameric ligand-gated ion channels that are ubiquitously expressed throughout the brain, including the nucleus accumbens.158,159 Multiple subtypes of AMPA receptors with distinct pharmacological profiles are composed from different combinations of subunits termed GluR1–4 (or GluRA–D), GluRδ1, and GluRδ2.151,192 AMPA receptor subunits exist in two functionally different variants, flip or flop, that are generated by alternative splicing, differently expressed throughout the brain and characterized by distinct desensitization kinetics.193–195 In contrast to NMDA receptors, AMPA receptor activation is voltage independent. AMPA receptors are permeable to cations, including Ca2+, Na+, and K+. The conversion of a glutamine (Q) codon to an arginine (R) renders GluR2-containing AMPA receptors impermeable to calcium.196–198 Because most GluR2 subunits are edited in this manner, GluR2-containing AMPA receptors are calcium impermeable.199 Interestingly, the relative expression of GluR2 subunit mRNA and protein in neurons is not static and may be remodeled by administration of cocaine.200,201 In the nucleus accumbens, GluR1 and GluR2 subunits are expressed in virtually all medium spiny neurons.202 In contrast, GluR3 and GluR4 subunit expression in the nucleus accumbens is relatively low.202–204
Similar to AMPA receptors, kainate receptors are heterotetrameric ligand-gated ion channels that mediate fast excitatory synaptic transmission.205,206 Kainate receptors are voltage-independent protein complexes that are composed of combinations of five subunits: GluR5–7, KA1, and KA2.151,207 Functional homomeric or heteromeric kainate receptors are formed from combinations of GluR5–7, whereas KA1 and KA2 form heteromeric receptors only by partnering with any of the GluR5–7 subunits.205,207 Kainate receptors are permeable to Na+; K+; and, depending on editing and/or alternative splicing of GluR5 and GluR6 subunit mRNAs, Ca2+.208–211 In addition to influencing cation permeability, mRNA editing and alternative splicing mechanisms regulate trafficking and subcellular localization of kainate receptor subunits.212–216 Kainate receptors are expressed presynaptically and postsynaptically in the nucleus accumbens, where they regulate neurotransmitter release and neuronal excitability, respectively.158,159,205
In contrast to AMPA receptors, the precise role of kainate receptors in synaptic plasticity remains unclear; however, there is emerging evidence that kainate receptors mediate some forms short- and long-term synaptic plasticity in the brain.205,207,211,217,218 Moreover, a specific role for kainate receptors in drug-induced plasticity is difficult to determine because of a lack of pharmacological compounds that discriminate between these two classes of ionotropic glutamate receptors.219 The most commonly used non-NMDA glutamate receptor antagonist, CNQX, exhibits relatively little selectivity between kainate and AMPA receptors and, at higher concentrations, binds to the glycine site on NMDA receptors.220–222 Recently, selective kainate receptor agonists and antagonists have been developed,219,223,224 which should prompt studies into the specific role of kainate receptors in the reinforcing effects of cocaine as well as cocaine craving and relapse.
Role of nucleus accumbens AMPA/kainate receptors in cocaine self-administration and extinction
Modulation of AMPA/kainate glutamate receptors influences cocaine self-administration behavior, indicating that the reinforcing and rewarding effects of cocaine are mediated, in part, through AMPA receptor–mediated signaling. Thus, systemic administration of an AMPA/kainate receptor antagonist decreases or has no effect on responding for cocaine on an FR schedule of reinforcement.143,175 Similarly, infusion of an AMPA/kainate receptor antagonist directly into the core, but not the shell, decreases cocaine self-administration under a second-order schedule of reinforcement, suggesting that AMPA receptors in these two subregions of the nucleus accumbens have dissociable roles in cue-induced cocaine seeking.225 However, intra-accumbal core administration of an AMPA receptor agonist also decreased responding for cocaine on an FR schedule of reinforcement, suggesting that increased accumbens AMPA receptor signaling augments the reinforcing effects of cocaine.143 Thus, future studies are required to elucidate the precise role of intra-accumbal AMPA/kainate receptors in the reinforcing effects of cocaine and whether basal levels of extracellular glutamate influence the reinforcing efficacy of cocaine in these behavioral models.143
During extinction, when animals learn that drug-seeking behavior is no longer reinforced in the absence of drug reward, AMPA receptor subunit expression is altered, suggesting that extinction training induces plasticity in glutamatergic systems that influence cocaine reinstatement.226 For example, GluR1 and GlurR2/3 subunit expression was increased in the nucleus accumbens shell of cocaine-experienced rats that had undergone 1 week of extinction training.227 Viral-mediated overexpression of GluR1 and GluR2 AMPA receptor subunits in the nucleus accumbens shell facilitated extinction of lever responding for cocaine and was sufficient to attenuated stress-induced reinstatement of cocaine seeking.227 These data indicate that extinction-induced plasticity in the nucleus accumbens shell is a compensatory response to decreased basal extracellular glutamate levels during withdrawal from cocaine. However, in rats that undergo withdrawal from cocaine, rather than active extinction, GluR1 subunit expression is decreased in the accumbens shell.172 Collectively, these results suggest that increases in AMPA receptor expression in the nucleus accumbens shell during extinction opposes stress-induced reinstatement of cocaine seeking and that active inhibitory learning, not passive withdrawal, is required for these neuroadaptations.
Effect of repeated cocaine exposure on AMPA receptor expression in the nucleus accumbens
Studies examining glutamate receptor subunit mRNA and protein expression in the nucleus accumbens of animals receiving noncontingent injections of cocaine and in animals self-administering intravenous injections of cocaine report inconsistent findings.30 For example, some reports have demonstrated no change in the expression of GluR1–2 or kainate receptor subunits in the nucleus accumbens after 24 h of withdrawal from repeated cocaine exposure,164,165,201 whereas others have reported decreased expression of accumbal GluR3/4 subunits at similar time points.166,167 In contrast, GluR1 and GluR2/3 subunit expression is increased in the nucleus accumbens after longer periods of forced abstinence in animals that developed behavioral sensitization.164,166,168,201 After protracted periods of forced abstinence among animals that previously self-administered cocaine, nucleus accumbens GluR1, GluR2, and GluR2/3 subunit expression are increased after protracted withdrawal periods in animals that previously self-administered cocaine.160,169 Similar results were observed after the extinction of cocaine self-administration behavior.227 Cocaine self-administration followed by a period of forced abstinence is also associated with increases in phospo-GluR1 in the accumbens core and shell, although the magnitude of this increase is less than that occurring after the acute administration of cocaine.172 Consistent with these preclinical studies, postmortem studies of human cocaine overdose victims reveals increased expression of GluR2/3 and a trend toward increased GluR1 subunit proteins in the nucleus accumbens.173 Collectively, these studies indicate that repeated exposure to cocaine followed by a period of forced abstinence, when extracellular glutamate levels are decreased in the nucleus accumbens,136,140 is associated with increases in the expression of AMPA receptor subunits in the nucleus accumbens, which may result in a predisposition toward cocaine craving and relapse.
Nucleus accumbens AMPA/kainate receptors and the reinstatement of cocaine seeking
Several studies have shown that accumbal AMPA/kainate receptors contribute significantly to the reinstatement of cocaine seeking. Thus, administration of an AMPA receptor agonist directly into the nucleus accumbens promotes reinstatement of cocaine seeking, whereas intra-accumbal administration of an AMPA/kainate receptor antagonist blocks reinstatement induced by a systemic priming injection of cocaine143,144,228 or conditioned stimuli previously paired with cocaine taking.229 Although these microinjection studies did not distinguish between the core and shell subregions of the nucleus accumbens, there is evidence that increased glutamate transmission in both the core and shell contributes to the reinstatement of cocaine seeking. Thus, increased extracellular glutamate release in the nucleus accumbens core was observed during cocaine priming–induced reinstatement of drug seeking,108 whereas administration of an AMPA/kainate receptor antagonist into the accumbens shell inhibited the reinstatement of cocaine seeking prompted by administration of cocaine into the mPFC.105 Recent evidence indicated that administration of an AMPA/kainate receptor antagonist into the accumbens core or shell attenuated the reinstatement of cocaine seeking.230 Consistent with these results, microinjection of AMPA directly into the accumbens core or shell reinstates cocaine seeking.231 Moreover, suppression of GluR1 transcription in either the accumbens core or shell impaired the reinstatement of drug seeking induced by a cocaine priming injection.231 Collectively, these data indicate that increased glutamate transmission through AMPA/kainate receptors in both the core and shell of the nucleus accumbens promotes the reinstatement of cocaine-seeking behavior.
AMPA receptor trafficking and cocaine-induced plasticity
A growing body of evidence indicates that the dynamic trafficking of AMPA receptors plays a critical role in neuronal plasticity.27,192,232–235 In terms of cocaine-induced neuronal plasticity, the ratio of cell surface to intracellular GluR1 and GluR2/3 AMPA receptor subunits in the nucleus accumbens is increased after 3 weeks, but not 1 day, after the last of a series of repeated cocaine injections.236 Increases in synaptic insertion of GluR1, GluR2, and possibly GluR3 subunits in the nucleus accumbens should contribute to cocaine-induced behavioral plasticity as well as augment long-term potentiation (LTP),27 a change in synaptic plasticity that has been demonstrated in the nucleus accumbens after repeated cocaine injections.237 However, repeated cocaine injections and cocaine self-administration also decrease the magnitude of long-term depression (LTD) in the nucleus accumbens.238–240 More recent studies suggest that cocaine-induced changes in the synaptic strength of excitatory cortico-accumbal synapses are bidirectional.204,241 That is, although repeated cocaine administration, followed by a period of forced abstinence, enhances AMPA receptor-mediated synaptic transmission241 and AMPA receptor transport to the cell surface in the nucleus accumbens,204 these effects are reversed 24 h after a systemic challenge injection of cocaine.204,241 Consistent with these findings, cocaine self-administration followed by extinction results in decreased GluR2–pSer880 in the nucleus accumbens shell, where as a cocaine challenge injection prompts an increase in accumbens shell GluR2–pSer880.230 Recent results suggest a behavioral correlate of this form of bidirectional plasticity in that the expression of behavioral sensitization to cocaine is associated with transient decreases in the behavioral hyperactivity induced by intra-accumbal AMPA administration.242 Taken together, these findings highlight one mechanism by which cocaine-induced plasticity in the nucleus accumbens regulates expression of sensitization to the incentive motivational effects of cocaine.204,236
Cocaine-induced bidirectional plasticity in the synaptic strength of excitatory cortico-accumbal synapses indicates that a prior history of cocaine alters the magnitude or direction of plasticity within a given neuron or synapse in response to a subsequent priming injection of cocaine, a process referred to as metaplasticity.243,244 Furthermore, dynamic regulation of AMPA receptors by intracellular proteins that regulate subunit trafficking and synaptic plasticity controls Ca2+ permeability of synaptic AMPA receptors.245 Experience-dependent modification of neural circuitry, including neural adaptations to drugs of abuse, are believed to underlie all forms of behavioral plasticity and are mediated, in part, by AMPA receptor trafficking.23,192,246–249 Thus, recent findings suggest that cocaine-induced plasticity in excitatory synapses within the nucleus accumbens initiates adaptive changes in neuronal ensembles that lead to drug-seeking behavior and alters subsequent physiological responses to cocaine, including increased trafficking and surface expression of AMPA receptors, during extended withdrawal.
AMPA receptor trafficking and cocaine-induced reinstatement of drug seeking
Blocking AMPA glutamate receptor–mediated signaling in the nucleus accumbens core or shell attenuates cocaine priming–induced reinstatement of drug seeking.105,108,230 Consistent with these results, decreased GluR1 subunit mRNA expression in the nucleus accumbens core or shell blocks cocaine priming–induced reinstatement.231 However, cocaine priming–induced reinstatement of drug-seeking behavior was associated with increased phosphorylation of GluR1 AMPA receptor subunits on Ser831, an amino acid residue phosphorylated by calcium/calmodulin-dependent kinase II (CaMKII) and protein kinase C (PKC), and enhanced cell surface expression of GluR1-containing AMPA receptors in the accumbens shell.23 Consonant with these findings, impairing the transport of GluR1-containing AMPA receptors to the cell surface in the nucleus accumbens shell attenuated the ability of a priming injection of cocaine to reinstate drug-seeking behavior.23 The reinstatement of cocaine seeking is also associated with increased phosphorylation of GluR2 AMPA receptor subunits at Ser880, a PKC phosphorylation site, in the accumbens shell.230 PKC-induced phosphorylation of GluR2 subunits at Ser880 and the subsequent association of GluR2 with protein interacting with C kinase (PICK1) results in rapid internalization of GluR2-containing AMPA receptors.246,250–253 However, there is a growing body of evidence that PICK1 also contributes significantly to the insertion of GluR2-containing AMPA receptors into synapses under certain circumstances.245,254 Although these results appear contradictory, these findings were observed in different cell types in the hippocampus and cerebellum. For example, activity-dependent interactions between GluR2 and PICK1 result in endocytosis of GluR2-containing AMPA receptors within Purkinje cells in the cerebellum.250,255 In contrast, PICK1 regulates the insertion of GluR2-containing AMPA receptors into synapses in cerebellar stellate cells.245,254 Disrupting interactions between GluR2 AMPA receptor subunits and PICK1 in the nucleus accumbens shell with a peptide that mimics C terminus residues of GluR2 subunits, including Ser880, attenuates cocaine-seeking behavior, which suggests that impairing the trafficking of GluR2-containing AMPA receptors in the nucleus accumbens disrupts the reinstatement of drug seeking.230 Similarly, intra-accumbal administration of a peptide that specifically blocks activity-dependent but not constitutive endocytosis of GluR2-containing AMPA receptors attenuates the expression of behavioral sensitization to amphetamine.256 These results indicate that enhanced behavioral responses after repeated cocaine exposure (reinstatement or behavioral sensitization) are associated with the internalization of GluR2-containing AMPA receptors in the core and shell of the nucleus accumbens. In contrast, the reinstatement of cocaine seeking was coincident with increases in the surface expression of GluR1-containing AMPA receptors in the nucleus accumbens shell.23 Thus, removal of GluR2-containing AMPA receptors from synapses in the nucleus accumbens shell attenuates cocaine-seeking behavior,230 and increases in GluR1-mediated excitatory transmission in the accumbens shell promote cocaine priming– and cue–induced reinstatement of drug seeking.23,257 Taken together, these findings suggest that the reinstatement of cocaine-seeking behavior is mediated by different trafficking of AMPA receptor subunits in the nucleus accumbens shell, including increased surface expression of GluR2-lacking AMPA receptors.257
Metabotropic glutamate receptors
Metabotropic glutamate receptors (mGluRs) are coupled to intracellular signaling pathways via G proteins and upon activation generate slow synaptic responses and regulate neuronal plasticity.258,259 Eight different mGluR subunits have been identified to date and classified into three main subfamilies on the basis of sequence homology, pharmacology, and coupling to intracellular effectors.260,261 These functional subfamilies include group I mGluRs (mGluR1 and 5), which stimulate phospholipase C (PLC), resulting in the generation of diacylglycerol (DAG) and inositol triphosphate (IP3), which activate PKC and Ca2+ release from intracellular stores; group II mGluRs (mGluR2 and 3); and group III mGluRs (mGluR4, 6–8), which inhibit adenylate cyclase activity and subsequently decrease cAMP levels.262,263 Group I and II mGluRs are widely distributed throughout the brain, including the nucleus accumbens.264–273 Group I mGluRs are expressed predominantly on postsynaptic membranes, just lateral to the postsynaptic density; however, there is some evidence for presynaptic localization of mGluR1 and mGluR5.274–278 mGluR2s are generally expressed at extrasynaptic sites on presynaptic terminals, where they have been demonstrated to attenuate excitatory amino acid neurotransmission.279–282 In contrast, mGluR3s are localized on both pre- and post-synaptic locations on neurons as well as more widespread distribution in glial cells.282,283 Recent studies have demonstrated that altered mGluR signaling mediates, in part, cocaine-induced neuroadaptations284–288 and that activation of mGluR signaling may reverse cocaine-induced synaptic plasticity.289
Effect of repeated cocaine exposure on mGluR expression in the nucleus accumbens
Several studies have examined mGluR mRNA and protein expression in the nucleus accumbens after repeated exposure to cocaine. For example, expression of mGluR5 mRNA is increased and expression of mGluR2/3 is decreased in the nucleus accumbens after 3 weeks of withdrawal from repeated, but not acute, cocaine administration.166,290 Because extracellular basal glutamate levels are decreased after repeated cocaine administration,136 it is likely that increased mGluR5 expression and decreased mGluR2/3 expression in withdrawn animals reflects a compensatory change in response to hypoglutamatergic transmission. These results are consistent with recent findings demonstrating that an acute injection of cocaine did not alter total accumbal expression of mGluR5 protein but was sufficient to reduce surface expression of mGluR5 in the nucleus accumbens, which suggests that trafficking of mGluRs plays a critical role in cocaine-induced synaptic plasticity.284 Consistent with this hypothesis, recent evidence indicates that mGluR2/3 and mGluR5 proteins are redistributed to the synaptosomal membrane fraction after a period of extended, but not acute, forced abstinence.290
Nucleus accumbens mGluRs and cocaine reinforcement
A few studies have assessed the role of mGluRs in cocaine self-administration behavior. Systemic administration of an mGluR2/3 glutamate receptor agonist attenuates cocaine self-administration through a mechanism that probably involves decreased synaptic glutamate transmission after stimulation of presynaptic mGluR2/3s.291 Constitutive mGluR5–knockout mice do not self-administer cocaine and are insensitive to the locomotor stimulant properties of cocaine.292 Similarly, administration of the mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) decreases self-administration of cocaine, suggesting that mGluRs may be viable targets for the development of therapeutics for cocaine addiction.293–297
Nucleus accumbens mGluRs and reinstatement of cocaine seeking
Recent evidence suggests that mGluRs may also contribute to cocaine priming–induced reinstatement of drug seeking. Systemic administration of an mGluR5 antagonist attenuated the ability of a priming injection of cocaine295 or cocaine-associated cues298 to reinstate cocaine seeking. Consistent with this finding, intra-accumbal shell administration of an mGluR5 antagonist attenuated cocaine priming–induced reinstatement.299 mGluR2/3s have also been shown to play a role in cocaine seeking. For example, systemic administration of an mGluR2/3 agonist attenuates cue-induced300 and cocaine priming–induced291,301 reinstatement of drug-seeking behavior. However, systemic and intra-accumbal core administration of an mGluR2/3 receptor agonist attenuates food seeking as well, suggesting that activation of accumbal mGluR2/3s impairs general responding for drugs of abuse and natural reinforcers.301
Role of the cystine–glutamate antiporter in cocaine seeking
In addition to decreasing basal levels of extracellular glutamate in the nucleus accumbens, withdrawal from repeated cocaine exposure decreases activity of the cystine–glutamate exchanger, an antiporter protein expressed on glial cells that exchanges extracellular cystine for intracellular glutamate.140,302 These results indicate that one potential mechanism underlying cocaine priming– induced reinstatement is decreased cystine–glutamate exchanger activity, a neuroadaptation that results in decreased extracellular glutamate levels.303 Normalization of exchanger activity by administering cystine directly into the nucleus accumbens or NAC (a cystine prodrug) systemically attenuates the ability of a priming injection of cocaine to reinstate drug-seeking behavior.140,304 Similarly, inhibiting cysteine–glutamate exchange in the nucleus accumbens promotes cocaine-induced drug seeking.303 Moreover, increasing cystine–glutamate exchanger activity prevented both the decrease in basal extracellular glutamate levels during withdrawal from repeated cocaine and the subsequent increase in glutamate levels and the reinstatement of cocaine seeking associated with a systemic priming injection of cocaine.140 Mechanistically, the reduction in extracellular glutamate levels in the nucleus accumbens core after repeated exposure to cocaine provides less tonic activation of mGluR2/3 autoreceptors on glutamatergic terminals in the nucleus accumbens, such that synaptic levels of glutamate are increased after a challenge injection of cocaine owing to less mGluR2/3-mediated inhibitor feedback.305,306 Taken together, these results indicate that the reinstatement of cocaine seeking is promoted, in part, by reducing cystine–glutamate exchange in the nucleus accumbens.
Cocaine-induced metaplasticity, N-acetylcysteine, and mGluRs
The finding that withdrawal from repeated cocaine administration and subsequent reexposure to cocaine results in bidirectional synaptic plasticity in the nucleus accumbens204,241 suggests that cocaine craving and relapse are regulated, in part, by cocaine-induced metaplasticity in excitatory synapses within the accumbens. Metaplasticity, as it relates to addiction, may involve drug-induced neuroadaptations in the physiological or biochemical state of glutamatergic networks or synapses that ultimately alters their ability to generate synaptic plasticity, such as LTP and LTD.244,307 Recently, it was shown that withdrawal from repeated cocaine administration alters the capacity of subsequent stimuli to induce neuroplasticity at excitatory synapses in the nucleus accumbens.287 This study demonstrated that the ability of PFC stimulation to produce LTP or LTD in nucleus accumbens core GABAergic projection neurons is impaired after 3 weeks of extinction training in cocaine-experienced animals, indicating that withdrawal from repeated cocaine exposure shifts the threshold necessary for generating a plastic response in the accumbens.287 Furthermore, systemic administration of NAC reversed cocaine-induced metaplasticity in the accumbens and restored the capacity of PFC stimulation to induce LTP and LTD at excitatory synapses in the accumbens core.287 The ability of NAC to restore synaptic plasticity and reverse cocaine-induced metaplasticity in cortico-accumbal synapses is due to presynaptic mGluR2/3- and post-synaptic mGluR5-mediated effects on LTP and LTD, respectively.287,308 Taken together, these results suggest that drugs, such as NAC that reverse cocaine-induced metaplasticity, may prevent cocaine craving and relapse. However, the precise relationship between altered synaptic plasticity and susceptibility to relapse remains to be determined.
Homer, mGluR signaling, and repeated cocaine
Intracellular scaffolding proteins called Homer proteins regulate mGluR1/5 signaling and trafficking in the brain.309–311 Homer proteins are enriched at excitatory synapses, where they bind to several synaptic proteins in the postsynaptic density and link mGluR1/5s to ionotropic glutamate receptors.311–313 After 3 weeks of withdrawal, mGluR1/5s, Homer1b/c, and Homer2a/b protein expression were decreased in the medial nucleus accumbens shell of cocaine-experienced rodents.278,314 Constitutive Homer1- or Homer2-knockout mice have behavioral phenotypes similar to those of animals pretreated with repeated injections of cocaine.315 Moreover, Homer2-knockout mice acquired cocaine self-administration behavior faster than wild-type control subjects and had reduced basal extracellular glutamate levels in the accumbens because of altered function of mGluR1s and the cystine–glutamate exchanger.315 Interestingly, Homer2 function converges with regulators of G protein signaling (RGS) function at synapses in the striatum, indicating that altered expression of AGS3 and Homer proteins during withdrawal from repeated cocaine use mediates persistent changes in cortico-accumbal glutamatergic projections.316–318 The behavioral and neurochemical parallels between constitutive Homer2-knockout mice and wild-type mice with a history of repeated cocaine administration suggests that Homer2 plays a critical role in regulating accumbens glutamate levels and cocaine-induced behavioral sensitization.319,320 Consistent with these results, overexpressing Homer isoforms in the nucleus accumbens attenuates expression of cocaine-induced behavioral sensitization as well as increased extracellular accumbens glutamate levels after a challenge injection of cocaine.321 Expression of Homer isoforms in the nucleus accumbens is differently regulated by acute versus chronic cocaine injections, and this cellular response is mediated by D1-like dopamine receptors, and not D2-like dopamine, AMPA, or NMDA receptors.322 Thus, Homer proteins regulate signaling and trafficking of metabotropic and ionotropic glutamate receptors in the nucleus accumbens, as well as extracellular levels of glutamate. Homer proteins may contribute to enduring molecular plasticity in excitatory synapses in the accumbens after repeated cocaine exposure.
Nucleus accumbens synaptic plasticity and reinstatement of cocaine-seeking behavior
As previously described, increases in dopamine and glutamate transmission in the nucleus accumbens independently promote the reinstatement of cocaine seeking.35,85 Although the downstream signaling effects and neuroadaptations that contribute to this behavior are not well defined, there has been recent progress in this area, which is outlined in the following sections.
Cocaine seeking and interactions between accumbens dopamine and glutamate systems
The cellular mechanisms underlying D1-like dopamine receptor–mediated reinstatement of cocaine seeking in the nucleus accumbens are poorly defined. Stimulation of G protein-coupled D1-like dopamine receptors increases synthesis of cAMP and activates protein kinase A (PKA), which contribute to the reinforcing effects of cocaine and reinstatement of cocaine-seeking behavior.323,324 One intracellular effector targeted by PKA is the L-type Ca2+ channel, which plays a critical role in psychostimulant-induced behavioral and neuronal plasticity.325,326 L-type Ca2+ channels in turn activate a family of protein kinases including CaMKII, an enzyme that regulates both the initiation and expression of psychostimulant-induced behavioral sensitization.325,327 In striatal neurons, activation of D1-like dopamine receptors enhances AMPA receptor–mediated excitatory postsynaptic potentials,328 an effect that is suppressed by administration of an L-type Ca2+ channel antagonist.329 Consistent with these findings, blocking L-type Ca2+ channels decreases glutamate-mediated burst firing of accumbal neurons.330 Collectively, these results suggest that cocaine-induced neuronal plasticity in dopamine and glutamate systems is mediated, in part, through activation of L-type Ca2+ channels and CaMKII.
A recent study examined this hypothesis and demonstrated that stimulating D1-like dopamine receptors in the medial nucleus accumbens shell promotes the reinstatement of cocaine seeking by serially stimulating L-type Ca2+ channels and phosphorylation of CaMKII on Thr286.23 Furthermore, reinstatement of cocaine-seeking behavior was associated with an increase in phosphorylation of the AMPA receptor subunit GluR1 on Ser831, an amino acid residue phosphorylated by CaMKII and PKC, and enhanced cell surface expression of GluR1-containing AMPA receptors in the accumbens shell.23 Consistent with these findings, impairing the transport of GluR1-containing AMPA receptors to the cell surface within the nucleus accumbens shell attenuated the ability of a priming injection of cocaine to reinstate drug-seeking behavior.23 These results indicate that D1-like dopamine receptor stimulation–dependent activation of L-type Ca2+ channels and CaMKII facilitates the reinstatement of cocaine seeking by promoting the transport of GluR1-containing AMPA receptors in the nucleus accumbens shell to the plasma membrane (Fig. 3). The mechanisms underlying CaMKII-dependent AMPA receptor transport, however, are unclear and may include targets other than GluR1.249,331 Thus, CaMKII activity in the nucleus accumbens shell may be an essential link between dopamine and glutamate systems involved in the neuronal plasticity underlying cocaine craving and relapse.
Figure 3.
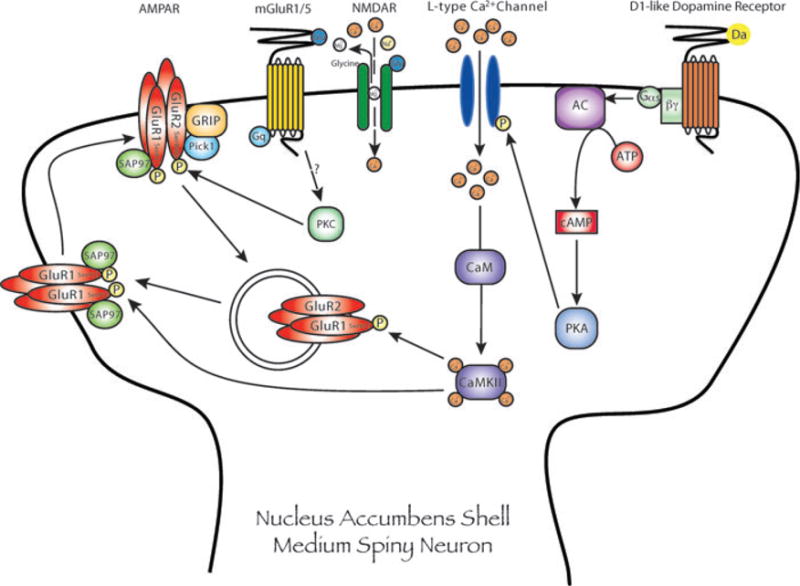
Link between nucleus accumbens shell dopamine and glutamate systems, via L-type Ca2+ channels and Ca2+/calmodulin kinase II (CaMKII), which is proposed to underlie the reinstatement of cocaine seeking. In brief: stimulation of D1-like dopamine receptors serially activates L-type Ca2+ channels and CaMKII. In addition to phosphorylation of CaMKII, reinstatement of cocaine seeking is associated with phosphorylation of GluR1 AMPA receptor subunits at Ser831, a known CaMKII and protein kinase C (PKC) phosphorylation site, as well as increased surface expression of GluR1-containing AMPA receptors in the nucleus accumbens shell. However, cocaine priming–induced reinstatement was not associated with an increase in GluR1 phosphorylation on Ser845, a known protein kinase A (PKA) phosphorylation site. Interfering with PDZ domain–containing proteins, such as synapse-associated protein (SAP) 97, and GluR1 subunits impairs trafficking of GluR1-containing AMPA receptors to the cell surface and attenuates cocaine seeking. Reinstatement of drug seeking is also associated with increased phosphorylation of GluR2 subunits at Ser880, a known PKC phosphorylation site that promotes internalization of GluR2-containing AMPA receptors. Although the receptor systems that activate PKC signaling during the reinstatement are unknown, one possibility is mGluR1/5s that are coupled to PKC via PLC. Consistent with the theory that PKC phosphorylation promotes internalization of GluR2-containing AMPA receptors after a priming injection of cocaine, disruption of accumbens shell protein interacting with C kinase (PICK1) function, which involves binding to GluR2 subunits and their rapid internalization, attenuates the reinstatement of cocaine seeking. Taken together, these results suggest that the reinstatement of cocaine-seeking behavior is associated with dynamic trafficking of AMPA receptor subunits between the cell surface and cytoplasmic compartments within the accumbens and that these molecular adaptations underlie cocaine-induced synaptic plasticity. (In color in Annals online.)
Nucleus accumbens, cocaine-induced molecular neuroplasticity, and the reinstatement of cocaine seeking
Repeated cocaine administration has profound cellular and molecular effects on nucleus accumbens dopamine and glutamate systems. Cocaine-induced neuroadaptations in reward-related circuitry mediate maladaptive behaviors, including the reinstatement of drug-seeking behavior.35 Aberrant neuroplasticity in learning and memory circuits within corticolimbic-striatal networks plays a critical role in the development and persistence of addictive behaviors.16,17,20,27,134,332 In addition to altered expression of ionotropic and mGluR subtypes in the nucleus accumbens, chronic cocaine exposure also regulates expression and function of intracellular effectors that mediate synaptic plasticity and more permanent modifications in chromatin structure and protein expression. Recent studies have begun to examine the cellular and molecular components that mediate enduring cocaine-induced molecular plasticity in nucleus accumbens glutamate systems and how these alterations influence reinstatement of drug-seeking behavior.
Emerging evidence indicates that pre- and post-synaptic adaptations in glutamatergic projections from the mPFC to the nucleus accumbens facilitate glutamate release in response to a priming injection of cocaine and promote reinstatement of drug seeking. Preclinical studies suggest that repeated cocaine administration reduces signaling through Giα-coupled receptors and that this deficit plays a critical role in cocaine addiction.333–336 Stimulation of transmembrane metabotropic G protein-coupled receptors activates heterotrimeric G proteins that, in turn, regulate intracellular effectors and transmit neuronal signals across plasma membranes. RGS proteins are accessory proteins that modulate signal transfer from G protein-coupled receptors to G protein and/or the activation state of Gα proteins by stimulating GTPase activity or blocking activation of signal transduction cascades by G proteins.337–339 Chronic cocaine administration increases RGS9 protein expression in the nucleus accumbens.317 Furthermore, withdrawal from chronic cocaine exposure increases expression of AGS3, an activator of G protein-coupled signaling that binds Giα and thus decreases signaling through Giα-mediated signaling cascades, in the mPFC and accumbens core.316 AGS3 antisense oligonucleotides administered directly into the mPFC reversibly inhibit AGS3 expression and restore D2-like dopamine receptor–mediated Giα signaling in the mPFC after chronic cocaine exposure, which provides further evidence that AGS3 reduces Giα signaling in the mPFC.316 Moreover, AGS3 anti-sense administration during extinction training attenuated cocaine priming–induced reinstatement of drug seeking.316 Taken together, these results suggest that elevated AGS3 levels during withdrawal decrease Giα-mediated signaling (D2-like dopamine receptors, mGluR2/3s, and μ opioid receptors) and shift the signaling bias in the mPFC to favor Gsα-mediated signaling (D1-like dopamine receptors, β-adrenergic receptors, and corticotropin-releasing factor receptors).340 Thus, persistent changes in RGS proteins in the mPFC indicate that stable molecular changes occur in glutamatergic projection neurons to the nucleus accumbens and that these adaptations contribute to the propensity to reinstate cocaine-seeking behavior after withdrawal from cocaine self-administration.
Repeated noncontingent cocaine injections also increases adenylyl cyclase and cyclic-AMP dependent PKA expression in the nucleus accumbens of rodents341 and nonhuman primates.342 Consistent with these results, PKA expression was increased in the nucleus accumbens or rats after withdrawal from cocaine self-administration.160 Administration of a PKA inhibitor directly into the nucleus accumbens reinstates cocaine seeking.323 Although administration of a PKA activator into the accumbens facilitates cocaine reinstatement, this effect may reflect a generalized increase in behavioral activation, because operant responding was increased on both active and inactive levers323; however, see Ref. 324. In contrast, administration of a PKA inhibitor decreased cocaine self-administration under a progressive-ratio schedule of reinforcement.324 These discrepant findings may be due to different dosing regimens and/or schedules of reinforcement that reflect motivational or regulatory aspects of drug-taking behavior.343 Furthermore, prominent sex differences exist in basal and cocaine-induced alterations in PKA signaling within the nucleus accumbens.344,345 Regardless, it is difficult to interpret the role of PKA signaling in priming-induced reinstatement because these behavioral effects could be modulated by pre- and/or postsynaptic effects that modulate dopamine and glutamate transmission in the accumbens.323,346,347 It is clear that stimulation of D1-like dopamine receptors in the nucleus accumbens activates PKA and increases insertion of AMPA receptor subunits into the plasma membrane.235,348,349 Thus, cocaine-induced neuroadaptations in PKA-mediated signaling probably promote dysfunctional synaptic plasticity by altering trafficking and surface expression of AMPA receptors in the nucleus accumbens.
Recent evidence indicates that PKC may mediate drug-induced neuroadaptations in synaptic plasticity within the nucleus accumbens during the reinstatement of cocaine seeking. PKC-dependent increases in phosphorylation of GluR2 subunits in the accumbens shell, and possibly core, are associated with cocaine-seeking behavior and suggest that internalization/trafficking of GluR2-containing AMPA receptors in the accumbens is one mechanism underlying cocaine-induced metaplasticity.230 These findings support those of previous studies demonstrating a role for PKC in psychostimulant-mediated behaviors. For example, repeated cocaine administration increases the phosphorylation of some, but not all, isoforms of PKC in the nucleus accumbens.350,351 Furthermore, intra-accumbal administration of a PKC inhibitor attenuated amphetamine-induced conditioned place preference (CPP)352 and systemic administration of a PKC inhibitor attenuated cocaine-induced CPP.353 Similarly, administration of a PKC inhibitor directly into the accumbens blocked the expression of behavioral sensitization to cocaine.354 PKC mRNA expression is increased in limbic areas, including the accumbens, after 5 days of withdrawal from self-administered cocaine.355 Although the precise role of accumbens PKC isoforms in cocaine priming–induced reinstatement of drug seeking is unknown, preliminary studies suggest that persistent changes in PKC expression after repeated cocaine exposure may lead to enduring changes in glutamate transmission. In addition to regulating AMPA receptor trafficking, psychostimulants may also influence the trafficking and functional regulation of dopamine,356–358 serotonin,359,360 and nore-pinephrine361,362 transporters through PKC- and PKA-dependent signaling mechanisms, although this hypothesis as it relates to cocaine craving and relapse has yet to be tested.
CaMKs have also been demonstrated to play a role in the persistent behavioral effects of repeated cocaine administration. For example, administration of a CaMKII inhibitor directly into the nucleus accumbens blocks expression of cocaine-induced behavioral sensitization.327,354 Furthermore, reinstatement of cocaine-seeking behavior is associated with CaMKII-mediated phosphorylation and surface expression of GluR1 subunits in the nucleus accumbens.23 The molecular bases for the physiological and behavioral effects of psychostimulant-induced activation of CaMKII may involve disruption of D2-like dopamine receptor–NR2B signaling interactions363 and/or increased trafficking of GluR1-containing AMPA receptors to the cell surface.23,364 Recent evidence suggests that CaMKIV also regulates cocaine-mediated behaviors. For example, mice selectively lacking CaMKIV expression in dopaminergic neurons display increased cocaine-induced CPP and behavioral sensitization.365 Moreover, a significant association between a single-nucleotide polymorphism in the human CaMKIV promoter and cocaine addiction has been identified, further supporting a role for this enzyme in cocaine craving and relapse.365 These results indicate that CaMKII and CaMKIV activity may have distinct influences on addictive behaviors, effects that are due, in part, to different regulation of CREB protein–dependent transcription.365–367 However, the precise role of CaMK proteins in the long-term plasticity associated with vulnerability to relapse in human cocaine addicts remains to be determined.
Repeated cocaine administration produces enduring neuroadaptations in several intracellular effectors that mediate dopamine and/or glutamate signaling in the nucleus accumbens. In addition to the aforementioned proteins, long-term molecular and synaptic plasticity in the accumbens after chronic cocaine exposure are also mediated, in part, by changes in the extracellular signal–regulated kinase signaling pathway,135,368,369 brain-derived neurotrophic factor,135,370 cyclin-dependent kinase 5,371,372 and gene expression.373,374 Identifying and reversing these and other cocaine-induced neuroadaptations may lead to more targeted pharmacotherapies that enhance or block specific forms of neuroplasticity that underlie maladaptive learning and memory processes, such as cocaine craving and addiction.
Glutamate-modulating drugs, cocaine craving, and relapse
Collectively, the studies presented here indicate that altered glutamate transmission in the nucleus accumbens mediates the reinforcing effects of cocaine as well as the reinstatement of cocaine-seeking behavior. With the importance of cocaine-induced neuroadaptations in neuronal and synaptic plasticity within glutamatergic circuits that mediate normal reward learning,17,27 recent approaches to developing novel pharmacotherapies for cocainead-diction have focused on drugs that inhibit/modulate glutamate transmission.134,375 The results of these clinical and preclinical studies are summarized in the following.
Cystine–glutamate exchanger substrate: N-acetylcysteine
The efficacy of NAC, a drug commonly used to treat acetaminophen overdose,376 in treating addictive behaviors has been tested for cocaine relapse,377,378 nicotine addiction,379 and gambling.380 As reviewed in the preceding, NAC has been shown to normalize decreased glutamate levels in the nucleus accumbens of cocaine-experienced animals during withdrawal from drug use and attenuate reinstatement of cocaine-seeking behavior.139,304 Recent clinical trials demonstrate that NAC is well tolerated; reduces cocaine use; and, according to subjective patient reports, decreases desire to use cocaine.377,378 However, NAC treatment does not significantly reduce cocaine craving and thus may not prevent relapse in abstinent cocaine addicts.381 Although the results of these preliminary studies are promising, clinical trials with larger sample sizes are needed to fully realize the therapeutic efficacy of NAC in treating maladaptive, compulsive behaviors, such as drug addiction.
Modafinil
Modafinil is a stimulant that is currently approved by the U.S. Food and Drug Administration (FDA) for the treatment of narcolepsy, shift-work sleep disorder, and obstructive sleep apnea.382,383 Preclinical studies of modafinil suggest a similar pharmacological profile to psychostimulants including amphetamines; however, not all its neurochemical and behavioral effects overlap with those of amphetamine.384 For example, modafinil binds to sites on both dopamine and norepinephrine transporters in vivo, and clinically relevant doses of modafinil increase extracellular dopamine levels.385,386 The molecular mechanisms that regulate the wake-promoting effects of modafinil have recently been attributed to activation of D1-like and D2-like dopamine receptors.387 Furthermore, modafinil increases histamine release,388 stimulates hypothalamic orexin neurons,389,390 increases glutamate release, and inhibits both GABA release391 and firing of midbrain dopamine neurons through a D2-like dopamine receptor–mediated mechanism.392 Although humans will self-administer modafinil more than placebo under certain conditions,393 most clinical studies suggest that modafinil has low abuse liability even among current drug users.394–396 Modafinil pretreatment does not affect cocaine self-administration in rodents, which suggests that this compound does not have reinforcing effects.397 In contrast, very high doses of modafinil produced reinforcing and discriminative stimulus effects in non-human primates.398 However, the effects of chronic modafinil administration on the reinforcing effects of cocaine have not been studied using animal models.
Modafinil pretreatment reduces cocaine-induced euphoria and craving in human cocaine users without producing adverse effects.399–401 Furthermore, cocaine-dependent patients reported that modafinil decreased cocaine-associated subjective measures after a drug-taking event.402 A recent clinical study demonstrated that modafinil pretreatment decreased high-dose cocaine self-administration as well as the intoxicating and cardiovascular effects of smoked cocaine.403 Collectively, these clinical results are promising and need to be confirmed by larger studies.
Partial NMDA receptor agonist: D-cycloserine
D-Cycloserine is an antibiotic that crosses the blood–brain barrier, binds with high affinity to the glycine modulatory site on the NMDA receptor and thus functions as a partial NMDA receptor agonist in vivo.404–406 D-Cycloserine facilitates fear extinction in laboratory animals and humans patients with anxiety disorders.407–409 Consistent with these results, administration of D-cycloserine enhances extinction of cocaine-induced CPP, a behavioral effect that was persistent.410–412 Taken together, these results suggest that D-cycloserine administration reduces the conditioned reinforcing properties of drug-associated stimuli through facilitation of extinction learning. However, a recent study demonstrates that administration of D-cycloserine directly into the basolateral amygdala augments cue-induced reinstatement of cocaine seeking.413 This study indicates that D-cycloserine administration enhances reconsolidation of cocaine-associated memories and thereby promotes reinstatement of drug-seeking behavior.413 Moreover, high doses of D-cycloserine reinstated cocaine-induced CPP.412 It remains to be determined how different dosing regimens of D-cycloserine affect addictive behaviors in preclinical as well as clinical studies.
Noncompetitive NMDA receptor antagonists: memantine and amantadine
Memantine is a noncompetitive NMDA receptor antagonist that is used to treat cognitive decline associated with Alzheimer’s disease.414,415 In addition to blocking NMDA receptors, memantine also blocks 5-HT3 receptors.416 Memantine does not have reinforcing effects in cocaine-dependent humans, which suggests that it does not have abuse liability.417 Memantine also increases the subjective and cardiovascular effects of cocaine without altering the choice to self-administer cocaine.418,419 Administration of memantine attenuates cocaine self-administration in rats177 and nonhuman primates.420 However, memantine administration does not attenuate the reinforcing effects of cocaine in mice,178 and higher doses of memantine increase cocaine self-administration behavior in nonhuman primates.420 Memantine may not be a likely pharmacotherapy for cocaine craving and relapse on the basis of its inability to decrease cocaine self-administration behavior and its potentiation of the subjective effects of cocaine. Large-scale clinical trials are needed to determine the efficacy of meman-tine administration in treating cocaine addiction.
Amantadine is a noncompetitive NMDA receptor antagonist with weak dopaminergic effects that is used to treat Parkinson’s disease and influenza A.421 Amantadine has been proposed as a therapeutic for cocaine dependence.422 Amantadine induces presynaptic release of dopamine and norepinephrine in the brain and blocks reuptake of these monoamines.423 In addition to these neurochemical effects, amantadine inhibits the release of acetyl-choline in the striatum424 and increases extracellular dopamine levels in the striatum425 through NMDA receptor–mediated mechanisms. Amantadine attenuates cocaine-mediated behaviors in animals undergoing withdrawal from continuous cocaine exposure.426 Although preliminary, clinical studies have demonstrated the amantadine treatment may reduce cocaine craving and use in current cocaine users427,428 (but see Refs. 429 and 430). However, amantadine’s therapeutic benefit may be realized only in cocaine addicts experiencing severe withdrawal symptoms.431
Anticonvulsants: topiramate and lamotrigine
Topiramate is an anticonvulsant drug approved by the FDA for treating migraines.432 In addition to activating GABAA receptors and blocking voltage-gated Na+ and Ca2+ channels, topiramate blocks mGluR5-containing AMPA receptors.433–436 When tested in rodents, topiramate administration did not substitute for the discriminative-stimulus properties of cocaine, nor did it attenuate the reinforcing effects of cocaine.437 Recent clinical studies demonstrate that cocaine-dependent patients receiving topiramate were more likely to abstain from cocaine use than control subjects.438 Further studies are needed to determine the efficacy of topiramate in preventing relapse of cocaine-taking behavior and the neurochemical basis for these behavioral effects.
Similar to topiramate, lamotrigine is an anticonvulsant drug that blocks voltage-gated Na+ and Ca2+ channels.433,439,440 By inhibiting presynaptic Na+ and Ca2+ channels, lamotrigine prevents the release of neurotransmitters including glutamate.441–445 Recent clinical studies demonstrate that lamotrigine reduces cocaine craving,446–448 although this treatment does not alter the subjective effects of cocaine.449 In light of the growing literature demonstrating a role for glutamate in cocaine priming–induced reinstatement, these results indicate that one mechanism by which anticonvulsants reduce cocaine craving and relapse in human addicts is through their ability to inhibit glutamate release.
Gabapentin
Gabapentin is an anticonvulsant that is currently FDA approved for treating seizures, anxiety, and neuropathic pain.450 Similar to other anticonvulsants, gabapentin inhibits presynaptic voltage-gated Na+ and Ca2+ channels and thus prevents release of neurotransmitters, including glutamate.433,451–454 Gabapentin is also an agonist that binds to specific GABAB receptor subtypes in the brain.455 Preclinical and clinical studies of gabapentin indicate that it may be of little clinical use for treating cocaine addition. For instance, gabapentin administration does not affect cocaine-induced behavioral sensitization,456,457 the reinforcing effects of cocaine, or cocaine-induced reinstatement of drug seeking.458,459 Although some clinical studies have shown that cocaine-dependent subjects treated with gabapentin have less cocaine use and craving,460–462 other studies demonstrated that gabapentin treatment did not affect cocaine use.448,463–466 Furthermore, only high doses of gabapentin decrease the discriminative stimulus effects of low doses of smoked cocaine, which suggests that gabapentin is not an efficacious treatment in human cocaine addicts.467
L-type Ca2+ channel antagonist: diltiazem
Diltiazem is an L-type Ca2+ channel blocker that is commonly prescribed to treat hypertension, angina, and selective cardiac arrhythmias.468 Calcium influx through L-type Ca2+ channels plays an important role in psychostimulant-induced behavioral and neuronal plasticity.327 Several studies have shown that L-type Ca2+ channels modulate cocaine-regulated behaviors other than behavioral sensitization. For example, systemic administration of L-type Ca2+ channel antagonists impairs cocaine self-administration,469–471 cocaine-induced CPP,472 and psychostimulant-induced behavioral sensitization.326,354,473,474 Consistent with these results, repeated administration of an L-type Ca2+ channel agonist directly into the ventral tegmental area cross-sensitizes to a subsequent challenge injection of cocaine.475 However, systemic administration of L-type Ca2+ channel antagonists does not attenuate the reinforcing effects of cocaine in nonhuman primates.471,476 Furthermore, injection of diltiazem directly into the nucleus accumbens shell facilitates cocaine-induced CPP and suggests that different brain regions mediate the rewarding and aversive effects of L-type Ca2+ channels.477 These results highlight the important role of calcium influx through L-type Ca2+ channels in a broad range of behaviors regulated by cocaine.
Recent evidence suggests that L-type Ca2+ channels and CaMKII may be an essential link between nucleus accumbens dopamine and glutamate systems involved in the neuronal plasticity underlying cocaine carving and relapse.23 With the importance of L-type Ca2+ channels in cocaine-induced behavioral and neuronal plasticity, it is likely that these receptors mediate glutamate transmission and cocaine seeking. Blocking L-type Ca2+ channels decreases glutamate-mediated burst firing of accumbal neurons,330 a physiological effect that would block cocaine priming–induced reinstatement of drug seeking by inhibiting increased glutamate signaling in the accumbens after a priming injection of cocaine.108 Thus, L-type Ca2+ channel antagonists may represent a potential class of pharmacotherapies for cocaine craving and addiction.
Acamprosate
Acamprosate is a derivative of homotaurine (a nonspecific GABA receptor agonist) that was originally developed to treat alcohol dependency and relapse.478–481 Despite structural similarities to the neurotransmitter GABA, there is no direct evidence indicating that acamprosate binds to recombinant GABAA receptors or enhances GABAA receptor function.482–484 However, acamprosate may indirectly affect GABAA receptor–mediated signaling by blocking presynaptic GABAB autoreceptors483 and/or increasing extracellular levels of taurine, an endogenous amino acid that potentiates GABAA receptor responses.485 Acamprosate also modulates glutamate transmission, specifically through NMDA receptor– and mGluR5–mediated mechanisms.484,486 Overall, acamprosate may function, in part, to restore homeostasis between excitatory and inhibitory neurotransmitter systems by attenuating withdrawal-induced hyperglutamatergic tone in brains exposed to chronic alcohol.484
Recent preclinical studies indicate that acamprosate may be beneficial in treating cocaine craving and relapse. In mice, acamprosate administration attenuates cocaine-induced CPP and cocaine priming–induced reinstatement of psychostimulant CPP.487,488 In rats trained to self-administer cocaine, acamprosate pretreatment attenuates both cocaine-and cue-induced reinstatement of drug seeking.489 Taken together, these results indicate that acamprosate may prevent relapse in human cocaine addicts. A phase II clinical trial examining the efficacy of acamprosate on cocaine use and craving in human is currently under way.30
Group I metabotropic glutamate receptor (mGluR1/5) antagonists: MPEP, MTEP, and EMQMCM
Recent preclinical studies have demonstrated that mGluR5 antagonists may have potential as pharmacotherapies for treating cocaine craving and relapse. The mGluR5 antagonists MPEP and MTEP were originally developed to study mGluR5 distribution and pharmacological properties in the brain.490 In addition to negatively modulating mGluR5s, MPEP is also an antagonist of NMDA receptors and it inhibits monoamine oxidase A activity.491 In contrast to MPEP, MTEP is a more selective mGluR5 antagonist with better pharmacological properties, including higher in vivo potency.490–492 As mentioned previously, constitutive mGluR5–knockout mice do not acquire cocaine self-administration behavior or exhibit cocaine-induced hyperlocomotor activity.292 Consistent with these results, MPEP pretreatment reduces the locomotor-stimulant properties of cocaine493 cocaine-induced CPP494 (however, see Ref. 495), as well as cocaine self-administration behavior in wild-type mice,292 rats,294,496,497 and nonhuman primates.295,498 Systemic administration of MPEP also attenuates cocaine- and cue-induced reinstatement of drug-seeking behavior in nonhuman primates295 and rats.298,299 Similar to the behavioral effects of MPEP, MTEP administration inhibits cocaine- and cue-induced reinstatement of cocaine seeking.297,299 Interestingly, systemic administration of the mGluR1–selective antagonist EMQMCM, but not the mGluR5–selective antagonist MTEP, blocked cocaine-induced behavioral sensitization, which suggests that subtypes of the group I family of mGluRs (mGluR1 and mGluR5) have distinct functional roles in cocaine-mediated behaviors.499 Collectively, these findings suggest that mGluR1/5s play critical roles in regulating cocaine-mediated behaviors. Results from reinstatement studies are promising in that mGluR5 antagonists may be useful pharmacotherapies for treating cocaine craving and relapse.
Group II metabotropic glutamate receptor (mGluR2/3) agonists: LY379268
LY379268 is a potent and selective agonist of presynaptic mGluR2/3s that has been shown to have anxiolytic- and antipsychotic-like behavioral effects in animal models.500,501 Administration of LY379268 increases extracellular dopamine levels in the mPFC, nucleus accumbens, and dorsal striatum.502,503 Furthermore, administration of LY379268 directly into the nucleus accumbens shell, but not core, reduces extracellular dopamine levels, indicating a different neurochemical effect within the accumbens subregions.504 Repeated amphetamine injections increase extracellular levels of dopamine and glutamate in the nucleus accumbens, and these effects are blocked by systemic administration of LY379268.505 Taken together, these results suggest that one neurochemical mechanism whereby LY379268 may block drug-seeking behavior is by modulating dopamine and glutamate transmission in the nucleus accumbens.
A growing body of evidence suggests that inhibiting cocaine-induced extracellular glutamate release with mGluR2/3 agonists may block the reinstatement of drug seeking.506 Whereas moderate doses of LY379268 administered systemically or directly into the nucleus accumbens core selectively attenuate the reinstatement of cocaine-seeking behavior,300,301 administration of high doses of LY379268 blocks food-seeking behavior.301 Similarly, systemic and intra-amygdala injections of LY379268 block incubation of cocaine and food seeking, suggesting that activation of mGluR2/3s has nonspecific effects on responding for drugs of abuse and natural rewards.507,508 However, the aforementioned studies did not examine the role of nucleus accumbens shell mGluR2/3s in the reinstatement of cocaine seeking. Recent findings suggest that LY379268 decreases the propensity to reinstate drug-seeking behavior in rodents through anxiolytic-like behavioral effects that reduce the saliency of stressful stimuli. Clinical studies of LY379268 in cocaine-dependent addicts are required to determine its efficacy in treating cocaine craving and relapse.
Conclusions
The results summarized in this review indicate that repeated exposure to cocaine produces profound changes in glutamate transmission in limbic nuclei, particularly the nucleus accumbens. Indeed, cocaine administration appears to influence virtually every aspect of glutamate transmission, including release, reuptake, receptor expression, receptor trafficking, and intracellular signaling. Thus, preclinical studies have identified many potential targets for the development of therapeutics for cocaine addiction. The ubiquity of glutamate systems in the nervous system, and particularly the important role glutamate plays in various forms of learning and memory, represents a substantial challenge to identifying effective therapeutic glutamate modulators with few serious side effects. Nonetheless, several modulators of glutamate transmission are being tested clinically as antiaddiction therapies, with some success. Ongoing and future preclinical studies will lead to a greater refinement of the cellular and molecular mechanisms that mediate cocaine-induced changes in synaptic plasticity, metaplasticity, and glutamate-mediated signal transduction, which will provide further insight into the pathology of addiction and identify novel therapeutic targets for cocaine addiction.
Acknowledgments
This work was supported by grants from the National Institutes of Health to R.C.P. (R01 DA15214, R01 DA22339, and K02 DA18678).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2007 National Survey on Drug Use and Health. National Findings Office of Applied Studies; Rockville, MD: 2008. [Google Scholar]
- 2.Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- 4.Carter BL, Tiffany ST. Cue-reactivity and the future of addiction research. Addiction. 1999;94:349–351. [PubMed] [Google Scholar]
- 5.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe JH, et al. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- 7.Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien CP, et al. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 9.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacol Biochem Behav. 1975;3:1055–1061. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- 11.Self DW, et al. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- 12.Spealman RD, et al. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–336. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- 13.Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- 14.Epstein DH, et al. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- 19.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 20.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 21.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 23.Anderson SM, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- 24.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 25.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, et al. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 27.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 28.Kelley AE, et al. Glutamate-mediated plasticity in corticostriatal networks: role in adaptive motor learning. Ann N Y Acad Sci. 2003;1003:159–168. doi: 10.1196/annals.1300.061. [DOI] [PubMed] [Google Scholar]
- 29.Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 30.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uys JD, LaLumiere RT. Glutamate: the new frontier in pharmacotherapy for cocaine addiction. CNS Neurol Disord Drug Targets. 2008;7:482–491. doi: 10.2174/187152708786927868. [DOI] [PubMed] [Google Scholar]
- 32.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Groenewegen HJ, Trimble M. The ventral striatum as an interface between the limbic and motor systems. CNS Spectr. 2007;12:887–892. doi: 10.1017/s1092852900015650. [DOI] [PubMed] [Google Scholar]
- 34.Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure–activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt HD, et al. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 36.Heimer L, et al. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- 37.Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- 38.Meredith GE, et al. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- 40.Heimer L, et al. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 41.Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- 42.Smeets WJ, Medina L. The efferent connections of the nucleus accumbens in the lizard Gekko gecko. A combined tract-tracing/transmitter-immunohistochemical study. Anat Embryol (Berl) 1995;191:73–81. doi: 10.1007/BF00215299. [DOI] [PubMed] [Google Scholar]
- 43.Groenewegen HJ, et al. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 44.Groenewegen HJ. The basal ganglia and motor control. Neural Plast. 2003;10:107–120. doi: 10.1155/NP.2003.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shim I, Wirtshafter D. Fos-like immunore-activity in the mamillary body and thalamus following injections of muscimol into the ventral tegmental nucleus of Gudden in the rat. Brain Res. 1996;712:173–178. doi: 10.1016/0006-8993(95)01301-6. [DOI] [PubMed] [Google Scholar]
- 46.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 47.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 48.Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9:223–227. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- 49.Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- 50.Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 51.Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry. 2003;160:1726–1739. doi: 10.1176/appi.ajp.160.10.1726. [DOI] [PubMed] [Google Scholar]
- 52.Napier TC, Maslowski-Cobuzzi RJ. Electro-physiological verification of the presence of D1 and D2 dopamine receptors within the ventral pallidum. Synapse. 1994;17:160–166. doi: 10.1002/syn.890170304. [DOI] [PubMed] [Google Scholar]
- 53.Carr DB, Sesack SR. Terminals from the rat prefrontal cortex synapse on mesoaccumbens VTA neurons. Ann N Y Acad Sci. 1999;877:676–678. doi: 10.1111/j.1749-6632.1999.tb09299.x. [DOI] [PubMed] [Google Scholar]
- 54.Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- 55.Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- 56.Sesack SR, et al. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- 57.Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaborszky L, et al. Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neuroscience. 1985;14:427–453. doi: 10.1016/0306-4522(85)90302-1. [DOI] [PubMed] [Google Scholar]
- 59.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 60.Meredith GE, et al. Morphological differences between projection neurons of the core and shell in the nucleus accumbens of the rat. Neuroscience. 1992;50:149–162. doi: 10.1016/0306-4522(92)90389-j. [DOI] [PubMed] [Google Scholar]
- 61.Meredith GE, Blank B, Groenewegen HJ. The distribution and compartmental organization of the cholinergic neurons in nucleus accumbens of the rat. Neuroscience. 1989;31:327–345. doi: 10.1016/0306-4522(89)90377-1. [DOI] [PubMed] [Google Scholar]
- 62.Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: immunohistochemical distribution of enkephalin, substance P, dopamine, and calcium-binding protein. J Comp Neurol. 1989;289:189–201. doi: 10.1002/cne.902890202. [DOI] [PubMed] [Google Scholar]
- 63.Brog JS, et al. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 64.Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- 66.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liechti ME, et al. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shin R, et al. Intracranial self-administration of MDMA into the ventral striatum of the rat: differential roles of the nucleus accumbens shell, core, and olfactory tubercle. Psychopharmacology (Berl) 2008;198:261–270. doi: 10.1007/s00213-008-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sellings LH, et al. Rewarding and aversive effects of nicotine are segregated within the nucleus accumbens. Eur J Neurosci. 2008;28:342–352. doi: 10.1111/j.1460-9568.2008.06341.x. [DOI] [PubMed] [Google Scholar]
- 70.Aragona BJ, et al. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Di Chiara G, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl. 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 73.Ghitza UE, et al. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23:7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parkinson JA, et al. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- 76.Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47(Suppl. 1):202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 77.Fuchs RA, et al. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- 78.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 79.Ito R, et al. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Floresco SB, et al. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci. 2006;26:2449–2457. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 82.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 83.Groenewegen HJ, Galis-de Graaf Y, Smeets WJ. Integration and segregation of limbic corticostriatal loops at the thalamic level: an experimental tracing study in rats. J Chem Neuroanat. 1999;16:167–185. doi: 10.1016/s0891-0618(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 84.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 85.Bossert JM, et al. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 86.Shaham Y, et al. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 87.De Vries TJ, et al. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- 88.De Vries TJ, et al. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology (Berl) 1999;143:254–260. doi: 10.1007/s002130050944. [DOI] [PubMed] [Google Scholar]
- 89.Fuchs RA, et al. Effects of 7-OH-DPAT on cocaine-seeking behavior and on re-establishment of cocaine self-administration. Pharmacol Biochem Behav. 2002;72:623–632. doi: 10.1016/s0091-3057(02)00731-1. [DOI] [PubMed] [Google Scholar]
- 90.Khroyan TV, et al. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- 91.Bachtell RK, et al. Effects of intranucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- 92.Wise RA, Murray A, Bozarth MA. Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology (Berl) 1990;100:355–360. doi: 10.1007/BF02244606. [DOI] [PubMed] [Google Scholar]
- 93.Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006;142:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 95.Schenk S, Gittings D. Effects of SCH 23390 and eticlopride on cocaine-seeking produced by cocaine and WIN35,428 in rats. Psychopharmacology(Berl) 2003;168:118–123. doi: 10.1007/s00213-002-1276-y. [DOI] [PubMed] [Google Scholar]
- 96.Vorel SR, et al. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- 98.Peng XQ, et al. The preferential dopamine D(3) receptor antagonist S33138 inhibits cocaine reward and cocaine-triggered relapse to drug-seeking behavior in rats. Neuropharmacology. 2009;56:752–760. doi: 10.1016/j.neuropharm.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Self DW, Karanian DA, Spencer JJ. Effects of the novel D1 dopamine receptor agonist ABT-431 on cocaine self-administration and reinstatement. Ann N Y Acad Sci. 2000;909:133–144. doi: 10.1111/j.1749-6632.2000.tb06679.x. [DOI] [PubMed] [Google Scholar]
- 100.Alleweireldt AT, et al. D1-receptor drugs and cocaine-seeking behavior: investigation of receptor mediation and behavioral disruption in rats. Psychopharmacology (Berl) 2003;168:109–117. doi: 10.1007/s00213-002-1305-x. [DOI] [PubMed] [Google Scholar]
- 101.Khroyan TV, et al. Attenuation of relapse to cocaine seeking by dopamine D1 receptor agonists and antagonists in non-human primates. Psychopharmacology (Berl) 2003;168:124–131. doi: 10.1007/s00213-002-1365-y. [DOI] [PubMed] [Google Scholar]
- 102.Norman AB, et al. Priming threshold: a novel quantitative measure of the reinstatement of cocaine self-administration. Brain Res. 1999;831:165–174. doi: 10.1016/s0006-8993(99)01423-7. [DOI] [PubMed] [Google Scholar]
- 103.Capriles N, et al. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- 104.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park WK, et al. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- 107.Sanchez CJ, et al. Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress- and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience. 2003;119:497–505. doi: 10.1016/s0306-4522(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 108.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90:226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 110.Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- 111.Ding DC, Gabbott PL, Totterdell S. Differences in the laminar origin of projections from the medial prefrontal cortex to the nucleus accumbens shell and core regions in the rat. Brain Res. 2001;917:81–89. doi: 10.1016/s0006-8993(01)02912-2. [DOI] [PubMed] [Google Scholar]
- 112.Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience. 1985;16:275–296. doi: 10.1016/0306-4522(85)90002-8. [DOI] [PubMed] [Google Scholar]
- 113.Wright CI, Groenewegen HJ. Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. J Comp Neurol. 1995;361:383–403. doi: 10.1002/cne.903610304. [DOI] [PubMed] [Google Scholar]
- 114.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 115.Dube L, Smith AD, Bolam JP. Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-size spiny neurons in the rat neostriatum. J Comp Neurol. 1988;267:455–471. doi: 10.1002/cne.902670402. [DOI] [PubMed] [Google Scholar]
- 116.Pickel VM, et al. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 1981;225:373–385. doi: 10.1016/0006-8993(81)90843-x. [DOI] [PubMed] [Google Scholar]
- 117.Wilson CJ. Morphology and synaptic connections of crossed corticostriatal neurons in the rat. J Comp Neurol. 1987;263:567–580. doi: 10.1002/cne.902630408. [DOI] [PubMed] [Google Scholar]
- 118.Pinto A, Jankowski M, Sesack SR. Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: ultrastructural characteristics and spatial relationships with dopamine afferents. J Comp Neurol. 2003;459:142–155. doi: 10.1002/cne.10596. [DOI] [PubMed] [Google Scholar]
- 119.Jentsch JD, Roth RH, Taylor JR. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog Brain Res. 2000;126:433–453. doi: 10.1016/S0079-6123(00)26028-7. [DOI] [PubMed] [Google Scholar]
- 120.Bamford NS, et al. Dopamine modulates release from corticostriatal terminals. J Neurosci. 2004;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bamford NS, et al. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- 122.Dani JA, Zhou FM. Selective dopamine filter of glutamate striatal afferents. Neuron. 2004;42:522–524. doi: 10.1016/j.neuron.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 123.Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. J Comp Neurol. 2002;442:392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- 124.Wang Z, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 125.Brady AM, O’Donnell P. Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci. 2004;24:1040–1049. doi: 10.1523/JNEUROSCI.4178-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Siegel GJ, et al. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Elsevier Academic Press; Burlington, MA: 2006. [Google Scholar]
- 127.Takamori S. VGLUTs: ‘exciting’ times for glutamatergic research? Neurosci Res. 2006;55:343–351. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 128.Fremeau RT, Jr, et al. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- 129.Herzog E, et al. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123:983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 130.Gras C, et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- 132.Beart PM, O’Shea RD. Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2007;150:5–17. doi: 10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baker DA, et al. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 135.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pierce RC, et al. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miguens M, et al. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology (Berl) 2008;196:303–313. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- 138.Smith JA, et al. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683:264–269. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- 139.Baker DA, Shen H, Kalivas PW. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids. 2002;23:161–162. doi: 10.1007/s00726-001-0122-6. [DOI] [PubMed] [Google Scholar]
- 140.Baker DA, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 141.Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- 142.Reid MS, Berger SP. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport. 1996;7:1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- 143.Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- 144.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kozell B, Meshul K. Alterations in nerve terminal glutamate immunoreactivity in the nucleus accumbens and ventral tegmental area following single and repeated doses of cocaine. Psychopharmacology (Berl) 2003;165:337–345. doi: 10.1007/s00213-002-1296-7. [DOI] [PubMed] [Google Scholar]
- 146.Kozell LB, Meshul CK. Nerve terminal glutamate immunoreactivity in the rat nucleus accumbens and ventral tegmental area after a short withdrawal from cocaine. Synapse. 2004;51:224–232. doi: 10.1002/syn.10304. [DOI] [PubMed] [Google Scholar]
- 147.Kozell LB, Meshul CK. The effects of acute or repeated cocaine administration on nerve terminal glutamate within the rat mesolimbic system. Neuroscience. 2001;106:15–25. doi: 10.1016/s0306-4522(01)00274-3. [DOI] [PubMed] [Google Scholar]
- 148.Torregrossa MM, Tang XC, Kalivas PW. The glutamatergic projection from the prefrontal cortex to the nucleus accumbens core is required for cocaine-induced decreases in ventral pallidal GABA. Neurosci Lett. 2008;438:142–145. doi: 10.1016/j.neulet.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Volkow ND, et al. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- 150.Volkow ND, et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dingledine R, et al. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 152.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 153.Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- 154.Nowak L, et al. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 155.Verkhratsky A, Kirchhoff F. NMDA receptors in glia. Neuroscientist. 2007;13:28–37. doi: 10.1177/1073858406294270. [DOI] [PubMed] [Google Scholar]
- 156.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 157.Karadottir R, et al. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tarazi FI, Baldessarini RJ. Regional localization of dopamine and ionotropic glutamate receptor subtypes in striatolimbic brain regions. J Neurosci Res. 1999;55:401–410. doi: 10.1002/(SICI)1097-4547(19990215)55:4<401::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 159.Tarazi FI, et al. Localization of ionotropic glutamate receptors in caudate-putamen and nucleus accumbens septi of rat brain: comparison of NMDA, AMPA, and kainate receptors. Synapse. 1998;30:227–235. doi: 10.1002/(SICI)1098-2396(199810)30:2<227::AID-SYN13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 160.Lu L, et al. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- 161.Famous KR, Schmidt HD, Pierce RC. When administered into the nucleus accumbens core or shell, the NMDA receptor antagonist AP-5 reinstates cocaine-seeking behavior in the rat. Neurosci Lett. 2007;420:169–173. doi: 10.1016/j.neulet.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Engblom D, et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 163.Castellano C, Cestari V, Ciamei A. NMDA receptors and learning and memory processes. Curr Drug Targets. 2001;2:273–283. doi: 10.2174/1389450013348515. [DOI] [PubMed] [Google Scholar]
- 164.Churchill L, et al. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J Neurochem. 1999;72:2397–2403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- 165.Hemby SE, Horman B, Tang W. Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain Res. 2005;1064:75–82. doi: 10.1016/j.brainres.2005.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ghasemzadeh MB, et al. Neuroadaptations in ionotropic and metabotropic glutamate receptor mRNA produced by cocaine treatment. J Neurochem. 1999;72:157–165. doi: 10.1046/j.1471-4159.1999.0720157.x. [DOI] [PubMed] [Google Scholar]
- 167.Yamaguchi M, et al. Repeated cocaine administration differentially affects NMDA receptor subunit (NR1, NR2A-C) mRNAs in rat brain. Synapse. 2002;46:157–169. doi: 10.1002/syn.10132. [DOI] [PubMed] [Google Scholar]
- 168.Scheggi S, et al. Dizocilpine infusion has a different effect in the development of morphine and cocaine sensitization: behavioral and neurochemical aspects. Neuroscience. 2002;109:267–274. doi: 10.1016/s0306-4522(01)00483-3. [DOI] [PubMed] [Google Scholar]
- 169.Tang W, et al. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. J Neurochem. 2004;89:1021–1033. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Loftis JM, Janowsky A. Regulation of NMDA receptor subunits and nitric oxide synthase expression during cocaine withdrawal. J Neurochem. 2000;75:2040–2050. doi: 10.1046/j.1471-4159.2000.0752040.x. [DOI] [PubMed] [Google Scholar]
- 171.Zhang X, et al. Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacology. 2007;32:377–387. doi: 10.1038/sj.npp.1301101. [DOI] [PubMed] [Google Scholar]
- 172.Edwards S, et al. Region-specific tolerance to cocaine-regulated cAMP-dependent protein phosphorylation following chronic self-administration. Eur J Neurosci. 2007;25:2201–2213. doi: 10.1111/j.1460-9568.2007.05473.x. [DOI] [PubMed] [Google Scholar]
- 173.Hemby SE, et al. Cocaine-induced alterations in nucleus accumbens ionotropic glutamate receptor subunits in human and non-human primates. J Neurochem. 2005;95:1785–1793. doi: 10.1111/j.1471-4159.2005.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schenk S, et al. Blockade of the acquisition of cocaine self-administration by the NMDA antagonist MK-801 (dizocilpine) Behav Pharmacol. 1993;4:652–659. [PubMed] [Google Scholar]
- 175.Pierce RC, Meil WM, Kalivas PW. The NMDA antagonist, dizocilpine, enhances cocaine reinforcement without influencing mesoaccumbens dopamine transmission. Psychopharmacology (Berl) 1997;133:188–195. doi: 10.1007/s002130050390. [DOI] [PubMed] [Google Scholar]
- 176.Pulvirenti L, Balducci C, Koob GF. Dextromethorphan reduces intravenous cocaine self-administration in the rat. Eur J Pharmacol. 1997;321:279–283. doi: 10.1016/s0014-2999(96)00970-3. [DOI] [PubMed] [Google Scholar]
- 177.Hyytia P, Backstrom P, Liljequist S. Site-specific NMDA receptor antagonists produce differential effects on cocaine self-administration in rats. Eur J Pharmacol. 1999;378:9–16. doi: 10.1016/s0014-2999(99)00446-x. [DOI] [PubMed] [Google Scholar]
- 178.Blokhina EA, et al. Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. Eur Neuropsychopharmacol. 2005;15:219–225. doi: 10.1016/j.euroneuro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 179.Ranaldi R, French E, Roberts DC. Systemic pretreatment with MK-801 (dizocilpine) increases breaking points for self-administration of cocaine on a progressive-ratio schedule in rats. Psychopharmacology (Berl) 1996;128:83–88. doi: 10.1007/s002130050113. [DOI] [PubMed] [Google Scholar]
- 180.Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 181.Pulvirenti L, Maldonado-Lopez R, Koob GF. NMDA receptors in the nucleus accumbens modulate intravenous cocaine but not heroin self-administration in the rat. Brain Res. 1992;594:327–330. doi: 10.1016/0006-8993(92)91145-5. [DOI] [PubMed] [Google Scholar]
- 182.Allen RM, et al. Effects of the competitive N-methyl-D-aspartate receptor antagonist, LY235959 [(−)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid], on responding for cocaine under both fixed and progressive ratio schedules of reinforcement. J Pharmacol Exp Ther. 2005;315:449–457. doi: 10.1124/jpet.105.086355. [DOI] [PubMed] [Google Scholar]
- 183.De Vries TJ, et al. MK-801 reinstates drug-seeking behaviour in cocaine-trained rats. Neuroreport. 1998;9:637–640. doi: 10.1097/00001756-199803090-00014. [DOI] [PubMed] [Google Scholar]
- 184.Day JC, et al. Cocaine-induced increase in cortical acetylcholine release: interaction with the hypothalamo-pituitary-adrenal axis. Eur J Neurosci. 1997;9:1130–1136. doi: 10.1111/j.1460-9568.1997.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 185.Neigh-McCandless G, et al. Stimulation of cortical acetylcholine release following blockade of ionotropic glutamate receptors in nucleus accumbens. Eur J Neurosci. 2002;16:1259–1266. doi: 10.1046/j.1460-9568.2002.02201.x. [DOI] [PubMed] [Google Scholar]
- 186.Zmarowski A, Sarter M, Bruno JP. Glutamate receptors in nucleus accumbens mediate regionally selective increases in cortical acetylcholine release. Synapse. 2007;61:115–123. doi: 10.1002/syn.20354. [DOI] [PubMed] [Google Scholar]
- 187.Zmarowski A, Sarter M, Bruno JP. NMDA and dopamine interactions in the nucleus accumbens modulate cortical acetylcholine release. Eur J Neurosci. 2005;22:1731–1740. doi: 10.1111/j.1460-9568.2005.04333.x. [DOI] [PubMed] [Google Scholar]
- 188.Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McCullough LD, Salamone JD. Increases in extracellular dopamine levels and locomotor activity after direct infusion of phencyclidine into the nucleus accumbens. Brain Res. 1992;577:1–9. doi: 10.1016/0006-8993(92)90530-m. [DOI] [PubMed] [Google Scholar]
- 190.Hondo H, et al. Effect of phencyclidine on dopamine release in the rat prefrontal cortex; an in vivo microdialysis study. Brain Res. 1994;633:337–342. doi: 10.1016/0006-8993(94)91558-x. [DOI] [PubMed] [Google Scholar]
- 191.Carboni E, et al. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- 192.Derkach VA, et al. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 193.Gomes AR, et al. Characterization of alternatively spliced isoforms of AMPA receptor subunits encoding truncated receptors. Mol Cell Neurosci. 2008;37:323–334. doi: 10.1016/j.mcn.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 194.Coleman SK, et al. Isoform-specific early trafficking of AMPA receptor flip and flop variants. J Neurosci. 2006;26:11220–11229. doi: 10.1523/JNEUROSCI.2301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Quirk JC, Siuda ER, Nisenbaum ES. Molecular determinants responsible for differences in desensitization kinetics of AMPA receptor splice variants. J Neurosci. 2004;24:11416–11420. doi: 10.1523/JNEUROSCI.2464-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hume RI, Dingledine R, Heinemann SF. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991;253:1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- 197.Rueter SM, et al. Glutamate receptor RNA editing in vitro by enzymatic conversion of adenosine to inosine. Science. 1995;267:1491–1494. doi: 10.1126/science.7878468. [DOI] [PubMed] [Google Scholar]
- 198.Seeburg PH. The role of RNA editing in controlling glutamate receptor channel properties. J Neurochem. 1996;66:1–5. doi: 10.1046/j.1471-4159.1996.66010001.x. [DOI] [PubMed] [Google Scholar]
- 199.Tanaka H, et al. The AMPAR subunit GluR2: still front and center-stage. Brain Res. 2000;886:190–207. doi: 10.1016/s0006-8993(00)02951-6. [DOI] [PubMed] [Google Scholar]
- 200.Ortiz J, et al. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- 201.Fitzgerald LW, et al. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gold SJ, et al. Stoichiometries of AMPA receptor subunit mRNAs in rat brain fall into discrete categories. J Comp Neurol. 1997;385:491–502. doi: 10.1002/(sici)1096-9861(19970908)385:4<491::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 204.Boudreau AC, et al. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pinheiro P, Mulle C. Kainate receptors. Cell Tissue Res. 2006;326:457–482. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- 206.Chittajallu R, et al. Kainate receptors: subunits, synaptic localization and function. Trends Pharmacol Sci. 1999;20:26–35. doi: 10.1016/s0165-6147(98)01286-3. [DOI] [PubMed] [Google Scholar]
- 207.Lerma J. Kainate receptor physiology. Curr Opin Pharmacol. 2006;6:89–97. doi: 10.1016/j.coph.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 208.Kohler M, et al. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- 209.Howe JR. Homomeric and heteromeric ion channels formed from the kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. J Neurophysiol. 1996;76:510–519. doi: 10.1152/jn.1996.76.1.510. [DOI] [PubMed] [Google Scholar]
- 210.Swanson GT, et al. Effect of RNA editing and subunit co-assembly single-channel properties of recombinant kainate receptors. J Physiol. 1996;492(Pt 1):129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci. 2003;4:481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- 212.Jaskolski F, et al. Subunit composition and alternative splicing regulate membrane delivery of kainate receptors. J Neurosci. 2004;24:2506–2515. doi: 10.1523/JNEUROSCI.5116-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Coussen F, et al. Co-assembly of two GluR6 kainate receptor splice variants within a functional protein complex. Neuron. 2005;47:555–566. doi: 10.1016/j.neuron.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 214.Jaskolski F, Coussen F, Mulle C. Subcellular localization and trafficking of kainate receptors. Trends Pharmacol Sci. 2005;26:20–26. doi: 10.1016/j.tips.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 215.Jaskolski F, et al. Differential trafficking of GluR7 kainate receptor subunit splice variants. J Biol Chem. 2005;280:22968–22976. doi: 10.1074/jbc.M413166200. [DOI] [PubMed] [Google Scholar]
- 216.Isaac JT, et al. Kainate receptor trafficking: physiological roles and molecular mechanisms. Pharmacol Ther. 2004;104:163–172. doi: 10.1016/j.pharmthera.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 217.Huettner JE. Kainate receptors and synaptic transmission. Prog Neurobiol. 2003;70:387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 218.Bortolotto ZA, et al. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- 219.Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- 220.Birch PJ, Grossman CJ, Hayes AG. 6,7-Dinitro-quinoxaline-2,3-dion and 6-nitro,7-cyanoquinoxaline-2,3-dion antagonise responses to NMDA in the rat spinal cord via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988;156:177–180. doi: 10.1016/0014-2999(88)90163-x. [DOI] [PubMed] [Google Scholar]
- 221.Fletcher EJ, Lodge D. New developments in the molecular pharmacology of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate and kainate receptors. Pharmacol Ther. 1996;70:65–89. doi: 10.1016/0163-7258(96)00014-9. [DOI] [PubMed] [Google Scholar]
- 222.Honore T, et al. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988;241:701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- 223.Lerma J, et al. Molecular physiology of kainate receptors. Physiol Rev. 2001;81:971–998. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- 224.Bleakman D, et al. Kainate receptor agonists, antagonists and allosteric modulators. Curr Pharm Des. 2002;8:873–885. doi: 10.2174/1381612024607108. [DOI] [PubMed] [Google Scholar]
- 225.Di Ciano P, et al. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Self DW, Choi KH. Extinction-induced neuroplasticity attenuates stress-induced cocaine seeking: a state-dependent learning hypothesis. Stress. 2004;7:145–155. doi: 10.1080/10253890400012677. [DOI] [PubMed] [Google Scholar]
- 227.Sutton MA, et al. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 228.Suto N, et al. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–2159. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- 229.Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- 230.Famous KR, et al. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ping A, et al. Contributions of nucleus accumbens core and shell GluR1 containing AMPA receptors in AMPA- and cocaine-primed reinstatement of cocaine-seeking behavior. Brain Res. 2008;1215:173–182. doi: 10.1016/j.brainres.2008.03.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 235.Wolf ME, Mangiavacchi S, Sun X. Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann N Y Acad Sci. 2003;1003:241–249. doi: 10.1196/annals.1300.015. [DOI] [PubMed] [Google Scholar]
- 236.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yao WD, et al. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- 238.Thomas MJ, et al. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 239.Beurrier C, Malenka RC. Enhanced inhibition of synaptic transmission by dopamine in the nucleus accumbens during behavioral sensitization to cocaine. J Neurosci. 2002;22:5817–5822. doi: 10.1523/JNEUROSCI.22-14-05817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Martin M, et al. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- 241.Kourrich S, et al. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bachtell RK, Self DW. Renewed cocaine exposure produces transient alterations in nucleus accumbens AMPA receptor-mediated behavior. J Neurosci. 2008;28:12808–12814. doi: 10.1523/JNEUROSCI.2060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bear MF. Bidirectional synaptic plasticity: from theory to reality. Philos Trans R Soc Lond B Biol Sci. 2003;358:649–655. doi: 10.1098/rstb.2002.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 245.Liu SJ, Cull-Candy SG. Subunit interaction with PICK and GRIP controls Ca2+ permeability of AMPARs at cerebellar synapses. Nat Neurosci. 2005;8:768–775. doi: 10.1038/nn1468. [DOI] [PubMed] [Google Scholar]
- 246.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 247.Carroll RC, et al. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- 248.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 249.Wolf ME, et al. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47(Suppl. 1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 250.Chung HJ, et al. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Perez JL, et al. PICK1 targets activated protein kinase C alpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 253.Terashima A, et al. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci. 2004;24:5381–5390. doi: 10.1523/JNEUROSCI.4378-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gardner SM, et al. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 255.Xia J, et al. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 256.Brebner K, et al. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310:1340–1343. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- 257.Conrad KL, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bellone C, Luscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci. 2008;65:2913–2923. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Grueter BA, McElligott ZA, Winder DG. Group I mGluRs and long-term depression: potential roles in addiction? Mol Neurobiol. 2007;36:232–244. doi: 10.1007/s12035-007-0037-7. [DOI] [PubMed] [Google Scholar]
- 260.Conn PJ. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann N Y Acad Sci. 2003;1003:12–21. doi: 10.1196/annals.1300.002. [DOI] [PubMed] [Google Scholar]
- 261.Schoepp DD, Conn PJ. Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol Sci. 1993;14:13–20. doi: 10.1016/0165-6147(93)90107-u. [DOI] [PubMed] [Google Scholar]
- 262.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 263.Kim CH, et al. Metabotropic glutamate receptors: phosphorylation and receptor signaling. J Neurosci Res. 2008;86:1–10. doi: 10.1002/jnr.21437. [DOI] [PubMed] [Google Scholar]
- 264.Testa CM, et al. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J Comp Neurol. 1998;390:5–19. [PubMed] [Google Scholar]
- 265.Testa CM, et al. Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ohishi H, et al. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- 267.Ohishi H, et al. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 268.Petralia RS, et al. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 269.Wright RA, et al. [3H]LY341495 binding to group II metabotropic glutamate receptors in rat brain. J Pharmacol Exp Ther. 2001;298:453–460. [PubMed] [Google Scholar]
- 270.Shigemoto R, et al. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- 271.Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of glutamate receptor subunit/subtype messenger RNAS for NMDAR1, GLuR1, GLuR2 and mGLuR5 by accumbal projection neurons. Brain Res Mol Brain Res. 1999;63:287–296. doi: 10.1016/s0169-328x(98)00288-5. [DOI] [PubMed] [Google Scholar]
- 272.Tallaksen-Greene SJ, et al. Localization of mGluR1a-like immunoreactivity and mGluR5-like immunoreactivity in identified populations of striatal neurons. Brain Res. 1998;780:210–217. doi: 10.1016/s0006-8993(97)01141-4. [DOI] [PubMed] [Google Scholar]
- 273.Ohishi H, Neki A, Mizuno N. Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci Res. 1998;30:65–82. doi: 10.1016/s0168-0102(97)00120-x. [DOI] [PubMed] [Google Scholar]
- 274.Muly EC, Maddox M, Smith Y. Distribution of mGluR1alpha and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol. 2003;467:521–535. doi: 10.1002/cne.10937. [DOI] [PubMed] [Google Scholar]
- 275.Fotuhi M, et al. Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J Neurosci. 1993;13:2001–2012. doi: 10.1523/JNEUROSCI.13-05-02001.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Moroni F, et al. Presynaptic mGlu1 type receptors potentiate transmitter output in the rat cortex. Eur J Pharmacol. 1998;347:189–195. doi: 10.1016/s0014-2999(98)00124-1. [DOI] [PubMed] [Google Scholar]
- 277.Rouse ST, et al. Distribution and roles of metabotropic glutamate receptors in the basal ganglia motor circuit: implications for treatment of Parkinson’s disease and related disorders. Pharmacol Ther. 2000;88:427–435. doi: 10.1016/s0163-7258(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 278.Swanson CJ, et al. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 280.Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 281.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- 282.Witkin JM, et al. Metabotropic glutamate receptors in the control of mood disorders. CNS Neurol Disord Drug Targets. 2007;6:87–100. doi: 10.2174/187152707780363302. [DOI] [PubMed] [Google Scholar]
- 283.Tamaru Y, et al. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- 284.Fourgeaud L, et al. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Freeman WM, et al. Cocaine-responsive gene expression changes in rat hippocampus. Neuroscience. 2001;108:371–380. doi: 10.1016/s0306-4522(01)00432-8. [DOI] [PubMed] [Google Scholar]
- 286.Grueter BA, et al. Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration. J Neurosci. 2006;26:3210–3219. doi: 10.1523/JNEUROSCI.0170-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Moussawi K, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ghasemzadeh MB, et al. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci Lett. 2009;452:167–171. doi: 10.1016/j.neulet.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 290.Ghasemzadeh MB, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience. 2009;159:414–426. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 291.Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group ii metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- 292.Chiamulera C, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 293.Kenny PJ, et al. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005;179:247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- 294.Kenny PJ, et al. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- 295.Lee B, et al. Attenuation of behavioral effects of cocaine by the metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- 296.Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- 297.Iso Y, et al. Synthesis and structure-activity relationships of 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine analogues as potent, noncompetitive metabotropic glutamate receptor subtype 5 antagonists; search for cocaine medications. J Med Chem. 2006;49:1080–1100. doi: 10.1021/jm050570f. [DOI] [PubMed] [Google Scholar]
- 298.Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- 299.Kumaresan V, et al. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 302.Kalivas PW, et al. Glutamate transmission and addiction to cocaine. Ann N Y Acad Sci. 2003;1003:169–175. doi: 10.1196/annals.1300.009. [DOI] [PubMed] [Google Scholar]
- 303.Kau KS, et al. Blunted cystine-glutamate antiporter function in the nucleus accumbens promotes cocaine-induced drug seeking. Neuroscience. 2008;155:530–537. doi: 10.1016/j.neuroscience.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Baker DA, et al. N-Acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci. 2003;1003:349–351. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- 305.Moran MM, et al. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Moran MM, et al. Cystine/glutamate antiporter regulation of vesicular glutamate release. Ann N Y Acad Sci. 2003;1003:445–447. doi: 10.1196/annals.1300.048. [DOI] [PubMed] [Google Scholar]
- 307.Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 308.Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- 309.Ango F, et al. Homer-dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol Cell Neurosci. 2002;20:323–329. doi: 10.1006/mcne.2002.1100. [DOI] [PubMed] [Google Scholar]
- 310.Roche KW, et al. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J Biol Chem. 1999;274:25953–25957. doi: 10.1074/jbc.274.36.25953. [DOI] [PubMed] [Google Scholar]
- 311.Tu JC, et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 312.Tu JC, et al. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 313.Xiao B, et al. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 314.Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: a two-species comparison. Brain Res. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 315.Szumlinski KK, et al. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 316.Bowers MS, et al. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Rahman Z, et al. RGS9 modulates dopamine signaling in the basal ganglia. Neuron. 2003;38:941–952. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- 318.Shin DM, et al. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. J Cell Biol. 2003;162:293–303. doi: 10.1083/jcb.200210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ghasemzadeh MB, et al. Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur J Neurosci. 2003;18:1645–1651. doi: 10.1046/j.1460-9568.2003.02880.x. [DOI] [PubMed] [Google Scholar]
- 320.Kalivas PW, Szumlinski KK, Worley P. Homer2 gene deletion in mice produces a phenotype similar to chronic cocaine treated rats. Neurotox Res. 2004;6:385–387. doi: 10.1007/BF03033313. [DOI] [PubMed] [Google Scholar]
- 321.Szumlinski KK, et al. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacology. 2006;31:768–777. doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- 322.Ghasemzadeh MB, et al. Cocaine activates Homer1 immediate early gene transcription in the mesocorticolimbic circuit: differential regulation by dopamine and glutamate signaling. Synapse. 2009;63:42–53. doi: 10.1002/syn.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Self DW, et al. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lynch WJ, Taylor JR. Persistent changes in motivation to self-administer cocaine following modulation of cyclic AMP-dependent protein kinase A (PKA) activity in the nucleus accumbens. Eur J Neurosci. 2005;22:1214–1220. doi: 10.1111/j.1460-9568.2005.04305.x. [DOI] [PubMed] [Google Scholar]
- 325.Gnegy ME. Ca2+/calmodulin signaling in NMDA-induced synaptic plasticity. Crit Rev Neurobiol. 2000;14:91–129. [PubMed] [Google Scholar]
- 326.Licata SC, Schmidt HD, Pierce RC. Suppressing calcium/calmodulin-dependent protein kinase II activity in the ventral tegmental area enhances the acute behavioural response to cocaine but attenuates the initiation of cocaine-induced behavioural sensitization in rats. Eur J Neurosci. 2004;19:405–414. doi: 10.1111/j.0953-816x.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 327.Licata SC, Pierce RC. The roles of calcium/calmodulin-dependent and Ras/mitogen-activated protein kinases in the development of psychostimulant-induced behavioral sensitization. J Neurochem. 2003;85:14–22. doi: 10.1046/j.1471-4159.2003.01662.x. [DOI] [PubMed] [Google Scholar]
- 328.Yan Z, et al. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- 329.Galarraga E, et al. Dopamine facilitates striatal EPSPs through an L-type Ca2 +conductance. Neuroreport. 1997;8:2183–2186. doi: 10.1097/00001756-199707070-00019. [DOI] [PubMed] [Google Scholar]
- 330.Cooper DC, White FJ. L-type calcium channels modulate glutamate-driven bursting activity in the nucleus accumbens in vivo. Brain Res. 2000;880:212–218. doi: 10.1016/s0006-8993(00)02868-7. [DOI] [PubMed] [Google Scholar]
- 331.Boehm J, Malinow R. AMPA receptor phosphorylation during synaptic plasticity. Biochem Soc Trans. 2005;33:1354–1356. doi: 10.1042/BST0331354. [DOI] [PubMed] [Google Scholar]
- 332.Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 333.Nestler EJ, et al. Chronic cocaine treatment decreases levels of the G protein subunits Gi alpha and Go alpha in discrete regions of rat brain. J Neurochem. 1990;55:1079–1082. doi: 10.1111/j.1471-4159.1990.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 334.Striplin CD, Kalivas PW. Robustness of G protein changes in cocaine sensitization shown with immunoblotting. Synapse. 1993;14:10–15. doi: 10.1002/syn.890140103. [DOI] [PubMed] [Google Scholar]
- 335.Xi ZX, et al. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- 336.Xi ZX, et al. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J Neurosci. 2003;23:3498–3505. doi: 10.1523/JNEUROSCI.23-08-03498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.De Vries L, et al. The regulator of G protein signaling family. Annu Rev Pharmacol Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- 338.Blumer JB, Lanier SM. Accessory proteins for G protein-signaling systems: activators of G protein signaling and other nonreceptor proteins influencing the activation state of G proteins. Receptors Channels. 2003;9:195–204. [PubMed] [Google Scholar]
- 339.Ribas C, et al. Pertussis toxin-insensitive activation of the heterotrimeric G-proteins Gi/Go by the NG108–15 G-protein activator. J Biol Chem. 2002;277:50223–50225. doi: 10.1074/jbc.C200567200. [DOI] [PubMed] [Google Scholar]
- 340.Kelley AE, Schiltz CA. Accessories to addiction: G protein regulators play a key role in cocaine seeking and neuroplasticity. Neuron. 2004;42:181–183. doi: 10.1016/s0896-6273(04)00223-5. [DOI] [PubMed] [Google Scholar]
- 341.Terwilliger RZ, et al. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- 342.Freeman WM, et al. Chronic cocaine-mediated changes in non-human primate nucleus accumbens gene expression. J Neurochem. 2001;77:542–549. doi: 10.1046/j.1471-4159.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 343.Arnsten AF, et al. Protein kinase A as a therapeutic target for memory disorders: rationale and challenges. Trends Mol Med. 2005;11:121–128. doi: 10.1016/j.molmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 344.Lynch WJ, et al. Effect of cocaine self-administration on striatal PKA-regulated signaling in male and female rats. Psychopharmacology (Berl) 2007;191:263–271. doi: 10.1007/s00213-006-0656-0. [DOI] [PubMed] [Google Scholar]
- 345.Nazarian A, et al. Sex differences in basal and cocaine-induced alterations in PKA and CREB proteins in the nucleus accumbens. Psychopharmacology (Berl) 2009;203:641–650. doi: 10.1007/s00213-008-1411-5. [DOI] [PubMed] [Google Scholar]
- 346.Chavez-Noriega LE, Stevens CF. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. J Neurosci. 1994;14:310–317. doi: 10.1523/JNEUROSCI.14-01-00310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Santiago M, Westerink BH. Role of adenylate cyclase in the modulation of the release of dopamine: a microdialysis study in the striatum of the rat. J Neurochem. 1990;55:169–174. doi: 10.1111/j.1471-4159.1990.tb08835.x. [DOI] [PubMed] [Google Scholar]
- 348.Chao SZ, et al. D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J Neurochem. 2002;83:704–712. doi: 10.1046/j.1471-4159.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- 349.Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- 350.Steketee JD, Rowe LA, Chandler LJ. The effects of acute and repeated cocaine injections on protein kinase C activity and isoform levels in dopaminergic brain regions. Neuropharmacology. 1998;37:339–347. doi: 10.1016/s0028-3908(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 351.Chen Q, et al. Reversal of cocaine sensitization-induced behavioral sensitization normalizes GAD67 and GABAA receptor alpha2 subunit expression, and PKC zeta activity. Biochem Biophys Res Commun. 2007;356:733–738. doi: 10.1016/j.bbrc.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Aujla H, Beninger RJ. Intra-accumbens protein kinase C inhibitor NPC 15437 blocks amphetamine-produced conditioned place preference in rats. Behav Brain Res. 2003;147:41–48. doi: 10.1016/s0166-4328(03)00136-0. [DOI] [PubMed] [Google Scholar]
- 353.Cervo L, et al. Protein kinases A and C are involved in the mechanisms underlying consolidation of cocaine place conditioning. Brain Res. 1997;775:30–36. doi: 10.1016/s0006-8993(97)00866-4. [DOI] [PubMed] [Google Scholar]
- 354.Pierce RC, et al. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- 355.Thomas KL, Everitt BJ. Limbic-cortical-ventral striatal activation during retrieval of a discrete cocaine-associated stimulus: a cellular imaging study with gamma protein kinase C expression. J Neurosci. 2001;21:2526–2535. doi: 10.1523/JNEUROSCI.21-07-02526.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Pristupa ZB, et al. Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse. 1998;30:79–87. doi: 10.1002/(SICI)1098-2396(199809)30:1<79::AID-SYN10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 357.Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- 358.Melikian HE, Buckley KM. Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci. 1999;19:7699–7710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Qian Y, et al. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Whitworth TL, Herndon LC, Quick MW. Psychostimulants differentially regulate serotonin transporter expression in thalamocortical neurons. J Neurosci. 2002;22:RC192. doi: 10.1523/JNEUROSCI.22-01-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Jayanthi LD, Samuvel DJ, Ramamoorthy S. Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J Biol Chem. 2004;279:19315–19326. doi: 10.1074/jbc.M311172200. [DOI] [PubMed] [Google Scholar]
- 362.Jayanthi LD, et al. Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J Biol Chem. 2006;281:23326–23340. doi: 10.1074/jbc.M601156200. [DOI] [PubMed] [Google Scholar]
- 363.Liu XY, et al. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 364.Snyder GL, et al. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Bilbao A, et al. Loss of the Ca2+/calmodulin-dependent protein kinase type IV in dopaminoceptive neurons enhances behavioral effects of cocaine. Proc Natl Acad Sci USA. 2008;105:17549–17554. doi: 10.1073/pnas.0803959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Matthews RP, et al. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sun P, et al. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- 368.Lu L, et al. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 369.Zhai H, et al. Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: implications for reinforcement and reinstatement. Cell Mol Neurobiol. 2008;28:157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Corominas M, et al. Brain-derived neurotrophic factor and its intracellular signaling pathways in cocaine addiction. Neuropsychobiology. 2007;55:2–13. doi: 10.1159/000103570. [DOI] [PubMed] [Google Scholar]
- 371.Benavides DR, Bibb JA. Role of Cdk5 in drug abuse and plasticity. Ann N Y Acad Sci. 2004;1025:335–344. doi: 10.1196/annals.1316.041. [DOI] [PubMed] [Google Scholar]
- 372.Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J. 2005;7:E353–360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- 374.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Dackis C, O’Brien C. Glutamatergic agents for cocaine dependence. Ann N Y Acad Sci. 2003;1003:328–345. doi: 10.1196/annals.1300.021. [DOI] [PubMed] [Google Scholar]
- 376.Smilkstein MJ, et al. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 377.LaRowe SD, et al. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 378.Mardikian PN, et al. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:389–394. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 379.Knackstedt LA, et al. The role of cystine–glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62:652–657. doi: 10.1016/j.biopsych.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 381.LaRowe SD, et al. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 2006;15:105–110. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Kumar R. Approved and investigational uses of modafinil : an evidence-based review. Drugs. 2008;68:1803–1839. doi: 10.2165/00003495-200868130-00003. [DOI] [PubMed] [Google Scholar]
- 383.Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 384.Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 385.Mignot E, et al. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep. 1994;17:436–437. doi: 10.1093/sleep/17.5.436. [DOI] [PubMed] [Google Scholar]
- 386.Madras BK, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- 387.Qu WM, et al. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–8469. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Ishizuka T, Murakami M, Yamatodani A. Involvement of central histaminergic systems in modafinil-induced but not methylphenidate-induced increases in locomotor activity in rats. Eur J Pharmacol. 2008;578:209–215. doi: 10.1016/j.ejphar.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 389.Scammell TE, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20:8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Chemelli RM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 391.Ferraro L, et al. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology. 1999;20:346–356. doi: 10.1016/S0893-133X(98)00085-2. [DOI] [PubMed] [Google Scholar]
- 392.Korotkova TM, et al. Modafinil inhibits rat midbrain dopaminergic neurons through D2-like receptors. Neuropharmacology. 2007;52:626–633. doi: 10.1016/j.neuropharm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 393.Stoops WW, et al. Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology (Berl) 2005;182:186–193. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- 394.Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14:53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- 395.Rush CR, et al. Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug Alcohol Depend. 2002;67:311–322. doi: 10.1016/s0376-8716(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 396.Rush CR, et al. Acute behavioral and physiological effects of modafinil in drug abusers. Behav Pharmacol. 2002;13:105–115. doi: 10.1097/00008877-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 397.Deroche-Gamonet V, et al. Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology (Berl) 2002;161:387–395. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- 398.Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology (Berl) 1996;126:286–292. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- 399.Dackis CA, et al. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 400.Dackis CA, et al. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- 401.Donovan JL, et al. Modafinil influences the pharmacokinetics of intravenous cocaine in healthy cocaine-dependent volunteers. Clin Pharmacokinet. 2005;44:753–765. doi: 10.2165/00003088-200544070-00006. [DOI] [PubMed] [Google Scholar]
- 402.Malcolm R, et al. Modafinil and cocaine interactions. Am J Drug Alcohol Abuse. 2006;32:577–587. doi: 10.1080/00952990600920425. [DOI] [PubMed] [Google Scholar]
- 403.Hart CL, et al. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- 404.Monahan JB, et al. d-Cycloserine, a positive modulator of the N-methyl-d-aspartate receptor, enhances performance of learning tasks in rats. Pharmacol Biochem Behav. 1989;34:649–653. doi: 10.1016/0091-3057(89)90571-6. [DOI] [PubMed] [Google Scholar]
- 405.Hood WF, Compton RP, Monahan JB. d-Cycloserine: a ligand for the N-methyl-d-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett. 1989;98:91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- 406.Emmett MR, et al. Actions of d-cycloserine at the N-methyl-d-aspartate-associated glycine receptor site in vivo. Neuropharmacology. 1991;30:1167–1171. doi: 10.1016/0028-3908(91)90161-4. [DOI] [PubMed] [Google Scholar]
- 407.Davis M, et al. Effects of d-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 408.Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by d-cycloserine: theoretical and clinical implications. Learn Mem. 2004;11:510–516. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- 409.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of d-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 410.Paolone G, Botreau F, Stewart J. The facilitative effects of d-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202:403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- 411.Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 412.Thanos PK, et al. d-Cycloserine accelerates the extinction of cocaine-induced conditioned place preference in C57bL/c mice. Behav Brain Res. 2009;199:345–349. doi: 10.1016/j.bbr.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Lee JL, et al. d-cycloserine potentiates the reconsolidation of cocaine-associated memories. Learn Mem. 2009;16:82–85. doi: 10.1101/lm.1186609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Lipton SA. The molecular basis of memantine action in Alzheimer’s disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Curr Alzheimer Res. 2005;2:155–165. doi: 10.2174/1567205053585846. [DOI] [PubMed] [Google Scholar]
- 415.Parsons CG, Stoffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system–too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 416.Rammes G, et al. The N-methyl-d-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neurosci Lett. 2001;306:81–84. doi: 10.1016/s0304-3940(01)01872-9. [DOI] [PubMed] [Google Scholar]
- 417.Vosburg SK, et al. An evaluation of the reinforcing effects of memantine in cocaine-dependent humans. Drug Alcohol Depend. 2005;79:257–260. doi: 10.1016/j.drugalcdep.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 418.Collins ED, et al. The effects of acute pretreatment with high-dose memantine on the cardiovascular and behavioral effects of cocaine in humans. Exp Clin Psychopharmacol. 2007;15:228–237. doi: 10.1037/1064-1297.15.3.228. [DOI] [PubMed] [Google Scholar]
- 419.Collins ED, et al. The effects of memantine on the subjective, reinforcing and cardiovascular effects of cocaine in humans. Behav Pharmacol. 1998;9:587–598. doi: 10.1097/00008877-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 420.Newman JL, Beardsley PM. Effects of memantine, haloperidol, and cocaine on primary and conditioned reinforcement associated with cocaine in rhesus monkeys. Psychopharmacology (Berl) 2006;185:142–149. doi: 10.1007/s00213-005-0282-2. [DOI] [PubMed] [Google Scholar]
- 421.Aoki FY, Sitar DS. Clinical pharmacokinetics of amantadine hydrochloride. Clin Pharmacokinet. 1988;14:35–51. doi: 10.2165/00003088-198814010-00003. [DOI] [PubMed] [Google Scholar]
- 422.Sofuoglu M, Kosten TR. Novel approaches to the treatment of cocaine addiction. CNS Drugs. 2005;19:13–25. doi: 10.2165/00023210-200519010-00002. [DOI] [PubMed] [Google Scholar]
- 423.Herblin WF. Amantadine and catecholamine up-take. Biochem Pharmacol. 1972;21:1993–1995. doi: 10.1016/0006-2952(72)90013-5. [DOI] [PubMed] [Google Scholar]
- 424.Stoof JC, et al. The anti-parkinsonian drug amantadine inhibits the N-methyl-d-aspartic acid–evoked release of acetylcholine from rat neostriatum in a non-competitive way. Eur J Pharmacol. 1992;213:439–443. doi: 10.1016/0014-2999(92)90634-g. [DOI] [PubMed] [Google Scholar]
- 425.Mizoguchi K, et al. Amantadine increases the extracellular dopamine levels in the striatum by re-uptake inhibition and by N-methyl-d-aspartate antagonism. Brain Res. 1994;662:255–258. doi: 10.1016/0006-8993(94)90821-4. [DOI] [PubMed] [Google Scholar]
- 426.King GR, Joyner C, Ellinwood EH., Jr Continuous or intermittent cocaine administration: effects of amantadine treatment during withdrawal. Pharmacol Biochem Behav. 1994;47:451–457. doi: 10.1016/0091-3057(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 427.Alterman AI, et al. Amantadine may facilitate detoxification of cocaine addicts. Drug Alcohol Depend. 1992;31:19–29. doi: 10.1016/0376-8716(92)90004-v. [DOI] [PubMed] [Google Scholar]
- 428.Shoptaw S, et al. A screening trial of amantadine as a medication for cocaine dependence. Drug Alcohol Depend. 2002;66:217–224. doi: 10.1016/s0376-8716(01)00205-8. [DOI] [PubMed] [Google Scholar]
- 429.Weddington WW, Jr, et al. Comparison of amantadine and desipramine combined with psychotherapy for treatment of cocaine dependence. Am J Drug Alcohol Abuse. 1991;17:137–152. doi: 10.3109/00952999108992817. [DOI] [PubMed] [Google Scholar]
- 430.Kampman K, et al. Amantadine in the early treatment of cocaine dependence: a double-blind, placebo-controlled trial. Drug Alcohol Depend. 1996;41:25–33. doi: 10.1016/0376-8716(96)01225-2. [DOI] [PubMed] [Google Scholar]
- 431.Kampman KM, et al. Amantadine in the treatment of cocaine-dependent patients with severe withdrawal symptoms. Am J Psychiatry. 2000;157:2052–2054. doi: 10.1176/appi.ajp.157.12.2052. [DOI] [PubMed] [Google Scholar]
- 432.Silberstein S, et al. Epidemiology, risk factors, and treatment of chronic migraine: a focus on topiramate. Headache. 2008;48:1087–1095. doi: 10.1111/j.1526-4610.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 433.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- 434.Dickenson AH, Ghandehari J. Anticonvulsants and anti-depressants. Handb Exp Pharmacol. 2007;177:145–177. doi: 10.1007/978-3-540-33823-9_6. [DOI] [PubMed] [Google Scholar]
- 435.Gryder DS, Rogawski MA. Selective antagonism of GluR5 kainate-receptor–mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J Neurosci. 2003;23:7069–7074. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Kaminski RM, Banerjee M, Rogawski MA. Topiramate selectively protects against seizures induced by ATPA, a GluR5 kainate receptor agonist. Neuropharmacology. 2004;46:1097–1104. doi: 10.1016/j.neuropharm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 437.Le Foll B, et al. Topiramate does not alter nicotine or cocaine discrimination in rats. Behav Pharmacol. 2008;19:13–20. doi: 10.1097/FBP.0b013e3282f3cf84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Kampman KM, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 439.Wang SJ, et al. Presynaptic inhibition of excitatory neurotransmission by lamotrigine in the rat amygdalar neurons. Synapse. 1996;24:248–255. doi: 10.1002/(SICI)1098-2396(199611)24:3<248::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 440.Leach MJ, Marden CM, Miller AA. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action. Epilepsia. 1986;27:490–497. doi: 10.1111/j.1528-1157.1986.tb03573.x. [DOI] [PubMed] [Google Scholar]
- 441.Lee CY, et al. Lamotrigine inhibits postsynaptic AMPA receptor and glutamate release in the dentate gyrus. Epilepsia. 2008;49:888–897. doi: 10.1111/j.1528-1167.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 442.Sitges M, et al. Effects of carbamazepine, phenytoin, lamotrigine, oxcarbazepine, topiramate and vinpocetine on Na+ channel-mediated release of [3H]glutamate in hippocampal nerve endings. Neuropharmacology. 2007;52:598–605. doi: 10.1016/j.neuropharm.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 443.Ahmad S, Fowler LJ, Whitton PS. Effects of acute and chronic lamotrigine treatment on basal and stimulated extracellular amino acids in the hippocampus of freely moving rats. Brain Res. 2004;1029:41–47. doi: 10.1016/j.brainres.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 444.Cunningham MO, Jones RS. The anticonvulsant, lamotrigine decreases spontaneous glutamate release but increases spontaneous GABA release in the rat entorhinal cortex in vitro. Neuropharmacology. 2000;39:2139–2146. doi: 10.1016/s0028-3908(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 445.Waldmeier PC, et al. Similar potency of carbamazepine, oxcarbazepine, and lamotrigine in inhibiting the release of glutamate and other neurotransmitters. Neurology. 1995;45:1907–1913. doi: 10.1212/wnl.45.10.1907. [DOI] [PubMed] [Google Scholar]
- 446.Brown ES, et al. Lamotrigine in patients with bipolar disorder and cocaine dependence. J Clin Psychiatry. 2003;64:197–201. doi: 10.4088/jcp.v64n0213. [DOI] [PubMed] [Google Scholar]
- 447.Margolin A, et al. A preliminary investigation of lamotrigine for cocaine abuse in HIV-seropositive patients. Am J Drug Alcohol Abuse. 1998;24:85–101. doi: 10.3109/00952999809001700. [DOI] [PubMed] [Google Scholar]
- 448.Berger SP, et al. A medication screening trial evaluation of reserpine, gabapentin and lamotrigine pharmacotherapy of cocaine dependence. Addiction. 2005;100(Suppl. 1):58–67. doi: 10.1111/j.1360-0443.2005.00983.x. [DOI] [PubMed] [Google Scholar]
- 449.Winther LC, et al. Effects of lamotrigine on behavioral and cardiovascular responses to cocaine in human subjects. Am J Drug Alcohol Abuse. 2000;26:47–59. doi: 10.1081/ada-100100590. [DOI] [PubMed] [Google Scholar]
- 450.Maneuf YP, et al. Cellular and molecular action of the putative GABA-mimetic, gabapentin. Cell Mol Life Sci. 2003;60:742–750. doi: 10.1007/s00018-003-2108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Shimoyama M, Shimoyama N, Hori Y. Gabapentin affects glutamatergic excitatory neurotransmission in the rat dorsal horn. Pain. 2000;85:405–414. doi: 10.1016/S0304-3959(99)00283-3. [DOI] [PubMed] [Google Scholar]
- 452.Dooley DJ, Mieske CA, Borosky SA. Inhibition of K(+)-evoked glutamate release from rat neocortical and hippocampal slices by gabapentin. Neurosci Lett. 2000;280:107–110. doi: 10.1016/s0304-3940(00)00769-2. [DOI] [PubMed] [Google Scholar]
- 453.Maneuf YP, McKnight AT. Block by gabapentin of the facilitation of glutamate release from rat trigeminal nucleus following activation of protein kinase C or adenylyl cyclase. Br J Pharmacol. 2001;134:237–240. doi: 10.1038/sj.bjp.0704227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Maneuf YP, et al. Reduction by gabapentin of K+-evoked release of [3H]-glutamate from the caudal trigeminal nucleus of the streptozotocin-treated rat. Br J Pharmacol. 2004;141:574–579. doi: 10.1038/sj.bjp.0705579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Ng GY, et al. Gamma-aminobutyric acid type B receptors with specific heterodimer composition and postsynaptic actions in hippocampal neurons are targets of anticonvulsant gabapentin action. Mol Pharmacol. 2001;59:144–152. [PubMed] [Google Scholar]
- 456.Itzhak Y, Martin JL. Effect of riluzole and gabapentin on cocaine- and methamphetamine-induced behavioral sensitization in mice. Psychopharmacology (Berl) 2000;151:226–233. doi: 10.1007/s002130000394. [DOI] [PubMed] [Google Scholar]
- 457.Filip M, et al. Various GABA-mimetic drugs differently affect cocaine-evoked hyperlocomotion and sensitization. Eur J Pharmacol. 2006;541:163–170. doi: 10.1016/j.ejphar.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 458.Filip M, et al. Diverse effects of GABA-mimetic drugs on cocaine-evoked self-administration and discriminative stimulus effects in rats. Psychopharmacology (Berl) 2007;192:17–26. doi: 10.1007/s00213-006-0694-7. [DOI] [PubMed] [Google Scholar]
- 459.Peng XQ, et al. Effects of gabapentin on cocaine self-administration, cocaine-triggered relapse and cocaine-enhanced nucleus accumbens dopamine in rats. Drug Alcohol Depend. 2008;97:207–215. doi: 10.1016/j.drugalcdep.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Myrick H, et al. Gabapentin in the treatment of cocaine dependence: a case series. J Clin Psychiatry. 2001;62:19–23. doi: 10.4088/jcp.v62n0105. [DOI] [PubMed] [Google Scholar]
- 461.Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65:84–86. doi: 10.4088/jcp.v65n0114. [DOI] [PubMed] [Google Scholar]
- 462.Raby WN. Gabapentin therapy for cocaine cravings. Am J Psychiatry. 2000;157:2058–2059. doi: 10.1176/appi.ajp.157.12.2058-a. [DOI] [PubMed] [Google Scholar]
- 463.Bisaga A, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend. 2006;81:267–274. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 464.Gonzalez G, et al. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone-treated patients. Drug Alcohol Depend. 2007;87:1–9. doi: 10.1016/j.drugalcdep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 465.Hart CL, et al. Gabapentin does not reduce smoked cocaine self-administration: employment of a novel self-administration procedure. Behav Pharmacol. 2007;18:71–75. doi: 10.1097/FBP.0b013e328014139d. [DOI] [PubMed] [Google Scholar]
- 466.Hart CL, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86:274–277. doi: 10.1016/j.drugalcdep.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 467.Haney M, et al. Smoked cocaine discrimination in humans: effects of gabapentin. Drug Alcohol Depend. 2005;80:53–61. doi: 10.1016/j.drugalcdep.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 468.Triggle DJ. Calcium channel antagonists: clinical uses–past, present and future. Biochem Pharmacol. 2007;74:1–9. doi: 10.1016/j.bcp.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 469.Kuzmin A, et al. Calcium antagonists isradipine and nimodipine suppress cocaine and morphine intravenous self-administration in drug-naive mice. Pharmacol Biochem Behav. 1992;41:497–500. doi: 10.1016/0091-3057(92)90363-k. [DOI] [PubMed] [Google Scholar]
- 470.Martellotta MC, et al. Effects of the calcium antagonist isradipine on cocaine intravenous self-administration in rats. Psychopharmacology (Berl) 1994;113:378–380. doi: 10.1007/BF02245212. [DOI] [PubMed] [Google Scholar]
- 471.Schindler CW, et al. Calcium channel blockers antagonize some of cocaine’s cardiovascular effects, but fail to alter cocaine’s behavioral effects. J Pharmacol Exp Ther. 1995;272:791–798. [PubMed] [Google Scholar]
- 472.Pani L, et al. The calcium antagonist PN 200–110 inhibits the reinforcing properties of cocaine. Brain Res Bull. 1991;26:445–447. doi: 10.1016/0361-9230(91)90022-c. [DOI] [PubMed] [Google Scholar]
- 473.Karler R, et al. Calcium channel blockers and behavioral sensitization. Life Sci. 1991;49:165–170. doi: 10.1016/0024-3205(91)90029-b. [DOI] [PubMed] [Google Scholar]
- 474.Reimer AR, Martin-Iverson MT. Nimodipine and haloperidol attenuate behavioural sensitization to cocaine but only nimodipine blocks the establishment of conditioned locomotion induced by cocaine. Psychopharmacology (Berl) 1994;113:404–410. doi: 10.1007/BF02245216. [DOI] [PubMed] [Google Scholar]
- 475.Licata SC, et al. Repeated stimulation of L-type calcium channels in the rat ventral tegmental area mimics the initiation of behavioral sensitization to cocaine. Psychopharmacology (Berl) 2000;152:110–118. doi: 10.1007/s002130000518. [DOI] [PubMed] [Google Scholar]
- 476.Rosenzweig-Lipson S, Barrett JE. Modification of the behavioral effects of (+/−)BAY k 8644, cocaine and d-amphetamine by L-type calcium channel blockers in squirrel monkeys. J Pharmacol Exp Ther. 1995;274:842–851. [PubMed] [Google Scholar]
- 477.Chartoff EH, Pliakas AM, Carlezon WA., Jr Microinjection of the L-type calcium channel antagonist diltiazem into the ventral nucleus accumbens shell facilitates cocaine-induced conditioned place preferences. Biol Psychiatry. 2006;59:1236–1239. doi: 10.1016/j.biopsych.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 478.Lhuintre JP, et al. Ability of calcium bis acetyl homotaurine, a GABA agonist, to prevent relapse in weaned alcoholics. Lancet. 1985;1:1014–1016. doi: 10.1016/s0140-6736(85)91615-0. [DOI] [PubMed] [Google Scholar]
- 479.Mann K. Pharmacotherapy of alcohol dependence: a review of the clinical data. CNS Drugs. 2004;18:485–504. doi: 10.2165/00023210-200418080-00002. [DOI] [PubMed] [Google Scholar]
- 480.Boothby LA, Doering PL. Acamprosate for the treatment of alcohol dependence. Clin Ther. 2005;27:695–714. doi: 10.1016/j.clinthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 481.Mason BJ. Acamprosate in the treatment of alcohol dependence. Expert Opin Pharmacother. 2005;6:2103–2115. doi: 10.1517/14656566.6.12.2103. [DOI] [PubMed] [Google Scholar]
- 482.Madamba SG, et al. Acamprosate (calcium acetylhomotaurinate) enhances the N-methyl-d-aspartate component of excitatory neurotransmission in rat hippocampal CA1 neurons in vitro. Alcohol Clin Exp Res. 1996;20:651–658. doi: 10.1111/j.1530-0277.1996.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 483.Berton F, et al. Acamprosate enhances N-methyl-daspartate receptor–mediated neurotransmission but inhibits presynaptic GABA(B) receptors in nucleus accumbens neurons. Alcohol Clin Exp Res. 1998;22:183–191. [PubMed] [Google Scholar]
- 484.Mann K, et al. Acamprosate: recent findings and future research directions. Alcohol Clin Exp Res. 2008;32:1105–1110. doi: 10.1111/j.1530-0277.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 485.Dahchour A, De Witte P. Ethanol and amino acids in the central nervous system: assessment of the pharmacological actions of acamprosate. Prog Neurobiol. 2000;60:343–362. doi: 10.1016/s0301-0082(99)00031-3. [DOI] [PubMed] [Google Scholar]
- 486.De Witte P, et al. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs. 2005;19:517–537. doi: 10.2165/00023210-200519060-00004. [DOI] [PubMed] [Google Scholar]
- 487.McGeehan AJ, Olive MF. The anti-relapse compound acamprosate inhibits the development of a conditioned place preference to ethanol and cocaine but not morphine. Br J Pharmacol. 2003;138:9–12. doi: 10.1038/sj.bjp.0705059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.McGeehan AJ, Olive MF. Attenuation of cocaine-induced reinstatement of cocaine conditioned place preference by acamprosate. Behav Pharmacol. 2006;17:363–367. doi: 10.1097/01.fbp.0000224384.01863.5f. [DOI] [PubMed] [Google Scholar]
- 489.Bowers MS, et al. Acamprosate attenuates cocaine- and cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2007;195:397–406. doi: 10.1007/s00213-007-0904-y. [DOI] [PubMed] [Google Scholar]
- 490.Carroll FI. Antagonists at metabotropic glutamate receptor subtype 5: structure activity relationships and therapeutic potential for addiction. Ann N Y Acad Sci. 2008;1141:221–232. doi: 10.1196/annals.1441.015. [DOI] [PubMed] [Google Scholar]
- 491.Cosford ND, et al. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J Med Chem. 2003;46:204–206. doi: 10.1021/jm025570j. [DOI] [PubMed] [Google Scholar]
- 492.Lea PM, 4th, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12:149–166. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.McGeehan AJ, Janak PH, Olive MF. Effect of the mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine (MPEP) on the acute locomotor stimulant properties of cocaine, D-amphetamine, and the dopamine reuptake inhibitor GBR12909 in mice. Psychopharmacology (Berl) 2004;174:266–273. doi: 10.1007/s00213-003-1733-2. [DOI] [PubMed] [Google Scholar]
- 494.McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- 495.Herzig V, Schmidt WJ. Effects of MPEP on locomotion, sensitization and conditioned reward induced by cocaine or morphine. Neuropharmacology. 2004;47:973–984. doi: 10.1016/j.neuropharm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 496.Kenny PJ, et al. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005;179:247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- 497.Tessari M, et al. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 498.Platt DM, Rowlett JK, Spealman RD. Attenuation of cocaine self-administration in squirrel monkeys following repeated administration of the mGluR5 antagonist MPEP: comparison with dizocilpine. Psychopharmacology (Berl) 2008;200:167–176. doi: 10.1007/s00213-008-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Dravolina OA, Danysz W, Bespalov AY. Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology (Berl) 2006;187:397–404. doi: 10.1007/s00213-006-0440-1. [DOI] [PubMed] [Google Scholar]
- 500.Swanson CJ, Schoepp DD. A role for noradrenergic transmission in the actions of phencyclidine and the antipsychotic and antistress effects of mGlu2/3 receptor agonists. Ann N Y Acad Sci. 2003;1003:309–317. doi: 10.1196/annals.1300.019. [DOI] [PubMed] [Google Scholar]
- 501.Monn JA, et al. Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J Med Chem. 1997;40:528–537. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- 502.Cartmell J, et al. Dopamine and 5-HT turnover are increased by the mGlu2/3 receptor agonist LY379268 in rat medial prefrontal cortex, nucleus accumbens and striatum. Brain Res. 2000;887:378–384. doi: 10.1016/s0006-8993(00)03067-5. [DOI] [PubMed] [Google Scholar]
- 503.Cartmell J, et al. The potent, selective mGlu2/3 receptor agonist LY379268 increases extracellular levels of dopamine, 3,4-dihydroxyphenylacetic acid, homovanillic acid, and 5-hydroxyindole-3-acetic acid in the medial prefrontal cortex of the freely moving rat. J Neurochem. 2000;75:1147–1154. doi: 10.1046/j.1471-4159.2000.0751147.x. [DOI] [PubMed] [Google Scholar]
- 504.Greenslade RG, Mitchell SN. Selective action of (−)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY379268), a group II metabotropic glutamate receptor agonist, on basal and phencyclidine-induced dopamine release in the nucleus accumbens shell. Neuropharmacology. 2004;47:1–8. doi: 10.1016/j.neuropharm.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 505.Kim JH, et al. Activation of group II mGlu receptors blocks the enhanced drug taking induced by previous exposure to amphetamine. Eur J Neurosci. 2005;21:295–300. doi: 10.1111/j.1460-9568.2004.03822.x. [DOI] [PubMed] [Google Scholar]
- 506.Imre G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev. 2007;13:444–464. doi: 10.1111/j.1527-3458.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Uejima JL, et al. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res. 2007;181:292–296. doi: 10.1016/j.bbr.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 508.Lu L, et al. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]


