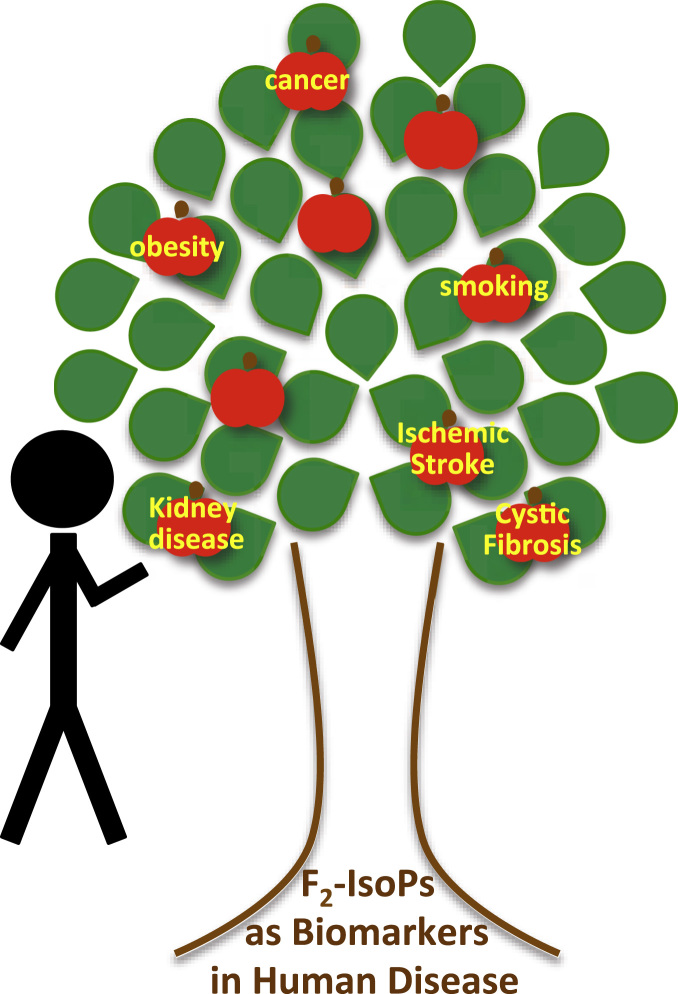
Oxidative stress as classified by F2-Isoprostanes (F2-IsoPs) in Human Disease: Grab the Low-Hanging Fruit – The utility of F2-IsoPs as a biomarker of oxidative injury in human disease is complex. Careful consideration of the formation, metabolism, and measurement of this biomarker is imperative in the design of clinical trials. Conditions with significantly elevated levels of F2-IsoPs are ideal candidates for targeting new generation antioxidant therapies in clinically relevant situations.
Oxidative damage to lipids, particularly during the storage of oils, dates to antiquity. The first evidence of lipid peroxidation was described by Senebier in 1791 when he described that olive oil kept in air lost its fluidity and obtained a bad smell while another sample not exposed air remain unchanged [1]. Yet, it was nearly 200 years later that specific products generated during the oxidation of polyunsaturated fatty acids (PUFAs) were first identified and the importance of PUFA oxidation in biological systems was illuminated. Tappel first introduced the free radical theory of aging in 1968 when he proposed that antioxidants would slow the aging process [2]. In 1985, Sies and Cadenas first introduced the term ‘oxidative stress’ and defined it as ‘a disturbance in the prooxidant-antioxidant balance in favor of the former, leading to potential damage [3], [4].’ Sies and Jones have since updated the definition to ‘an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage [5].’ A primary target of molecular damage resulting from oxidative stress is PUFA.
One crucial stumbling block to the study of oxidative stress in human physiology and pathophysiology has been the availability of effective biomarkers to not only assess oxidative injury but to also identify the effectiveness of potential therapies to decrease damage. Many of the common early markers of lipid peroxidation, including TBARS, malondialdehyde (MDA), and lipid hydroperoxides (LOOHs), are now considered ineffective as biomarkers due to their reactivity, metabolism, or unreliable methods of quantification [6]. Following the accidental discovery of the F2-isoprostanes (F2-IsoPs) by Morrow and Roberts in 1991, the field rapidly embraced the measurement of these compounds, as it seemed that these molecules fit the magic bullet description of the perfect biomarker [7], [8], [9], [10]. First and foremost, the F2-IsoPs are relatively chemical stable, especially compared to TBARS, MDA, and LOOHs. Importantly, they are ubiquitous in human plasma and urine and, consequently, normal levels could be defined. Further, early studies showed increases in these molecules in diseases and lifestyle factors classically associated with oxidative stress (ie. atherosclerosis, hyperlipidemia, smoking, etc.) Yet, despite more than 4000 published works on IsoPs in the past 25 years, F2-IsoPs are still not considered a clinically relevant biomarker.
The current report by van ‘t Erve and colleagues is incredibly timely in an era when the utility of lipid biomarkers of oxidative injury are being re-imagined [11]. Therein, the authors performed the first systematic meta-analysis of published human clinical trials in which F2-IsoPs were quantified as a biomarker of oxidative injury. The authors specifically compared levels of the isomer 8-iso-PGF2α in cases and appropriately matched controls across 50 different health outcomes in order to rank the conditions by level of oxidative injury, and the findings were quite surprising. For example, oxidative stress has classically been linked with cardiovascular disease (ie. coronary artery disease, hypertension, hypercholesterolemia, etc.) and associated with lifestyle factors such as obesity and smoking. Yet, in this analysis, the association of oxidative damage with these conditions was much less significant in comparison to a variety of other diseases. Cystic fibrosis, pulmonary arterial hypertension, chronic renal insufficiency, bronchiectasis, and Rett Syndrome were the top 5 diseases found to be associated with the greatest levels of oxidative damage. Interestingly, exposure to second-hand smoke had a higher injury level than current smokers, and type 1 diabetes had a much more significant association than type 2 diabetes. Further, cancer, which is often considered to be a disease with significant oxidative damage, had one of the lowest associations with increased levels of 8-iso-PGF2α.
These findings are consistent with the increasing evidence that suggests that the formation and metabolism of the F2-IsoPs is significantly more complex than originally appreciated. Reviews of the literature by Halliwell, Galano, and others suggest several factors that need to be considered when designing clinical trials in which F2-IsoPs are to be quantified [12], [13], [14], [15]. For example, it is imperative to recognize that F2-IsoPs are initially formed esterified in cellular phospholipids and are then enzymatically hydrolyzed to the free acid form [12]. Platelet activating factor acetylhydrolase (PAF-AH) is one enzyme that hydrolyzes F2-IsoPs in the plasma yet the rate of hydrolysis of these molecules from phospholipids remains largely unexplored [16]. PAF-AH activity is altered in some disease states and could affect levels of free F2-IsoPs in the plasma. Also, 64 different stereoisomers of F2-IsoPs are generated from the oxidation of arachidonic acid [7]. 8-iso-PGF2α (also known as 15-F2t-IsoP or iPF2α-III) is most often quantified in biological samples as it is one of the most abundant of the 64 stereoisomers in vivo and was the first of the isomers to be synthesized. 8-iso-PGF2α, however, is generated not only via the free radical induced oxidation of arachidonic acid but also from enzymatic oxidation via the cyclooxygenase (COX) enzymes. When using this molecule to assess non-enzymatic oxidative injury, it has been suggested that it is most prudent to examine the ratio of 8-iso-PGF2α to the COX-derived PGF2α [17]. Quantification of other abundant isomers, such as 5-iPF2α-VI or 8,12-isoiPF2α-VI, that are only produced non-enzymatically has also been recommended [18], [19], [20]. In addition to these issues surrounding F2-IsoP formation, these molecules are metabolized via β-oxidation, conjugation, and/or reduction to yield urinary products that are also potential biomarkers of oxidative injury [10]. Finally, PUFA other than arachidonic acid can be oxidized to yield F2-IsoP-like products that have promising biomarker potential, particularly in diseases of the central nervous system [13], [15]. Combined, these data demonstrate the use of F2-IsoPs and related metabolites as biomarkers of oxidative injury requires rigorous consideration of sample matrix and timing of sample collection as well as comprehensive evaluation of particularly molecular species to be measured.
While this study considers only this one particular biomarker of lipid peroxidation in oxidative stress and offers only a snapshot of the current literature on F2-IsoPs in human disease, this type of meta-analysis is particularly relevant in directing the field toward a clinically relevant future, in which personalized medicine is an attainable goal. The conditions presented in this meta-analysis as having the most significantly elevated levels of 8-iso-PGF2α are ideal candidates for targeting new generation antioxidant therapies in clinically relevant situations. Next steps will be to introduce the necessary rigor and validation to move isoprostanes from a research tool to the basis of a clinical test that can be used in the new redox biology based therapeutics that are emerging as a focus in precision medicine. This is indeed the coming of age for the development of reliable biomarkers in redox biology.
Acknowledgements
G.L.M. is supported by NIDDK Grant DK-20593.
Footnotes
Featured Article: Classifying Oxidative Stress by F2-Isoprostane Levels Across Human Disease: A Meta-Analysis. Redox Biology, 2017, Volume 28: pp 582-599 Thomas van ’t Erve, Maria Kadiiska, Stephanie London, Ronald Mason.
References
- 1.J. Senebier, Physiologievégétale.Encyclopédieméthodique t.1, Paris, 1791.
- 2.Tappel A.L. Will antioxidant nutrients slow aging processes? Geriatrics. 1968;23:97–105. [PubMed] [Google Scholar]
- 3.Sies H., Cadenas E. Oxidative stress: damage to intact cells and organs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985;311:617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- 4.Cadenas E., Sies H. Oxidative stress: excited oxygen species and enzyme activity. Adv. Enzyme Regul. 1985;23:217–237. doi: 10.1016/0065-2571(85)90049-4. [DOI] [PubMed] [Google Scholar]
- 5.Jones D.P., Sies H. The Redox Code. Antioxid. Redox Signal. 2015;23:734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forman H.J., Augusto O., Brigelius-Flohe R., Dennery P.A., Kalyanaraman B., Ischiropoulos H., Mann G.E., Radi R., Roberts L.J., 2nd, Vina J., Davies K.J. Even free radicals should follow some rules: a guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015;78:233–235. doi: 10.1016/j.freeradbiomed.2014.10.504. [DOI] [PubMed] [Google Scholar]
- 7.Morrow J.D., Hill K.E., Burk R.F., Nammour T.M., Badr K.F., Roberts L.J., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. USA. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrow J.D., Chen Y., Brame C.J., Yang J., Sanchez S.C., Xu J., Zackert W.E., Awad J.A., Roberts L.J. The isoprostanes: unique prostaglandin-like products of free-radical-initiated lipid peroxidation. Drug Metab. Rev. 1999;31:117–139. doi: 10.1081/dmr-100101910. [DOI] [PubMed] [Google Scholar]
- 9.Milne G.L., Yin H., Hardy K.D., Davies S.S., Roberts L.J., 2nd Isoprostane generation and function. Chem. Rev. 2011;111(10):5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milne G.L., Dai Q., Roberts L.J., 2nd The isoprostanes–25 years later. Biochim. Biophys. Acta. 2015;1851:433–445. doi: 10.1016/j.bbalip.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van 't Erve T.J., Kadiiska M.B., London S.J., Mason R.P. Classifying oxidative stress by F2-isoprostane levels across human diseases: a meta-analysis. Redox Biol. 2017;12:582–599. doi: 10.1016/j.redox.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halliwell B., Lee C.Y. Using isoprostanes as biomarkers of oxidative stress: some rarely considered issues. Antioxid. Redox Signal. 2010;13:145–156. doi: 10.1089/ars.2009.2934. [DOI] [PubMed] [Google Scholar]
- 13.Galano J.M., Lee Y.Y., Durand T., Lee J.C. Special Issue on "analytical Methods for Oxidized Biomolecules and antioxidants" The use of isoprostanoids as biomarkers of oxidative damage, and their role in human dietary intervention studies. Free Radic. Res. 2015;49:583–598. doi: 10.3109/10715762.2015.1007969. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y.Y., Galano J.M., Oger C., Vigor C., Guillaume R., Roy J., Le Guennec J.Y., Durand T., Lee J.C. Assessment of Isoprostanes in human plasma: technical considerations and the use of mass spectrometry. Lipids. 2016;51:1217–1229. doi: 10.1007/s11745-016-4198-x. [DOI] [PubMed] [Google Scholar]
- 15.Yen H.C., Wei H.J., Lin C.L. Unresolved issues in the analysis of F2-isoprostanes, F4-neuroprostanes, isofurans, neurofurans, and F2-dihomo-isoprostanes in body fluids and tissue using gas chromatography/negative-ion chemical-ionization mass spectrometry. Free Radic. Res. 2015;49:861–880. doi: 10.3109/10715762.2015.1014812. [DOI] [PubMed] [Google Scholar]
- 16.Stafforini D.M., Sheller J.R., Blackwell T.S., Sapirstein A., Yull F.E., McIntyre T.M., Bonventre J.V., Prescott S.M., Roberts L.J., 2nd Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J. Biol. Chem. 2006;281:4616–4623. doi: 10.1074/jbc.M507340200. [DOI] [PubMed] [Google Scholar]
- 17.van 't Erve T.J., Lih F.B., Kadiiska M.B., Deterding L.J., Eling T.E., Mason R.P. Reinterpreting the best biomarker of oxidative stress: the 8-iso-PGF(2α)/PGF(2α) ratio distinguishes chemical from enzymatic lipid peroxidation. Free Radic. Biol. Med. 2015;83:245–251. doi: 10.1016/j.freeradbiomed.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labuschagne C.F., van den Broek N.J., Postma P., Berger R., Brenkman A.B. A protocol for quantifying lipid peroxidation in cellular systems by F2-isoprostane analysis. PLoS One. 2013;8:e80935. [Google Scholar]
- 19.Lawson J.A., Li H., Rokach J., Adiyaman M., Hwang S.W., Khanapure S.P., FitzGerald G.A. Identification of two major F2 isoprostanes, 8,12-iso- and 5-epi-8, 12-isoisoprostane F2alpha-VI, in human urine. J. Biol. Chem. 1998;273:29295–29301. doi: 10.1074/jbc.273.45.29295. [DOI] [PubMed] [Google Scholar]
- 20.Sicilia T., Mally A., Schauer U., Pähler A., Völkel W. LC-MS/MS methods for the detection of isoprostanes (iPF2alpha-III and 8,12-isoiPF2alpha-VI) as biomarkers of CCl4-induced oxidative damage to hepatic tissue. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008;861:48–55. doi: 10.1016/j.jchromb.2007.11.021. [DOI] [PubMed] [Google Scholar]