Abstract
Introduction
Dementia is a prevalent condition in older adults associated with decline in cognitive and functional abilities and substantial burden. This study assessed the prevalence and impact of subjective memory impairment in the United States.
Methods
The 2011 to 2014 National Health and Nutrition Examination Survey, a population-based, nationally representative survey, was analyzed. Data included medical examinations, self-reported cognitive and functional limitations, and health care utilization over 1 year. Participants were aged ≥65 years and completed both interview and medical examination components. Descriptive analyses of patient characteristics were performed, and complex survey regression models were used to test associations.
Results
Of 2431 survey participants included, 53.1% had no memory impairment, 40.1% had early-stage memory impairment, and 6.6% had late-stage memory impairment. In adjusted analyses, late-stage versus no impairment was associated with more functional limitations (odds ratio [OR] = 7.26, P < .001), greater health care utilization (OR = 2.46, P < .001), and higher likelihood of seeing a mental health specialist (OR = 3.06, P = .001).
Discussion
Consistent with previous research, individuals with late-stage memory impairment had significantly greater functional limitations and higher health care utilization versus individuals with early-stage or no memory impairment.
Keywords: Dementia, Memory impairment, Functional limitation, Health care resource utilization, Population health
1. Introduction
Dementia is a collection of symptoms including memory loss, personality change, and impaired intellectual functions resulting from disease or trauma to the brain. In 2016, an estimated 47 million people worldwide were living with dementia [1]. Alzheimer's disease (AD) is the most common cause of dementia, affecting approximately 5.4 million individuals in 2016 in the United States, of which an estimated 5.2 million are aged ≥65 years [2], [3]. AD is a disease characterized by memory loss and degeneration of mental abilities that are serious enough to interfere with daily activities [2].
AD is the sixth-leading cause of death in the United States, and most patients live an average of 8 years after their symptoms become noticeable; however, the range in survival is between 4 and 20 years due to the influence of age, genetics, and lifestyle [2]. Because of the costs of health care, long-term care, and hospice for individuals with AD and other dementias, the economic burden of AD/dementia is substantial. In 2016, total direct costs for all individuals with AD/dementia in the United States are estimated at $236 billion; Medicare and Medicaid will cover an estimated at $160 billion (68%) of these costs [2]. Among Medicare beneficiaries (aged ≥65 years) with AD/dementia, 2015 per-person total Medicare health care payments ($49,126) were over three times higher than Medicare spending for Medicare beneficiaries without AD/dementia ($15,550) [2].
In patients with AD, the progression of symptoms from mild to moderate to severe varies from person to person; however, disease progression is associated with a decline in cognitive and functional abilities. In the more advanced stages of dementia, patients are reported to need help with basic activities of daily living (ADLs), such as bathing, dressing, eating, and using the bathroom. Patients with advanced disease also lose their ability to communicate, fail to recognize loved ones, and often become confined to bed and reliant on full-time caregiving [2].
Studies indicate that many individuals report mild cognitive impairment (MCI), subjective memory impairment, and functional complaints years before the development of dementia and AD [4], [5]. MCI, which entails mild but measureable changes in cognitive function and is thought to be an intermediate stage in the trajectory from normal cognition to dementia, affects approximately 15% to 20% of individuals aged ≥65 years [2], [6]. Individuals with MCI, particularly those with MCI that involves memory impairment, progress to dementia at a higher rate than individuals with normal cognition [6]. In addition, self-reported cognitive and functional disabilities have been linked to poor outcomes in healthy adults [7]. Therefore, it is important to have knowledge about the prevalence and magnitude of memory impairment, as early diagnosis might delay further memory loss and disease progression. However, there is a paucity of published data describing population-based, nationally representative prevalence of memory impairment and associated functional limitations in US adults aged ≥65 years.
In 2011, the National Health and Nutrition Examination Survey (NHANES), a population-based health survey of noninstitutionalized US residents, introduced a new question to capture difficulty in memory or confusion. With the inclusion of this question in NHANES, the severity of impairment, which has often been difficult to assess, can now be evaluated at a population level. To our knowledge, studies evaluating the prevalence and magnitude of memory impairment using the NHANES data have not been published. To support broader understanding of dementia severity using nationally representative, self-reported data, this study aimed to estimate the prevalence of memory impairment and functional limitations by severity and to investigate the associated risk with health care resource utilization (HRU) in the US population.
2. Methods
2.1. Study design and data source
A retrospective analysis using cross-sectional survey data was conducted to assess the prevalence and impact of memory impairment and functional limitations by severity. NHANES, a program of studies conducted by the National Center for Health Statistics (NCHS), evaluates the health and nutritional status of a representative sample of about 5000 US adults and children each year. The NHANES program began in the early 1960s as a series of surveys focusing on different population groups or health topics, and in 1999, the survey became a continuous program [8]. NHANES data are used by federal agencies, research organizations, universities, health care providers, and educators for a variety of purposes (e.g., research across a wide variety of diseases, tracking trends related to policies, prevention programs, education programs, and developing national standards for such measurements as height, weight, and blood pressure) [8].
NHANES participants are selected by a complex, multistage probability design that combines interviews at the participant's home followed by physical examinations at a mobile examination center. The NHANES interview includes demographic, socioeconomic, dietary, and health-related questions. The examination component consists of medical, dental, and physiological measurements and laboratory tests administered by medical personnel. NHANES was approved by the NCHS Research Ethical Review Board, and this analysis was conducted using deidentified, publicly available data.
2.2. Participant selection
For the purposes of this study, NHANES participants aged ≥65 years who completed both interview and medical examination components for the 2011 to 2014 survey years were selected.
2.3. Outcome measures
2.3.1. Identification of memory impairment and severity
Participants with a positive response to the question “During the past 7 days, how often have you had trouble remembering where you put things like keys or wallet?” were considered to have memory impairment. Possible responses to this question included “never,” “about once,” “2 or 3 times,” “nearly everyday,” “several times a day,” “refused,” and “don't know.” With medical expert opinion, based on response, participants were grouped into impairment severity categories as displayed in Table 1.
Table 1.
Memory severity impairment using NHANES 2011–2014 memory question
| During the past 7 days, how often have you had trouble remembering where you put things like keys or wallet? | Memory impairment classification |
|---|---|
| Never | Never |
| About once | Early stage |
| 2 or 3 times | |
| Nearly everyday | Late stage |
| Several times a day | |
| Refused | Refused |
| Don't know | Don't know |
Abbreviation: NHANES, National Health and Nutrition Examination Survey.
2.3.2. Functional limitations
Self-reported functional limitations in NHANES were evaluated similarly to previous research [9]. Functional abilities were assessed using standardized questions in multiple functional domains, including ADLs (eating/dressing), instrumental activities of daily living (IADLs) (managing money/doing chores around the house/preparing meals), and social interaction activities (participating in social activities such as meetings or visiting friends). Possible responses for all functional domain questions included “no difficulty,” “some difficulty,” “much difficulty,” or “unable to do.” Participants who reported “much difficulty” or “unable to do” on ≥1 task within that domain were identified as having a functional limitation.
2.3.3. Patient demographics and clinical characteristics
Demographic characteristics included age, gender, race/ethnicity, body mass index (BMI), household income, and education level. Self-reported medical comorbidities were also assessed. Participants were classified as having 0 or 1+ comorbidities based on self-report of the following common chronic diseases/events: angina, arthritis, hypertension, coronary heart disease, heart attack, congestive heart failure, stroke, liver disease, diabetes, cancer, chronic pulmonary disease/emphysema, and asthma. Use of prescription medications for antidementia treatment (i.e., donepezil, rivastigmine, galantamine, and memantine) were assessed for each participant based on self-reports. In addition, the following concomitant medications were assessed: anticoagulants, antiplatelets, nonsteroidal anti-inflammatory drugs, antihistamines, prostate anticholinergics, medications for dyslipidemia, antidiabetics, antihypertensives, antiepileptics, antipsychotics, antiemetic neuroleptics and stimulants, medications for Parkinson's disease, antidepressants, benzodiazepines, narcotics, hormones, and other prescription medications. Because of data availability, estimates for prescription drug use were limited to the 2011 to 2012 survey. Self-reported current health status and current health status compared to 1 year ago were also captured.
2.3.4. Health care resource utilization
Frequency of HRU over 1 year and occurrence of mental health specialist visits (yes/no) were assessed. Measures of HRU included emergency services, outpatient services, hospitalizations, clinic use, and physician visits as collected in the surveys. The numbers and percentages of patients receiving each type of care were examined. In addition, the number of times health care was received over the past year, time since the last health care visit, overnight hospitalization, and having seen a mental health professional in the past year were captured.
2.4. Statistical analysis
NHANES 1999 to 2010 and 2011 to 2012 Analytic Guidelines were followed to determine the appropriate survey sample weights for analyses, combining 2-year weights to analyze multiyear samples, variance estimation, and appropriate procedures for subsetting the NHANES data [10], [11]. Nationally representative estimates of the civilian noninstitutionalized US population were estimated using the year 2000 population census, and corresponding correctly adjusted sampling weights developed by the Centers for Disease Control and Prevention were applied. Descriptive analyses were conducted to assess patient characteristics and included mean values and variance estimations for the continuous variables of interest and frequency distributions for the categorical variables. Differences between groups were analyzed using t-tests and chi-square tests as appropriate, following NHANES statistical guidelines. Separate analyses to calculate the risk of HRU in early- and late-stage impairment versus no impairment were conducted using analytical procedures (i.e., surveylogistic and surveyreg) to account for the complex sampling design of NHANES. The association between HRU (including seeing a mental health specialist and overnight hospitalization), prevalence of functional limitations, and level of memory impairment were assessed using weighted logistic regression (specifically, surveylogistic), with adjustments for age, gender, race, education, congestive heart failure/heart disease (coronary artery disease), diabetes mellitus, and BMI. The odds ratio between early- and late-stage impairment versus no impairment and HRU and functional limitations was also assessed.
3. Results
3.1. Participant characteristics
Of the 2556 participants aged ≥65 years from the 2011 to 2014 surveys, a total of 2431 meeting further inclusion criteria were included in these analyses, of whom 1102 reported some trouble remembering. The weighted prevalence of memory impairment among individuals aged ≥65 years was 47% (standard error [SE] = 1.39). This rate translates to 19,251,817 individuals in the United States with memory impairment. Based on responses to the question on difficulty with remembering, 53.1% of survey participants were categorized as having no memory impairment, 40.1% were classified as having early-stage memory impairment, and 6.6% were classified as having late-stage memory impairment. Participants with late-stage impairment were significantly more likely to have used a proxy respondent in NHANES (14.26%) versus participants with no impairment (2.42%) and participants with early-stage impairment (1.40%) (P < .001).
There were significant differences observed among the three memory impairment groups on participant characteristics. As shown in Table 2, mean age was highest among individuals with late-stage impairment, and, relatedly, more individuals with late-stage impairment were aged between 75 and 80 years versus individuals with early-stage and no impairment (P < .001). Mean number of reported comorbidities was higher in the late-stage group versus the groups with early-stage and no impairment (P < .001). The late-stage group was also more likely not to have completed high school and less likely to have graduated from college versus the other groups.
Table 2.
Demographic characteristics by severity of memory impairment classification
| Characteristic | No memory impairment | Memory impairment |
P-value | |
|---|---|---|---|---|
| Early stage | Late stage | |||
| Age | ||||
| Mean (SE) | 72.59 (0.20) | 73.42 (0.18) | 74.53 (0.54) | <.0001 |
| Age group (%) | ||||
| 65–74 years | 64.26 | 53.91 | 46.50 | <.0001 |
| 75–80 years | 35.74 | 46.09 | 53.50 | |
| Female (%) | 52.31 | 59.77 | 58.80 | .0266 |
| Race (%) | ||||
| Caucasian | 77.07 | 81.33 | 71.82 | .0012 |
| Black | 9.54 | 7.19 | 7.43 | |
| Other | 13.39 | 11.48 | 20.75 | |
| Comorbidity index | ||||
| Mean (SE) | 2.27 (0.08) | 2.54 (0.07) | 3.0 (0.17) | .0001 |
| Median household income (%) | ||||
| <$20,000 per year | 18.92 | 19.41 | 25.49 | .1385 |
| <$20,000–$55,000 per year | 40.91 | 43.71 | 40.54 | |
| >$55,000 per year | 36.34 | 33.80 | 24.86 | |
| Missing | 3.82 | 3.08 | 9.11 | |
| Education level (%) | ||||
| Not completed high school | 19.67 | 20.47 | 35.15 | .0075 |
| High school graduate | 21.39 | 23.21 | 25.53 | |
| Some college | 31.02 | 28.64 | 20.85 | |
| College graduate | 27.84 | 27.65 | 17.00 | |
| BMI groups (%) | ||||
| Normal (<25) | 26.38 | 29.16 | 38.62 | .0918 |
| Overweight (25–29) | 35.32 | 35.97 | 33.04 | |
| Obesity (≥30) | 35.71 | 33.13 | 23.64 | |
Abbreviations: SE, standard error; BMI, body mass index.
3.2. Functional limitations
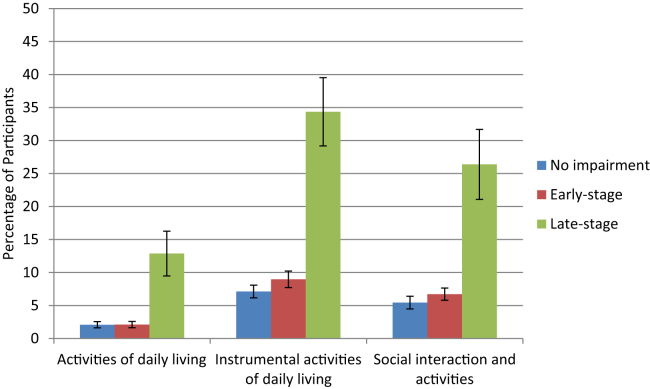
Fig. 1 shows the prevalence of functional limitations (ADLs, IADLS, social interactions) by severity of memory impairment. In all three functional areas, limitations were significantly greater in the late-stage group. In general, there was limited functional impairment in the groups with no memory impairment and early-stage memory impairment.
Fig. 1.
Prevalence of functional limitations by severity of memory impairment.
3.3. Medical comorbidities and medication use
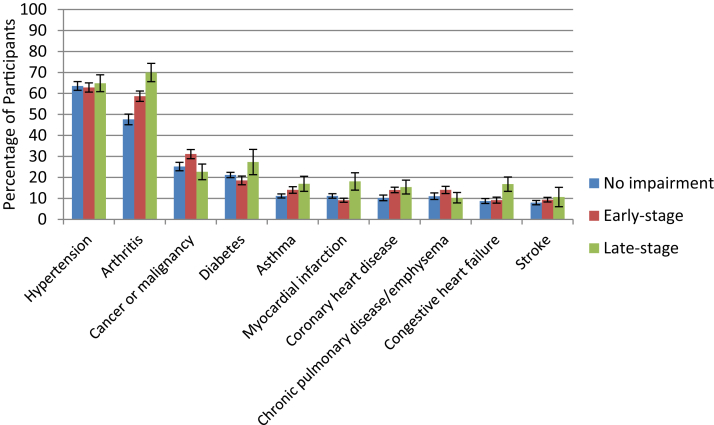
The percentage of participants with the 10 most commonly reported medical comorbidities by memory impairment severity are presented in Fig. 2. Significant group differences were observed on the percentage of survey participants who reported arthritis, coronary heart disease, myocardial infarction, stroke, and cancer/malignancy (all P < .050). With the exception of cancer/malignancy, these conditions were most commonly reported in the late-stage group. When comparing the early- and late-stage impairment groups, significant differences were observed on percentage of survey participants having arthritis (58.69% vs. 69.96%; P = .017), congestive heart failure (9.16% vs. 16.79%; P = .021), myocardial infarction (9.13% vs. 18.08%; P = .032), and stroke (9.39% vs. 20.66%; P = .026).
Fig. 2.
Top 10 self-reported medical comorbidities by severity of memory impairment.
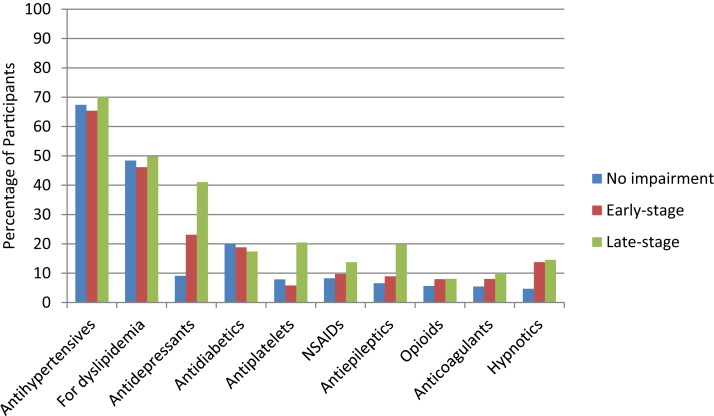
The percentage of participants with the 10 most commonly reported concomitant medications by memory impairment severity are presented in Fig. 3. Significant group differences were observed on the percentage of participants who reported use of antidepressants, antiplatelets, antiepileptics, and hypnotics (all P < .050). For each of these medication classes, the late-stage group reported the highest percentages of use. When comparing the early- versus late-stage impairment groups, significant differences were observed on use of antidepressants (23.12% vs. 41.04%; P = .049), antiplatelets (5.82% vs. 20.47%; P = .021), and antihistamines (3.20% vs. 1.35%; P = .036). Significantly greater use of two antidementia treatments (i.e., donepezil and memantine) was observed in the late-stage group, although use of antidementia medication was generally low in this group. Self-reported use of donepezil and memantine was 1.1% and 0.35%, respectively, in the no impairment group; 1.18% and 0.11%, respectively, in the early-stage group; and 13.71% and 8.26%, respectively, in the late-stage group.
Fig. 3.
Top 10 self-reported concomitant medications by severity of memory impairment. Abbreviation: NSAID, nonsteroidal anti-inflammatory drug.
3.4. Current health status and current health status compared to 1 year ago
Participants were asked to assess their current health status. The percentage of participants who rated their current health as “good,” “very good,” or “excellent” was 36.65% in the no impairment group, 35.44% in the early-stage group, and 27.85% in the late-stage group. The percentage of participants who rated their current health as “fair” or “poor” was 8.79% in the no impairment group, 9.50% in the early-stage group, and 18.40% in the late-stage group. The remaining percentage of participants in each group did not have current health status information. Participants also reported how their current health status compared with their health status 1 year ago. Most participants in each memory impairment group reported that their health status was the same as 1 year ago (72.65% no impairment group; 70.52% early-stage group; 62.47% late-stage group). The percentage of participants who reported worse health compared to a year ago was 12.87% in the no impairment group, 13.78% for the early-stage group, and 22.58% for the late-stage group.
3.5. Health care resource utilization
In the previous year, participants in all memory impairment severity groups most often sought health care in physician office/health maintenance organization settings (81.15% no impairment group; 84.75% early-stage group; 72.87% late-stage group), followed by clinic/health center settings (12.46% no impairment group; 10.42% early-stage group; 19.45% late-stage group). For all groups, hospital emergency rooms and outpatient departments were rarely used for routine health care. Among respondents with an overnight hospitalization, the mean number of overnight hospitalizations was significantly higher for the late-stage group (1.97, SE = 0.37), followed by the no impairment (1.44, SE = 0.06) and early-stage groups (1.28, SE = 0.06), P = .034.
3.6. Association of memory impairment severity and health care resource utilization and functional limitations
Adjusted logistic regression analyses examined the association between severity of memory impairment and overnight hospitalization in the previous year, the number of times health care was received in the previous year, use of a mental health professional in the previous year, and prevalence of functional limitations (Table 3). There were no significant differences in the odds of overnight hospitalizations between the no impairment group and either the early-stage or late-stage groups. No impairment versus late-stage impairment was significantly associated with the number of times health care was received over the past year (odds ratio [OR] = 2.46, P < .001). However, early-stage memory impairment was not significantly associated with number of times health care was received over the past year when compared with no impairment (OR = 1.04, P = .691). Compared to the no impairment group, the early-stage group was 1.54 (P = .070) times more likely to see a mental health specialist, and the late-stage group was 3.06 (P = .001) times more likely to see a mental health specialist. In terms of functional limitations, compared to the no impairment group, the late-stage group was 7.26 times more likely to report functional limitations (P < .001), whereas the likelihood of functional limitations did not differ significantly between the no impairment and early-impairment groups (P = .111).
Table 3.
Logistic regression analyses of the association between memory impairment and health care resource utilization and function limitations
| Outcomes | N | Adjusted odds ratio∗ | 95% confidence interval |
P-value | |
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| Overnight hospitalization | |||||
| Early-stage versus no impairment | 2180 | 1.29 | 0.98 | 1.68 | .0576 |
| Late-stage versus no impairment | 1465 | 1.27 | 0.72 | 2.23 | .3908 |
| Number of time health care received in the past year | |||||
| Early-stage versus no impairment | 2179 | 1.04 | 0.86 | 1.25 | .6908 |
| Late-stage versus no impairment | 1463 | 2.46 | 1.72 | 3.54 | <.0001 |
| Saw a mental health specialist in the past year | |||||
| Early-stage versus no impairment | 2180 | 1.54 | 0.95 | 2.50 | .0694 |
| Late-stage versus no impairment | 1464 | 3.06 | 1.53 | 6.14 | .0011 |
| Functional limitations | |||||
| Early-stage versus no impairment | 2180 | 1.32 | 0.93 | 1.87 | .1106 |
| Late-stage versus no impairment | 1465 | 7.26 | 3.34 | 15.78 | <.0001 |
Controlling for age, gender, race, education, congestive heart failure/heart disease (coronary artery disease), diabetes mellitus, and body mass index.
4. Discussion
This study provides an overview of the extent of subjective memory impairment in the US population. The results suggest that based on the NHANES survey question, prevalence of current memory impairment among individuals aged ≥65 years in the United States is 40% to 47%. Participants with late-stage impairment experienced more functional limitations compared with other impairment groups. Participants who were classified as having late-stage memory impairment received more health care resources than the no impairment and the early-stage groups. In addition, the late-stage group was significantly more likely to visit mental health specialists compared with the no impairment group.
These findings are consistent with previous literature that shows the significant societal and personal economic burden associated with HRU among individuals with memory impairment and dementias compared with individuals with normal cognitive function. Individuals with AD/dementia have more hospital stays, skilled nursing facility stays, and home health care visits as other older adults [2]. A study using data from the Health and Retirement Study linked with Medicare claims from the period 2000 to 2008 found that among community-dwelling elderly fee-for-service Medicare beneficiaries, those who had dementia were significantly more likely than those who did not to have a hospitalization (26.7% vs. 18.7%) and an emergency department visit (34.5% vs. 25.4%) in each year [12]. Fillit et al. [13] found health care utilization and costs to be higher among patients with AD compared with age- and gender-matched controls in a Medicare managed care plan; excess costs were driven by inpatient hospitalizations and use of skilled nursing facilities. In addition, this study found that later stage AD patients had substantially higher costs than earlier stage AD patients.
Even among individuals with MCI whose memory impairment is not significant enough to affect daily activities, health care utilization and costs are significantly higher compared with individuals without MCI. Zhu et al. [14] found that average annual costs per person for medical care were substantially higher for participants with MCI ($6499 vs. $2969), with hospitalizations being the greatest cost driver. Adjusted analyses from this study showed that after controlling for other covariates, direct medical costs were 44% higher for participants with MCI compared with those without MCI.
Research has also shown that resource utilization and costs increase with declining memory and cognitive function [2]. For example, in a study of 6991 US adults aged ≥70 years who participated in the 1998 wave of the Health and Retirement Study [15], participants were categorized into low, moderate, or high cognitive function groups. Participants in the low cognitive function group had fewer physician outpatient visits but more hospitalizations, including more nights hospitalized, versus the other groups.
Caspi et al. [15] also found that individuals in the low cognitive function group experienced more comorbid medical conditions. In the present study, of the 10 most commonly reported medical comorbidities reported by participants, prevalence for most conditions was highest in the late-stage group. Previous research has shown that comorbid medical conditions are more common among individuals with AD/dementia. For example, analyses of the National 5% Sample Medicare Fee-for-Service Beneficiaries data for 2014 [2] showed that Medicare beneficiaries with AD/dementia are more likely than those without AD/dementia to have other chronic conditions. Furthermore, individuals with AD/dementia and comorbid conditions incur higher health care costs. Analyses of annual per-person Medicare payments for seven conditions (coronary artery disease, diabetes, congestive heart failure, chronic kidney disease, chronic obstructive pulmonary disease, stroke, and cancer) showed that Medicare beneficiaries with dementia had higher average per-person payments in all categories (hospital care, physician care, skilled nursing facility care, home health care, hospice care) with the exception of hospital care payments for individuals with congestive heart failure, compared with Medicare beneficiaries without dementia [2].
In our study, significantly greater functional limitations (ADLs, IADLs, and social interactions) were observed in the late- versus early-stage memory impairment and no impairment groups, although adjusted results showed that only the comparison between the last-stage and no impairment groups differed significantly. Previous research has shown that functional limitations can be observed early in the course of cognitive and memory decline [16]. A recent systematic literature review of 37 studies [17] assessed IADL deficits (e.g., problems with medication intake, telephone use, keeping appointments, finding things at home, and using everyday technology) in patients with MCI compared with patients with normal cognition or dementia. In this review, all but two studies found IADL deficits in patients with MCI compared with control subjects without cognitive impairment. In general, patients with MCI had intermediate functional performance between healthy controls and patients with mild AD, particularly in more complex tasks with high cognitive demand [17]. Another population-based US study estimated the prevalence of self-reported confusion or memory loss over the previous 12 months among adults aged ≥60 years using the cognitive decline module in the 2011 Behavioral Risk Factor Surveillance System (BRFSS) survey [18]. Results showed that 12.7% of respondents responded affirmatively to a question about whether they had experienced increased confusion or memory loss in the preceding 12 months; of these, 35.2% reported that they had experienced functional difficulties as a consequence of their confusion or memory loss [18]. In our study, 62.35% of respondents classified as having late-stage impairment and 22.81% of respondents classified as having early-stage impairment responded “yes” to this same question in the NHANES survey compared with only 6.63% of respondents classified as having no impairment (P < .001).
In summary, functional limitations and HRU are substantial in late stages of dementia and increase with cognitive and memory decline, although functional limitations and increased HRU can be observed even in early stages of impairment. Currently, 1 in 9 people aged ≥65 years (11%) in the United States has AD, and given the anticipated increase in the number of older adults, projections suggest that the burden of AD will increase substantially in coming decades [3]. Therefore, early recognition of impairment or functional deficits is essential for clinical practice and the identification of individuals who may be at increased risk of progression to dementia and AD. One component of the National Alzheimer's Project Act [19] is to identify early symptomatic stages of AD. The use of brief questions such as those used in NHANES on memory or functional limitations may serve as indicators of health status for health care practitioners [9], and early interventions may help to delay progression of memory impairment and, consequently, functional limitations. Previous research supports the use of self-reported health status measures, which correlate with both objective health status and mortality [20], [21].
4.1. Limitations
Some study limitations should be noted. Information specific to dementia is not captured as part of the NHANES survey; therefore, the question used in this study to assess memory impairment is only a proxy that provides some initial insight into the extent of memory impairment in the US population. Study results did show that antidementia medication use was higher among the late-stage memory impairment group, which helps to corroborate the severity of memory impairment. Trends from clinical practice show that patients with memory complaints are prescribed antidementia treatment despite a lack of diagnosis or years before a diagnosis is made [22]. Further research is needed to understand the association between the use of this measure of memory impairment severity in NHANES surveys and clinical assessments of cognition and memory function.
In addition, NHANES is based on self-reported information, which may be subject to recall bias and even more so in a population with memory complaints. However, it is important to note that self-reported health status is common in population-based health assessments (e.g., NHANES, BRFSS). In NHANES, respondents may use proxies for certain components of the assessment, including the family interview, sample person (SP) questionnaire, and the computer-assisted personal interviewing (CAPI) component of the mobile exam center (MEC) assessment. In our study, participants in the late-stage memory impairment group were significantly more likely to have a proxy respondent for both the SP (9.73%) and MEC CAPI (9.22%) survey components compared with participants in the no impairment and early-stage impairment groups (proxy use was 0.17% to 1.78% on any survey component). Furthermore, respondents are also able to answer “Don't know” to each question and would therefore be excluded from memory impairment classification with this response. Finally, medication data may be subject to misclassification, and our findings regarding drug utilization over the study period were limited due to the availability of medication data in only the 2011 to 2012 NHANES.
5. Conclusions
The estimated prevalence of reported memory impairment among individuals aged ≥65 years in the United States is 40% to 47%. Consistent with previous research, individuals with late-stage memory impairment had significantly greater functional limitations and higher health care use compared with individuals with no or early-stage memory impairment. NHANES assesses a large representative sample of the US population and includes brief questions on memory or functional limitations that may serve as indicators of health status. Such information may help with early identification of dementia and earlier access to available treatment, education, and support services for individuals with dementia, their families, and their caregivers.
Research in Context.
-
1.
Systematic review: Authors reviewed literature on US population–based estimates of memory impairment among older adults, the economic burden of memory impairment, and the association of memory impairment severity on functional abilities and health care resource use.
-
2.
Interpretation: Based on a National Health and Nutrition Examination Survey (NHANES) survey question, estimated US prevalence of subjective memory impairment among individuals aged ≥65 years is 40% to 47%. Participants with late-stage impairment experienced more functional limitations and health care use versus other impairment groups, which is consistent with previous research.
-
3.
Future directions: Information specific to dementia is not captured as part of the NHANES survey; therefore, the question used in this study to assess memory impairment and severity is a proxy that provides some initial insight into the extent of memory impairment in older US adults. Further research is needed to understand the association between this measure of memory impairment and clinical assessments of cognition and memory function.
Acknowledgments
Editorial support for the preparation of this article was provided by Rupali Naik, PhD, and Ann Cameron, PhD, of Xcenda LLC, and funded by Otsuka America Pharmaceutical, Inc., Princeton, NJ, USA. The sponsor participated in the study design, data analysis and interpretation, article preparation, and the decision to submit this article for publication.
Myrlene Sanon Aigbogun, Robert Stellhorn, Holly Krasa, and Dusan Kostic are employees of Otsuka America Pharmaceuticals, Inc., which provided funding for this research.
References
- 1.Alzheimer's Disease International, World alzheimer report 2016: Improving healthcare for people living with dementia: coverage, quality and costs now and in the future. Available at: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf. Accessed August 25, 2016.
- 2.Alzheimer's Association 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Hebert L.E., Weuve J., Scherr P.A., Evans D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jessen F., Wiese B., Bachmann C., Eifflaender-Gorfer S., Haller F., Kölsch H. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell A.J., Beaumont H., Ferguson D., Yadegarfar M., Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130:439–451. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- 6.Roberts R., Knopman D.S. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29:753–772. doi: 10.1016/j.cger.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reisberg B., Shulman M.B., Torossian C., Leng L., Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics . National Center for Health Statistics; Hyattsville, MD: 2014. National Health and Nutrition Examination Survey, 2013–2014: Overview. [Google Scholar]
- 9.Hajjar I., Wharton W., Mack W.J., Levey A.I., Goldstein F.C. Racial disparity in cognitive and functional disability in hypertension and all-cause mortality. Am J Hypertens. 2016;29:185–193. doi: 10.1093/ajh/hpv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; Hyattsville, MD: 2013. National Health and Nutrition Examination Survey: Analytic Guidelines, 1999-2010.http://www.cdc.gov/nchs/data/series/sr_02/sr02_161.pdf Available at: Accessed August 25, 2016. [Google Scholar]
- 11.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; Hyattsville, MD: 2013. National Health and Nutrition Examination Survey: Analytic Guidelines, 2011-2012.http://www.cdc.gov/nchs/data/nhanes/analytic_guidelines_11_12.pdf Available at: Accessed August 25, 2016. [Google Scholar]
- 12.Feng Z., Coots L.A., Kaganova Y., Wiener J.M. Hospital and ED use among Medicare beneficiaries with dementia varies by setting and proximity to death. Health Aff. 2014;33:683–690. doi: 10.1377/hlthaff.2013.1179. [DOI] [PubMed] [Google Scholar]
- 13.Fillit H., Hill J.W., Futterman R. Health care utilization and costs of Alzheimer's disease: the role of co-morbid conditions, disease stage, and pharmacotherapy. Fam Med. 2002;34:528–535. [PubMed] [Google Scholar]
- 14.Zhu C.W., Sano M., Ferris S.H., Whitehouse P.J., Patterson M.B., Aisen P.S. Health-related resource use and costs in elderly adults with and without mild cognitive impairment. J Am Geriatr Soc. 2013;61:396–402. doi: 10.1111/jgs.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caspi E., Silverstein N.M., Porell F., Kwan N. Physician outpatient contacts and hospitalizations among cognitively impaired elderly. Alzheimers Dement. 2009;5:30–42. doi: 10.1016/j.jalz.2008.05.2493. [DOI] [PubMed] [Google Scholar]
- 16.Brown P.J., Devanand D.P., Liu X., Caccappolo E. Alzheimer's Disease Neuroimaging Initiative, functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011;68:617–626. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jekel K., Damian M., Wattmo C., Hausner L., Bullock R., Connelly P.J. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimers Res Ther. 2015;7:17. doi: 10.1186/s13195-015-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Self-reported increased confusion or memory loss and associated functional difficulties among adults aged ≥60 years—21 states, 2011. Morb Mortal Wkly Rep. 2013;62:347–350. [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services . US Dept of Health and Human Services; Washington, DC: 2016. National Plan to Address Alzheimer's Disease: 2016 Update.https://alzheimers.acl.gov/pdf/NationalPlantoAddressAlzheimersDisease.pdf Available at: Accessed September 1, 2016. [Google Scholar]
- 20.Idler E.L., Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 21.Meng Q., Xie Z., Zhang T. A single-item self-rated health measure correlates with objective health status in the elderly: a survey in suburban Beijing. Front Public Health. 2014;2:27. doi: 10.3389/fpubh.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider L.S., Insel P.S., Weiner M.W. Alzheimer's Disease Neuroimaging Initiative, Treatment with cholinesterase inhibitors and memantine of patients in the Alzheimer's Disease Neuroimaging Initiative. Arch Neurol. 2011;68:58–66. doi: 10.1001/archneurol.2010.343. [DOI] [PMC free article] [PubMed] [Google Scholar]