Abstract
Alcohol use disorders are common both in the United States and globally, and are associated with a variety of co-morbid, inflammation-linked diseases. The pathogenesis of many of these ailments are driven by the activation of the NLRP3 inflammasome, a multi-protein intracellular pattern recognition receptor complex that facilitates the cleavage and secretion of the pro-inflammatory cytokines IL-1β and IL-18. We hypothesized that protracted exposure of leukocytes to ethanol would amplify inflammasome activation, which would help to implicate mechanisms involved in diseases associated with both alcoholism and aberrant NLRP3 inflammasome activation. Here we show that long-term ethanol exposure of human peripheral blood mononuclear cells and a mouse macrophage cell line (J774) amplifies IL-1β secretion following stimulation with NLRP3 agonists, but not with AIM2 or NLRP1b agonists. The augmented NRLP3 activation was mediated by increases in iNOS expression and NO production, in conjunction with increases in mitochondrial membrane depolarization, oxygen consumption rate, and ROS generation in J774 cells chronically exposed to ethanol (CE cells), effects that could be inhibited by the iNOS inhibitor SEITU, the NO scavenger carboxy-PTIO, and the mitochondrial ROS scavenger MitoQ. Chronic ethanol exposure did not alter K+ efflux or Zn2+ homeostasis in CE cells, although it did result in a lower intracellular concentration of NAD+. Prolonged administration of acetaldehyde, the product of alcohol dehydrogenase (ADH) mediated metabolism of ethanol, mimicked chronic ethanol exposure, whereas ADH inhibition prevented ethanol-induced IL-1β hypersecretion. Together, these results indicate that increases in iNOS and mitochondrial ROS production are critical for chronic ethanol-induced IL-1β hypersecretion, and that protracted exposure to the products of ethanol metabolism are probable mediators of NLRP3 inflammasome hyperactivation.
Abbreviations: 4-MP, 4-methylpyrazole; AA, Acetaldehyde; ADH, Alcohol dehydrogenase; ALDH, Aldehyde dehydrogenase; ARDS, Acute respiratory distress syndrome; ASC, Apoptosis-associated speck like protein containing a CARD; AUD, Alcohol use disorder; Casp-1, caspase-1;CE, Chronic ethanol-exposed J774 cells; CNS, Central nervous system; CYP2E1, cytochrome P450 2E1; DAMP, Danger associated molecular pattern; DCF-DA, 2’,7’-Dichlorofluorescindiacetate; ECAR, Extracellular acidification rate; EtOH, Ethanol; hPBMC, Human peripheral blood mononuclear cells; ICP-OES, Inductively coupled plasma optical emission spectroscopy; IL-6, Interleukin-6; IL-1α, Interleukin-1α; IL-1β, Interleukin-1β; iNOS, Inducible nitric oxide synthase; LT, Anthrax lethal toxin; Lys, Lysate; NLR, Nucleotide Oligomerization Domain (NOD)-Like Receptor; NLRP3, Nucleotide-Binding Oligomerization Domain, Leucine Rich Repeat And Pyrin Domain Containing Protein 3 (NLR family pyrin domain containing 3); NO, Nitric oxide; OCR, Oxygen consumption rate; PAMP, Pathogen associated molecular pattern; PRR, Pattern recognition receptor; RNS, Reactive nitrogen species; ROS, Reactive oxygen species; SEITU, S-ethyl-isothiourea; Sup, Supernatant; TNF, Tumor necrosis factor; UT, Untreated J774 cells
Keywords: Inflammasome, IL-1β, Ethanol, Inflammation
Graphical abstract
Highlights
-
•
Chronic ethanol exposure amplifies NLRP3 inflammasome-induced IL-1β secretion.
-
•
NO and mitochondrial ROS mediate chronic ethanol-augmented IL-1β secretion.
-
•
Alcohol dehydrogenase-generated metabolites cause NLRP3 inflammasome over-activation.
1. Introduction
Alcoholism is a common but problematic syndrome affecting nearly every organ system [1]. In the year 2014, approximately 6.8%, or 16.4 million adults and 855,000 adolescents in the U. S., were diagnosed with an alcohol use disorder (AUD) [2]. AUDs were estimated to be the third most common non-genetic cause of mortality in the U.S. in the year 2000, with opportunistic infections and organ damage (such as brain and liver) being the two most prominent co-morbidities [3]. The pattern of drinking has divergent effects on the complications of alcohol abuse. Binge, or acute, alcohol consumption leads to transient immunosuppression, while prolonged alcohol consumption promotes the development of chronic inflammation and is associated with increases in the production of pro-inflammatory cytokines such as TNF and IL-1β [1], [4], [5], [6], [7]. This chronic inflammatory state has been implicated to play a causal role in the initiation or pathogenesis of many diseases associated with AUDs, such as gout, acute respiratory distress syndrome (ARDS), cirrhosis, diabetes, central nervous system (CNS) atrophy, chronic pancreatitis, and several forms of cancer [8], [9], [10], [11], [12], [13], [14], [15], [16], [17].
Chronic ethanol exposure has direct and indirect effects on cells that can initiate an inflammatory state within a host. It promotes the generation of reactive oxygen species (ROS) in the cytosol and mitochondria via the impairment of antioxidant systems, metabolism by cytochrome P450 2E1 (CYP2E1), and enhanced electron transport chain activity accompanied by electron leakage from complexes I and III [18], [19], [20]. Ethanol metabolism by CYP2E1 and alcohol dehydrogenase (ADH) results in the generation of acetaldehyde, which induces oxidative stress and the formation of DNA and protein adducts [21], [22], [23]. Ethanol exposure has also been shown to promote inducible nitric oxide synthase (iNOS) expression and the subsequent production of NO [24], [25]. Together, the ROS, reactive nitrogen species (RNS), and acetaldehyde produced during chronic ethanol exposure can induce nuclear and mitochondrial DNA damage and initiate ER stress via the accumulation of misfolded proteins [25], [26], [27]. Specifically, oxidized mitochondrial DNA and ER stress stimulate signaling pathways such as NF-κB and the NRLP3 inflammasome, but it is not known whether these factors contribute to inflammasome activation during chronic ethanol consumption [28], [29].
Inflammasomes are a family of large multi-protein intracellular pattern recognition receptors (PRRs) that respond to a wide variety of exogenous pathogen associated molecular patterns (PAMPs) and endogenous danger associated molecular patterns (DAMPs), leading to the release of the pro-inflammatory cytokines, IL-1β and IL-18, and culminating in a form of pro-inflammatory death known as pyroptosis [30]. Unlike many innate immune pathways, stimulation of a functional inflammasome requires two events, priming and activation. During priming (step 1), activation of the transcription factor NF-κB downstream of the stimulation of many PRRs, facilitates the transcription and translation of many inflammasome components (as well as the secretion of pro-inflammatory cytokines such as TNF and IL-6) [31]. Activation of the inflammasome complex (step 2) requires the exposure of cells to a separate set of PAMPs and DAMPs, which work through unique signaling pathways to induce the oligomerization of one of several different Nucleotide Oligomerization Domain (NOD)-Like Receptor (NLR) proteins, the adaptor protein Apoptosis-associated Speck-like protein containing a CARD (ASC), and pro-caspase-1, into an organized inflammasome complex [32]. This oligomerization causes the rapid and irreversible formation of inflammasome complexes, mediated by ASC speck oligomerization, which allows for the conversion of pro-caspase-1 into an active caspase-1 enzyme that cleaves pro-IL-1β and pro-IL-18 into their mature, secreted forms and initiates pyroptosis. These cytokines then function to promote vasodilation, attract and stimulate neutrophils, induce fever, and activate the acute phase response, while pyroptosis amplifies inflammation by releasing DAMPs and fully formed ASC specks [33], [34].
The NLRP3 inflammasome is assembled in response to a particularly diverse set of PAMPs and DAMPs, including ATP, nigericin, alum, asbestos, silica, and cholesterol crystals [35], [36], [37]. Although these agonists activate the inflammasome through disparate upstream pathways, such as membrane pore formation and lysosomal rupture, they eventually converge on K+ efflux, ROS production, mitochondrial dysfunction and ASC phosphorylation and multimerization [29], [38], [39], [40], [41], [42], [43]. NLRP3 activation plays a vital role in immune homeostasis and while it is expressed predominantly by macrophages, it can also function within many other leukocyte and non-leukocyte cell types [44]. While necessary for generating an effective immune response to numerous pathogens, over-activation of the NLRP3 inflammasome has been implicated in the pathogenesis of numerous inflammation-related diseases, many of which are also common among patients with alcohol use disorders, such as gout, diabetes, and chronic pancreatitis [45], [46], [47].
The objective of our studies was to investigate whether chronic ethanol exposure modulates activation of the NLRP3 inflammasome and thus secretion of the highly pro-inflammatory cytokine, IL-1β, and through which mechanisms it may occur. In doing so, we sought to better understand the mechanisms behind alcohol-associated inflammation that can promote the development of alcoholism-associated diseases, and help to identify therapeutic targets for the treatment of diseases caused by AUDs.
2. Materials and methods
2.1. Reagents
LPS, sodium orthovanadate, SEITU and monoclonal anti-mouse β-actin antibodies were from Sigma-Aldrich (St. Louis, MO). Anti-mouse ASC and caspase-1 antibodies were from Santa Cruz Biotechnology Inc. (Dallas, TX), and anti-mouse and human IL-1β antibodies were from R&D Systems (Minneapolis, MN). Biotin conjugated anti- mouse and rabbit secondary antibodies were from GE Healthcare UK Limited (Little Chalfont, Buckinghamshire, UK) and anti-goat IgG was from Jackson ImmunoResearch (West Grove, PA). For inflammasome stimulation, nigericin and poly (dA: dT) were from Invivogen (San Diego, CA), ATP and anthrax lethal factor and protective antigen were from Amersham Biosciences (Piscatawy, NJ) and BEI Resources (Manassas, VA), respectively. Ethanol and carboxy-PTIO were from Pharmco AAPER (Brookfield, CT) and Life Technologies Corporation (Carlsbad, CA). NAD+ was from Cayman Chamical (Ann Arbor, Michigan), and acetaldehyde and 4-methylpyrazole (4-MP) were from Acros Organics (Geel, Belgium). Dr. Michael P. Murphy (Medical Research Council Mitochondrial Biology Unit, University of Cambridge, Cambridge, UK) kindly provided MitoQ.
2.2. Cell culture
J774 cells from American Type Culture Collection (ATCC, Manassas, VA) were maintained in DMEM (Gibco, Grand Island, NY) supplemented with 10% FBS (Gibco), 1% l-Glutamine (Gibco), and 1× Primocin (Invivogen). Cells were not used beyond passage 20 to reduce variability between the experiments and although cells at a lower passage demonstrated more robust cytokine secretion, the pattern of responsiveness remained the same.
For experiments in which cell supernatants were examined by ELISA, cells were plated at 2.5x105 cells/well in 250 µl of media in a 48-well plate and allowed to grow overnight. The following day, the media was removed, fresh media was added and cells were treated as indicated within the figure legends for each experiment. Cell supernatants were harvested at the end of each experiment, spun down at 3,300xg for 10 min to pellet cellular debris, transferred to new tubes, and frozen at −20 °C until analysis. For the measurement of intracellular IL-1β, the adherent cells were washed twice in PBS and lysed in 250 µl of NP-40 lysis buffer (50 mM Tris pH 8, 150 mM NaCl, 1% NP-40) for 10 min on ice with scraping. The cell lysates were spun down at 3,300xg for 10 min to pellet cellular debris, transferred to new tubes, and frozen at −20 °C until analysis.
For experiments analyzed through western blotting, J774 cells were plated at 3x106 cells/well in 2 ml of media in 6-well plates and allowed to grow overnight. The next day, the media was removed, cells were washed twice in warm PBS and placed in 1 ml of serum free media. The cells were treated as indicated within the figure legend for each experiment. Supernatants were spun down at 3300×g, transferred to new tubes, and frozen at −20 °C. The cells were then washed twice with PBS and lysed in RIPA buffer (50 mM Tris pH 8, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS) containing 1 mM sodium orthovanadate (Sigma-Aldrich), 1x protease cocktail inhibitor (Sigma-Aldrich), and 1x PMSF (Sigma-Aldrich) on ice for 10 min with scraping. Cellular debris was removed through centrifugation at 3300×g for 10 min, the lysates were transferred to new tubes, and frozen at −20 °C. Before running on a gel, protein concentrations in the lysates were quantified by a detergent compatible protein assay. In all experiments using J774 cells, the previously determined dose of LPS for half-maximal IL-1β secretion, upon stimulation, was used (37 ng/ml) [5].
2.3. Generation of J774 cells exposed to prolonged ethanol or acetaldehyde treatment
J774 cells were cultured in T-25 flasks at a density of 2x105 cells/ml in 5 ml of media with no additional treatments or with ethanol, acetaldehyde, or ethanol and 4-MP as indicated for 24 h. The media was then removed and fresh media was added to each flask with the adherent cells. Treatments were re-added to the fresh media for 24 h and the media and treatment change was repeated once more for a total of 72 h of exposure. The adherent cells were then scraped and spun down at 200xg for 10 min. The cells were then resuspended in media, counted and plated at 2.5x105 cells/well in 250 µl of media in a 48-well plate and then treated with LPS and ATP as indicated in the figure legends.
2.4. Generation of chronic ethanol (CE) J774 cells
CE J774 cells were grown, starting at passage 7, in the presence of 1.2% (947 mg/dL) ethanol/media, with media/ethanol changes every 48 h. CE cells were grown for a minimum of 2 weeks in the presence of ethanol before being used for experiments and were compared to cells unexposed to ethanol grown from the same lot, which were referred to as untreated (UT) cells.
2.5. Isolation and treatment of human PBMCs (hPBMCs)
Heparinized blood was diluted 1:1 in PBS, and 4 ml of diluted blood was layered over 3 ml of lymphocyte separation medium (LSM) (MP Biomedicals) and centrifuged at 400×g for 15 min at room temperature. The top layer of plasma was aspirated and discarded, leaving 2–3 mm above the buffy coat, and the buffy coat and half of the lower LSM layer was aspirated, mixed with PBS, and centrifuged at 200×g for 10 min at room temperature. The cells were washed once in PBS, resuspended in DMEM, and plated at 1.25x106 cells/ml in 6-well plates in triplicate in 2.5 ml of media with 0 mg/dL, 387 mg/dL, 947 mg/dL, and 1418 mg/dL ethanol for 24 h. The media and non-adherent cells were then removed and 1 ml of fresh media was added to each well with the adherent cells. Non-adherent cells were spun down at 200×g for 10 min, resuspended in fresh media, and added back to their original wells at a total volume of 2.5 ml. Ethanol was re-added to the fresh media at 0 mg/dL, 387 mg/dL, 947 mg/dL, and 1418 mg/dL for 24 h and the media and ethanol change was repeated for a total of 72 h of exposure to ethanol. The adherent cells were then scraped, triplicate wells were pooled, and adherent and non-adherent cells were spun down at 200×g for 10 min. The hPBMCs were then resuspended in media, counted, plated at 2.5x105 cells/well in 250 µl of media in a 48-well plate, and treated as indicated with LPS and ATP in the figure legends.
2.6. Measurement of total protein, IL-1β, TNF and IL-6
Total protein levels from cell lysates were measured using the Lowry assay according to manufacturer's protocols (Bio-Rad). Mouse IL-1β and TNF (BD Biosciences, San Jose, CA) and mouse IL-6 and human IL-1β, TNF and IL-6 (R&D Systems) ELISAs were conducted according to manufacturer's protocols.
2.7. Measurement of extracellular nitrite
Extracellular nitrite concentration was determined by mixing nitrite standard or cell supernatants with Griess reagent (0.2% naphthylethylenediamine dihydrochloride, 2% sulphanilamide, 5% phosphoric acid in water) (Sigma-Aldrich) in a 1:1 ratio and measuring the absorbance at 572 nm on a Synergy HTX Multi-Mode plate reader (BioTek, Winooski, VT) using the program Gen5 1.1.
2.8. Measurement of secreted lactate
Extracellular lactate was measured from the supernatants of UT and CE cells treated as indicated in the figure legends using an L-Lactate Assay Kit according to the manufacturer's protocols (Cayman Chemical).
2.9. Measurement of extracellular ethanol
To measure the average concentration of ethanol that CE cells were exposed to over a 48-h period, cells were placed in 947 mg/dL ethanol in 15 ml of media and 100 µl of cell culture supernatant was removed from the tissue culture flask every 4 h. The concentration of ethanol in these samples was measured by an ethanol assay kit according to the manufacturer's protocols (Megazyme, Bray, Ireland) and the average concentration of ethanol in the media over 48-h was calculated.
2.10. Chloroform-methanol protein precipitation from cell supernatants and western blots
Chloroform and methanol protein precipitation from cell supernatants and western blots were performed as described previously [5].
2.11. Inductively coupled plasma optical emission spectroscopy (ICP-OES) measurements for intracellular potassium, sodium, and zinc
ICP-OES protocols were adapted from those previously described [5]. Briefly, J774 cells were plated at 3x106 cells/well in 2 ml of media in 6-well plates, were allowed to grow overnight and were then treated as indicated. Following stimulation, the supernatants were removed and cells were washed twice with sterile saline (0.9% NaCl), and lysed in 30% HNO3 overnight. The samples were then diluted in Milli-Q water to a final concentration of 3% HNO3 for potassium (K) and zinc (Zn) measurement, and were diluted tenfold further in 3% HNO3 for sodium (Na) measurement. Analyses were performed on a PerkinElmer Optima 7000DV ICP optical emission spectrometer (OES) with a cyclonic spray chamber, concentric nebulizer, CCD array detector, a PerkinElmer S10 autosampler, and WinLab32 software (PerkinElmer, Shelton, CT). The axial detection mode was utilized for measurement of K, Zn, and Na in all standards and samples. Concentrated K, Zn, and Na standard solutions for ICP (1000 mg/L, TraceCERT®) were both obtained from Fluka Analytical (Buchs, Switzerland). The concentrated yttrium (Y) standard solution for atomic spectroscopy (1000 mg/L) was obtained from PerkinElmer. Triplicate external calibration was performed using combined K, Zn, and Na standards in the range of 0 – 20 ppm (mg/L) for K and Zn, and 0 – 10 ppm for Na. Yttrium (0.1 ppm) was used as an internal standard in all calibration standards and samples. The standards were prepared in 3% HNO3 in Milli-Q water to matrix match with the samples. Concentrations were quantified based on the emission wavelengths at 766.478 nm, 206.200 nm, 589.600 nm, and 371.029 nm for K, Zn, Na, and Y, respectively. All samples were measured in triplicate and averaged.
2.12. Measurement of extracellular flux
Real-time changes in extracellular acidification rates (ECARs, as a measure of lactate production) and oxygen consumption rates (OCRs, as a measure of oxidative phosphorylation) were analyzed using extracellular flux analysis (Seahorse Bioscience, North Billerica, MA) as described previously [48]. Briefly, UT and CE J774 cells were plated at 1.5x105 cells per well in 50 µl of media (unbuffered RPMI 1640, 10 mM glucose, 10% FCS) (Sigma-Aldrich) and allowed to adhere at 37 °C. Once adhered, 200 µl of additional media was added to each well, and the J774 cells were analyzed per the manufacturer's instructions to obtain real-time measurements of baseline OCRs and ECARs. Where indicated, ECARs and/or OCRs were analyzed in response to 1 μM oligomycin, 1.5 μM fluoro-carbonyl cyanide phenylhydrazone, and 100 nM rotenone plus 1 μM antimycin A (Sigma-Aldrich).
2.13. Measurement of intracellular NAD+
Intracellular NAD+ was measured from J774 UT and CE cells treated as indicated in the figure legends with an NAD/NADH Cell Based Assay Kit (Cayman Chemical) according to the manufacturer's protocol.
2.14. Measurement of mitochondrial ROS
J774.1 UT and CE macrophages were plated overnight in 12-well plates at a density of 106 cells/ml and 500 µl/well. The next day, cells remained unchallenged or were challenged with 1 μg/ml LPS for 5 h at 37 °C. Fifty-five µl/well of a 50 μM solution of MitoSOX Red in DMSO (Invitrogen) was added to the cells to give a final concentration of 5 μM and the cells were incubated for 30 min at 37 °C. Some samples were treated with DMSO instead of MitoSOX Red loading (negative control) or were loaded with MitoSOX Red and simultaneously treated with 2 μM of the mitochondrial superoxide generator Antimycin A (Sigma-Aldrich; positive control). Experimental samples were incubated with 1 mM ATP (to activate the NLRP3 inflammasome) during the MitoSOX Red loading. Media was then aspirated and the cells were gently washed with 500 µl/well PBS. The PBS was aspirated and 200 µl/well of PBS+0.1% FBS was added to the cells. The cells were scraped and collected into flow tubes to which 800 µl of PBS+0.1% FBS was added, and the cells were centrifuged at 200×g for 5 min. The cells were resuspended and washed once in 1 ml of PBS+0.1% FBS. Following centrifugation, cells were stained with 1 µg/ml DAPI (Thermo Scientific) in 200 µl PBS+0.1% FBS for 10 min at 4 °C. Following the addition of 800 µl of PBS+0.1% FBS, cells were centrifuged at 200×g for 5 min, resuspended in 200 µl of 2% PFA for 10 min, washed in an additional 800 µl of PBS+0.1% FBS, resuspended in 180 µl of PBS+0.1% FBS, transferred into wells of a 96-well round-bottom plate, and analyzed using a MACSQuant VYB flow cytometer (Miltenyi Biotec) with DAPI analyzed at 360/460 nm and MitoSOX Red analyzed at 488/575 nm (excitation/emission, respectively). FCS files were analyzed using FlowJo software (Tree Star, Ashland, OR) from which the MitoSOX Red fluorescence intensity of the live (DAPI-negative), single-cell population was determined. Experiments were performed in triplicate and the study was repeated.
2.15. Measurement of intracellular ROS
Intracellular ROS concentrations were measured by 2’,7’-dichlorofluorescindiacetate (DCF-DA) assay as previously described [5]. Briefly, J774 cells were plated at 1.5x105 cells/well in a clear bottom and black-walled 96-well tissue culture plate overnight. The next day, the cells were washed and treated as indicated with LPS (1 μg/ml) in serum and phenol red free media for 3 h and with MitoQ (0.3–1.2 μM) for 2 additional hours. The media was then removed and replaced with fresh serum and phenol red free media containing 2.5 mM probenecid (Tocris Bioscience, Bristol, UK) with and without 10 μM DCF-DA (Molecular Probes, Eugene, OR) for 40 min at room temperature. The cells were subsequently washed in HBSS containing 2.5 mM probenecid for 5 min at 37 °C. The cells were then placed in serum-free and phenol red-free media with and without MitoQ (0.3–1.2 μM), read on a Synergy HTX Multi-Mode plate reader (BioTek) at 485Ex/520Em for 2 min, treated as indicated with ATP (1 mM) and read on the plate reader for an additional 30 min with one read per minute. The baseline read for each well and the average fluorescence of the group that was not treated with DCF-DA were subtracted from each subsequent reading and the areas under the curves were calculated using GraphPad Prism 7 for Windows (La Jolla, CA).
2.16. Quantitative RT-PCR
UT and CE J774 cells were plated at 1.5x106 cells per well in 1 ml of media in a 12-well plate overnight. The following day, the media was removed and replaced with fresh media with and without LPS (37 ng/ml) for 4 h. After this, the supernatants were removed and the cells were washed twice in cold PBS before total RNA was extracted using the PrepEase RNA Isolation Kit (USB Corp., Cleveland, OH). Primers were designed for mouse Arg1 (5’-CCACAGTCTGGCAGTTGGAAG-3’ and 5’-GGTTGTCAGGGGAGTGTTGAT-3’), Asc (5’-ACAGAAGTGGACGGAGTGCT-3’ and 5’-CTCCAGGTCCATCACCAAGT-3’), Casp1 (5’-CACAGCTCTGGAGATGGTGA-3’ and 5’-TCTTTCAAGCTTGGGCACTT-3’), Il6 (5’-CCGGAGAGGAGACTTCACAG-3’ and 5’-GAGCATTGGAAATTGGGGTA-3’), Il18 (5’-CTGCCTGCTGGCTGGAGCTG-3’ and 5’-TGGTCTGGGGTTCACTGGCACT-3’), Il1a (5’-CCTAAAGAACTGTTACAGTGAAAACG-3’ and 5’-TGGTCAATGGCAGAACTGTAGTCT-3’), Il1b (5’-GCCCATCCTCTGTGACTCAT-3’ and 5’-AGGCCACAGGTATTTTGTCG-3’), Nlrp3 (5’-ATGCTGCTTCGACATCTCCT-3’ and 5’-AACCAATGCGAGATCCTGAC-3’), Nos2 (5’-AGTTCGTCCCCTTCTCCTGT-3’ and 5’-CCTTGTTCAGCTACGCCTTC-3’), Sod1 (5’-CCAGTGCAGGACCTCATTTT-3’ and 5’-AATCCCAATCACTCCACAGG-3’), and Tnf (5’-TCCCAGGTTCTCTTCAAGGGA-3’ and 5’-GGTGAGGAGCACGTAGTCGG-3’) using NCBI Primer BLAST and synthesized by Integrated DNA Technologies (Coralville, IA). Quantitative RT-PCR was performed using SYBR Green Supermix (Bio-Rad, Hercules, CA) and normalized to Gapdh (5’-ACGACCCCTTCATTGACCTC-3’ and 5’-TTCACACCCATCACAAACAT-3’) using the ∆∆CT method as previously described [49].
2.17. Statistical calculations
All experiments were performed in triplicate and representative results are presented. Results were analyzed by unpaired t-test or one-way ANOVA and Tukey's post hoc test using GraphPad Prism 7 for Windows. A p value <0.05 was considered statistically significant.
3. Results
3.1. Prolonged and chronic ethanol exposure leads to IL-1β hypersecretion, which is inhibitable by acute ethanol administration
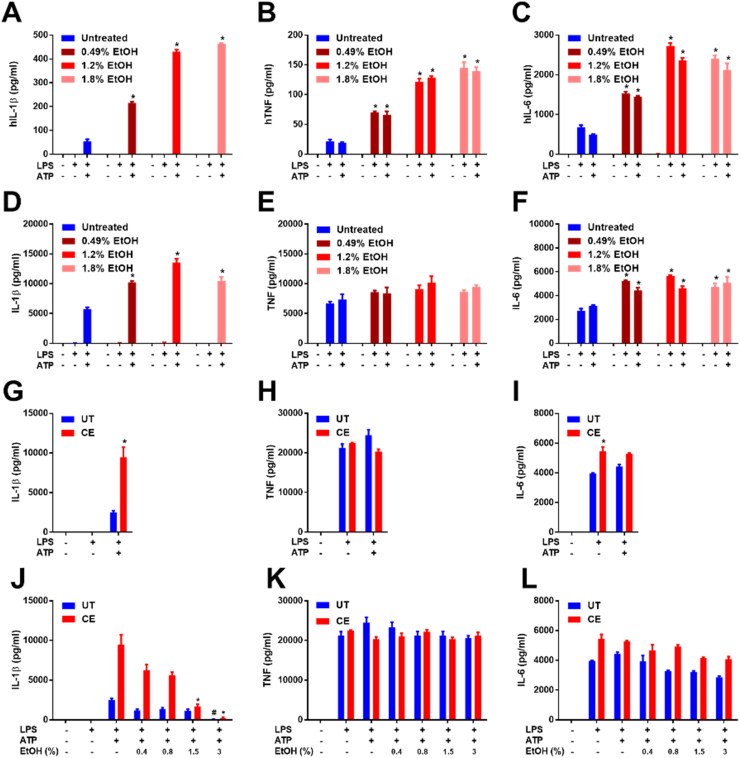
To test the effects of protracted ethanol exposure on NLRP3 inflammasome activation, human PBMCs were treated once per day with ethanol (387–1418 mg/dL) for 72 h and subsequently primed with LPS for 8 h and stimulated with ATP for 1 h in ethanol free media. PBMCs exposed to ethanol secreted higher levels of IL-1β, TNF, and IL-6 in a dose dependent manner relative to controls (Fig. 1A-C). Similarly, J774 cells, a mouse macrophage cell line, demonstrated increased IL-1β and IL-6, but not TNF secretion in response to prolonged ethanol exposure (Fig. 1D-F). Since LPS and ATP mediated IL-1β and TNF and IL-6 release were increased by long-term exposure to ethanol, this indicates that NF-κB and NLRP3 inflammasome activation are amplified during chronic ethanol exposure.
Fig. 1.
Protracted and chronic ethanol exposure augment NLRP3 inflammasome dependent IL-1β secretion from macrophages. IL-1β (A), TNF (B), and IL-6 (C) ELISAs from hPBMCs treated with ethanol (0.49, 1.2, or 1.8% =387, 947, or 1418 mg/dL, respectively) for 72 h at the indicated concentrations with media/ethanol changes every 24 h, removed from ethanol and treated for 4 h with LPS (10 ng/ml) and for 1 h with ATP (5 mM). IL-1β (D), TNF (E), and IL-6 (F) ELISAs from J774 cells treated with ethanol (0.49, 1.2, or 1.8% =387, 947, or 1418 mg/dL, respectively) for 72 h at the indicated concentrations with media/ethanol changes every 24 h, removed from ethanol and treated for 8 h with LPS (37 ng/ml) and for 1 h with ATP (5 mM). IL-1β (G), TNF (H), and IL-6 (I) ELISAs from UT and CE J774 cells removed from ethanol and treated for 8 h with LPS (37 ng/ml) and for 1 h with ATP (5 mM). IL-1β (J), TNF (K), and IL-6 (L) ELISAs from UT and CE J774 cells removed from ethanol and treated for 8 h with LPS (37 ng/ml) and for 1 h with ethanol (0.4–3% vol/vol =315–2360 mg/dL) and ATP (5 mM). *<0.0001 by a Tukey's post hoc test following a one-way ANOVA relative to the untreated LPS+ATP (A and D), untreated LPS (B, C, E, F), UT LPS+ATP (G), UT LPS (H and I) group. *<0.0001 by a Tukey's post hoc test following a one-way ANOVA comparing CE LPS+ATP+EtOH (0.4–3%) groups relative to the CE LPS+ATP group (J-L). #<0.0001 by a Tukey's post hoc test following a one-way ANOVA comparing UT LPS+ATP+EtOH (0.4–3%) groups relative to the UT LPS+ATP group (J-L). All experiments were performed in triplicate. EtOH = ethanol, UT = untreated, CE = chronic ethanol.
Chronic alcoholism is characterized by months to years of heavy drinking. Therefore, to create a model of chronic alcohol exposure in cell culture, J774 cells were grown continuously in 947 mg/dL ethanol (205.5 mM) for at least 14 days, with replenishment of media and ethanol every 48 h (these chronic ethanol treated cells are designated as CE cells) simultaneously with untreated cells (designated as UT cells). Over the course of the 48 h the average level of ethanol was measured to be 460 mg/dL (100 mM) in the culture supernatants with a nadir at 48 h of 354 mg/dL ethanol (77 mM) (data not shown). As the level of ethanol in the blood can reach 100 mM during periods of heavy drinking, this concentration was considered to be relevant for modeling the effects of severe human alcoholism [50], [51], [52]. However, it should be noted that as the legal blood alcohol concentration limit for driving in the United States is 80 mg/dL, the doses of ethanol used in these experiments are higher than casual drinkers, or many alcoholics will likely experience.
LPS and ATP treatment generated markedly increased IL-1β secretion from CE cells as compared to UT cells (Fig. 1G). However, in contrast to cells exposed to ethanol for only 72 h, CE cells did not secrete increased amounts of TNF or IL-6 (Fig. 1H and I). These results suggest that, in contrast to prolonged ethanol treatment, chronic ethanol exposure augments NLRP3 inflammasome activation independent of enhancing priming.
We have previously shown that acute exposure to ethanol inhibits NLRP3 activation through a reduction in ROS production and the direct stimulation of protein tyrosine phosphatases [5]. To determine whether chronic exposure to ethanol can confer resistance to NLRP3 inflammasome inhibition by acute ethanol, UT and CE cells were primed with LPS for 8 h and treated with ethanol (315–2360 mg/dL) and ATP simultaneously for an additional hour. Acute ethanol administration substantially inhibited IL-1β secretion from both UT and CE cells, demonstrating that CE cells maintain sensitivity to the effects of acute ethanol (Fig. 1J). Acute ethanol did not have a significant effect on TNF or IL-6 secretion (Fig. 1K and L), as has been previously reported [5].
3.2. IL-1β secretion from CE cells is not the result of enhanced priming
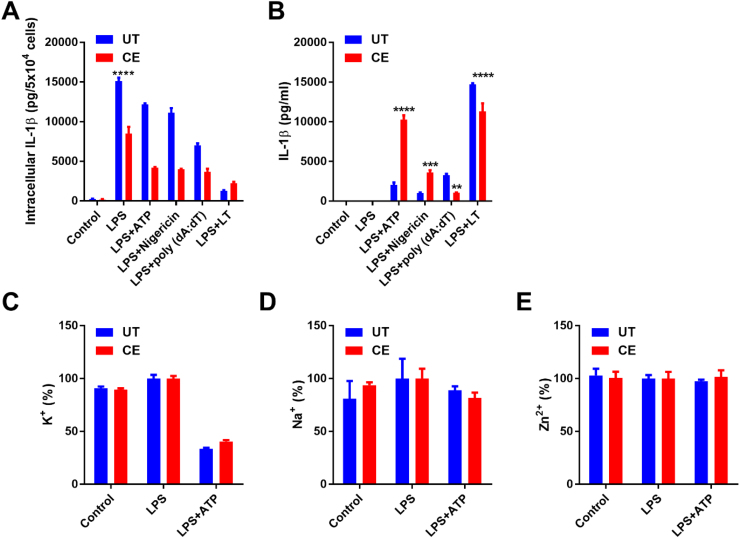
Having observed increased IL-1β secretion from CE cells, it was next determined whether this was a result of increased priming and production of inflammasome components, or more effective secretion of IL-1β subsequent to inflammasome activation. Therefore, to examine whether chronic ethanol exposure augments macrophage priming, UT and CE cells were treated with the TLR4 agonist LPS for 3 h to induce NF-κB activation, and mRNA was extracted for the analysis of NF-κB responsive genes. It was determined that CE cells produced significantly less Casp1, Nlrp3, Il1b, Il18, Il1a, Il6, and Tnf mRNA than UT cells in response to LPS stimulation (Table 1). To further rule out increased pro-IL-1β protein production as a cause for the exaggerated mature IL-1β secretion from CE cells, UT and CE cells were treated with LPS for 8 h, lysed, and the concentration of intracellular IL-1β protein was measured by ELISA. In keeping with there being blunted Il1b gene expression, there was also less intracellular IL-1β present after LPS priming in CE cells than in UT cells (Fig. 2A). In addition, when normalized to the levels of intracellular IL-1β within LPS primed cells, CE cells secreted significantly more of their intracellular pool of IL-1β than UT cells after LPS and ATP (UT: 20.6±1.8%, CE: 51.9±2.0% p<0.01), or LPS and nigericin stimulation (UT: 27.4±6.6%, CE: 53.0±1.0% p<0.05) (Fig. 2A). These results indicate that cells exposed to chronic ethanol have enhanced NLRP3 activation and IL-1β secretion, independent of priming.
Table 1.
Chronic ethanol exposure does not induce greater priming in J774 cells. RT Q-PCR from UT and CE J774 cells removed from ethanol and treated with and without LPS (37 ng/ml) for 4 h. ****<0.0001, ***<0.001, **<0.01, *<0.05 by a Tukey's post hoc test following a one-way ANOVA relative to the UT group. ####<0.0001, ###<0.001, ##<0.01, #<0.05 by a Tukey's post hoc test following a one-way ANOVA relative to the UT+LPS group. All experiments were performed in triplicate. UT = untreated, CE = chronic ethanol.
| Gene |
Relative Gene Expression |
|||
|---|---|---|---|---|
| UT | CE | UT+LPS | CE+LPS | |
| Asc | 1.00±0.19 | 0.95±0.54 | 0.43±0.03 | 0.39±0.09 |
| Casp1 | 1.07±0.22 | 0.74±0.49 | 3.21±0.21 *** | 1.09±0.21 ### |
| Il6 | 0.93±0.21 | 13.38±10.29 | 30288.36±1131.05 **** | 8242.33±1190.51 **** #### |
| Il18 | 1.08±0.14 | 0.51±0.23 | 3.63±0.14 **** | 0.72±0.15 #### |
| Il1a | 1.04±0.12 | 1.32±0.90 | 31315.28±4342.58 **** | 12070.08±1760.61 ** #### |
| Il1b | 1.08±0.32 | 0.70±0.29 | 9203.57±974.49 **** | 2691.73±375.94 ** #### |
| Nlrp3 | 1.13±0.24 | 0.96±0.18 | 13.46±1.08 **** | 6.92±1.37 *** #### |
| Tnf | 1.05±0.18 | 1.08±0.25 | 96.00±7.70 **** | 29.99±2.95 *** #### |
Fig. 2.
Chronic ethanol treatment results in more effective IL-1β secretion in response to NLRP3 inflammasome agonists, independent of alterations in ion flux. IL-1β ELISAs from the lysates (A) and supernatants (B) of UT and CE J774 cells treated with LPS (37 ng/ml) for 8 h and ATP (5 mM) or nigericin (5 μM) for 1 h and poly (dA: dT) (1 µg/ml) and LT (1 µg/ml PA+1 µg/ml LF) for 18 and 3 h respectively. ICP-OES measurement of intracellular K (C), Na (D), and Zn (E) from cells treated with LPS (37 ng/ml) for 8 h and ATP (5 mM) for 1 h. ****<0.0001, ***<0.001, **<0.01, *<0.05 by a Tukey's post hoc test following a one-way ANOVA relative to the UT groups. All experiments were performed in triplicate. UT = untreated, CE = chronic ethanol.
3.3. NLRP3, but not AIM2 or NLRP1b inflammasome activation is amplified by chronic ethanol exposure
There are several different types of inflammasome complexes that can facilitate IL-1β secretion, each with differing agonists and mechanisms of activation [53], [54]. To determine whether increased secretion of IL-1β due to chronic ethanol exposure was specific to the NLRP3 inflammasome, UT and CE cells were treated with ATP, nigericin, poly (dA: dT), or anthrax lethal toxin (LT), agonists of the NLRP3 inflammsome, AIM2, or NLRP1b inflammasome respectively. While both ATP and nigericin treated CE cells secreted significantly more IL-1β than UT cells, CE cells stimulated with poly (dA: dT) or LT secreted significantly less of the cytokine than their UT counterparts (Fig. 2A and B). This indicates that chronic ethanol exposure specifically sensitizes the NLRP3 inflammasome to hyper-activation, and is therefore acting on a pathway specific the stimulation of this inflammasome complex.
3.4. IL-1β hypersecretion is independent from alterations in ion homeostasis
Since activation of the NLRP3 inflammasome has been shown to be dependent on K+ efflux [55], intracellular K+ was measured by ICP-OES from UT and CE cells at baseline, after LPS priming, and subsequent to inflammasome activation with LPS and ATP. In contrast to expectations, there was no greater efflux of potassium from CE cells subsequent to NLRP3 inflammasome activation than from UT cells (Fig. 2C). Sodium efflux does not occur during NLRP3 inflammasome activation, and its intracellular levels did not change subsequent to inflammasome activation (Fig. 2D). It is therefore likely that chronic ethanol exposure acts on a part of the pathway downstream or independent of this necessary point of the NLRP3 inflammasome activation cascade.
Additionally, prolonged exposure to ethanol can deplete intracellular Zn2+, and treatment of macrophages with Zn2+ can inhibit inflammasome activation [56], [57]. To examine whether a reduction of Zn2+ within CE cells might be sensitizing them to inflammasome activation, intracellular levels of this ion were also measured by ICP-OES. However, there was no change or difference in the levels Zn2+ due to LPS and ATP treatment or between CE and UT cells (Fig. 2E).
3.5. Chronic ethanol exposure leads to the hypersecretion of NLRP3 inflammasome components
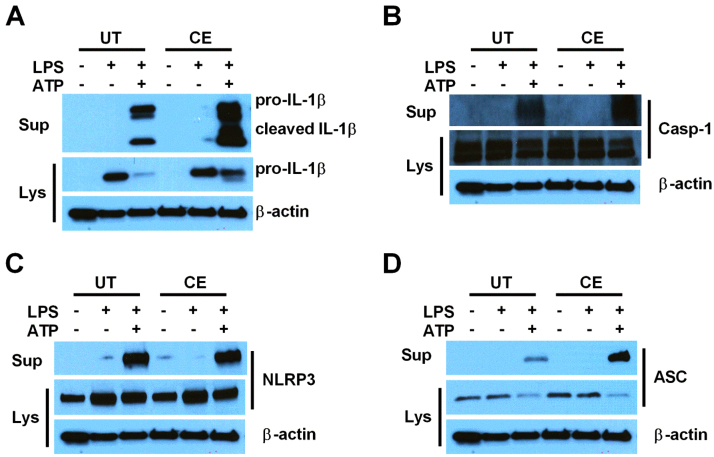
Complete inflammasome activation results in the secretion of not only IL-1β and IL-18, but also inflammasome components including caspase-1, ASC, and NLRP3 [34], [58]. To determine whether these proteins are also released at a greater magnitude from CE cells, Western blots were performed from cell lysates and supernatants of UT and CE cells at baseline, and after treatment with LPS or LPS and ATP (Fig. 3A-D). Consistent with more effective NLRP3 inflammasome activation, CE cells secreted more IL-1β, caspase-1, and ASC, although not NLRP3, than UT cells after stimulation with LPS and ATP. In addition to secreting more classically cleaved 17 kDa mature IL-1β, CE cells secreted more of the alternatively cleaved 20 kDa form of IL-1β (Fig. 3A).
Fig. 3.
Chronic ethanol treatment causes hyper-secretion of proteins associated with NLRP3 inflammasome activation upon stimulation. IL-1β (A), caspase-1 (B), NLRP3 (C), and ASC (D) western blots from the lysates and supernatants of UT and CE J774 cells treated with LPS (37 ng/ml) for 8 h and ATP (5 mM) for 1 h. All experiments were performed in triplicate. UT = untreated, CE = chronic ethanol, sup = supernatant, lys = lysate, casp-1 = caspase-1.
3.6. Prolonged treatment with acetaldehyde mimics ethanol-promoted IL-1β hypersecretion
Acetaldehyde is a toxic metabolite of ethanol that can damage mitochondria and promote mitochondrial ROS production [59], [60], [61]. To determine whether prolonged exposure to this metabolite could, like ethanol, promote the release of IL-1β upon NLRP3 inflammasome activation, J774 cells were treated for 72 h with media alone, 0.1 or 1 mM acetaldehyde, or 947 mg/dL ethanol, followed by stimulation with LPS or LPS and ATP in treatment free media. Similar to ethanol, prolonged acetaldehyde treatment leads to higher levels of IL-1β secretion after inflammasome activation (Fig. 4A). However, while ethanol prompted an increase in IL-6 production after LPS priming, acetaldehyde promoted TNF release (Fig. 4B and C).
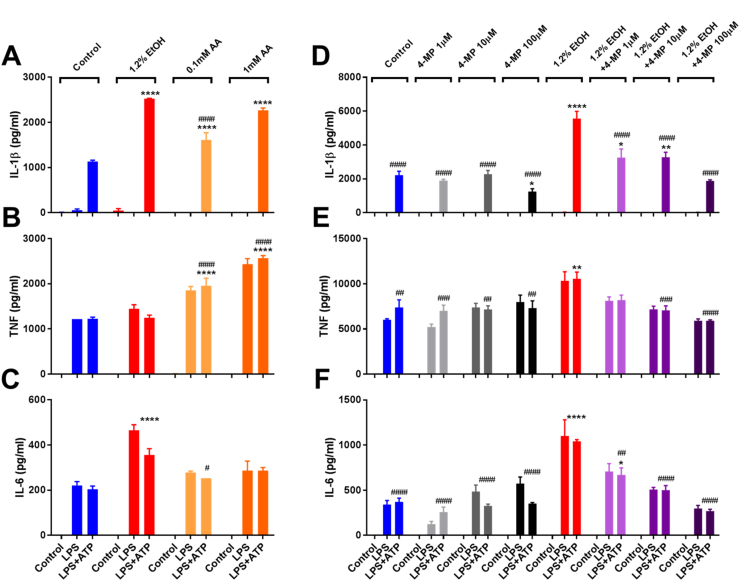
Fig. 4.
Acetaldehyde supplementation mimics and inhibition of alcohol dehydrogenase prevents protracted ethanol exposure-induced IL-1β hypersecretion. IL-1β (A), TNF (B), and IL-6 (C) ELISAs from J774 cells treated with nothing, ethanol (1.2% =947 mg/dL), or acetaldehyde (0.1 or 1 mM) for 72 h with media/treatment changes every 24 h, removed from treatments and primed for 8 h with LPS (37 ng/ml) and stimulated for 1 h with ATP (5 mM). IL-1β (D), TNF (E), and IL-6 (F) ELISAs from J774 cells treated with nothing, 4-MP (1–100 µM), ethanol (1.2%), or ethanol (1.2%) +4-MP (1–100 µM) for 72 h with media/treatment changes every 24 h, removed from treatments and primed for 8 h with LPS (37 ng/ml) and stimulated for 1 h with ATP (5 mM). ****<0.0001, ***<0.001, **<0.01, *<0.05 by a Tukey's post hoc test following a one-way ANOVA relative to the control LPS+ATP group (A-F). ####<0.0001, ###<0.001, ##<0.01, #<0.05 by a Tukey's post hoc test following a one-way ANOVA relative to the 947 mg/dL ethanol LPS+ATP group (A-F). All experiments were performed in triplicate. EtOH = ethanol, AA = acetaldehyde, 4-MP =4-methylpyrazole.
3.7. Inhibition of alcohol dehydrogenase enzymatic activity prevents prolonged ethanol-induced IL-1β hypersecretion
Since acetaldehyde was shown to mimic the effects of ethanol on inflammasome activation, macrophages were treated with 947 mg/dL ethanol for 72 h in the presence and absence of the alcohol dehydrogenase inhibitor, 4-MP. While 33% of the original 947 mg/dL ethanol dose was metabolized by J774 cells after 24 h, the highest dose of 4-MP used (100 µM) reduced this to 12%, demonstrating that this drug effectively inhibited the metabolism of ethanol to acetaldehyde (data not shown). After stimulation with LPS or LPS and ATP in treatment free media, J774 cells grown in the presence of ethanol and 4-MP (1–100 µM) displayed a dose dependent decrease in IL-1β, TNF and IL-6 secretion to levels comparable to control cells (Fig. 4D-F). These results indicate that either the process of ethanol metabolism to acetaldehyde, direct actions of acetaldehyde within the cell, or both, are necessary for chronic ethanol treatment to promote NLRP3 inflammasome hyperactivation.
3.8. Chronic ethanol-induced IL-1β hypersecretion is mediated by increased iNOS activity and nitric oxide production
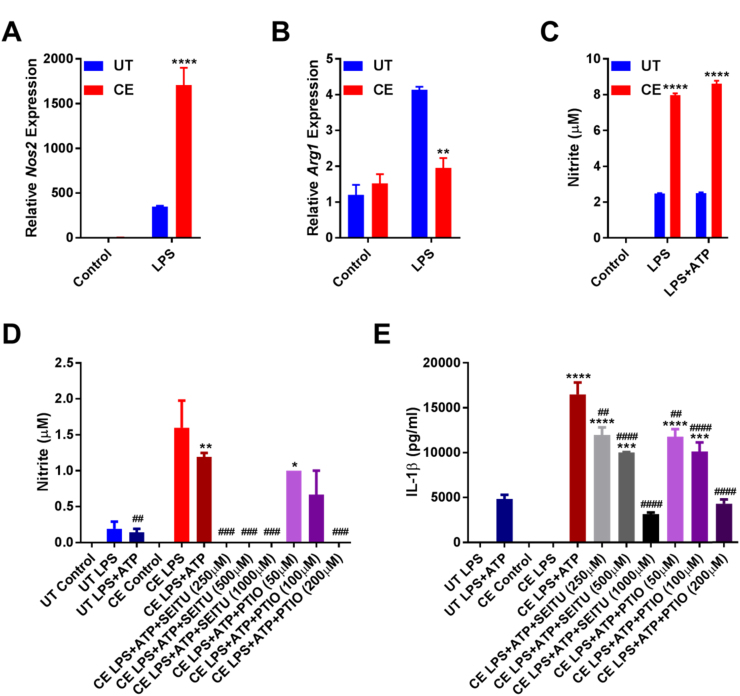
Long-term treatment with ethanol in vivo and in vitro promotes iNOS activation and aberrant NO production [24], [62]. To examine whether CE cells display a similar phenotype, Nos2 and Arg1 expression were measured by RT-qPCR using cDNA generated from mRNA extracted from the lysates of UT and CE cells with or without 4 h of LPS stimulation. While there was no difference in the expression of Nos2 or Arg1 from unstimulated macrophages, following LPS treatment the CE cells demonstrated significantly higher Nos2 and lower Arg1 expression than their counterparts (Fig. 5A and B). Furthermore, the concentration of NO was significantly higher in the supernatants of CE cells that were primed with LPS or treated with LPS and ATP (Fig. 5C).
Fig. 5.
Chronic ethanol-induced increases in NO production promote IL-1β hypersecretion. RT Q-PCR for Nos2 (A) and Arg1 (B) from UT and CE J774 cells removed from ethanol and treated with and without LPS (37 ng/ml) for 4 h. Griess assay (C) of supernatants from UT and CE J774 cells treated with LPS (37 ng/ml) for 8 h and ATP (5 mM) for 1 h. Griess assay (D) and IL-1β ELISA (E) of supernatants from UT and CE J774 cells treated with LPS (37 ng/ml), SEITU (250–1000 µM), and carboxy-PTIO (50–200 µM) for 8 h and ATP (5 mM) for 1 h. ****<0.0001, ***<0.001, **<0.01, *<0.05 by a Tukey's post hoc test following a one-way ANOVA relative to the UT groups (A and B), or the UT LPS+ATP group (C-D). ####<0.0001, ###<0.001, ##<0.01, #<0.05 by a Tukey's post hoc test following a one-way ANOVA relative to the CE LPS+ATP group (D and E). All experiments were performed in triplicate. UT = untreated, CE = chronic ethanol, SEITU = S-ethyl-isothiourea.
To determine the importance of amplified NO production on IL-1β hypersecretion, CE cells were treated with an iNOS inhibitor (SEITU) or a NO scavenger (carboxy-PTIO) during LPS priming. Both SEITU and carboxy-PTIO treatment dose-dependently decreased the concentrations of NO in cell culture media, but also IL-1β secretion from CE cells (Fig. 5D and E). Collectively, these results demonstrate that iNOS-induced NO production contributes to the augmented IL-1β secretion by cells chronically exposed to ethanol.
3.9. Chronic ethanol exposure increases lactate secretion and depletes intracellular NAD+ stores
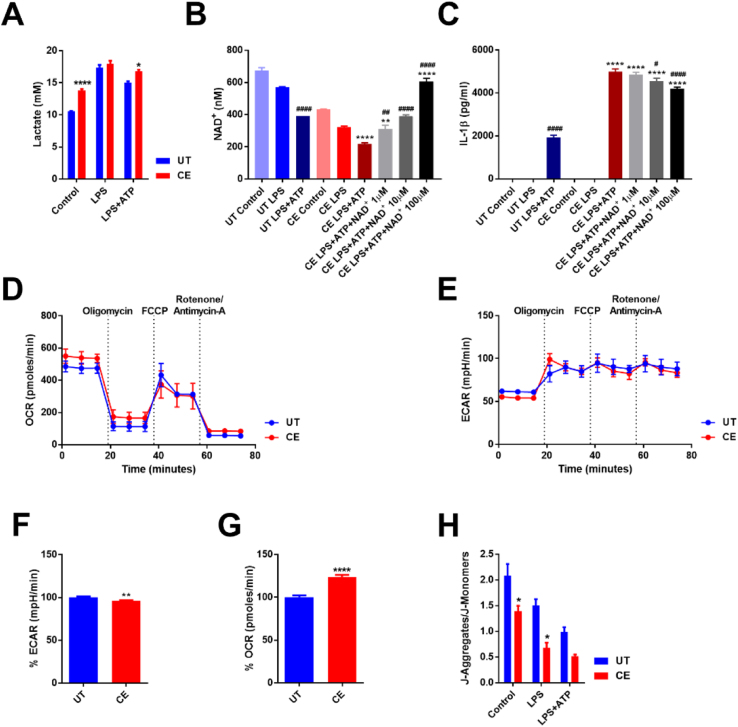
Alcoholism is associated with increases in circulating lactate levels, which is caused by a decrease in NAD+ during the metabolism of ethanol to acetaldehyde and acetate, and therefore an inability to convert lactate to pyruvate [63]. Moreover, lactate has also been shown to induce IL-1β secretion from the NLRP3 inflammasome [64]. Consequently, CE cells were found to have higher levels of lactate in their supernatants after 8 h of incubation, which was most pronounced in unstimulated cells (Fig. 6A).
Fig. 6.
Chronic ethanol treatment of macrophages induces mitochondrial dysfunction. Lactate assay of supernatants from UT and CE J774 cells treated with LPS (37 ng/ml) for 8 h and ATP (5 mM) for 1 h (A). NAD/NADH assay of intracellular NAD+ from J774 UT and CE cells primed with LPS (37 ng/ml) for 6 h, treated with NAD+(1–100 µM) and ATP (5 mM) for 1 h (B). IL-1β ELISA from the supernatants of J774 UT and CE cells primed with LPS (37 ng/ml) for 6 h, treated with NAD+(1–100 µM) and ATP (5 mM) for 1 h (C). Mitochondrial function of UT and CE cells was assessed by an extracellular flux analyzer assay (D and E). Average basal ECAR (F) and OCR (G) were measured from extracellular flux assays of UT and CE J774 cells relative to UT J774 cells. Ratio of J-aggregates to J-monomers from a JC-1 assay on UT and CE J774 cells treated with LPS (1 μg/ml) for 5 h and ATP (1 mM) for 30 min (H). ****<0.0001, ***<0.001, **<0.01, *<0.05 by a Tukey's post hoc test following a one-way ANOVA relative to the UT groups (A and H) and relative to the UT LPS+ATP group (B and C). ####<0.0001, ###<0.001, ##<0.01, #<0.05 by a Tukey's post hoc test following a one-way ANOVA relative to the CE LPS+ATP group (B and C). ****<0.0001, ***<0.001, **<0.01, *<0.05 by an unpaired t-test test relative to the UT group (F and G). All experiments were performed in triplicate. UT = untreated, CE = chronic ethanol.
Since a reduction in intracellular NAD+ is thought to play an important role in the final stages of NLRP3 inflammasome activation [65], the levels of this co-factor were measured in UT and CE macrophages at baseline, during LPS priming and after NLRP3 inflammasome activation with LPS and ATP. A decrease in intracellular NAD+ was observed during priming as well as following inflammasome activation in both UT and CE cells. Moreover, there was a substantial decrease in intracellular NAD+ within macrophages chronically treated with ethanol at baseline compared to UT cells (Fig. 6B). Exogenous administration of NAD+ to CE cells restored their intracellular levels of the co-factor to those of UT cells, but only minimally reduced IL-1β secretion when CE cells were subsequently stimulated to activate the NLRP3 inflammasome (Fig. 6B and C).
3.10. Chronic ethanol treatment leads to mitochondrial dysfunction
Having observed CE mediated alterations in lactate production and NAD+ levels, to further examine the consequences of ethanol exposure on the mitochondria and the role that these organelles might play in inflammasome activation, extracellular acidification rates (ECAR) and oxygen consumption rates (OCR) were measured on unstimulated CE and UT cells using an XF extracellular flux analyzer assay. CE cells did not display a significant difference in their response to a mitostress test compared to UT cells (Fig. 6D and E), but were shown to have a slightly lower ECAR and a significantly higher OCR compared to UT cells (Fig. 6F and G).
Mitochondrial membrane depolarization occurs during NLRP3 inflammasome activation as a consequence of K+ efflux and results in mitochondrial ROS production [29]. The degree of mitochondrial membrane polarization in both cell types was measured by staining with JC-1 in live cells at baseline, during priming, or after NLRP3 inflammasome activation. As has been previously shown, both LPS priming and particularly inflammasome activation increased mitochondrial membrane depolarization [29]. Importantly, the mitochondrial membranes of CE cells displayed a substantially lower mitochondrial membrane potential than UT cells, which further decreased following activation of the inflammasome (Fig. 6H). These results suggest that chronic ethanol exposure damages the mitochondria of CE cells and leads to alterations in mitochondrial functioning.
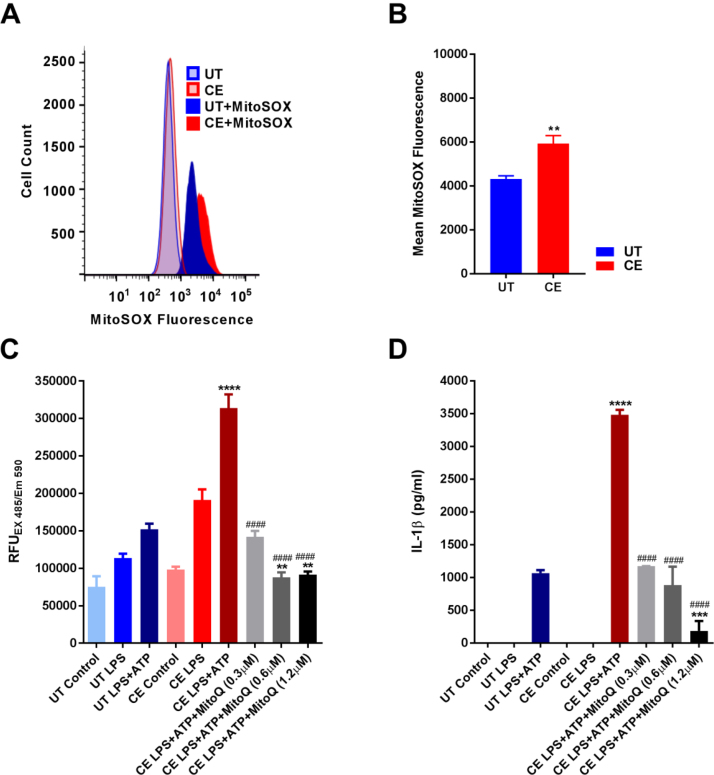
3.11. IL-1β hypersecretion promoted by chronic ethanol treatment is dependent upon mitochondrial ROS production
The production of mitochondrial ROS is thought to be one of the final steps in NLRP3 inflammasome activation due to its ability to generate oxidized mitochondrial DNA [29], [66], [67]. Since CE cells show signs of mitochondrial damage, mitochondrial ROS production was quantified in UT and CE cells at baseline by MitoSox fluorescence. From these results, CE cells were confirmed to produce more mitochondrial ROS than UT cells (Fig. 7A and B). To determine whether this increase in mitochondrial ROS production in CE cells was important for their amplified release of IL-1β, CE cells were pretreated with the mitochondrial ROS scavenger MitoQ after LPS priming and before ATP stimulation. A DCF-DA assay was then used to record the amount of ROS production in live cells during the course of ATP treatment and inflammasome activation. MitoQ was found to dose-dependently inhibit both ROS production and IL-1β secretion from CE cells. Furthermore, doses of MitoQ that reduced ROS production from CE cells to those of UT cells also restored IL-1β release to levels secreted by UT cells following stimulation with LPS and ATP (Fig. 7C and D).
Fig. 7.
MitoQ ameliorates chronic ethanol-induced ROS production and IL-1β hyper-secretion. MitoSox staining of J774 UT and CE cells measured by flow cytometry (A and B). DCF-DA assay AUC (C) from UT and CE J774 cells treated with LPS (1 μg/ml) for 3 h, MitoQ (0.3–1.2 μM) for 2 h, and ATP (1 mM) for 30 min. IL-1β ELISA from UT and CE J774 cells treated with LPS (1 μM) for 3 h, MitoQ (0.3–1.2 μM) for 2 h, and ATP (1 mM) for 30 min (D). ****<0.0001, ***<0.001, **<0.01, *<0.05 by an unpaired t-test test relative to the UT group (B). ****<0.0001, ***<0.001, **<0.01, *<0.05 by a Tukey's post hoc test following a one-way ANOVA relative to the UT LPS+ATP group (C and D). ####<0.0001, ###<0.001, ##<0.01, #<0.05 by a Tukey's post hoc test following a one-way ANOVA relative to the CE LPS+ATP group (C and D). All experiments were performed in triplicate. UT = untreated, CE = chronic ethanol, AUC = area under the curve.
4. Discussion
Chronic consumption of ethanol has long been associated with an increased severity and prevalence of inflammation associated ailments [1]. The NLRP3 inflammasome, through IL-1β and IL-18 production, is implicated in the pathogenesis of many of these diseases, including chronic pancreatitis, gout, and diabetes [45], [46], [47]. The results of this study show that prolonged and chronic exposure of hPBMCs and J774 cells to ethanol amplifies the activation of the NRLP3 inflammasome, which may be associated with AUD related diseases (Fig. 1, Fig. 2, Fig. 3). Long-term ethanol treatment does not promote IL-1β release from unstimulated monocytes and macrophages, implying that the alcohol does not act as a direct inflammasome agonist in these cell types. Additionally, in chronic ethanol (CE) exposed J774 cells, IL-1β hypersecretion occurs despite no increase in TNF or IL-6 secretion, and regardless of decreased production of inflammasome components following LPS priming (Fig. 1, Fig. 2, and Table 1). Instead, CE cells appear to more effectively cleave and secrete the pro-IL-1β that they contain. Furthermore, we show here that CE cells only hypersecrete IL-1β when stimulated with NLRP3, and not AIM2 or NLRP1b agonists, implying that ethanol likely acts on pathways specific to NLRP3 inflammasome activation.
Alcoholics frequently have an increased level of circulating lactate, which occurs because the metabolism of ethanol is accompanied by the conversion of NAD+ to NADH by ADH and ALDH respectively, depleting intracellular NAD+ stores and preventing the conversion of lactate to pyruvate [63]. In agreement, we observed that CE cells produce more lactate than UT cells at baseline as well as after LPS and ATP stimulation (Fig. 6A). This increase in lactate accumulation might be responsible for the enhanced secretion of alternatively cleaved 20 kDa IL-1β from CE cells (Fig. 3A), since lactate has been shown to promote the cleavage of this 20 kDa isoform through the activation of the protease cathepsin D [64].
All known mechanisms that lead to activation of the NLRP3 inflammasome converge and are dependent upon K+ efflux, which leads to mitochondrial membrane depolarization and ROS production [29], [55]. It is therefore conceivable that ethanol amplifies inflammasome activation by promoting the release of this ion. However, UT and CE cells release equivalent amounts of their intracellular K+ stores during inflammasome activation, making it unlikely that ethanol acts in this manner (Fig. 2C). Zn2+ depletion activates, and its supplementation inhibits, NLRP3 inflammasome activation [56]. Moreover, ethanol can diminish intracellular Zn2+ stores by altering the production of Zn2+ transporting channels [57]. However, since UT and CE cells display equivalent concentrations of intracellular Zn2+, we conclude that Zn2+ is not responsible for the hypersecretion of IL-1β during chronic ethanol exposure (Fig. 2E).
Protracted treatment with ethanol increases the expression of iNOS and promotes NO production in vitro and in vivo [24], [25]. Similarly, CE cells stimulated with LPS display higher Nos2 and lower Arg1 expression, and produce significantly more NO than UT cells (Fig. 5A-C). Treatment with an iNOS inhibitor (SEITU) or an NO scavenger (carboxy-PTIO) both dose dependently inhibit IL-1β release from CE cells to the levels of control cells, underlining the importance of amplified NO production on their ability to promote IL-1β secretion (Fig. 5E). Yet, NO has been previously shown to inhibit NLRP3 inflammasome activation through the S-nitrosylation of NLRP3 and caspase-1 [68], [69]. It is possible that the effects of NO on inflammasome activation could display an inverse U-shaped curve, since the concentrations of NO found to be inhibitory were induced by high concentrations of the NO donor (SNAP), which likely resulted in NO levels far higher than those produced by our CE cells. NO produced during ethanol exposure can combine with ROS to form peroxynitrite, which can directly damage mitochondria [70]. Mitochondrial damage and the resultant production of mitochondrial ROS are critical for NLRP3 inflammasome activation via the generation of oxidized mitochondrial DNA, which is thought to bind and activate NLRP3 [29]. It is thus possible that chronic ethanol treatment promotes the activation of this PRR by causing NO and peroxynitrite-induced damage to mitochondria.
Ethanol consumption perturbs mitochondrial functioning by driving electron transport chain activity through the over-production of NADH [19]. In agreement with this, our CE cells display a much higher OCR than UT cells (Fig. 6G). A byproduct of electron transport chain activity is the generation of ROS [19]. Therefore, the ethanol-induced increase in oxygen consumption could help drive mitochondrial ROS production and NLRP3 inflammasome activation (Fig. 7A and B). The loss of mitochondrial membrane potential is another critical step in NLRP3 inflammasome activation, which occurs in response to K+ efflux and leads to mitochondrial ROS production [29]. Importantly, we show here that CE cells at baseline have a lower mitochondrial membrane potential than UT cells (Fig. 6H). This is likely a consequence of chronic ethanol-induced damage to the mitochondria, and could allow the cells to be more readily activated when exposed to NLRP3 inflammasome agonists.
NAD+ depletion occurs in the final stages of NLRP3 inflammasome activation subsequent to depolarization of the mitochondrial membrane [65]. While CE cells display marked reductions in intracellular NAD+, supplementation with exogenous NAD+ fails to return IL-1β secretion to control levels despite effectively restoring intracellular NAD+ stores. Because of this, we speculate that the depletion of this co-factor plays only a minimal role in ethanol induced IL-1β hypersecretion (Fig. 6B and C). During classical NLRP3 inflammasome stimulation, the decrease in NAD+ results from a decline in mitochondrial activity following mitochondrial membrane depolarization [65]. This inactivates the protein deacetylase sirtuin 2 (SIRT2) by depleting its co-factor, and enables NLRP3 and ASC to be transported via microtubules to the perinuclear region, where they can then form a complete inflammasome complex [65]. Since the intracellular NAD+ reduction seen in CE cells occurs chronically rather than acutely, it is possible that CE cells are able to adapt over time to the lack of this co-factor.
Aberrant mitochondrial ROS production is critical to NLRP3 inflammasome activation via the oxidation of mitochondrial DNA [29]. Treatment with the mitochondrial ROS scavenger, MitoQ, has been shown to inhibit ethanol-induced hepatosteatosis through its ability to scavenge mitochondrial ROS and RNS, and has been proposed as a potential treatment for non-alcoholic and alcoholic fatty liver disease [71]. In agreement, MitoQ dose dependently inhibits both the elevated ROS and IL-1β release by CE cells during inflammasome activation (Fig. 7C and D). Based on this result, we speculate that mitochondrial damage and ROS production is a major mechanism through which chronic ethanol treatment is able to promote NLRP3 stimulation.
Long-term exposure to ethanol also results in increased ethanol catabolism and the accumulation of direct metabolites within cells. Many of the effects that we have observed in our CE cells could also be attributed to ethanol's primary metabolite, acetaldehyde [59], [60], [61]. Here we show that prolonged treatment with acetaldehyde recapitulates the IL-1β hypersecretion induced by prolonged ethanol exposure (Fig. 4A). Moreover, co-treatment of cells with ethanol and the ADH inhibitor, 4-MP, over 72 h dose dependently inhibits the amplified IL-1β secretion caused by prolonged ethanol exposure (Fig. 4D). This indicates that the metabolite of ethanol, acetaldehyde, rather than ethanol itself leads to hyper-activation of the NLRP3 inflammasome.
There is a dichotomy between the effects of acute and chronic ethanol exposure on the immune system. Acute, or binge, ethanol consumption leads to transient immunosuppression, whereas long-term ethanol exposure promotes the development of inflammation [1], [4], [6]. We have shown previously that acute exposure of leukocytes to ethanol inhibits inflammasome activation through the direct activation of protein tyrosine phosphatases [5]. In contrast, we show here that chronic ethanol exposure up-regulates the secretion of IL-1β from activated monocytes and macrophages. Since the inhibition of ADH prevented chronic ethanol exposure from inducing IL-1β hypersecretion, despite there being increased amounts of ethanol present in the cell culture, we conclude that ethanol itself does not promote inflammation, but rather its metabolite, acetaldehyde, is responsible for augmented NLRP3 inflammasome activatability and subsequent IL-1β secretion. It is possible that acute ethanol exposure does not allow enough time for significant amounts of acetaldehyde to accumulate, allowing the immunosuppressive properties of ethanol to dominate. However, during chronic ethanol exposure, a substantial quantity of ethanol is converted into acetaldehyde, shifting the equilibrium away from immunosuppression and towards inflammation. We report here that acute ethanol administration can override the effects of chronic ethanol and inhibit NLRP3 inflammasome activation (Fig. 1), helping to explain why alcoholics who have a background of chronic inflammatory symptoms still experience transient immunosuppression after binge drinking.
In conclusion, long-term exposure to ethanol amplifies the release of IL-1β upon NLRP3, but not AIM2 or NLRP1b, inflammasome activation. The effects of ethanol on this inflammasome are mediated by increased iNOS expression and NO production in conjunction with mitochondrial dysfunction and elevated ROS generation. Prolonged acetaldehyde administration mimics, while treatment with an ADH inhibitor prevents ethanol-induced IL-1β hypersecretion, indicating that the metabolites of ethanol are the probable mediators of over-activation of this inflammasome. By identifying iNOS activity, NO and mitochondrial ROS production, and ADH activity as being critical for chronic ethanol-induced NLRP3 inflammasome hyperactivation, we provide new mechanistic insights into the development of alcoholism-associated inflammation and identify potential treatment targets for its associated diseases.
Acknowledgements
We thank Dr. Michael P. Murphy (Medical Research Council Mitochondrial Biology Unit, University of Cambridge, Cambridge, UK) for kindly providing MitoQ.
Footnotes
This work was funded by National Institute of Health grants R01 HL107291, P30 GM103532, P20 GM103496, R01 HL133920, a UVM/State of Vermont Pre-Seed Fund grant (Grant no. 071270-17-02), and grants from the UVM Office of Undergraduate Research.
References
- 1.Gonzalez-Reimers E., Santolaria-Fernandez F., Martin-Gonzalez M.C., Fernandez-Rodriguez C.M., Quintero-Platt G. Alcoholism: a systemic proinflammatory condition. World J. Gastroenterol. 2014;20:14660–14671. doi: 10.3748/wjg.v20.i40.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beresford T.P., Wongngamnit N., Temple B.A. Alcoholism: diagnosis, prognosis, epidemiology, and burden of the disease. Handb. Clin. Neurol. 2014;125:3–13. doi: 10.1016/B978-0-444-62619-6.00001-X. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad A.H., Marks J.S., Stroup D.F., Gerberding J.L. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 4.Dai Q., Pruett S.B. Different effects of acute and chronic ethanol on LPS-induced cytokine production and TLR4 receptor behavior in mouse peritoneal macrophages. J. Immunotoxicol. 2006;3:217–225. doi: 10.1080/15476910601080156. [DOI] [PubMed] [Google Scholar]
- 5.Hoyt L.R., Ather J.L., Randall M.J., DePuccio D.P., Landry C.C., Wewers M.D., Gavrilin M.A., Poynter M.E. Ethanol and other short-chain alcohols inhibit NLRP3 inflammasome activation through protein tyrosine phosphatase stimulation. J. Immunol. 2016;197:1322–1334. doi: 10.4049/jimmunol.1600406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manzardo A.M., Poje A.B., Penick E.C., Butler M.G. Multiplex immunoassay of plasma cytokine levels in men with alcoholism and the relationship to psychiatric assessments. Int J. Mol. Sci. 2016;17:472. doi: 10.3390/ijms17040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandrekar P., Bala S., Catalano D., Kodys K., Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J. Immunol. 2009;183:1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkowitz D.M., Danai P.A., Eaton S., Moss M., Martin G.S. Alcohol abuse enhances pulmonary edema in acute respiratory distress syndrome. Alcohol Clin. Exp. Res. 2009;33:1690–1696. doi: 10.1111/j.1530-0277.2009.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.al-Jarallah K.F., Shehab D.K., Buchanan W.W. Rheumatic complications of alcohol abuse. Semin Arthritis Rheum. 1992;22:162–171. doi: 10.1016/0049-0172(92)90016-7. [DOI] [PubMed] [Google Scholar]
- 10.Rehm J., Taylor B., Mohapatra S., Irving H., Baliunas D., Patra J., Roerecke M. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 2010;29:437–445. doi: 10.1111/j.1465-3362.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim S.J., Kim D.J. Alcoholism and diabetes mellitus. Diabetes Metab. J. 2012;36:108–115. doi: 10.4093/dmj.2012.36.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung K.I., Ju A., Lee H.M., Lee S.S., Song C.H., Won W.Y., Jeong J.S., Hong O.K., Kim J.H., Kim D.J. Chronic ethanol ingestion, type 2 diabetes mellitus, and brain-derived neurotrophic factor (BDNF) in rats. Neurosci. Lett. 2011;487:149–152. doi: 10.1016/j.neulet.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Tarnowska-Dziduszko E., Bertrand E., Szpak G.M. Morphological changes in the corpus callosum in chronic alcoholism. Folia Neuropathol. 1995;33:25–29. [PubMed] [Google Scholar]
- 14.Samokhvalov A.V., Rehm J., Roerecke M. Alcohol consumption as a risk factor for acute and chronic pancreatitis: a systematic review and a series of meta-analyses. EBioMedicine. 2015;2:1996–2002. doi: 10.1016/j.ebiom.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linhart K., Bartsch H., Seitz H.K. The role of reactive oxygen species (ROS) and cytochrome P-450 2E1 in the generation of carcinogenic etheno-DNA adducts. Redox Biol. 2014;3:56–62. doi: 10.1016/j.redox.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceni E., Mello T., Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J. Gastroenterol. 2014;20:17756–17772. doi: 10.3748/wjg.v20.i47.17756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seitz H.K., Mueller S. Alcohol and cancer: an overview with special emphasis on the role of acetaldehyde and cytochrome P450 2E1. Adv. Exp. Med Biol. 2015;815:59–70. doi: 10.1007/978-3-319-09614-8_4. [DOI] [PubMed] [Google Scholar]
- 18.Rao P.S., Kumar S. Chronic effects of ethanol and/or darunavir/ritonavir on U937 monocytic cells: regulation of cytochrome P450 and antioxidant enzymes, oxidative stress, and cytotoxicity. Alcohol Clin. Exp. Res. 2016;40:73–82. doi: 10.1111/acer.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey S.M., Cunningham C.C. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28:1318–1326. doi: 10.1002/hep.510280521. [DOI] [PubMed] [Google Scholar]
- 20.Zhong Y., Dong G., Luo H., Cao J., Wang C., Wu J., Feng Y.Q., Yue J. Induction of brain CYP2E1 by chronic ethanol treatment and related oxidative stress in hippocampus, cerebellum, and brainstem. Toxicology. 2012;302:275–284. doi: 10.1016/j.tox.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Balbo S., Brooks P.J. Implications of acetaldehyde-derived DNA adducts for understanding alcohol-related carcinogenesis. Adv. Exp. Med. Biol. 2015;815:71–88. doi: 10.1007/978-3-319-09614-8_5. [DOI] [PubMed] [Google Scholar]
- 22.Setshedi M., Wands J.R., Monte S.M. Acetaldehyde adducts in alcoholic liver disease. Oxid. Med. Cell Longev. 2010;3:178–185. doi: 10.4161/oxim.3.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan T., Zhao Y., Zhang X. Acetaldehyde induces cytotoxicity of SH-SY5Y cells via inhibition of Akt activation and induction of oxidative stress. Oxid. Med. Cell Longev. 2016;2016:4512309. doi: 10.1155/2016/4512309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C., Wang S., Qin J., Lv Y., Ma X., Liu C. Ethanol upregulates iNOS expression in colon through activation of nuclear factor-kappa B in rats. Alcohol Clin. Exp. Res. 2010;34:57–63. doi: 10.1111/j.1530-0277.2009.01066.x. [DOI] [PubMed] [Google Scholar]
- 25.Venkatraman A., Shiva S., Wigley A., Ulasova E., Chhieng D., Bailey S.M., Darley-Usmar V.M. The role of iNOS in alcohol-dependent hepatotoxicity and mitochondrial dysfunction in mice. Hepatology. 2004;40:565–573. doi: 10.1002/hep.20326. [DOI] [PubMed] [Google Scholar]
- 26.Alli A.A., Brewer E.M., Montgomery D.S., Ghant M.S., Eaton D.C., Brown L.A., Helms M.N. Chronic ethanol exposure alters the lung proteome and leads to mitochondrial dysfunction in alveolar type 2 cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;306:L1026–L1035. doi: 10.1152/ajplung.00287.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji C. New insights into the pathogenesis of alcohol-induced ER stress and liver diseases. Int. J. Hepatol. 2014;2014:513787. doi: 10.1155/2014/513787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebeaupin C., Proics E., de Bieville C.H., Rousseau D., Bonnafous S., Patouraux S., Adam G., Lavallard V.J., Rovere C., Le Thuc O., Saint-Paul M.C., Anty R., Schneck A.S., Iannelli A., Gugenheim J., Tran A., Gual P., Bailly-Maitre B. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015;6:e1879. doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimada K., Crother T.R., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V.K., Wolf A.J., Vergnes L., Ojcius D.M., Rentsendorj A., Vargas M., Guerrero C., Wang Y., Fitzgerald K.A., Underhill D.M., Town T., Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He W.T., Wan H., Hu L., Chen P., Wang X., Huang Z., Yang Z.H., Zhong C.Q., Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J.S., Kim K.D., Na H.S., Jeong S.Y., Park H.R., Kim S., Chung J. Tumor necrosis factor-alpha and interleukin-1beta expression pathway induced by Streptococcus mutans in macrophage cell line RAW 264.7. Mol. Oral Microbiol. 2012;27:149–159. doi: 10.1111/j.2041-1014.2012.00639.x. [DOI] [PubMed] [Google Scholar]
- 32.Narayanan K.B., Park H.H. Purification and analysis of the interactions of caspase-1 and ASC for assembly of the inflammasome. Appl. Biochem. Biotechnol. 2015;175:2883–2894. doi: 10.1007/s12010-014-1471-4. [DOI] [PubMed] [Google Scholar]
- 33.LaRock C.N., Cookson B.T. The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe. 2012;12:799–805. doi: 10.1016/j.chom.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franklin B.S., Bossaller L., De Nardo D., Ratter J.M., Stutz A., Engels G., Brenker C., Nordhoff M., Mirandola S.R., Al-Amoudi A., Mangan M.S., Zimmer S., Monks B.G., Fricke M., Schmidt R.E., Espevik T., Jones B., Jarnicki A.G., Hansbro P.M., Busto P., Marshak-Rothstein A., Hornemann S., Aguzzi A., Kastenmuller W., Latz E. The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation. Nat. Immunol. 2014;15:727–737. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao P.C., Chao L.K., Chou J.C., Dong W.C., Lin C.N., Lin C.Y., Chen A., Ka S.M., Ho C.L., Hua K.F. Lipopolysaccharide/adenosine triphosphate-mediated signal transduction in the regulation of NLRP3 protein expression and caspase-1-mediated interleukin-1beta secretion. Inflamm. Res. 2013;62:89–96. doi: 10.1007/s00011-012-0555-2. [DOI] [PubMed] [Google Scholar]
- 36.Perregaux D., Barberia J., Lanzetti A.J., Geoghegan K.F., Carty T.J., Gabel C.A. IL-1 beta maturation: evidence that mature cytokine formation can be induced specifically by nigericin. J. Immunol. 1992;149:1294–1303. [PubMed] [Google Scholar]
- 37.McKee A.S., Munks M.W., MacLeod M.K., Fleenor C.J., Van Rooijen N., Kappler J.W., Marrack P. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hara H., Tsuchiya K., Kawamura I., Fang R., Hernandez-Cuellar E., Shen Y., Mizuguchi J., Schweighoffer E., Tybulewicz V., Mitsuyama M. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat. Immunol. 2013;14:1247–1255. doi: 10.1038/ni.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin Y.C., Huang D.Y., Wang J.S., Lin Y.L., Hsieh S.L., Huang K.C., Lin W.W. Syk is involved in NLRP3 inflammasome-mediated caspase-1 activation through adaptor ASC phosphorylation and enhanced oligomerization. J. Leukoc. Biol. 2015 doi: 10.1189/jlb.3HI0814-371RR. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 40.Moore S.F., MacKenzie A.B. NADPH oxidase NOX2 mediates rapid cellular oxidation following ATP stimulation of endotoxin-primed macrophages. J. Immunol. 2009;183:3302–3308. doi: 10.4049/jimmunol.0900394. [DOI] [PubMed] [Google Scholar]
- 41.Sun B., Wang X., Ji Z., Wang M., Liao Y.P., Chang C.H., Li R., Zhang H., Nel A.E., Xia T. NADPH oxidase-dependent NLRP3 inflammasome activation and its important role in lung fibrosis by multiwalled carbon nanotubes. Small. 2015;11:2087–2097. doi: 10.1002/smll.201402859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 43.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 44.Guarda G., Zenger M., Yazdi A.S., Schroder K., Ferrero I., Menu P., Tardivel A., Mattmann C., Tschopp J. Differential expression of NLRP3 among hematopoietic cells. J. Immunol. 2011;186:2529–2534. doi: 10.4049/jimmunol.1002720. [DOI] [PubMed] [Google Scholar]
- 45.Amaral F.A., Costa V.V., Tavares L.D., Sachs D., Coelho F.M., Fagundes C.T., Soriani F.M., Silveira T.N., Cunha L.D., Zamboni D.S., Quesniaux V., Peres R.S., Cunha T.M., Cunha F.Q., Ryffel B., Souza D.G., Teixeira M.M. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum. 2012;64:474–484. doi: 10.1002/art.33355. [DOI] [PubMed] [Google Scholar]
- 46.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M.T., Brickey W.J., Ting J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanak M.A., Shahbazov R., Yoshimatsu G., Levy M.F., Lawrence M.C., Naziruddin B. A small molecule inhibitor of NFkappaB blocks ER stress and the NLRP3 inflammasome and prevents progression of pancreatitis. J. Gastroenterol. 2016 doi: 10.1007/s00535-016-1238-5. [DOI] [PubMed] [Google Scholar]
- 48.Amiel E., Everts B., Fritz D., Beauchamp S., Ge B., Pearce E.L., Pearce E.J. Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function. J. Immunol. 2014;193:2821–2830. doi: 10.4049/jimmunol.1302498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin R.A., Ather J.L., Daggett R., Hoyt L., Alcorn J.F., Suratt B.T., Weiss D.J., Lundblad L.K., Poynter M.E. The endogenous Th17 response in NO2-promoted allergic airway disease is dispensable for airway hyperresponsiveness and distinct from Th17 adoptive transfer. PLoS One. 2013;8:e74730. doi: 10.1371/journal.pone.0074730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nurmi K., Virkanen J., Rajamaki K., Niemi K., Kovanen P.T., Eklund K.K. Ethanol inhibits activation of NLRP3 and AIM2 inflammasomes in human macrophages--a novel anti-inflammatory action of alcohol. PLoS One. 2013;8:e78537. doi: 10.1371/journal.pone.0078537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell M.C., Jr., Teigen E.L., Ramchandani V.A. Absorption and peak blood alcohol concentration after drinking beer, wine, or spirits. Alcohol Clin. Exp. Res. 2014;38:1200–1204. doi: 10.1111/acer.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rae C.D., Davidson J.E., Maher A.D., Rowlands B.D., Kashem M.A., Nasrallah F.A., Rallapalli S.K., Cook J.M., Balcar V.J. Ethanol, not detectably metabolized in brain, significantly reduces brain metabolism, probably via action at specific GABA(A) receptors and has measureable metabolic effects at very low concentrations. J. Neurochem. 2014;129:304–314. doi: 10.1111/jnc.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Opdenbosch N., Gurung P., Vande Walle L., Fossoul A., Kanneganti T.D., Lamkanfi M. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat. Commun. 2014;5:3209. doi: 10.1038/ncomms4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanamsagar R., Aldrich A., Kielian T. Critical role for the AIM2 inflammasome during acute CNS bacterial infection. J. Neurochem. 2014;129:704–711. doi: 10.1111/jnc.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munoz-Planillo R., Kuffa P., Martinez-Colon G., Smith B.L., Rajendiran T.M., Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Summersgill H., England H., Lopez-Castejon G., Lawrence C.B., Luheshi N.M., Pahle J., Mendes P., Brough D. Zinc depletion regulates the processing and secretion of IL-1beta. Cell Death Dis. 2014;5:e1040. doi: 10.1038/cddis.2013.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joshi P.C., Mehta A., Jabber W.S., Fan X., Guidot D.M. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am. J. Respir. Cell Mol. Biol. 2009;41:207–216. doi: 10.1165/rcmb.2008-0209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baroja-Mazo A., Martin-Sanchez F., Gomez A.I., Martinez C.M., Amores-Iniesta J., Compan V., Barbera-Cremades M., Yague J., Ruiz-Ortiz E., Anton J., Bujan S., Couillin I., Brough D., Arostegui J.I., Pelegrin P. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 2014;15:738–748. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- 59.Tamura M., Ito H., Matsui H., Hyodo I. Acetaldehyde is an oxidative stressor for gastric epithelial cells. J. Clin. Biochem. Nutr. 2014;55:26–31. doi: 10.3164/jcbn.14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farfan Labonne B.E., Gutierrez M., Gomez-Quiroz L.E., Konigsberg Fainstein M., Bucio L., Souza V., Flores O., Ortiz V., Hernandez E., Kershenobich D., Gutierrez-Ruiz M.C. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol. Toxicol. 2009;25:599–609. doi: 10.1007/s10565-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 61.Liang Y., Harris F.L., Brown L.A. Alcohol induced mitochondrial oxidative stress and alveolar macrophage dysfunction. Biomed. Res. Int. 2014;2014:371593. doi: 10.1155/2014/371593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finnerty N., O'Riordan S.L., Klamer D., Lowry J., Palsson E. Increased brain nitric oxide levels following ethanol administration. Nitric Oxide. 2015;47:52–57. doi: 10.1016/j.niox.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Mezey E. Metabolic effects of alcohol. Fed. Proc. 1985;44:134–138. [PubMed] [Google Scholar]
- 64.Edye M.E., Lopez-Castejon G., Allan S.M., Brough D. Acidosis drives damage-associated molecular pattern (DAMP)-induced interleukin-1 secretion via a caspase-1-independent pathway. J. Biol. Chem. 2013;288:30485–30494. doi: 10.1074/jbc.M113.478941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Misawa T., Takahama M., Kozaki T., Lee H., Zou J., Saitoh T., Akira S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 66.Kim S.R., Kim D.I., Kim S.H., Lee H., Lee K.S., Cho S.H., Lee Y.C. NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death Dis. 2014;5:e1498. doi: 10.1038/cddis.2014.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu J.W., Lee M.S. Mitochondria and the NLRP3 inflammasome: physiological and pathological relevance. Arch. Pharm. Res. 2016;39:1503–1518. doi: 10.1007/s12272-016-0827-4. [DOI] [PubMed] [Google Scholar]
- 68.Hernandez-Cuellar E., Tsuchiya K., Hara H., Fang R., Sakai S., Kawamura I., Akira S., Mitsuyama M. Cutting edge: nitric oxide inhibits the NLRP3 inflammasome. J. Immunol. 2012;189:5113–5117. doi: 10.4049/jimmunol.1202479. [DOI] [PubMed] [Google Scholar]
- 69.Mishra B.B., Rathinam V.A., Martens G.W., Martinot A.J., Kornfeld H., Fitzgerald K.A., Sassetti C.M. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat. Immunol. 2013;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang E.S., Lee J.H., Park J.W. Ethanol induces peroxynitrite-mediated toxicity through inactivation of NADP+-dependent isocitrate dehydrogenase and superoxide dismutase. Biochimie. 2008;90:1316–1324. doi: 10.1016/j.biochi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Chacko B.K., Srivastava A., Johnson M.S., Benavides G.A., Chang M.J., Ye Y., Jhala N., Murphy M.P., Kalyanaraman B., Darley-Usmar V.M. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54:153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]