Abstract
Background:
Liver cancer makes up a huge percentage of cancer mortality worldwide. Irreversible electroporation (IRE) is a relatively new minimally invasive nonthermal ablation technique for tumors that applies short pulses of high frequency electrical energy to irreversibly destabilize cell membrane to induce tumor cell apoptosis.
Methods:
This review aims to investigate the studies regarding the use of IRE treatment in liver tumors and metastases to liver. We searched PubMed for all of IRE relevant English language articles published up to September 2016. They included clinical trials, experimental studies, observational studies, and reviews. This review manuscript is nothing with ethics issues and ethical approval is not provided.
Results:
In recent years, increasingly more studies in both preclinical and clinical settings have been conducted to examine the safety and efficacy of this new technique, shedding light on the crucial advantages and disadvantages that IRE possesses. Unlike the current leading thermal ablation techniques, such as radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation, IRE requires shorter ablation time without damaging adjacent important vital structures.
Conclusion:
Although IRE has successfully claimed its valuable status in the field of hepatic cancer treatment both preclinical and clinical settings. In order to systemically test and establish its safety and efficacy for clinical applications, more studies still need to be conducted.
Keywords: hepatocellular carcinoma, irreversible electroporation, liver metastases, primary liver tumor, tumor ablation
1. Introduction
Liver cancer can be divided into 2 categories based on the primary tumor site: primary liver cancer and metastatic cancer to liver (liver metastases). Primary liver cancer is the 3rd leading cause of cancer mortality worldwide[1] and the 7th leading cause of cancer-related death in the United States.[2] Among the types of primary liver cancers, hepatocellular carcinoma (HCC) is the predominant histologic form[3] and has an incidence similar to the US liver cancer mortality rate, indicating a poor prognosis for HCC patients.[4] According to the data from Surveillance, Epidemiology, and End Results Program of National Cancer Institute, both the HCC incidence rate and the US liver cancer mortality rate increased over recent years, from 3.1 to 5.1 per 100,000 persons and from 3.3 to 4.0 per 100,000 persons, respectively.[5] Furthermore, survival rate in patients with advanced HCC is not significantly improved by systemic chemotherapy.[6,7] Only 20% to 30% of patients are eligible candidates for surgical tumor resection when they are diagnosed. Safer and more efficient procedures are desperately needed for liver tumors and metastases treatment.[8] This review compares irreversible electroporation (IRE) with other traditional ablation techniques and includes research studies in both preclinical and clinical settings published in recent years focused on investigating safety and/or efficacy of IRE technique, aiming to summarize these studies to provide an update of IRE application and improve the clinical practice of this new technique.
We searched PubMed for all of IRE relevant English language articles published up to September 2016. This review manuscript is nothing with ethics issues and ethical approval is not necessary.
2. IRE and other local ablation techniques
Local ablation techniques have been crucial and useful for treating benign or malignant tumors in the past several decades. Partial liver resection or liver transplantation is still the most preferred method for treating malignant tumors for its potential of curing the cancer. However, the application of liver resection or transplantation is largely affected by several factors including patient's general condition, liver function reserve, and tumor stage and so on. Also, it is constrained by the location of the tumor. In this case, few patients can be eligible candidates for this primary treatment. IRE is a comparatively new minimally invasive nonthermal ablation therapy for hepatic tumor where high frequency electrical energy is applied in short pulses to ablate tissue.[9,10] Electroporation induces increased permeability of cell membrane.[11,12] This can lead to an abnormal transmembrane electrical potential across the plasma membrane which will irreversibly open the plasma membrane leading to cell apoptosis.[13,14] However, while electroporation technology has been utilized for decades,[15–17] IRE is still an emerging field in clinical application.
In addition to IRE, several other alternatives have been developed for ablating liver cancer, such as cryoablation, radiofrequency ablation (RFA), and microwave ablation (MWA). Every technique has been proven effective under certain circumstances and possesses its own advantages and disadvantages. Cryoablation freezes malignant tissue with a low temperature probe, which can be guided accurately by intraoperative ultrasonography and thermally monitored precisely with thermocouple.[18] However, it is difficult to use cryoablation when the lesion is close to large vessels because the relative heat of circulating blood may warm cryoprobe and decrease treatment efficacy.[18] Also, cryoablation can cause variable damage at the margin of ablation zones and injury to adjacent structures.[19] RFA is another popular local ablation technique that thermally destroys the malignant tissue by placing a small electrode into the tumor to deliver directed radiofrequency energy. MWA is a form of thermal ablation used in interventional radiology to treat cancer. It uses electromagnetic waves in the microwave energy spectrum to produce tissue-heating effects. RFA and MWA are both widely used techniques, and in both techniques, the generated heat is difficult to control due to thermal fluctuation from blood circulation which affects the local heat.[18,20,21]
Unlike the other alternative techniques, IRE requires minimal energy input into the system and can induce cell death in a nonthermal way, giving IRE an unique advantage in treatment of liver cancer.[19,22,23] Typically, RFA procedures run in 375 to 500 kHz and MWA functions at 915 MHz or 2.45 GHz, while cryoablation requires cooling tumors to cytotoxic temperatures.[23,24] Compared to other alternatives, IRE only applies several series of electrical pulses (typically 90 pulses) ranging from 1500 to 3000 V. The nonthermal electrical characteristic of IRE prevents collateral damage to other tissues, such as vessels, ducts, and nerves, which often occur in thermal ablation techniques.[25,26] Furthermore, IRE creates a sharp edge between the treated and untreated tissues, thus providing a higher regional specificity in ablation which allows relatively easier monitoring and controlling.[27] In addition, IRE requires shorter time to operate than other traditional ablation techniques because of its nonthermal characteristic, making it a more efficient technique than others. Recently, Tam and Abdelsalam[28] evaluated the intratumoral uptake of nanoparticles used in combination with IRE or RFA in rabbit VX2 models, they found the nanoparticles in the tumor was most intense after RFA at 1 hour time point. However, the uptake of nanoparticles increased at 18-hour time point in IRE group, higher than RFA group, whose uptake decreased during the time range. This study suggested that IRE might have ability to accelerate the cellular internalization of molecules.
3. IRE of hepatic tumors in preclinical settings
In recent years, more and more animal models have been built to evaluate the safety and efficacy of IRE. Different approaches were created to test the effects of different parameter settings on animal models. Summary of recent animal liver models of IRE in preclinical settings is shown in Table 1.[25,29–34]
Table 1.
Summary of recent animal liver models of IRE in preclinical settings.
In 2012, Lee and Tafti[33] corroborated the safety and efficacy of IRE in treating large tumor models in vivo by a rabbit model. They implanted VX2 tumors in 35 New Zealand White Rabbits and divided them into control, single IRE (IRE-S), and multiple IRE (IRE-M) 3 groups. The rabbits in IRE-S group underwent single IRE application, and those in IRE-M group were treated with multiple IRE applications. Before IRE treatment, the intrahepatic VX2 tumors were allowed to grow up to 1 to 1.5 cm. The mean longest diameter of ablation zones in IRE-S group was 3.6 cm and in IRE-M group was 3.9 cm. Contrast-enhanced computed tomography (CT) scans were performed on all the animals before the procedure at 24 hours, 3 days, and 21 days after treatment. Outcomes from both IRE-S and IRE-M group showed a significant decrease in tumor volume compared with control group. Furthermore, complete ablation of tumor was observed in IRE-M group 21 days after procedure, indicating a promising efficacy of IRE. No complication was observed in the experiment either during or after IRE sessions. Lee and Prieto[25] also created another animal liver model in 16 Yorkshire pigs, which demonstrated IRE as a safe and effective ablative method. Fifty-five ablations were performed with real-time monitoring with ultrasound and followed by ultrasound, MRI, and CT. The mean longest diameter of the ablation zones was 3.4 ± .3 cm. Tumor cell death was observed with no complication in any of 16 animals and with full preservation of periablative zone structures including blood vessels, bile ducts, and neighboring tissues. The treatment areas were sharply demarcated, and areas of complete cell death stained positive for apoptotic markers (TUNEL, BCL-2 oncoprotein), suggesting involvement of the apoptotic process in the pathophysiology of cell death caused by IRE. In another swine model built by Charpentier et al,[32] 16 liver ablations and 4 ablations in the liver hilum were performed in 8 healthy pigs using 2 monopolar electrodes spaced 2 cm apart. The longest diameter of ablation zones ranged from 2.95 ± 0.31 cm to 4.45 ± 0.07 cm. Hemorrhagic necrosis of the hepatocytes in the ablation zone were found with no evidence of heat sink and no major complication. Bile ducts, portal veins, and hepatic arteries were well-preserved. According to the ablation results of these studies, IRE may have the potential to be superior to conventional thermal ablation for the treatment of small, unresectable liver tumors.
In addition to swine models, 30 Sprague-Dawley rat models were built by Guo et al[29] to examine the efficacy of IRE. In this model, significant differences in tumor size was found between the treated and untreated groups of HCC rat models with no postoperative complications in the treated group (Fig. 1). MR images showed a significant tumor size reduction within 15 days posttherapy, and histology correlation studies showed a clear progression from poorly differentiated viable hepatoma tissue pretherapy to extensive tumor necrosis and complete tumor regression in 9 of 10 treated rats 7 to 15 days after treatment.
Figure 1.
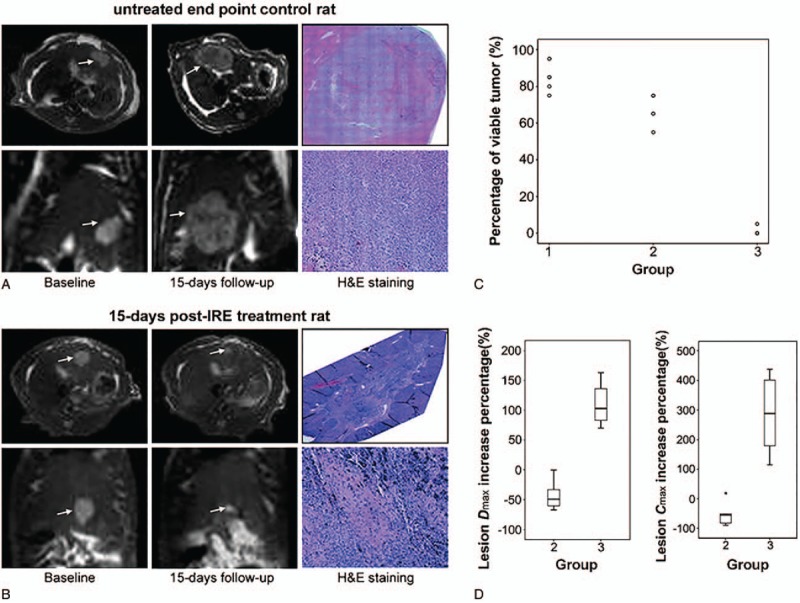
MRI images in axial and coronal orientations along with corresponding pathologic H&E slide images for an untreated 15-d end point control rat (A) and a 15-d post-IRE treatment rat (B).[29] A significant reduction of tumor size in IRE-treated animal (B) is shown compared to the untreated rat (A). Arrows indicate tumor positions. H&E pathology slides showed 70% viable tissue within the untreated tumor (A) and completed tumor regression within the IRE-treated rat (B). Scatter plot (C) shows the percentage of viable tumor tissue for 6 rats at baseline control interval (group 1), 6 untreated control rats following a 15-d growth period after original baseline scan (group 2), and 6 IRE-treated rats following the same 15-d growth period (group 3). Box plots (D) show the Dmax∗ increase (left) and Cmax∗ increase (right) for 15-d follow-up animals in untreated control group 2 and IRE-treated group 3. Dmax and Cmax increase for group 2 rats were significantly greater than Dmax and Cmax increase for group 3 rats (P = .004 for both comparisons using nonparametric Mann–Whitney U test). ∗Dmax, along the orientation bearing the largest tumor diameter; ∗Cmax, the cross-product of the maximum lesion diameter Dmax and largest diameter measured perpendicular to Dmax. IRE = irreversible electroporation, MRI = magnetic resonance imaging.
4. IRE of hepatic tumors in clinical settings
To date, 9 published case series have evaluated the safety and efficacy of IRE on human liver tumors. All of them adopted the NanoKnife system (AngioDynamics, New York), which consists of a footswitch, a control panel with a screen and a cardiac synchronizer, and a direct current generator connected with unipolar or bipolar needle electrodes. Table 2 shows summary of recent IRE studies on liver tumor treatment in clinical settings.[35–42]
Table 2.
Summary of recent IRE studies on liver tumor treatment in clinical settings.

In 2010, Ball et al[43] published a clinical trial report of IRE on evaluating the complications associated with IRE procedure which is the first report of IRE intervention in human subjects. They found IRE to be an encouraging new technique and most importantly, it spared adjacent tissues with no vascular damage. All complications were manageable and largely predicable.
Later, a single-center prospective nonrandomized cohort study was published by Thomson et al in 2011,[44] which included 38 patient volunteers with advanced liver, kidney, or lung malignancy. Clinical examination, biochemistry, and CT scans were obtained before, immediately after, 1 month after, and 3 months after IRE to examine the treated area. A total of 63 IRE ablations of liver tumors were performed in 25 patients, and immediate postprocedure CT scans showed rim enhancement with no enhancement of the ablated areas. Subsequent 1- and 3-month follow-up CT scans showed nonenhancement in both the ablated areas and the rims. Fifteen out of the 18 targeted tumors were successfully ablated in HCC patients. The IRE response rate reached 50% in liver metastases group. However, progressive disease was found in all individuals in this group and all metastases larger than 5 cm showed no response compared to control group. According to this study, Thomson found IRE to be safe for human clinical use when ECG-synchronized delivery was used. The author also suggested that there was no evidence of major vessel or bile duct injury when these structures were included in the IRE procedure zones.
A retrospective review study of patients treated with a total 31 separate IRE procedures over a period of 10 months was reported by Kingham et al.[45] The study included 28 patients with 65 tumors ranging from 0.5 to 5 cm with a median of 1 cm. The majority of patients had colorectal cancer liver metastases (75%), and HCC patients made 7% of the study population. Contrast-enhanced CT scans or MRI was performed in the immediate perioperative period at 1 to 3 months and 6 months. Overall, there were 3 local recurrences and 1 tumor with persistent disease – a combined local failure rate of 7.5%. Within the study, 41 tumors were located within 1 cm of a major hepatic vein or portal pedicle. Complications included 1 case of intraoperative arrhythmia and 1 case of postoperative portal vein thrombosis. The mortality rate was 3% with no treatment-associated mortality. As a result, the authors concluded that IRE technique was a safe treatment option for patients with perivascular malignant hepatic tumors.
Similar results were found by Cannon et al,[46] who published a study representing a multiinstitutional prospective registry of 44 patients undergoing 48 IRE procedures near vital structures in a period of 2 years for liver tumors including 20 colorectal metastases, 14 HCCs, and 10 other metastases. The study was specifically focused on the tumors which were in proximity to vital structures. Patients were followed up at the time of discharge or within 2 weeks of IRE procedure for safety consideration and then at 3-month intervals with CT or MRI evaluations. Initial success was achieved in 100% of the procedures, with an overall local recurrence free survival (LRFS) rate of 97.4%, 94.6%, and 59.5% at 3, 6, and 12 months, respectively. In addition, a trend toward higher recurrence rates was found for tumors larger than 4 cm (hazard ratio: 3.236, 95% CI: 0.585–17.891; P = .178) and a slight difference between the surgical and percutaneous approaches of IRE was observed 3 and 6 months postprocedure. There were 9 adverse events after 5 procedures. Three of these 9 adverse events including neurogenic bladder, abdominal pain, and flank pain may have been procedure related. All complications resolved within 30 days, and there were no treatment-related deaths.
Another retrospective study reported by Hosein and Loaiza-Bonilla[47] analyzed 29 patients (28 evaluable) who underwent 36 IRE sessions on 58 tumors for the management of colorectal liver metastasis between March 2010 and February 2013. All patients were assessed by CT scan immediately after IRE treatment, most had subsequent CT scans after 30 days according to patients’ condition. No biliary strictures, vessel stenosis, thrombosis, or shunting within or near the treatment zones were observed in the CT scan results. Two adverse events related to IRE procedures were found in 2 patients (7%) including 1 case of ventricular arrhythmia and 1 case of atrial fibrillation; both resolved without sequelae. According to the study, LRFS reached 79% at the 2-year time point. However, all patients (n = 19, 100%) showed progressive disease within 2 years.
Cheng et al[48] published a similar retrospective study in the following year focusing on 6 HCC patients who underwent a total of 6 IRE procedures before liver transplant. All tumors showed a complete response on follow-up cross-sectional imaging performed at 1 month and every 3 months until liver transplant. Complete pathologic necrosis was observed in 5 of 6 treated tumors with sharp edges and well-preserved bile duct.
Recently Padia and Yeung[49] published a retrospective study with 20 HCC patients treated with IRE which aimed to assess the postprocedure findings of IRE with MRI. The study reported a trend of decreasing signal intensity in the peripheral ablation zone on both T2-weighted (P = .01) and T1-weighted (P < .08) images acquired on postprocedure day 1, 30, and every 90 days thereafter. Complete response was observed in 18 patients (90%) with the other 2 patients showing a partial response (Fig. 2).
Figure 2.
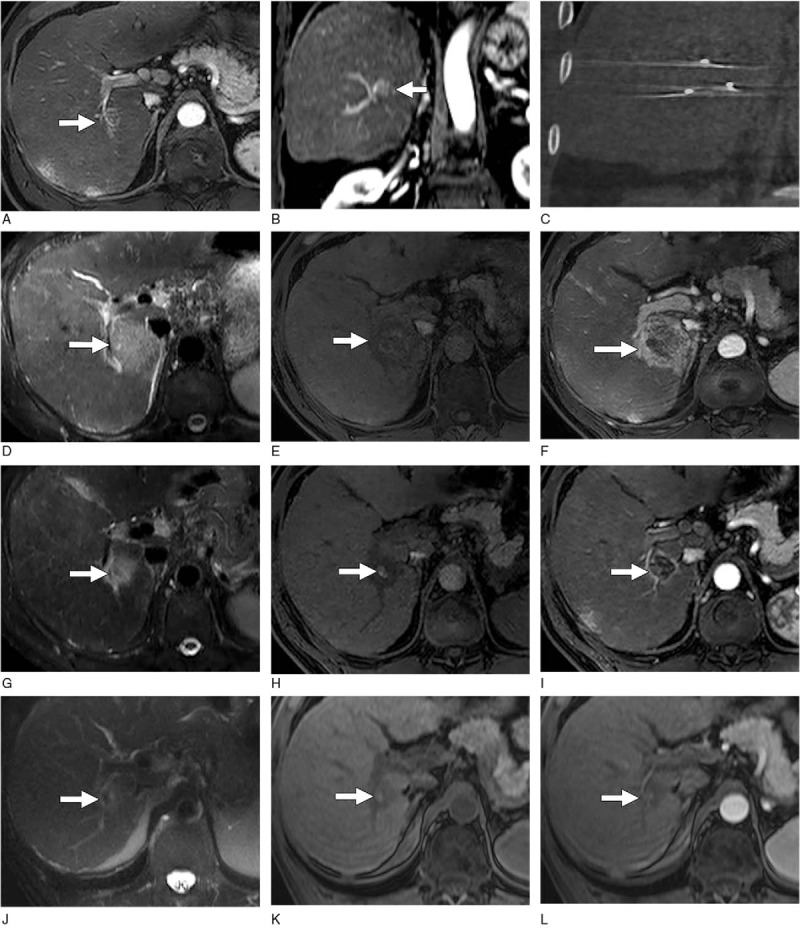
MR images of HCC after IRE in a 63-year-old man.[49] Baseline axial (A) and coronal (B) gadolinium contrast-enhanced T1-weighted MR images obtained in the arterial phase show a 2-cm HCC lesion (arrow) in the right hepatic lobe adjacent to the right portal vein. (C) Unenhanced coronal CT image obtained after percutaneous IRE shows 3 electrodes in a parallel configuration. (D) T2-weighted MR image obtained 24 hours after IRE shows a large ablation zone (arrow) of diffuse increased signal intensity. (E) Unenhanced T1-weighted MR image obtained 24 hours after IRE shows slight hyperintensity of the ablated tumor with associated decreased signal intensity in the ablative margin (arrow). (F) T1-weighted MR image obtained in the arterial phase 1 day after IRE shows marked persistent enhancement of the ablative margin (arrow). The rumor itself demonstrates no enhancement. (G) T2-weighted MR image obtained 30 days after IRE shows a marked reduction of the ablation zone (arrow). (H) Unenhanced T1-weighted MR image obtained 30 days after IRE shows that the increased signal intensity of the tumor (arrow) persists. The size of the tumor decreased compared with day 1 after IRE. (I) T1-weighted MR image obtained in the arterial phase 30 days after IRE shows a substantial decrease in the size of the ablation zone compared with day 1 and thinning of the ablative margin (arrow). Unenhanced (J) T2- and (K) T1-weighted MR images obtained 120 days after IRE show further involution of the ablation zone and persistent slightly increased signal intensity of the tumor (arrow). (L) MR image obtained in the arterial phase shows a lack of enhancement in the entire ablation zone. CT = computed tomography, HCC = hepatocellular carcinoma, IRE = irreversible electroporation, MR = magnetic resonance.
In 2015, Sugimoto and Kobayashi[50] conducted a prospective clinical trial to assess the safety of IRE in Japan including 5 patients with 6 HCCs enrolled. Within the study, 5 of the 6 tumors were successfully treated and no local recurrence at 244 ± 55 days of follow-up. No procedure-related complications were seen during or after IRE intervention but an increase of 78 and 36 mm Hg in systolic and diastolic blood pressure were observed as a minor complication.
In another prospective single-center study, Niessen and Pregler[51] found promising results on the therapeutic efficacy of IRE treatment for hepatic tumors. The study included 65 malignant tumors in 34 patients, of which 33 were HCCs, 22 were colorectal cancer metastasis, 5 were cholangiocellular carcinoma, 3 were neuroendocrine cancer metastasis, and 2 were testicular cancer metastasis. The primary follow-up imaging was performed within 24 hours via both contrast-enhanced CT scans and MR imaging after the IRE procedure. Twelve tumors were treated with a 2nd IRE procedure for incomplete ablation and local recurrence observed by follow-up imaging. LRFS was 87.4%, 79.8%, and 74.8% at 3, 6, and 12 months. However, the complication rate was 27.5% with 6 major complications including 1 diffuse intraperitoneal bleeding, 1 partial thrombosis of the portal vein, and 4 liver abscesses. The high complication rate drew significant concerns to its safety.
5. Discussion
As discussed above, IRE processes its own advantages especially including avoiding the heat sink and sparing vital structures adjacent to tumor. The tumor ablation efficacy is promising, especially for those tumors smaller than 5 cm. Vital structures were all well-preserved in the studies although they were really close to the ablated area.
However, IRE is still not the first preferred choice among the various ablation procedures for HCC patients due to its limitations. In most situations, only patients who have limited volume hepatic tumor and who are not candidates for liver transplantation or other thermal ablation surgeries will be enrolled in IRE treatment. As one of its shortcomings, IRE always requires general anesthesia and adequate neuromuscular blockage for its potential to cause muscle stimulation from high current during the procedure. Another important shortcoming of IRE is the inability to completely ablate large tumors, especially those larger than 5 cm, without repetition or repositioning the electrodes, which limits the number of qualified candidates.[52] In addition, IRE can cause unintended injury to other structures when electrodes are placed. Ball et al[43] reported 3 instances of pneumothorax during their study. One of these cases occurred after transabdominal placement of electrodes in the liver. No treatment was required for the other 2 cases and none of the 3 cases required urgent treatment. Also, the same group found 3 cases of pneumothorax in another clinical trial published in 2011.[44] One of them was caused directly by an electrode but resolved spontaneously with no delay in discharge. Authors suggested that electrodes could be placed during periods of apnea to minimize diaphragmatic movement if the procedure carried the risk of pneumothorax. Cardiac arrhythmia is also a notable serious complication from IRE due to the presence of high current close to the heart. Ventricular bigeminy, ventricular tachycardia, and atrial fibrillation were reported by Ball et al and Thomson et al.[43,44] According to the study, the most serious cardiac rhythm disturbance occurred during the ablation of a very large hepatic tumor which was directly beneath the diaphragm and close to the inferior cardiac border.[43]
In conclusion, although IRE has been introduced in the clinical arena within the past decade, it has successfully claimed its valuable status in the field of hepatic cancer treatment though it possesses its own disadvantages as discussed above. In order to systemically test and establish its safety and efficacy for clinical applications, more studies still need to be conducted.
Footnotes
Abbreviations: CT = computed tomography, HCC = hepatocellular carcinoma, IRE = irreversible electroporation, LRFS = local recurrence free survival, MWA = microwave ablation, RFA = radiofrequency ablation.
TL, XW, and ZS contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Balogh J, Victor D, 3rd, Asham EH, et al. J Hepatocell Carcinoma 2016;3:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Standard Populations (Millions) For Age-Adjustment. Surveillance, Epidemiology and End Results 2013; http://seer.cancer.gov/stdpopulations/. [Google Scholar]
- [3].Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016;122:1757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Altekruse SF, Henley SJ, Cucinelli JE, et al. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014;109:542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Altekruse SF. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Burroughs A. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol 2004;5:409–18. [DOI] [PubMed] [Google Scholar]
- [7].Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- [8].Dusheiko GM. Treatment of small hepatocellular carcinomas. Lancet 1992;340:285. [DOI] [PubMed] [Google Scholar]
- [9].Rubinsky B, et al. Irreversible electroporation: a new ablation modality-clinical implications. Technol Cancer Res Treat 2007;6:37–48. [DOI] [PubMed] [Google Scholar]
- [10].Al-Sakere B, Bernat C, et al. Tumor ablation with irreversible electroporation. PLoS One 2007;2:e1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Weaver JCCY. Theory of electroporation: a review. Bioelectrochem Bioenerg 1996;41:135–60. [Google Scholar]
- [12].Kourounis G, Paul Tabet P, Moris D, et al. Irreversible electroporation (Nanoknife® treatment) in the field of hepatobiliary surgery: Current status and future perspectives. J BUON 2017;22:141–9. [PubMed] [Google Scholar]
- [13].Weiss MJ, Wolfgang CL. Ireversible electroporation: a novel therapy for stage III pancreatic cancer. Adv Surg 2014;48:253–8. [DOI] [PubMed] [Google Scholar]
- [14].Davalos RV, et al. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005;33:223–31. [DOI] [PubMed] [Google Scholar]
- [15].R.S. Reversible electrical breakdown of the excitable membrane of a Ranvier node. Acad Bras Cienc 1958;30:57–63. [Google Scholar]
- [16].Neumann ERK. Permeability changes induced by electric impulses in vesicular membranes. J Membrane Biol 1972;10:279–90. [DOI] [PubMed] [Google Scholar]
- [17].Zimmermann UVJ, et al. Dielectric breakdown of cell membranes. Biophys J 1974;14:881–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kohli VCP-A. Cryoablation of liver tumours. Br J Surg 1998;85:1171–2. [DOI] [PubMed] [Google Scholar]
- [19].Onik GMP, et al. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat 2007;6:295–300. [DOI] [PubMed] [Google Scholar]
- [20].Joosten JRT. Local radiofrequency ablation techniques for liver metastases of colorectal cancer. Crit Rev Oncol Hematol 2007;62:153–63. [DOI] [PubMed] [Google Scholar]
- [21].Yoon JCJ, Kim N, et al. High-frequency microwave ablation method for enhanced cancer treatment with minimized collateral damage. Int J Cancer 2011;129:1970–8. [DOI] [PubMed] [Google Scholar]
- [22].Edd JFHL, et al. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng 2006;53:1409–15. [DOI] [PubMed] [Google Scholar]
- [23].Lencioni RBT, Martin RC. Image-guided ablation of malignant liver tumors: recommendations for clinical validation of novel thermal and non-thermal technologies – a western perspective. Liver Cancer 2015;4:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Knavel EMBC. Tumor ablation: common modalities and general practice. Tech Vasc Interventional Rad 2013;16:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lee EWCC, Prieto VE, et al. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology 2010;255:426–33. [DOI] [PubMed] [Google Scholar]
- [26].Maor ERB. Endovascular nonthermal irreversible electroporation: a finite element analysis. J Biomech Eng 2010;132. [DOI] [PubMed] [Google Scholar]
- [27].R L. Loco-Regional Treatment of Hepatocellular Carcinoma. Hepatology. 2010; 52:762–773. [DOI] [PubMed] [Google Scholar]
- [28].Tam ALMM, Abdelsalam M, et al. Imaging intratumoral nanoparticle uptake after combining nanoembolization with various ablative therapies in hepatic VX2 rabbit tumors. J Biomed Nanotechnol 2016;12:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Guo YZY, Klein R, et al. Irreversible electroporation therapy in the liver: longitudinal efficacy studies in a rat model of hepatocellular carcinoma. Cancer Res 2010;70:1555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang YGY, Ragin AB, et al. MR imaging to assess immediate response to irreversible electroporation for targeted ablation of liver tissues: preclinical feasibility studies in a rodent model. Radiology 2010;256:424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guo YZY, Klein A, et al. Irreversible electroporation in the liver: contrast-enhanced inversion-recovery MR imaging approaches to differentiate reversibly electroporated penumbra from irreversibly electroporated ablation zones. Radiology 2011;258:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Charpentier KPWF, Noble L, et al. Irreversible electroporation of the liver and liver hilum in swine. HPB 2011;13:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee EWWD, Tafti BA, et al. Irreversible electroporation in eradication of rabbit VX2 liver tumor. J Vasc Interv Radiol 2012;23:833–40. [DOI] [PubMed] [Google Scholar]
- [34].Zhang YWS, Nicolai JR, et al. Multimodality imaging to assess immediate response to irreversible electroporation in a rat liver tumor model. Radiology 2014;271:721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kenneth R, Thomson WC, Samantha J, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol 2011;22:611–21. [DOI] [PubMed] [Google Scholar]
- [36].T Peter Kingham AMK, Michael I D’Angelica, Peter J Allen, Ronald P DeMatteo, George I Getrojdman, Constantinos T Sofocleous, Stephen B Solomon, William R Jarnagin, Yuman Fong. Ablation of Perivascular Hepatic Malignant Tumors with Irreversible Electroporation. J Am Coll Surg. 2012; 215(3):379–387. [DOI] [PubMed] [Google Scholar]
- [37].Robert Cannon SE, David Hayes, Govindarajan Narayanan, et al. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol 2013;107:544–9. [DOI] [PubMed] [Google Scholar]
- [38].Peter J, Hosein AE, Arturo Loaiza-Bonilla, et al. Percutaneous irreversible electroporation for the treatment of colorectal cancer liver metastases with a proposal for a new response evaluation system. J Vasc Intervent Radiol 2014;25:1233–9. [DOI] [PubMed] [Google Scholar]
- [39].Rex G, Cheng RB, Matthew MYeh, et al. Irreversible electroporation can effectively ablate hepatocellular carcinoma to complete pathologic necrosis. J Vasc Intervent Radiol 2015;26:1184–8. [DOI] [PubMed] [Google Scholar]
- [40].Katsutoshi Sugimoto FM, Yoshiyuki Kobayashi, Kazuhiro Saito, et al. Irreversible electroporation for nonthermal tumor ablation in patients with hepatocellular carcinoma: initial clinical experience in Japan. Jpn J Radiol 2015;33:424–32. [DOI] [PubMed] [Google Scholar]
- [41].Siddharth A, Padia GEJ, Raymond S. Yeung, et al. Irreversible electroporation in patients with hepatocellular carcinoma: immediate versus delayed findings at MR imaging. Radiology 2016;278:285–94. [DOI] [PubMed] [Google Scholar]
- [42].Christoph Niessen LPB, Benedikt Pregler, Marco Dollinger, et al. Percutaneous ablation of hepatic tumors using irreversible electroporation: a prospective safety and midterm efficacy study in 34 patients. J Vasc Interv Radiol 2016;27:480–6. [DOI] [PubMed] [Google Scholar]
- [43].Ball CTK, et al. Irreversible electroporation: a new challenge in “out of operating theater” anesthesia. Anesth Analg 2010;110:1305–9. [DOI] [PubMed] [Google Scholar]
- [44].Thomson KRCW, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol 2011;22:611–21. [DOI] [PubMed] [Google Scholar]
- [45].Kingham TPKA, D’Angelica MI, et al. Ablation of perivascular hepatic malignant tumors with irreversible electroporation. J Am Coll Surg 2012;215:379–87. [DOI] [PubMed] [Google Scholar]
- [46].Cannon RES, Hayes D, et al. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol 2013;107:544–9. [DOI] [PubMed] [Google Scholar]
- [47].Hosein PJEA, Loaiza-Bonilla A. Percutaneous irreversible electroporation for the treatment of colorectal cancer liver metastases with a proposal for a new response evaluation system. J Vasc Interv Radiol 2014;25:1233–9. [DOI] [PubMed] [Google Scholar]
- [48].Cheng RGBR, et al. Irreversible electroporation can effectively ablate hepatocellular carcinoma to complete pathologic necrosis. J Vasc Interv Radiol 2015;26.:1184–8. [DOI] [PubMed] [Google Scholar]
- [49].Padia SAJG, Yeung RS. Irreversible electroporation in patients with hepatocellular carcinoma: immediate versus delayed findings at MR imaging. Radiology 2016;278:285–94. [DOI] [PubMed] [Google Scholar]
- [50].Sugimoto KMF, Kobayashi Y. Irreversible electroporation for nonthermal tumor ablation in patients with hepatocellular carcinoma: initial clinical experience in Japan. Jpn J Radiol 2015;33:424–32. [DOI] [PubMed] [Google Scholar]
- [51].Niessen CBL, Pregler B. Percutaneous ablation of hepatic tumors using irreversible electroporation: a prospective safety and midterm efficacy study in 34 patients. J Vasc Interv Radiol 2016;27:480–6. [DOI] [PubMed] [Google Scholar]
- [52].Jiang CDR, et al. A review of basic to clinical studies of irreversible electroporation therapy. IEEE Trans Biomed Eng 2014;62:4–20. [DOI] [PubMed] [Google Scholar]
- [53].Ben-David EAL, et al. Characterization of irreversible electroporation ablation in in vivo porcine liver. AJR Am J Roentgenol 2012;198:W62–8. [DOI] [PubMed] [Google Scholar]


