Supplemental Digital Content is available in the text
Keywords: economic impact, focus of bacteremia, hospital costs, multidrug-resistant microorganisms, nosocomial bacteremia
Abstract
To estimate the incremental cost of nosocomial bacteremia according to the causative focus and classified by the antibiotic sensitivity of the microorganism.
Patients admitted to Hospital del Mar in Barcelona from 2005 to 2012 were included. We analyzed the total hospital costs of patients with nosocomial bacteremia caused by microorganisms with a high prevalence and, often, with multidrug-resistance. A control group was defined by selecting patients without bacteremia in the same diagnosis-related group.
Our hospital has a cost accounting system (full-costing) that uses activity-based criteria to estimate per-patient costs. A logistic regression was fitted to estimate the probability of developing bacteremia (propensity score) and was used for propensity-score matching adjustment. This propensity score was included in an econometric model to adjust the incremental cost of patients with bacteremia with differentiation of the causative focus and antibiotic sensitivity.
The mean incremental cost was estimated at €15,526. The lowest incremental cost corresponded to bacteremia caused by multidrug-sensitive urinary infection (€6786) and the highest to primary or unknown sources of bacteremia caused by multidrug-resistant microorganisms (€29,186).
This is one of the first analyses to include all episodes of bacteremia produced during hospital stays in a single study. The study included accurate information about the focus and antibiotic sensitivity of the causative organism and actual hospital costs. It provides information that could be useful to improve, establish, and prioritize prevention strategies for nosocomial infections.
1. Introduction
Nosocomial infections are one of the main adverse effects of healthcare. A study of the costs of adverse events conducted in 12 Spanish hospitals reported that they represent 64.2% of the total cost of adverse events.[1] Bacteremia worsens the prognosis of infections, since it can trigger septic shock, with multiorgan failure, which increases mortality, hospital stay, and costs.
In Spanish hospitals, the prevalence of nosocomial infections in 2014 was 5.6% of admitted patients, and a substantial proportion of these infections (15.3%) were bloodstream infections (NBSI).[2] The cost increase of hospital care due to the presence of bacteremia oscillates between $5875 (€7814) and $86,500 (€115,045)[3] due to the wide variation in patient profiles, the type of infection, the causative organisms, and calculation methods. The few analyses including all patients in a hospital have reported excess costs of €11,916[4] and €12,853.[5] These costs increase when the causative microorganism is resistant to antimicrobials, since the costs of bacteremia episodes caused by multidrug-resistant microorganisms have been estimated to exceed those of episodes caused by multidrug-sensitive microorganisms from $18,588 to $29,069 (€24,722 to €38,662).[6]
The development of an infection and the consequent risk of bacteremia while a patient is receiving hospital care is often a problem of healthcare quality. Associated excess costs are used as a measure of the impact of these infections, although some authors have suggested that traditional ways of calculating these costs overestimate the increase due to the existence of various types of biases. Primarily, an episode of nosocomial bacteremia is partially related to length of exposure and, consequently, longer hospital stay increases the risk of infection. In addition, a hospital-acquired infection generates additional days of stay, turning length of hospital stay into a time-dependent bias. Other biases are related to uncomplete information on other confounding variables (default variable bias) or inclusion of an inadequate number of control cases in the sample (selection bias).[3,4,7–9]
Analytic accounting systems can be considered as the best cost calculation method, since they faithfully reflect variability in clinical practice,[10] even though, due to their complexity, they are implemented in a few hospitals.
Previous studies of the economic impact of adverse events have demonstrated the strengths of using full-costing accounting techniques compared with the secondary estimates employed in many studies.[1,4,7,9] Numerous studies have also shown the reduction of analytical biases using a propensity-score approach.[4,6,7,11] Consequently, this strategy may be useful to identify the cost of bloodstream infections, since it provides information for prioritizing resources to reduce this problem and allows evaluation of strategies for their reduction.
The main aim of this study was to calculate the incremental cost of nosocomial bacteremia according to the causative focus, classified by the antibiotic sensitivity of the causative organism.
2. Methods
This study was performed at Hospital del Mar, Barcelona, a tertiary teaching hospital with 400 beds providing medical and surgical care, with a catchment area of 300,000 inhabitants.
The study population consisted of acute care hospital admissions from 2005 to 2012. All discharges were classified with the all-patient refined-diagnosis-related group (APR-DRG) v. 24.0 grouper.[12] We selected admitted patients who developed nosocomial bacteremia caused by Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, or Pseudomonas aeruginosa. These microorganisms were analyzed because of their high prevalence, and, in Spain, are often multidrug-resistant. The control group included all patients in the same APR-DRG group if at least 10 cases without bacteremia were found in the same DRG.
We excluded admissions grouped into nonspecific APR-DRG. Patients with 2 or more episodes of bacteremia during the same hospital stay were also excluded because these patients have specific risk factors.[13]
The dependent variable was the cost of the hospitalization episode. The main explanatory variable was the presence of nosocomial bacteremia. Additional study variables were age and sex, type of admission (emergency or elective), admission diagnosis, type of treatment (medical or surgical), discharge status (alive or deceased), level of comorbidities as measured by the Elixhauser index,[14] the presence of complications, intensive care unit (ICU) admission, and per-patient cost before the bacteremia episode as a weighted measure of length of hospital stay (in days) prior to bacteremia detection and the cost weight of the adjacent APR-DRG group.
The total costs of hospital admissions with nosocomial bacteremia caused by the selected microorganisms and according to the source of the bacteremia were analyzed by taking into account the antimicrobial susceptibility of each microorganism. This group of patients was compared with the control group.
Definition of bacteremia and the criteria used to define the sources of infection causing bacteremia were based on Center for Disease Control and Prevention definitions.[15] Magiorakos criteria[16] were used to identify multidrug-resistant microorganisms.
Clinical information on the hospitalization process of each patient included in the study was obtained from the hospital information system, which has an established analytical cost accounting system in which cost distribution (full-costing) is realized according to activity-based-costing criteria.[10] Calculation of accurate per-day costs for each hospital admission was based on this cost-accounting system and on the standardized minimum basic dataset (MBDS), which provides information on the demographic characteristics of each patient, length of stay, type of admission, discharge destination, discharging department, and diagnoses and procedures coded using the international classification of diseases, ninth edition, clinical modification. All costs were adjusted to 2012 price levels according to the national price index published by Spain National Institute of Statistics.[17] Admissions with bacteremia were identified using the dataset collected by the infection control department, which prospectively follows up all bacteremia episodes.
A descriptive analysis was conducted of all variables related to admissions with and without bacteremia and to microorganism sensitivity. The chi-square test was used to compare categorical variables and mean values were compared using the analysis of variance test.
A generalized linear model with binomial distribution and logit function was fitted to estimate, for each admission, the probability of developing bacteremia (propensity score) and was used for propensity-score matching adjustment.[4,7,18] The model included the previously described variables, with logarithmic transformations for the APR-DRG cost weight and for prebacteremia cost (see Table 1 and Fig. 1, Supplemental Content which shows the results of propensity-score matching model and bias reduction between variables). Subsequently, the propensity score was included in a generalized linear model with the Gamma distribution and the log link function to adjust the incremental cost of patients who developed bacteremia, as well as differences in this cost, depending on the infection causing the bacteremia and whether it was classified by the antibiotic sensitivity of the causative organism. The econometric model included the same variables as those used to estimate the propensity score.
The incremental cost of developing a bacteremia episode was calculated by comparing the adjusted cost of admissions with and without bacteremia within the same APR-DRG group.
The study was approved by the Ethics Committee for Clinical Research of Hospital del Mar, Parc de Salut Mar. All patient-level data were anonymized, and no additional informed consent was required.
3. Results
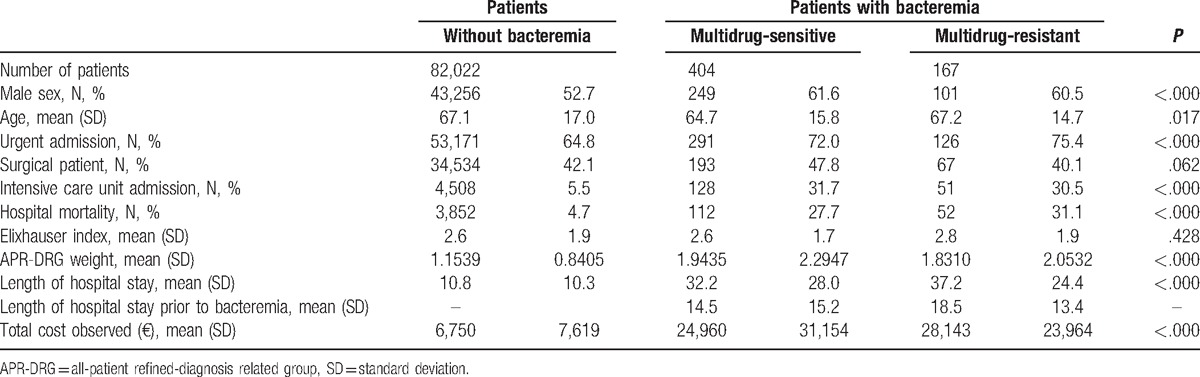
Of the 177,378 discharges included in the study, 1,703 (0.96%) developed an episode of bacteremia. Of these, 669 (39.3%) were caused by the previously defined microorganisms. Finally, 571 admissions with bacteremia met the inclusion criteria and 82,022 admissions treated in the same period and grouped in the same APR-DRG without nosocomial bacteremia were included in the control group. The characteristics of the selected episodes are shown in Table 1. At hospital admission, patients from both groups had similar levels of comorbidities (Elixhauser index) and requirements for surgical intervention, although patients who developed bacteremia were more likely to be admitted to the ICU, had higher mortality, longer length of stay, and greater severity (DRG weight), which clearly suggested a higher profile of hospital costs.
Table 1.
Characteristics of patients with and without bacteremiaand average cost of the episode.
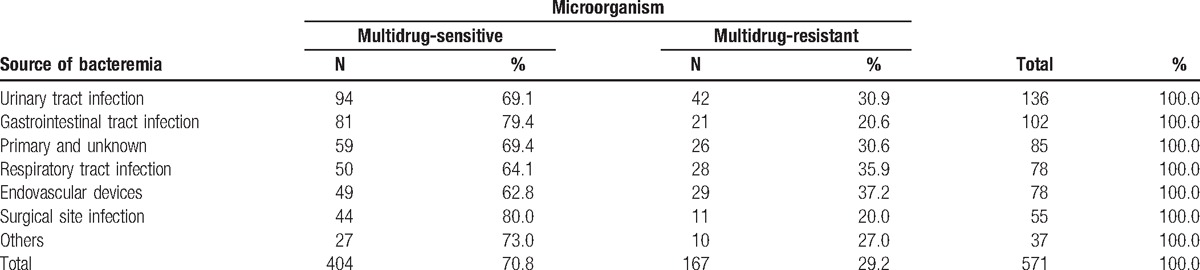
The most frequent focus of bacteremia was urinary tract infection (UTI), with 136 (23.8%) cases, followed by nonsurgical abdominal infection, with 102 (17.9%) cases. The least frequent, 37 episodes (6.5%), was grouped as other infections. Overall, 29.2% of causative microorganisms showed multidrug resistance. Infections related to endovascular devices were associated with the highest percentage (37.2%) of episodes with multidrug resistance, followed by respiratory infections (35.9%). As Table 2 shows, bacteremia caused by a surgical site infection showed the lowest percentage of multidrug resistance (20%) (see Table 2, Supplemental Content for additional descriptive results according to the causative organism and its antibiotic sensitivity).
Table 2.
Distribution by focus of bacteremia and antibiotic sensitivity of the causative organism.
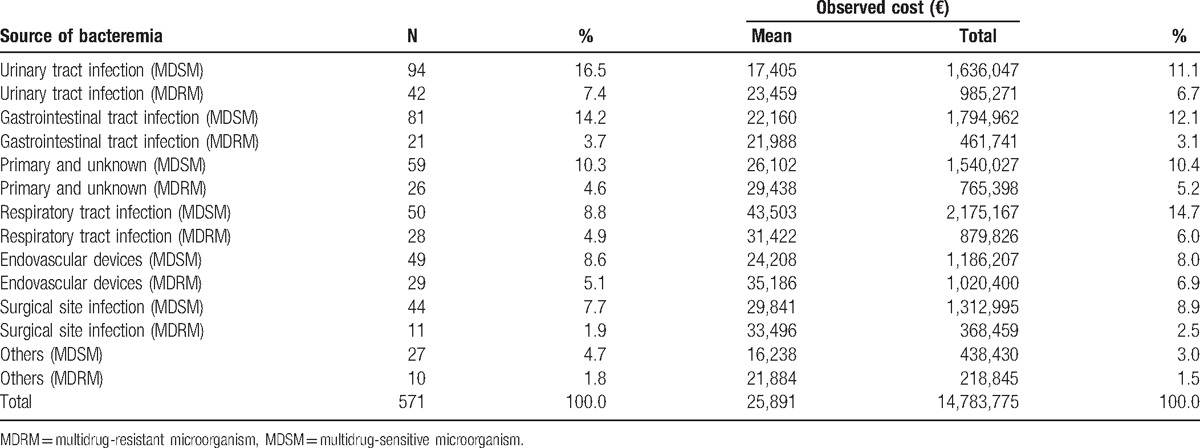
Average observed costs according to the focus of bacteremia and antibiotic sensitivity of the causative organism are shown in Table 3. Substantial differences were found according to the focus of bacteremia: those with a respiratory source with a multidrug-sensitive microorganism were the most costly, with a mean observed cost of €43,503; of the grouped foci, episodes caused by a multidrug-resistant microorganism had the lowest mean cost €16,238. The largest difference in the mean observed cost according to antibiotic sensitivity was detected for bacteremia caused by a respiratory infection: multisensitive microorganisms were €12,081 more costly (see Table 3, Supplemental Content which summarizes distribution of fixed, variable, and drug costs, according to the focus of bacteremia and antibiotic susceptibility).
Table 3.
Description of observed total hospital cost, according to the focus of bacteremia and antibiotic susceptibility of the causative organism.
The mean incremental cost was estimated at €15,526 (Table 4). The mean incremental cost was €6786 for antimicrobial-susceptible and €13,299 for multidrug-resistant UTI bacteremia. The highest incremental costs were found for primary or unknown foci of bacteremia: €26,082 for antimicrobial-susceptible bacteremia, €29,186 for multidrug-resistant bacteremia, and €25,292 when the source of bacteremia was a respiratory infection with a multidrug-resistant microorganism.
Table 4.
Mean incremental cost and global impact according to the focus of bacteremia and antibiotic sensitivity of the causative organism.
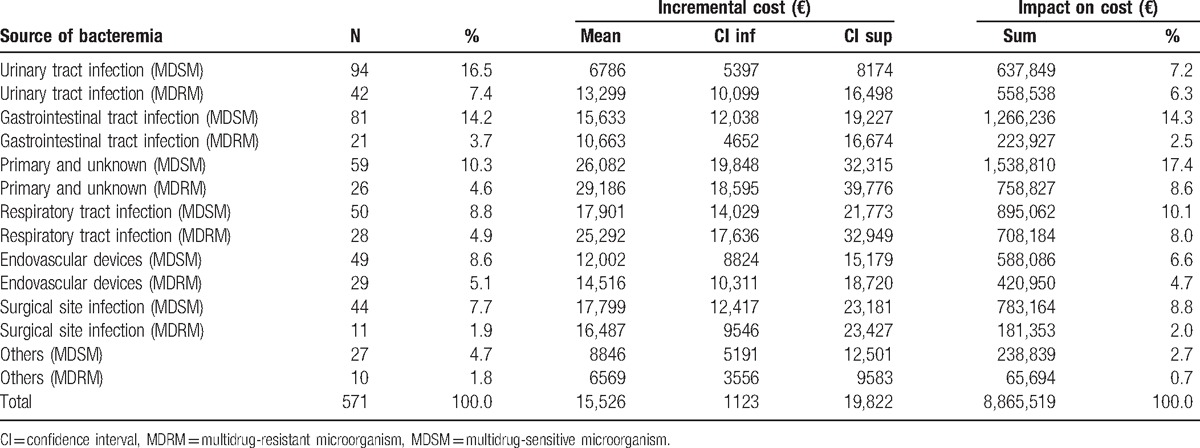
Bacteremia was associated with an annual increase of €1,108,190 over total hospital costs. Primary bacteremia due to a multidrug-resistant microorganism had a substantial impact on total costs of €192,351 per year, followed by bacteremia secondary to an infection of the gastrointestinal system caused by a multidrug-resistant microorganism, with a total of €158,280 per year.
4. Discussion
The present study analyzed the costs of all episodes of nosocomial bacteremia caused by the most common microorganisms in a tertiary university hospital. The aim was to provide information on the incremental cost of nosocomial bacteremia according to the causative focus, classified by the antibiotic sensitivity of the causative organism. Very little information is available in the literature on the foci causing nosocomial bacteremia, and even less information has been published on the costs associated with these episodes, except for costs associated with bacteremia due to vascular access devices frequently analyses that have focused in special care units on a single microorganism or outbreak used in special care units or analyses that have focused on a single microorganism or outbreak.[3,18] Moreover, only 15.4% of episodes of nosocomial bacteremia occur in ICUs, with most of these infections (67%) occurring in conventional units.[19]
The mean incremental cost of patients who develop bacteremia during admission was €15,526 per discharge, representing an annual increment in hospital cost of €1,108,190. The highest incremental cost was observed in bacteremia with a primary or unknown source caused by a multidrug-resistant microorganism, reaching €29,185, and the lowest corresponded to UTI bacteremia caused by a multidrug-sensitive microorganism (€6786) and those grouped in other foci, also due to multidrug-sensitive microorganisms (€6569).
In the nosocomial infections surveillance report of the Institut National de Santé Publique du Québec,[19] the most frequent source was vascular catheters, causing almost 22% of bloodstream infections, followed by those caused by a primary or unknown foci, and thirdly by those related to UTIs. This distribution differs from that found in the present study, in which the most frequent source was the urinary tract.
It is known that UTIs are the most frequent nosocomial infections, although they rarely give rise to an episode of bacteremia; it is estimated that bacteremia occurs in 3.5% of UTIs.[20] Even so, and due to their high frequency, UTI have been estimated to cause 21% of episodes of nosocomial bacteremia.[21] In our study, bacteremia episodes caused by UTI represented 23.8% of the total (n = 136), and we estimated that these patients generated an excess hospital cost of €1,196,387 in the period analyzed.
According to Torres et al,[22] nosocomial pneumonia is the 2nd most frequent nosocomial infection and leads to the highest morbidity, mortality, and costs. Other authors have reported that this entity causes 8.7% of cases of nosocomial bacteremia.[19] In the present study, nosocomial pneumonia caused 13.7% of the episodes of bacteremia analyzed, of which 36% were caused by a multidrug-resistant microorganism. The incremental cost was €17,901 if the causative microorganism was multidrug-sensitive and was €25,292 if multidrug-resistant. It is noteworthy that the average cost observed in those caused by a multidrug-sensitive microorganism was much higher than those caused by a multidrug-resistant microorganism, possibly because these patients have more severe illness and high mortality rates; this effect disappeared in the estimation of incremental cost, thus confirming it.
As previously mentioned, there is more information on the costs associated with vascular access-related bacteremia, and although most of these analyses have been performed in ICUs, the excess cost reported varied from $3170 to $39,219 (€4216 to €52,161),[3] and the most frequent value is described at around $16,000 (€21,280). Most of these estimates are higher than the findings of the present study, in which incremental costs were estimated at €12,002 for vascular access-related bacteremia caused by multidrug-sensitive microorganisms and €14,513 for those caused by multidrug-resistant microorganisms.
Some authors have calculated that 10.9% of nosocomial bacteremia episodes are due to a surgical site infection.[19] The cost increase of SSI has been calculated to be $11,876 (€15,795).[23] A much higher cost than ours has been described in patients developing bacteremia caused by S aureus after undergoing hip or knee replacement surgery, reaching $67,439 (€89,694), although these costs included the cost of outpatient follow-up and readmissions, if they occurred.[24]
This study has some limitations. First, we used information from a single center, which may hamper extrapolation of the results. Second, this study includes only hospital-acquired bacteremia. Other present-on-admission healthcare-associated bacteremia is always the result of a previous contact between the patient and healthcare and the hospital; all these hospitalization episodes have a cost that could have been reduced or eliminated. Finally, costs were calculated from the perspective of the hospital and the care of admitted patients.
This study aimed to assess the incremental cost of developing a bacteremia during hospital stay: for this reason, control cases were chosen from patients in the same APR-DRG group in order to take into account patients with infection but not developing bacteremia. A comparison with control cases without infection would have provided even higher estimates of incremental costs.
A strength of the study is that bacteremia was identified by the infection control team rather than through administrative databases, which have been estimated to identify only 20% of infections.[25] This allowed the identification of the total number of days of stay before the development of bacteremia. Moreover, this study analyzed actual observed costs through an activity-based accounting system, rather than by using estimated cost or tariffs or length of stay as a proxy of hospital costs.
Last, this study provides information on the cost of bacteremia according to the causative source, in a single study and using the same methodology, thus providing the cost relative to each of these foci in relation to the remaining foci and overcoming the limitation of analyzing infections in a single center.
In conclusion, this study shows that determination of the excess cost associated with nosocomial bacteremia, adjusted according to the causative focus and the antibiotic sensitivity of the causative microorganism, can be used to delineate the problem more clearly and thus adapt and prioritize strategies for its prevention according to its incidence.
Acknowledgments
The authors thank the team of infection control and microbiology laboratory, as well as all the hospital professionals, their magnificent daily work has allowed us to have the information necessary to perform this analysis.
Supplementary Material
Footnotes
Abbreviations: APR-DRG = all-patient refined-diagnosis-related group, ICU = intensive care unit, UTI = urinary tract infection.
Funding/support: The revision of the English version of this work has been funded by the RD12/0001/0015 REDISSEC Project (integrated in the National R + D + I and financed by the ISCIII-General Evaluation Branch and by the ERDF-European Regional Development Fund).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Allué N, Chiarello P, Bernal Delgado E, et al. Impacto económico de los eventos adversos en los hospitales españoles a partir del Conjunto Mínimo Básico de Datos. Gac Sanit 2014;28:48–54. [DOI] [PubMed] [Google Scholar]
- [2].EPINE: Evolución 1990-2014, Con Resumen de 2014; 2014. http://hws.vhebron.net/epine/Descargas/EPINE 1990-2014 web.pdf. Accessed November 2, 2015. [Google Scholar]
- [3].Fukuda H, Lee J, Imanaka Y. Variations in analytical methodology for estimating costs of hospital-acquired infections: a systematic review. J Hosp Infect 2011;77:93–105. [DOI] [PubMed] [Google Scholar]
- [4].Riu M, Chiarello P, Terradas R, et al. [Economic impact of nosocomial bacteraemia. A comparison of three calculation methods]. Enferm Infecc Microbiol Clin 2016;34:620–5. [DOI] [PubMed] [Google Scholar]
- [5].Pirson M, Dramaix M, Struelens M, et al. Costs associated with hospital-acquired bacteraemia in a Belgian hospital. J Hosp Infect 2005;59:33–40. [DOI] [PubMed] [Google Scholar]
- [6].Roberts RR, Hota B, Ahmad I, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 2009;49:1175–84. [DOI] [PubMed] [Google Scholar]
- [7].Riu M, Chiarello P, Terradas R, et al. Cost attributable to nosocomial bacteremia. analysis according to microorganism and antimicrobial sensitivity in a University Hospital in Barcelona. PLoS One 2016;11:e0153076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beyersmann J, Kneib T, Schumacher M, et al. Nosocomial infection, length of stay, and time-dependent bias. Infect Control Hosp Epidemiol 2009;30:273–6. [DOI] [PubMed] [Google Scholar]
- [9].Nelson RE, Samore MH, Jones M, et al. Reducing time-dependent bias in estimates of the attributable cost of health care-associated methicillin-resistant Staphylococcus aureus infections: a comparison of three estimation strategies. Med Care 2015;53:827–34. [DOI] [PubMed] [Google Scholar]
- [10].Cots F, Chiarello P, García-alzórriz E, et al. Cost de L’activitat Assistencial. Variable de Resultat per a La Gestió Clínica. Barcelona; 2010. https://www.rechosp.org/mybox/cms/1505. Accessed August 18, 2015. [Google Scholar]
- [11].Neidell MJ, Cohen B, Furuya Y, et al. Costs of healthcare- and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clin Infect Dis 2012;55:807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Averill RF, Goldfield N, Hughes JS, et al. (2003). All Patient Refined Diagnosis Related Groups (APR-DRGs): Methodology Overview. 3M Health Information Systems, 1–81. [Google Scholar]
- [13].Jensen US, Knudsen JD, Wehberg S, et al. Risk factors for recurrence and death after bacteraemia: a population-based study. Clin Microbiol Infect 2011;17:1148–54. [DOI] [PubMed] [Google Scholar]
- [14].AHRQ. Agency for Healthcare Research and Quality. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed August 12, 2015. [DOI] [PubMed] [Google Scholar]
- [15].Horan TC, Andrus M, Dudeck Ma. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–32. [DOI] [PubMed] [Google Scholar]
- [16].Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268–81. [DOI] [PubMed] [Google Scholar]
- [17].Instituto Nacional de Estadística. Índice de precios al consumo. http://www.ine.es/jaxi/menu.do?type=pcaxis&path=/t25/p138&file=inebase&L=0. Accessed October 10, 2014. [Google Scholar]
- [18].Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol 2006;163:262–70. [DOI] [PubMed] [Google Scholar]
- [19].Carignan A, Fortin E, Rocher I, et al. Bactériémies nosocomiales panhospitalières. Résultats de Surveillance 2012–2013. Surveillance Prov des Infect Nosocomiales 2013;14: Available at: http://wwhttps//www.inspq.qc.ca/pdf/publications/1782_ResultatsSurvBACTOT2012-13Vol1No4.pdfw.inspq.qc.ca. Accessed April 4, 2015. [Google Scholar]
- [20].Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control 2000;28:68–75. [DOI] [PubMed] [Google Scholar]
- [21].Fortin E, Rocher I, Frenette C, et al. Healthcare-associated bloodstream infections secondary to a urinary focus: the québec provincial surveillance results. Infect Control Hosp Epidemiol 2012;33:456–62. [DOI] [PubMed] [Google Scholar]
- [22].Torres A, Ferrer M, Badia JR. Treatment guidelines and outcomes of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis 2010;51suppl 1:S48–53. [DOI] [PubMed] [Google Scholar]
- [23].Schweizer ML, Cullen JJ, Perencevich EN, et al. Costs associated with surgical site infections in Veterans Affairs Hospitals. JAMA Surg 2014;52246:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chu VH, Crosslin DR, Friedman JY, et al. Staphylococcus aureus bacteremia in patients with prosthetic devices: costs and outcomes. Am J Med 2005;118:1416. [DOI] [PubMed] [Google Scholar]
- [25].Greene LLR. Healthcare-Associated Infections. The Regulatory Landscape. Phoenix; 2013. http://www.infectioncontroltoday.com/digital-issues/2013/06/healthcare-associated-infections.aspx. Accessed November 18, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


