Abstract
Controversy remains over whether random cervical biopsies and endocervical curettage (ECC) should be used in women with positive screening but negative colposcopy. Our paper aims to determine the indications for random biopsies and ECC among these screened positive women.
Three thousand two hundred thirteen women with any positive screening test result but negative colposcopy, who received random 4-quadrant biopsies, were pooled from 17 population-based cervical cancer screening studies done in China from 1999 to 2008. The detection rates of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and CIN grade 3 or worse (CIN3+) stratified by cytology and high-risk human papillomavirus (HR-HPV) status were assessed, as well as the false negative rates for CIN2+ and CIN3+ by random biopsies without ECC.
Compared with women with negative cytology and positive HR-HPV, those with atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesion (ASC-US/LSIL) and negative HR-HPV had the equivalent lower risks of CIN2+ and CIN3+, but ascending risks were observed in the groups of ASC-US/LSIL and positive HR-HPV, and atypical glandular cells/atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion/high-grade squamous intraepithelial lesion or worse (AGC/ASC-H/HSIL+). If random biopsies were only taken without ECC, 9.3% of CIN2+ and 18.5% of CIN3+ would have been missed.
For women with any positive screening but negative colposcopy, in areas with good cytological infrastructure, it was necessary to perform random biopsies plus ECC on those with cytological ASC-US/LSIL and positive HR-HPV, AGC, ASC-H, or HSIL+. In contrast, those with other results should be followed up.
Keywords: cervical intraepithelial neoplasia, colposcopy, endocervical curettage, random biopsy
1. Introduction
Cervical cancer is the fourth most common cancer in women worldwide, with an estimated 528,000 new cases and 266,000 deaths in 2012.[1] Due to the longer duration of precancerous lesions and several available screening methods, cervical cancer is the kind of malignant tumor fit for screening. Cervical cancer has a standardized screening and diagnosis procedure—that is screening, colposcopy, and biopsy in that order. During this procedure, the colposcopy examination is an indispensable technique. However, a colposcopy examination is subjective. Its accuracy to a great extent relies on the physician's experience, and there are large variations between physicians’ performances.[2] It is also affected by other factors, such as the number,[2] the size and scope of the lesions, cytological result before colposcopy, high-risk human papillomavirus (HR-HPV) testing result, the number of biopsies under colposcopy, the type of transformation zone, and so on.[3–6] Moreover, colposcopy has several limitations, such as the limited perspective for the lesions in the endocervical canal, the difficulty to identify infiltration under the presence of cervical epithelium, and the uncertainty of colposcopy image. Hence, these lead to a quantity of false negatives[7,8] and poor reproducibility of a colposcopy examination.[9,10] Because of the significant values of colposcopy in the management of cervical abnormalities, improving the sensitivity of colposcopy and detection rates for cervical cancer and precancerous lesions is one of key points when promoting screening quality.
Although some studies demonstrated that performing up to 4 random biopsies and/or endocervical curettage (ECC) increased the yield of cervical intraepithelial neoplasia grade 3 or worse (CIN3+) regardless of skill,[2,11] we cannot take multiple biopsies and ECC on every woman screened, so the indication of random biopsies and ECC need to be explored. Moreover, the most amount of controversy surrounds whether women with positive screening result but negative colposcopy (normal-appearing cervix) should take random 4-quadrant biopsies or not, or whether we should combine and use random 4-quadrant biopsies and ECC together or not. Currently, there is little direct research on the diagnostic value of random 4-quadrant biopsies and ECC in these screened positive women. Thus, we pooled the individual data from 17 population-based studies in China to explore the clinical indications related with the detection of CIN grade 2 or worse (CIN2+) or CIN3+ in women with positive screening result but negative colposcopy and to determine the necessity of random 4-quadrant biopsies and ECC in these screened positive women.
2. Materials and methods
2.1. Participants
From 1999 to 2008, Cancer Institute/Hospital, Chinese Academy of Medical Sciences (CICAMS) (Beijing, China), in collaboration with the Cleveland Clinic (Cleveland, OH), Program for Appropriate Technology in Health (PATH) (Seattle, WA), and International Agency for Research on Cancer (IARC) (Lyon, France), screened 30,371 women from 9 provinces (4 urban and 10 rural areas) of China in 17 population-based, cross-sectional, cervical cancer screening studies. The 17 studies were: 7 projects from the Shanxi Province Cervical Cancer Screening Study (SPOCCS 1; SPOCCS 2; SPOCCS 3-Xiangyuan, -Beijing, -Henan, -Xinjiang, and -Shanghai) performed with the Cleveland from 1999 to 2007,[2,12,13] 5 projects from the Screening Technologies to Advance Rapid Testing (START 2003, 2004, 2005, 2006, and 2007) performed with PATH from 2003 to 2007,[14–16] 2 projects performed with IARC in Yangcheng in 2004 (IARC-Yangcheng) and Shenzhen in 2005 (IARC-Shenzhen)[17,18], the fast HPV trial performed in 2007,[19] the Prevalence Survey performed in Jiangsu in 2008[20] and the Hybrid Capture 2 clinical trial (HC2 clinical trial) performed in 2008.[21] Eligible women were 15 to 59 years old, had sexual history, were not pregnant, had an intact uterus, and had no history of CINs, cervical cancer, or pelvic irradiation. Most women included had not been screened in the past 5 years. Written informed consent was obtained from all women. Seventeen population-based studies were approved by the institutional review boards of CICAMS and other cooperative institutions before implementation. This present paper only involved the women with positive screening result but negative colposcopy who had 4-quadrant random biopsies for analysis, some of whom received ECC concurrently.
2.2. Procedures
The main information of every individual study is listed in Table 1 and has been published in great detail.[22] All participants concurrently underwent liquid-based cytology (LBC; Sure-PathTM, BD Diagnostics, Franklin Lakes, NJ or ThinPrep, Hologic, Bedford, MA), HR-HPV DNA testing (HC2, Qiagen, Gaithersburg, MD), and visual inspection with acetic acid (VIA). Cytology results were graded according to the Bethesda system. The cytological classifications were: within normal limits (negative), atypical squamous cells of undetermined significance (ASC-US), atypical glandular cells (AGC), atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion (ASC-H), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), squamous cell carcinoma (SCC); adenocarcinoma in situ (AIS), or adenocarcinoma (ADC). HPV DNA testing was performed using the high-risk probe of the HC2 test. HPV DNA positive was defined according to the manufacturer's recommended positive cut point of 1.0 relative light units per cutoff (approximately equal to 1.0 pg DNA per mL). Positivity for VIA was defined as distinct, dense, non-moveable acetowhite areas in the transformation zone near the squamocolumnar junction, visible 1 minutes after application of 3% to 5% acetic acid. All visual inspection was performed by trained Chinese gynecologists.
Table 1.
Characteristics of pooled studies.
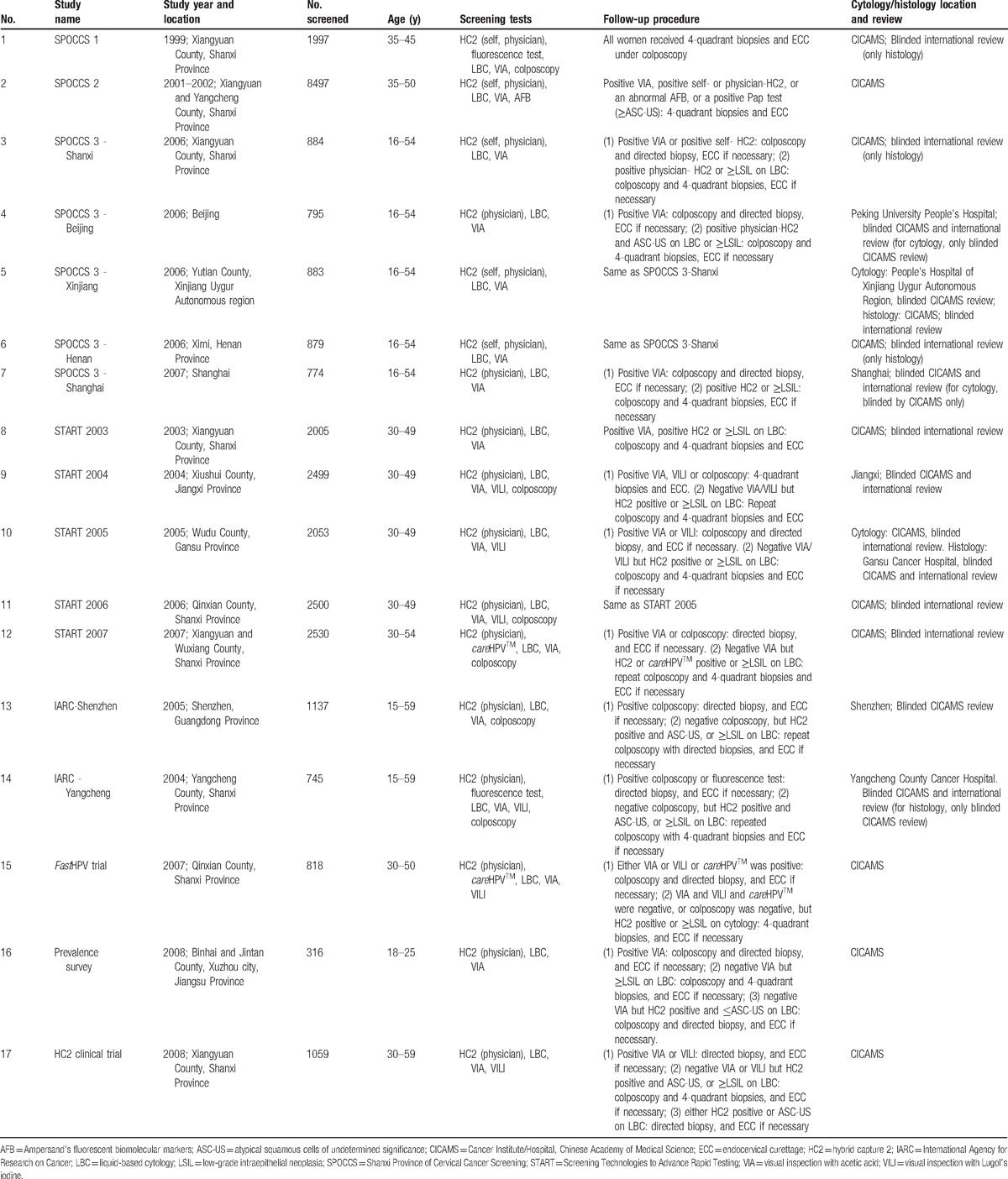
Women positive for any of the 3 screening tests received colposcopy and biopsy if necessary, which were performed by trained Chinese gynecologists. Electronic colposcopy (SLC-2000, Goldway, China) was used. The diagnosis of colposcopy was assessed based on color, turbidity, boundary, and outline of the white vinegar epithelium and vascular characteristics comprehensively. Each quadrant of the cervix was graded separately as negative (no lesion seen), low-grade (HPV or CIN1), high-grade (CIN2 or 3), cancer, or unsatisfied colposcopy. Colposcopy-directed biopsy was used in cases of visible lesions. When study protocol included random 4-quadrant punch biopsies (Table 1), biopsies were taken at positions of 2, 4, 8, and 10 o’clock at the squamocolumnar junction if the colposcopic examination showed no lesion in a quadrant. Cervical biopsy was performed using a 2-mm bronchoscopy biopsy instrument. In SPOCCS 1, SPOCCS 2, and START 2003 and 2004, women with 4-quadrant biopsies concurrently underwent ECC. In the other studies, ECC was performed on women if they had an unsatisfactory colposcopy (squamocolumnar junction could not be completely visualized), if the lesion extended into the endocervical canal in its entirety or was inaccessible to biopsy, or if the lesion was glandular. Histological diagnoses were graded by CIN terminology as normal (no CIN lesions), CIN1, CIN2, CIN3, SCC, AIS, or ADC. The worst histopathological result of biopsies and ECC was taken as the final diagnosis.
In all studies, laboratory personnel performing HC2 were blinded to other test results, and cytopathologists and histopathologists made diagnoses without knowledge of other test results. Colposcopists were blinded to the results of all screening tests but were aware 1 test was positive, except in SPOCCS 1, in which all women received colposcopy regardless of test positivity. In all of the studies, cytology and biopsy results were read or reviewed at CICAMS, although some cytologies or biopsies were read first by local pathologists. Cytology results in 6 studies and biopsy results in 11 studies were reviewed for quality control by international experts (Table 1).
2.3. Statistical methods
Firstly, the detection rates of CIN2+ and CIN3+ were calculated from different cytological groups. The odds ratios (OR) for CIN2+ and CIN3+ detection and their 95% confidence intervals (95% CIs) were also assessed by comparing with cytological negative. Secondly, the detection rates of CIN2+ and CIN3+ in different cytological groups were calculated, stratified by HR-HPV status. In each stratified group, the OR value and 95% CI compared with those with negative cytology and positive HR-HPV was evaluated. Thirdly, we used McNemar χ2 test to compare the detection rates of CIN2+ or CIN3+ by random 4-quadrant biopsies with or without ECC in women undergoing random 4-quadrant biopsies and ECC concurrently, and calculated the false negative rate for CIN2+ or CIN3+ by random biopsies alone without ECC. A P value less than .05 (2-sided) was considered to be statistically significant. SAS 9.2 (SAS Institute, Cary, NC) was used for all analyses.
3. Results
A total of 30,371 women were screened in 17 population-based studies. Of them, 27,158 women were excluded due to negative screening results (21,081, 77.6%), abnormal colposcopy or lack of colposcopy examination (4647, 17.1%), lack of random 4-quadrant biopsies (1379, 5.1%), and unsatisfactory cytology, or missing HR-HPV results (51, 18.8%). Thereby, 3213 women with any positive screening result (VIA, LBC, or HR-HPV) but negative colposcopy were included for final analysis, including 503 with 4-quadrant random biopsies but no ECC and 2710 with both random 4-quadrant biopsies and ECC (Fig. 1). Among these 3213 women, 77.3% (2484/3213) had postive HR-HPV, 53.7% (1726/3213) had abnormal cytology, and 12.9% (416/3213) had abnormal VIA. The average age of the women included was 40.7 ± 5.3 years (range: 20–57 years), and 0.7% (24/3213), 94.9% (3048/3213), and 4.4% (141/3213) were in 20 to 24, 25 to 49, ≥50 age groups, respectively. Most women were married (97.9%, 3144/3212) and had never smoked (97.0%, 3117/3211). 9.4% (293/3125) were menopause and only 1.1% (35/3211, 2 with missing data) had ever used oral contraceptive.
Figure 1.
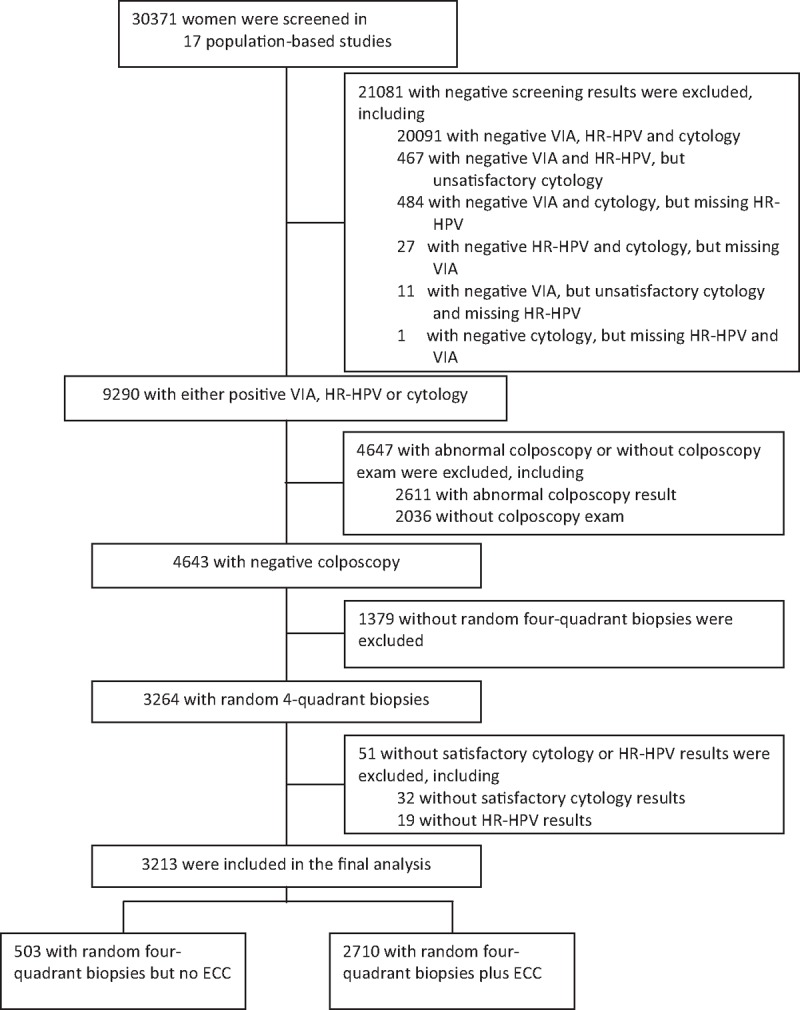
Flowchart of study participants. ECC = endocervical curettage, HR-HPV = high-risk human papillomavirus, VIA = visual inspection with acetic acid.
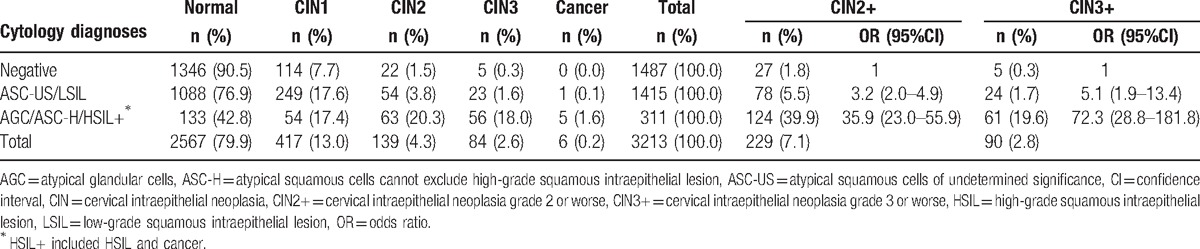
Of the 3213 women included in the analysis, 2567 (79.9%) were diagnosed as histological negative, 417 (13.0%) as CIN1, 139 (4.3%) as CIN2, 84 (2.6%) as CIN3, and 6 (0.2%) as cancer. The total detection rates of CIN2+ and CIN3+ were 7.1% (229/3213) and 2.8% (90/3213), respectively, which were increased with the severity of cytological results. Compared with cytological negative, ASC-US/LSIL, AGC/ASC-H/HSIL+, respectively had 3.2 (95%CI: 2.0–4.9) and 35.9 (95%CI: 23.0–55.9) times higher risks of CIN2+, and had 5.1 (95%CI: 1.9–13.4) and 72.3 (95%CI: 28.8–181.8) times higher risks of CIN3+ (Table 2). 12.9% (416/3213) of women had abnormal VIA, in which the detection rates of CIN2+and CIN3+ had no significant differences from those with negative VIA (CIN2+: 7.0% vs. 7.2%, CIN3+: 3.1% vs. 2.8%, all P > .05).
Table 2.
Concordance between cytology diagnoses and disease outcomes in women with abnormal screening results and negative colposcopy.
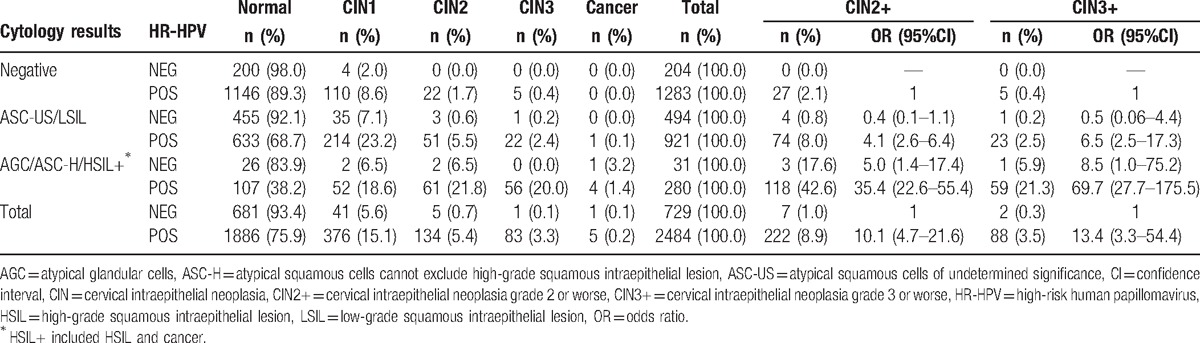
In 2484 women with positive HR-HPV but negative colposcopy, the detection rates of CIN2+ and CIN3+ were 8.9% (222/2484) and 3.5% (88/2484), respectively, which were significantly higher than those in 729 women with negative HR-HPV (CIN2+: 1.0% [7/729]; CIN3+: 0.3% [2/729], all P < .0001) (OR: 10.1 [95% CI: 4.7–21.6] for CIN2+; 13.4 [95% CI: 3.3–54.4] for CIN3+). Compared with women with negative cytology and positive HR-HPV, those with cytological ASC-US/LSIL and negative HR-HPV had the equivalent lower risk of CIN2+ and CIN3+ (OR:0.4 [95%CI: 0.1–1.1] for CIN2+, 0.5 [95%CI: 0.06–4.4] for CIN3+), but the ascending risks of CIN2+ and CIN3+ were observed in the groups of ASC-US/LSIL and positive HR-HPV, AGC/ASC-H/HSIL+ and negative HR-HPV, and AGC/ASC-H/HSIL+ and positive HR-HPV (OR: 4.1 [95%CI: 2.6–6.4], 5.0 [95%CI: 1.4–17.4], and 35.4 [95%CI: 22.6–55.4] for CIN2+, 6.5 [95%CI: 2.5–17.3], 8.5 [95%CI: 1.0–75.2], and 69.7 [95%CI: 27.7–175.5] for CIN3+) (Table 3).
Table 3.
Concordance between cytology diagnoses and disease outcomes stratified by high-risk HPV status in women with abnormal screening results and negative colposcopy.
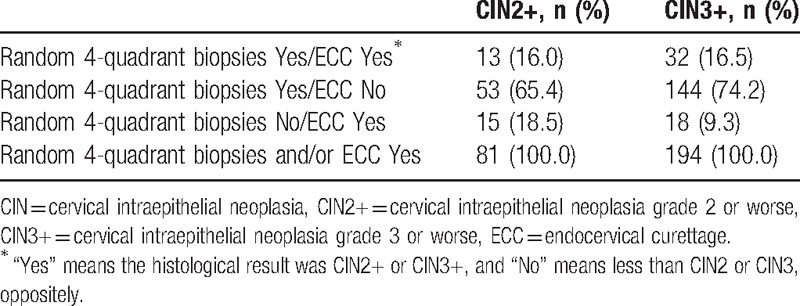
Of 2710 women with negative colposcopies and 4-quadrant biopsies plus ECC, 540 cases were confirmed as CINs or cancer, and 40 (7.4%, 40/540) cases had worse ECC diagnoses than random biopsies. Of the 40 cases, 14 CIN1, 10 CIN2, 14 CIN3, and 2 micro-invasive cervical cancers were diagnosed by ECC. Nearly, all 40 cases were HR-HPV positive, except for 1 which was cytological ASC-US and diagnosed as CIN1 by ECC. The detection rate of CIN2+ or CIN3+ by random 4-quadrant biopsies plus ECC was higher than that by random 4-quadrant biopsies alone (CIN2+: 7.2% vs. 6.5%; CIN3+: 3.0% vs. 2.4%; all P < .0001). If only random 4-quadrant biopsies were taken, the false negative rates for CIN2+ and CIN3+ would have, respectively, been 9.3% (18/194) and 18.5% (15/81) (Tables 4 and 5).
Table 4.
Comparison of histopathological diagnoses between random 4-quadrant biopsies and ECC and only random biopsy in women with 4-quadrant biopsies and ECC concurrently∗.
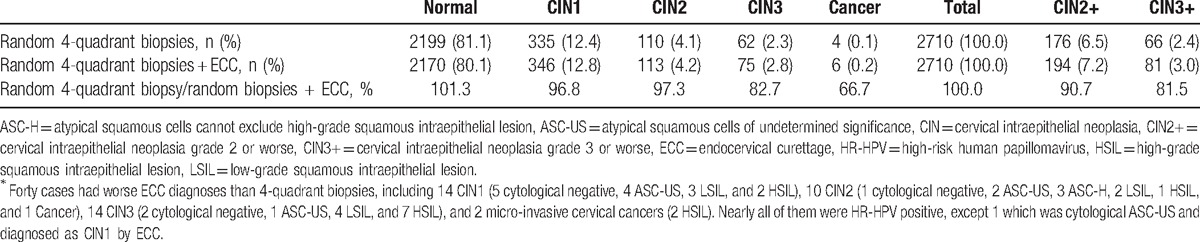
Table 5.
Proportions of random 4-quadrant biopsies and/or ECC showing CIN2+ and CIN3+.
4. Discussion
Our study which included 3213 women with positive screening result but negative colposcopy from 17 population-based studies performed in China, enabled us to evaluate the risks of CIN2+ or CIN3+ in the groups stratified by different screening results, and to further determine the clinical value of random 4-quadrant biopsies plus ECC in this screened positive population. The study demonstrated that the prevalences of CIN2+ and CIN3+ were 7.1% and 2.8%, respectively, detected by random 4-quadrant biopsies and ECC, and the risks of CIN2+ and CIN3+ among those with both ASC-US/LSIL and positive HR-HPV, or AGC/ASC-H/HSIL+, were significantly higher than the ones among those with cytological negative, or ASC-US/LSIL and negative HR-HPV. It was also confirmed that ECC could contribute to increasing the detection rates of CIN2+ and CIN3+. Therefore, performing random 4-quadrant biopsies plus ECC on high-risk populations among women with positive screening result but negative colposcopy could decrease the false negative rate for CIN lesions.
The sensitivity of colposcopy for CIN detection is influenced by many factors. It was reported that the false negative rate of colposcopy was 14%, and 0% to 8.9% of invasive carcinoma was potentially under-diagnosed, with an average of 2%.[23,24] The misdiagnosis rate for CIN2+ in women diagnosed as CIN1 by directed biopsy under colposcopy was 19% to 55%.[25,26] The accuracy of colposcopy impression was highly related with the number of cervical quadrants with lesions, and the accuracy of 1, 2, and 3/4 quadrant involved was, respectively, 13%, 44%, and 85%.[2] The possible reasons for false negative colposcopy were smaller lesion, thinner lesion epithelium, lower nuclear density, and/or no obvious boundary around the thinner epithelium.[27] Our pooled analysis found that 20.1% of CIN were missed by colposcopy, including 4.3% CIN2, 2.6% CIN3, and 0.2% invasive cervical cancer. These findings indicate that even if colposcopy was negative, the woman still had the risk of CIN or invasive cervical cancer, especially the CIN2+ lesions with smaller size and thinner epithelium, which could not be identified even by the experienced colposcopists and were likely to cause false negatives.[6,27,28]
The severity of cytological abnormality and HPV status before colposcopy were the risk factors of false negatives for high-grade lesions by colposcopy.[11,26,29,30] Alvarez RD reported that 84% to 97% of CIN2 were under-diagnosed in CIN1 but cytological HSIL.[29] The multivariate analysis showed that previous cytological HSIL/AGC was an independent risk factor (OR = 4.67).[26] Pretorius RG found that besides colposcopy-directed biopsy, 17.6% CIN2+ could still be detected in cytological HSIL or cancer group by random biopsies, but 1.7% CIN2+ in ASC-US and positive HR-HPV group (P < .001).[11] Our pooled study showed that the risk of CIN2+ or CIN3+ also increased with the severity of cytological abnormality in women with negative colposcopy. Meanwhile, our study also indicated women with negative cytological and positive HR-HPV, or ASC-US/LSIL and negative HR-HPV had a relatively lower absolute risk of CIN2+ or CIN3+. This finding implies that these women had a lower clinical value to perform random 4-quadrant biopsies plus ECC, and were fit for follow-up. However, women with cytological ASC-US/LSIL and positive HR-HPV, AGC, ASC-H or HSIL+ had a higher absolute risk of CIN2+ or CIN3+, and should be given more attention and receive 4-quadrant biopsies plus ECC.[31] These actions are in accord with the attention given to those with abnormal cytology in the recent guideline of American Society of Colposcopy & Cervical Pathology, which recommend that women with cytological negative but positive HR-HPV, or ASC-US/LSIL and negative HR-HPV, should be followed up.[32] Our findings also confirmed the necessity of ASC-US/LSIL triage with HR-HPV.
Several studies showed that random 4-quadrant biopsies plus ECC could greatly increase the detection rate of CIN2+ or CIN3+.[14,33,34] Pretorius RG found that random biopsy can detect 22.9% to 37.4% of CIN2+ lesions, and 2.4% to 5.5% of CIN2+ was detected by ECC alone.[35] He also suggests that in women with negative colposcopy, random biopsy was helpful in improving the detection rate of CIN2+, and ECC should be performed at the same time, even if the colposcopy was satisfied.[11] Other studies also recommended performing random biopsies and ECC in non-pregnant women aged >25 years old.[36,37] Our study showed that random 4-quadrant biopsies plus ECC could significantly raise the detection rate of CIN2+ or CIN3+ compared with random biopsies alone in women with negative colposcopy, and 9.3% of CIN2+ and 18.5% of CIN3+ would have been missed without ECC.
The current study has both strengths and limitations. The study pooled more than 3200 individual data with positive screening result and negative colposcopy from 17 population-based studies, which was conducted in 9 provinces and 14 field sites and had a larger sample size compared with other reported studies. Moreover, our study had the strict quality control for biopsy and cytology reading. The sensitivity of cytology in general women in our study (80.5% for CIN2+, test positivity at LSIL or worse[19]) was higher than the average level reported in a European and North American pooled study[38] (53.0% for CIN2+, test positivity at ASC-US or worse). This ensures the good internal validity of our study. On the other hand, the high accuracy of cytology limits the generalization of our research conclusion to the regions with poor cytological infrastructure; this is the limitation of our study. Therefore, the results of cytology and HPV testing are valuable to determine whether the random 4-quadrant biopsies and ECC are necessary among women with positive screening result but negative colposcopy in settings with good cytological infrastructure, otherwise a HR-HPV testing result, which is objective, should mainly be considered in settings without good cytological diagnosis level.
5. Conclusion
In summary, for women with any positive screening result but negative colposcopy, the risk of CIN2+ or CIN3+ was highly correlated with cytology and HR-HPV results. In the areas with good cytological infrastructure, it was necessary to perform random 4-quadrant biopsies plus ECC on women with cytological ASC-US/LSIL and positive HR-HPV, AGC, ASC-H, or HSIL+. In contrast, immediate random biopsies and ECC could not be performed on women with cytological negative and positive HR-HPV, or ASC-US/LSIL and negative HR-HPV, who should be followed up. For women in areas with poor cytological infrastructure, HR-HPV testing result and other potential biomarkers, for example, HPV genotyping, P16/Ki67 and E6/E7 oncoprotein could be considered to decide whether immediate random biopsies plus ECC or not. This strategy probably helps maximize screening benefits and minimize potential harms. Further studies on cost-effective analysis and prospective trials are required to test the role of random biopsies and ECC.[39]
Acknowledgments
Thanks for the generous support from the Cleveland Clinic, Program for Appropriate Technology in Health, the International Agency for Research on Cancer, and the Bill & Melinda Gates Foundation to Chinese cervical cancer prevention research for the 17 original studies. The authors also thank the women who participated in our studies from Beijing, Gansu, Guangdong, Jiangsu, Jiangxi, Henan, Shanghai, Shanxi, and Xinjiang, as well as the local health workers and the staffs in the research team for cervical cancer prevention in CICAMS. They also thank Dr. Jerome L. Belinson for his contributions in SPOCCS projects and the valuable advices on this paper. And, also Ayling Dominguez from University of Chicago for language editing.
Footnotes
Abbreviations: ADC = adenocarcinoma, AGC = atypical glandular cells, AIS = adenocarcinoma in situ, ASC-H = atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion, ASC-US = atypical squamous cells of undetermined significance, CICAMS = Cancer Institute/Hospital, Chinese Academy of Medical Sciences, CIN2+ = cervical intraepithelial neoplasia grade 2 or worse, CIN3+ = CIN grade 3 or worse, CIs = confidence intervals, ECC = endocervical curettage, HC2 = hybrid capture 2, HR-HPV = high-risk human papillomavirus, HSIL = high-grade squamous intraepithelial lesion, IARC = International Agency for Research on Cancer, LBC = liquid-based cytology, LSIL = low-grade squamous intraepithelial lesion, OR = odds ratios, PATH = Program for Appropriate Technology in Health, SCC = squamous cell carcinoma, SPOCCS = Shanxi Province Cervical Cancer Screening Study, START = Screening Technologies to Advance Rapid Testing, VIA = visual inspection with acetic acid.
S-YH, W-HZ have contributed equally to the article.
Funding Information: This pooled data analysis work was supported by the National Natural Science Foundation of China (Grant No. 81402748) and Chinese Academy of Medical Science Initiative for Innovative Medicine (2016-I2M-1-019).
The authors report no conflicts of interest.
References
- [1].Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer. 2013;Available at: http://globocan.iarc.fr. Accessed on February 5, 2015. [Google Scholar]
- [2].Pretorius RG, Belinson JL, Burchette RJ, et al. Regardless of skill, performing more biopsies increases the sensitivity of colposcopy. J Low Genit Tract Dis 2011;15:180–8. [DOI] [PubMed] [Google Scholar]
- [3].Belinson JL, Qiao YL, Pretorius RG, et al. Shanxi Province cervical cancer screening study II: self-sampling for high-risk human papillomavirus compared to direct sampling for human papillomavirus and liquid based cervical cytology. Int J Gynecol Cancer 2003;13:819–26. [DOI] [PubMed] [Google Scholar]
- [4].Pretorius RG, Belinson JL, Zhang WH, et al. The colposcopic impression. Is it influenced by the colposcopist's knowledge of the findings on the referral Papanicolaou smear? J Reprod Med 2001;46:724–8. [PubMed] [Google Scholar]
- [5].Qiao YL, Zhang WH, Li L. A cross-sectional comparative study of cervical cancer screening methods in Shanxi Province [in Chinese]. Acta Acad Med Sin 2002;24:50–3. [Google Scholar]
- [6].Kong BH, Zheng WX. Several problems on the diagnosis and treatment of cervical cancer and precancerous lesions in China [in Chinese]. Natl Med J China 2010;90:3027–30. [PubMed] [Google Scholar]
- [7].Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol 2006;108:264–72. [DOI] [PubMed] [Google Scholar]
- [8].Stoler MH, Vichnin MD, Ferenczy A, et al. The accuracy of colposcopic biopsy: Analyses from the placebo arm of the Gardasil clinical trials. Int J Cancer 2011;128:1354–62. [DOI] [PubMed] [Google Scholar]
- [9].Jeronimo J, Massad LS, Castle PE, et al. Interobserver agreement in the evaluation of digitized cervical images. Obstet Gynecol 2007;110:833–40. [DOI] [PubMed] [Google Scholar]
- [10].Massad LS, Jeronimo J, Schiffman M. Interobserver agreement in the assessment of components of colposcopic grading. Obstet Gynecol 2008;111:1279–84. [DOI] [PubMed] [Google Scholar]
- [11].Pretorius RG, Zhang WH, Belinson JL, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol 2004;191:430–4. [DOI] [PubMed] [Google Scholar]
- [12].Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001;83:439–44. [DOI] [PubMed] [Google Scholar]
- [13].Belinson JL, Hu S, Niyazi M, et al. Prevalence of typespecific human papillomavirus in endocervical, upper and lower vaginal, perineal and vaginal self-collected specimens: implications for vaginal self-collection. Int J Cancer 2010;127:1151–7. [DOI] [PubMed] [Google Scholar]
- [14].Cagle AJ, Hu SY, Sellors JW, et al. Use of an expanded gold standard to estimate the accuracy of colposcopy and visual inspection with acetic acid. Int J Cancer 2010;126:156–61. [DOI] [PubMed] [Google Scholar]
- [15].Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol 2008;9:929–36. [DOI] [PubMed] [Google Scholar]
- [16].Moy L, Zhao F, Li LY, et al. Human papillomavirus testing and cervical cytology in primary screening for cervical cancer among women in rural China: comparison of sensitivity, specifi city, and frequency of referral. Int J Cancer 2010;127:646–56. [DOI] [PubMed] [Google Scholar]
- [17].Dai M, Bao YP, Li N, et al. Human papillomavirus infection in Shanxi Province, People's Republic of China: a population-based study. Br J Cancer 2006;95:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li N, Shi JF, Franceschi S, et al. Different cervical cancer screening approaches in a Chinese multicentre study. Br J Cancer 2009;100:532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lin CQ, Chen F, Liu B, et al. A parallel study of careHPV and Hybrid Capture 2 human papillomavirus DNA testing for cervical cancer screening in rural China. J Virol Methods 2014;202:73–8. [DOI] [PubMed] [Google Scholar]
- [20].Hu SY, Hong Y, Zhao FH, et al. Prevalence of HPV infection and cervical intraepithelial neoplasia and attitudes towards HPV vaccination among Chinese women aged 18-25 in Jiangsu province. Chin J Cancer Res 2011;23:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu CY, Bian ML, Geng L. Clinical trial on evaluation of Hybrid Capture II for human papillomavirus in screening for cervical cancer and cervical intraepithelial neoplasia [in Chinese]. China Cancer 2009;18:1008–11. [Google Scholar]
- [22].Zhao FH, Lin MJ, Chen F, et al. Performance of high-risk human papillomavirus DNA testing as a primary screen for cervical cancer: a pooled analysis of individual patient data from 17 population-based studies from China. Lancet Oncol 2010;11:1160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang WH. The Principal Points of Diagnosis and Treatment for Cervical Lesions. 1st ed.Beijing: People's Medical Publishing House; 2006. [Google Scholar]
- [24].Li N, Zhang WH. Diagnosis and treatment of cervical intraepithelial neoplasia [in Chinese]. Pract J Cancer 2001;16:140–3. [Google Scholar]
- [25].Qian M, You ZX, Yao FF, et al. Analysis of missed CIN2+ in CIN1+ diagnosed by colposcopically directed biopsy [in Chinese]. Chin J Clin Obstet Gynecol 2013;14:403–5. [Google Scholar]
- [26].Cheng YF, Wang XY, Lü WG, et al. Cervical intraepithelial neoplasia 2+ in low-grade squamous intraepithelial lesion pathologically diagnosed by colposcopy-assisted biopsy [in Chinese]. Natl Med J China 2010;90:1882–5. [PubMed] [Google Scholar]
- [27].Yang B, Pretorius RG, Belinson JL, et al. False negative colposcopy is associated with thinner cervical intraepithelial neoplasia 2 and 3. Gynecol Oncol 2008;110:32–6. [DOI] [PubMed] [Google Scholar]
- [28].Chen XD, Shi HY. Morphological observation of cone biopsy and hysterectomy specimens of high-grade cervical intraepithelial neoplasia [in Chinese]. Zhonghua Fu Chan Ke Za Zhi 2008;43:429–32. [PubMed] [Google Scholar]
- [29].Alvarez RD, Wright TC. Optical Detection Group. Effective cervical neoplasia detection with a noveloptical detection system: a randomized trial. Gynecol Oncol 2007;104:281–9. [DOI] [PubMed] [Google Scholar]
- [30].Pan QJ, Hu SY, Guo HQ, et al. Liquid-based cytology and human papillomavirus testing: a pooled analysis using the data from 13 population-based cervical cancer screening studies from China. Gynecol Oncol 2014;133:172–9. [DOI] [PubMed] [Google Scholar]
- [31].Song Y, Zhao YQ, Zhang X, et al. Random biopsy in colposcopy-negative quadrant is not effective in women with positive colposcopy in practice. Cancer Epidemiol 2015;39:237–41. [DOI] [PubMed] [Google Scholar]
- [32].Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013;121:829–46. [DOI] [PubMed] [Google Scholar]
- [33].Nam K, Chung S, Kwak J, et al. Random biopsy after colposcopy-directed biopsy improves the diagnosis of cervical intraepithelial neoplasiagrade 2 or worse. J Low Genit Tract Dis 2010;14:346–51. [DOI] [PubMed] [Google Scholar]
- [34].Huh WK, Sideri M, Stoler M, et al. Relevance of random biopsy at the transformation zone when colposcopy is negative. Obstet Gynecol 2014;124:670–8. [DOI] [PubMed] [Google Scholar]
- [35].Zhang WH. Colposcopic Diagnostic Atlas. Beijing: People's Medical Publishing House; 2012. [Google Scholar]
- [36].Pretorius RG, Belinson JL, Azizi F, et al. Utility of random cervical biopsy and endocervical curettage in a low-risk population. J Low Genit Tract Dis 2012;16:333–8. [DOI] [PubMed] [Google Scholar]
- [37].Pretorius RG, Belinson JL, Peterson P, et al. Which colposcopies should include endocervical curettage? J Lower Genit Tract Dis 2015;19:278–81. [DOI] [PubMed] [Google Scholar]
- [38].Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primay cervical cancer screening. Int J Cancer 2006;119:1095–101. [DOI] [PubMed] [Google Scholar]
- [39].Massad LS. Selecting patients for endocervical curettage. J Low Genit Tract Dis 2015;19:271–2. [DOI] [PubMed] [Google Scholar]


